?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The purpose of this study was to investigate the effects of the European barbel Barbus barbus (L., 1758) invasion in the Tiber River basin (Italy) on the native Tiber barbel Barbus tyberinus Bonaparte, 1839, verifying whether the co-occurrence played a negative impact on growth rate and relative weight. Fish census data were collected during three periods (2000–2005, 2006–2010, 2011–2015) at 158 sampling sites. Since its first record in 1998, European barbel rapidly spread in the study area: it was present in more than 20% of the monitoring sites, where it is leading to the gradual replacement of Tiber barbel by widening its distribution in the Tiber River and in the downstream reaches of the main tributaries. By contrast, Tiber barbel has suffered from this competition, as demonstrated by the fact that the mean value of the relative weight was significantly higher where European barbel was absent. The results obtained suggested that this non-native species could be a serious threat to the conservation status of endemic Tiber barbel, and constitute the premise to underpin conservation strategies aiming to preserve native freshwater biodiversity.
Introduction
The water courses in the Mediterranean Basin are particularly rich in endemic species (Myers et al. Citation2000; Ribeiro & Leunda Citation2012). The introduction of exotic fish species (“non-native” or “introduced”, see Gozlan et al. Citation2010) in the aquatic ecosystems of this area can influence the functional composition of the fish communities (Marr et al. Citation2013) and can lead to the local extinction of the native species or to the decline of their abundances (Crivelli Citation1995; Vila-Gispert et al. Citation2002). These negative effects can be more evident on native species belonging to the same genus as those introduced, due to the occurrence of introgressive hybridisation (Vitule et al. Citation2009).
Knowledge of the spatial distributions and dispersal patterns of non-native species is essential to undertake proper management strategies to preserve native freshwater biodiversity (Gago et al. Citation2016). Some studies on the ecological impacts of non-native species, carried out in the Iberian peninsula (Elvira & Almodovar Citation2001; Clavero et al. Citation2004) and in Turkey (Innal & Erk’akan Citation2006), highlighted the importance of the Mediterranean area in terms of biodiversity conservation of freshwater fish fauna, and showed that the introduction of exotic fishes in rivers can represent a serious threat to the endemic species.
The Tyrrhenian side of the central Apennines is of crucial importance for the conservation of fish biodiversity in Italy, due to the presence of many endemic species that have very limited and fragmented ranges of distribution (Lorenzoni et al. Citation2014). In the middle stream reaches of this area the fish assemblages are characterised by the presence of the following endemic species typical of the “barbel zone” (Huet Citation1949): the Tiber barbel Barbus tyberinus Bonaparte, 1839 (Bianco Citation1993, Citation1995; Ketmaier et al. Citation2009), the Arno goby Padogobius nigricans (Canestrini, 1867), the Apennine roach Sarmarutilus rubilio (Bonaparte, 1837), the Italian riffle dace Telestes muticellus (Bonaparte, 1837) and the Etruscan chub Squalius lucumonis (Bianco, 1983). Some of them are of particular conservational interest: S. lucumonis is listed as a “species in critical risk of extinction” in the International Union for Conservation of Nature (IUCN) Red List of Italian Vertebrates (Rondinini et al. Citation2013) and “endangered” in the IUCN Red List of Threatened Species (Crivelli Citation2006), whereas B. tyberinus has been classified as “near threatened” in the IUCN Red List of Threatened Species (Freyhof Citation2013) and “vulnerable” in the IUCN Red List of Italian Vertebrates (Rondinini et al. Citation2013). The chub Squalius squalus (Bonaparte, 1837) also belongs to the group of native species of the barbel zone, and represents one of the most common and abundant freshwater species in Italy; due to its great plasticity and tolerance to adverse ecological conditions (Lorenzoni et al. Citation2011; Pompei et al. Citation2011), the species is not considered endangered and is listed as of “least concern” in the IUCN Red List of Italian Vertebrates (Rondinini et al. Citation2013). As in previous studies (Lorenzoni et al. Citation2006b; Carosi et al. Citation2015, Citation2016), the Padanian barbel Barbus plebejus Bonaparte, 1839 was considered also indigenous to the Tiber River basin, even if its original range is still uncertain (Bianco Citation2003). Currently, the natural composition of these fish communities is profoundly altered by the introduction of many alien species, including the European barbel, Barbus barbus (L., 1758) (Lorenzoni et al. Citation2006a; Carosi et al. Citation2015). The European barbel is native to Central and Eastern Europe and was introduced to Italy in 1994 (Bianco & Ketmaier Citation2001; Lorenzoni et al. Citation2006a; Meraner et al. Citation2013). Since its introduction, the European barbel has rapidly invaded many Italian water courses (Zerunian Citation2002), where it has quickly become established and caused negative impacts to native fish species, especially those belonging to the same genus. Recent studies have shown that the introduction of the European barbel to North Adriatic basins (Northern Italy) has caused introgressive hybridisation and the decline of endemic native populations of Barbus plebejus Bonaparte, 1839 (Meraner et al. Citation2013). The invasiveness of the species can be attributed to some of its biological and ecological characteristics, such as its high tolerance to environmental degradation and its more rapid growth in weight and length compared to native Barbus species (Lorenzoni et al. Citation2006a). Moreover, this species is characterised by its ability to move great distances through connections in the hydrographic system (Britton & Pegg Citation2011). In the Tiber River basin, the species was recorded for the first time in 1998, in the Paglia River (Mearelli et al. Citation2000). Given the great importance of B. barbus for sport fishing, its introduction was most likely due to intentionally unauthorised stocking activities carried out by anglers. Over the last two decades, it has become acclimatised and is rapidly expanding in the main course and in all of the major tributaries of the Tiber River, where it could pose a serious threat to the native Tiber barbel.
As for the Tiber barbel, the presence of the European barbel also characterises the barbel zone of rivers (Przybylski et al. Citation2004; Britton et al. Citation2013). It is, therefore, interesting in this context to verify the presence of differences in the ecological characteristics of these cyprinid species. The negative impacts of the European barbel on the Tiber barbel can occur due to competition for food and habitat, as well as introgressive hybridisation between the two species (Carosi et al. Citation2006; Kottelat & Freyhof Citation2007; Meraner et al. Citation2013; Geiger et al. Citation2016), with a consequent loss of genetic integrity in native populations.
The main purpose of this study was to investigate the effects of the European barbel invasion on the native barbel populations, verifying whether the ecological overlap (co-occurrence) may have a negative impact on growth rate and relative weight. Body condition indices, based on individual length–weight relationship, may be used as a way to assess the health and the fitness of a fish population, assuming that plump fish of a given length are in better condition than thinner ones (Blackwell et al. Citation2000). Previous studies carried out in the Tiber River basin (Carosi et al. Citation2006, Citation2016, Citation2017; Giannetto et al. Citation2012) and in Western Anatolia (Gaygusuz et al. Citation2013) have shown the effectiveness of body condition indices, as well as relative weight and condition factor, as tools to assess interactions between non-native and native species. This analysis did not take into account the Padanian barbel, because the presence of the species was limited to a few sites with very low population densities.
Moreover, it is known that the distribution and the life-history strategies of freshwater fishes are affected by the environmental characteristics (Vadas & Orth Citation2000; Smith & Kraft Citation2005), also in relation to the introduction of exotic species. In particular, physical-chemical and hydromorphologic characterisations are required to analyse the habitat descriptors that may explain the relation between invasive and native barbel species. Therefore, another aim of the present study was to assess the effect of environmental features and the role of abiotic factors on the distribution and abundance of these two species.
Study area
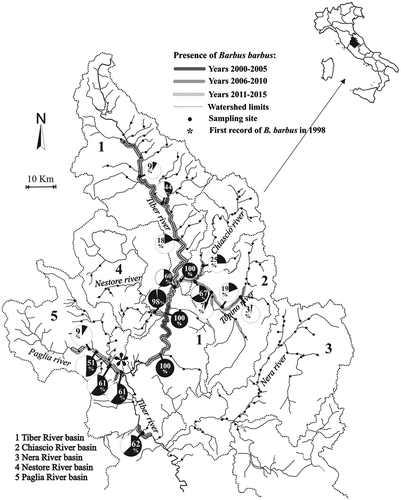
The Tiber is the second largest basin (total watershed = 12,692 km2) and the third longest river in Italy (405 km) (Lorenzoni et al. Citation2006b). The study area comprises the section of the Tiber River basin limited to the Umbria Region and encompasses 8412 km2 (). It was divided into five sub-watersheds: the Chiascio (watershed = 1974 km2), the Nera (750 km2), the Nestore (1033 km2), the Paglia (641 km2) and the Tiber River’s residual watershed (4014 km2). Environmental and biological data were collected from 158 sampling sites located within 84 water courses in total, during the periods 2000–2005, 2006–2010 and 2011–2015. All the sites were sampled once, in autumn, in each census period.
The sub-basins under study differ in regard to their geomorphological characteristics and water quality (Lorenzoni et al. Citation2006b). The main Tiber River basin is characterised by impermeable soil with a mountainous upper section, while the middle and downstream reaches flow in lowland areas. The Nestore and Paglia river basins are also characterised by impermeable soil and their water courses flow in hilly areas. All of these streams have torrential characteristics, with marked flow rate oscillations and a high susceptibility to periods of drought in summer, which is aggravated by the use of water abstraction for irrigation. The Nera River basin is mountainous and permeable; its hydrographic network is made up of a few watercourses of modest size. In this last case, the water supply is stable all year round as a result of the presence of many underground springs. The geomorphological features of the Chiascio River are intermediate.
Materials and methods
Fish sampling
Fish samplings were carried out using a continued or pulsed current electrofishing device with power ranging from 1.500 to 4.500 W, and applying the removal method (Moran Citation1951; Zippin Citation1956). Specimens were anaesthetised, identified and counted. For the barbel species, only specimens recognised as phenotypic Tiber barbel, European barbel and Padanian barbel were used in the present study, according to the morphologic characterisation carried out in a previous study (Lorenzoni et al. Citation2006a). For specimens smaller than 10 cm in total length (TL), the identification of the species was based on the margin shape of the dorsal fin (concave or convex, Kottelat & Freyhof Citation2007) and on the spinous last simple dorsal fin ray. For each individual, TL was measured to an accuracy of 0.1 cm, weight was measured with a digital balance to an accuracy of 0.1 g (Anderson & Neumann Citation1996), and a sample of scales was collected for age attribution. After the measurements were completed, all specimens were released at the site of capture. All scales were stored in ethanol (30%) and next observed in the laboratory through the use of a stereomicroscope equipped with a camera. The images of the scales have been archived using the image-analysis system IAS 2000 (QEA’s IASLab® software). The scalimetric method for age determination was applied by two different operators (Bagenal Citation1978) and was subsequently integrated with the analysis of the length-frequency distribution (Britton et al. Citation2004).
Environmental parameters
To characterise the analysed river stretches, 20 environmental parameters were measured (). Hydrologic variables were measured at the cross-sectional area of each sampling reach. The length of the sampling stretches, established as 10 times the wetted channel, was comprised within the range of 50–100 m. Field measurements of specific conductivity, pH, water temperature and dissolved oxygen were made with electronic meters at the same time as fish sampling, and the other chemical parameters of the water were determined subsequently in the laboratory (for more details, see Carosi et al. Citation2016). In order to evaluate the water quality on the basis of the presence of macroinvertebrates, the extended biotic index (EBI) was used (Ghetti Citation1986) (for more details, see Carosi et al. Citation2016).
Table I. For each fish species present in the study area, the table shows: (i) co-occurrence with the European barbel, calculated by the ratio between the number of sites in which the species was present in sympatry with the European barbel and the total number of sites in which the European barbel was present; (ii) total number of sites where the species was found in the three census periods; (iii) origin; and (iv) total number of individuals caught.
Table II. Constrained quadratic ordination (CQO) results: scores of the descriptors on the latent environmental variable. BOD5: biochemical oxygen demand; Cl: chlorides; COD: chemical oxygen demand; EBI: Extended Biotic Index; NNH3: ammonia; NNO2: nitrites; NNO3: nitrates; Ptot: total phosphorus; PPO4: phosphorous orthophosphate; SO4: sulphates.
Statistical analysis
Following Vilizzi et al. (Citation2012), constrained quadratic ordination (CQO) was used to analyse the relationships among the environmental and fish data matrices; moreover, this analysis allowed us to identify the ecological preferences of B. barbus and B. tyberinus in respect to the environmental changes that occur along the longitudinal gradient of the rivers. The choice of this multivariate parametric analysis was for the following reasons: (i) it has greater flexibility than other methods; (ii) the output is an ordination diagram that is easy to interpret; and (iii) it fits a symmetrically bell-shaped response curve (Yee Citation2004, Citation2006). The CQO analysis was processed with the VGAM package version 1.0–3 in the statistical framework R version 3.3.2 (R Development Core Team 2016). CQO analysis estimates the optimal linear combination of the environmental variables (which are condensed into the latent variable) and regresses the species data upon the latent variable axis using quadratic curves fitted across the species scores. The optimum of a certain fish species is the value along the longitudinal gradient at which the highest probability of occurrence for that species is recorded; for the non-native species recently introduced in the study area, such as B. barbus, R. sericeus and G. gobio, the term “optimum” refers strictly to the CQO analysis and to the research context (in terms of time and space), as it may not fully reflect the niche of the species. The tolerance measures the width of the response curve – that is, how much deviation the species can tolerate from its optimal microhabitat (Yee Citation2004). To minimise the potential biases affecting CQO due to differences between sampling periods and differences in sampling sites, and to make the data collected in the three census periods comparable, the fish and environmental data sets were restricted to 99 sites. All of the 99 sites were surveyed over the three periods (2000–2005, 2006–2010, 2011–2015). Four species, namely Salvelinus fontinalis (Mitchill, 1814), Thymallus thymallus (L., 1758), Ctenopharyngodon idellus (Valenciennes, 1844) and Blicca bjoerkna (L., 1758), were omitted from the analysis because their presence can be considered occasional in the study area, and these species were most likely not established. The environmental matrix included 20 variables and 297 observations (99 sampling sites × three census periods). The fish assemblage matrix included 36 variables (fish species densities) and 297 observations (99 sampling sites × three census periods).
Permutational multivariate analysis of variance (PERMANOVA; Anderson & Walsh Citation2013) on log-transformed data was used to: (i) identify the descriptors affecting the presence and distribution of B. tyberinus in co-occurrence with B. barbus in the study area, and (ii) evaluate the occurrence of temporal changes in the habitat use of the species. The PERMANOVA statistical analysis was performed to determine Sum of Square (SS), F and p values using 999 permutations with the package VEGAN version 2.4–2 (Oksanen et al. Citation2017).
Fish growth and body condition parameters
For B. barbus, the total length–weight relationship (LWR) for the total sample and for each sub-basin was estimated by the least-squares method (Ricker Citation1975), based on the logarithmic equation:
The standard error was calculated for the slope (b) of the relationship, and isometric growth was tested through t-test using the equation:
where Sb is the standard error of the slope, for a = 0.05 (Sokal & Rohlf Citation1987).
The theoretical growth was estimated for the total sample and for each sub-basin by the von Bertalanffy growth curve model (von Bertalanffy Citation1938), using the average total lengths for age class:
where TL is the total length of the fish at time t, L∞ is the theoretical maximum length (cm), k is the rate of approach to L∞, and t0 is the theoretical age at which TL = 0. Additionally, the index of growth performance (Φ’) was calculated using the equation of Pauly and Munro (Citation1984):
where k and L∞ are the growth parameters of the von Bertalanffy model.
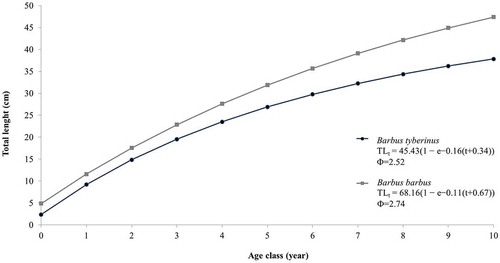
To compare the theoretical growth curves of the European barbel and the Tiber barbel, the equations calculated for 12 and 40 populations, respectively, of the Tiber River basin were used. The selection of these populations was based on the clear age structure and sufficient abundance to carry out the analysis. The comparison was carried out testing multiple models with and without common von Bertalanffy growth function (VBGF) parameters between the two species. Akaike’s information criterion (AIC; Burnham & Anderson Citation1998) was used to select the best candidate model to describe the theoretical growth.
To assess the interaction between the European barbel and the Tiber barbel, the relative weight, Wr, was estimated using the following equation:
where W = weight (g) and Ws = standard weight.
The relative weight (Wr) is a condition index based on the comparison between the real weight of an individual and the ideal weight of a specimen of the same species in good physiological condition (standard weight). Wr values lower than 95 indicate poor body condition. In the present study, the standard weight, Ws, was computed for the Tiber barbel with the empirical percentile (EmP) method, using the following equation, which is specific to the Tiber River populations (Angeli et al. Citation2010):
To test for general effects of age class and presence/absence of the European barbel on the Wr of the Tiber barbel, a PERMANOVA was used (Wheeler & Torchiano Citation2016; package: lmPerm version 2.1.0), followed by pairwise two-sample permutation tests (Mangiafico Citation2017; package: rcompanion). Only specimens with TL > 8 cm and belonging to the 1+–8+ age groups were included in the analysis.
Results
Distribution and abundance
The results of the fish census showed that the European barbel was present in 36 of the sampled sites (22.78% of the total number of sites) with a progressive increase of its relative abundance along the longitudinal gradient in the Tiber River and its major tributaries, i.e. Chiascio, Topino, Paglia rivers and the lower reaches of the Nestore River (). In three sites the barbel populations were represented exclusively by B. barbus. In more recent years (2011–2015), the highest population densities were reported in the Paglia River basin, where a considerable increase in abundance over time was observed (mean ± standard error, SE = 0.40 ± 0.07 ind. m−2), and the Nestore River basin (mean ± SE = 0.06 ± 0.12 ind. m−2). The spatial distribution of the European barbel densities in the three census periods showed a progressive invasion of the watersheds, starting from the sites where the species had been introduced the earliest, i.e. the middle reaches of the Tiber River (), with the highest density values found where the species has been present for more time.
Figure 1. Study area, distribution of the European barbel and location of the sampling sites. For the Tiber River and its main tributaries, the relative abundances are reported, which were calculated as percentages of the total barbel population density (European barbel = black pie slices + Tiber barbel = white pie slices).
Demographic characterisation
In total, 1174 specimens of European barbel were collected. The length of fish ranged from 2.0 to 62.0 cm (mean ± SE = 17.05 ± 0.28), and the weight from 0.5 to 2143.0 g (mean ± SE = 118.68 ± 5.46). Eleven age classes (0+–10+) were identified. Analysis of the age-based demographic trend of the European barbel was carried out in the four sampling sites (namely Topino, Nestore, Tiber and Paglia) where better structured populations resulted in terms of high number of age groups and presence of young individuals. The results showed that, except for the Paglia River in which a well-structured population in all three census periods was found (), in the other cases only in recent times were the populations characterised by the presence of many age classes with the prevalence of young specimens (data not shown). The comparison with the demographic trend of the Tiber barbel populations of the same sites showed, excluding the Topino River, a progressive decline over time of the population structure in terms of number and abundance of the age classes. The Paglia population is representative of the antithetical trend of the native barbel compared to the invasive one; in fact, in the three census periods, the number of age classes of B. tyberinus decreased from nine (0+–8+) to three (0+–2+) ().
Figure 2. Spatial distribution of European barbel densities in the years 2000–2005, 2006–2010 and 2011–2015.
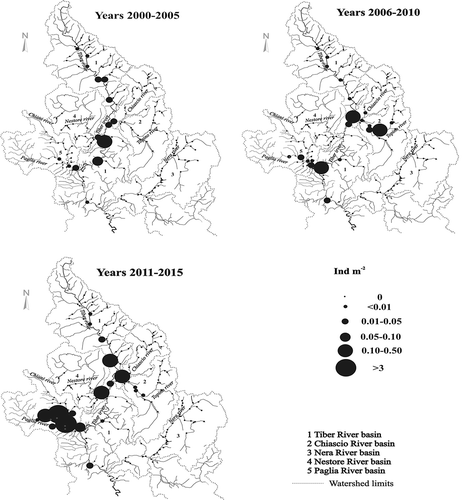
Figure 3. Age-based demographic trend for the European barbel in the three census periods in the Paglia River, and comparison with the Tiber barbel demographic trend in the same site.
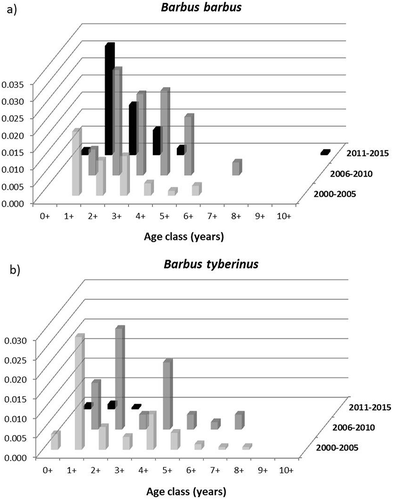
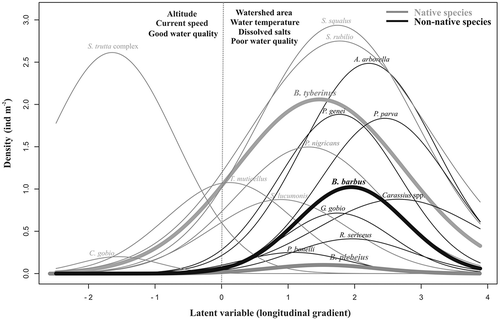
Figure 4. Constrained quadratic ordination (CQO) ordination plot for native (grey curves) and non-native (black curves) fish species densities in the Tiber River basin. A general description of the more significant environmental features is provided on each side of the latent variable range (separated by a dotted grey line) Only the response curves related to the barbel species (European barbel + Tiber barbel + Padanian barbel) and to the fish species with mean density values > 0.03 ind. m−2 and present in more than five sites are shown.
Fish community
A total of 41 fish species were found in the fish census (). The percentages of co-occurrence with B. barbus were higher for some rheophilous species, such as S. squalius (100.00%), S. rubilio (90.16%), B. tyberinus and P. nigricans (85.25%), and the Italian nase Protochondrostoma genei (Bonaparte, 1839) (83.61%); however, the percentages of co-occurrence with the European barbel were high even for other more limnophilous non-native species, such as the bleak Alburnus arborella (Bonaparte, 1841) (81.97%), the topmouth gudgeon Pseudorasbora parva (Temminck & Schlegel, 1846) (65.57%), the carp Cyprinus carpio L., 1758 (55.74%) and the goldfish Carassius spp. (45.90%).
For all of the fish species, unimodal bell-shaped curves were fitted through CQO analysis (). Except for Salmo trutta complex Linnaeus, 1758 and Cottus gobio Linnaeus, 1758, all profiles had an optimum at the right (positive) side of the latent variable. In the x-axis represents the longitudinal gradient of the water courses and describes the changes in environmental and biological characteristics occurring along the rivers. The fish assemblage composition varied along this longitudinal gradient and the European barbel was located more downstream than the Tiber barbel, as shown by the higher optimum value resulting for the alien species. For each species, the optimum and tolerance values on the range of the latent variable are provided in Appendix I. Negative scores resulted for altitude, pH, NNO3, SO4, PPO4, EBI and average current speed, indicating a higher value of the descriptor on the left-side axis of the CQO plot (, ). Positive scores resulted for all the other environmental variables, indicating a higher value of the descriptor on the right-side axis of the CQO plot. The Tiber barbel and the European barbel both prefer the downstream reaches and their presence was strongly associated with high water temperature, high contents of dissolved salts and a relatively poor water quality, as shown by the overlap of the response curves calculated for these two species. Moreover, the Tiber barbel showed a wider habitat range, as indicated by the higher tolerance value (1.27), compared to the European barbel (0.79).
Temporal changes in Barbus tyberinus habitat use in co-occurrence with B. barbus
The PERMANOVA results showed that, among environmental variables, some morphological parameters most affected the presence and distribution of B. tyberinus in the presence of B. barbus (distance from the source, watershed area and altitude), flow rate, water temperature, conductivity and sulphates (), with a significant change in habitat use over time. In particular, a gradually increasing trend in the three census periods resulted for the water temperature and the altitude. The opposite trend was noted for the watershed area, the distance from the source and some chemical parameters, such as the nitrates and the COD; these results suggested a gradual shift over time to upstream of B. tyberinus along the longitudinal gradient.
Table III. PERMANOVA results: descriptors affecting the presence and distribution of Barbus tyberinus in co-occurrence with B. barbus in the Tiber River basin. SO4: sulphates.
Growth
The LWR for the total sample was W = 0.012 ± 0.01TL2.907 ± 0.01 (R2 = 0.99). The b (slope) value of the LWR was lower than 3, but the differences between the calculated value (2.91) and the value of isometric growth (b = 3) were not statistically significant in the t-test analysis (t = 11.53; p > 0.05; Ricker Citation1975). In addition, the results of the regression coefficients calculated for each sub-basin showed that the b values were smaller than 3 in all cases (), also not statistically significant in the t-test analysis (p > 0.05).
Table IV. Parameters of the total length–weight relationship (LWR) and of theoretical growth for Barbus barbus.
Theoretical growth parameters of the European barbel in the Nestore River basin were faster than in the other populations (), exhibiting greater values of Φ. In comparison with the theoretical growth curves of Tiber barbel, the non-native species was distinguished by greater performance and larger size in all age classes ().
Estimation of relative weight (Wr) for Barbus tyberinus
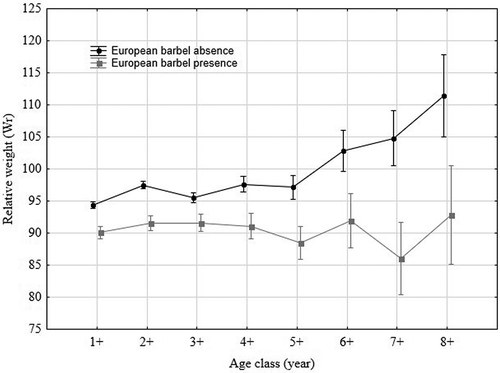
The difference between mean values of Wr calculated for the Tiber barbel at sites where the European barbel was present (mean ± SD = 89.15 ± 28.12) and absent (mean ± SD = 95.99 ± 14.57) were statistically significant (PERMANOVA, F = 234.23; p = 0.001), and far below the value of 95 in cases of sympatry, indicating poor body conditions. At the same time, the Wr mean values, broken down by age group and presence/absence of the European barbel, showed that the negative effects due to the presence of the non-native species were highly statistically significant (F = 14.43; p = 0.001), and more evident for the specimens belonging to the older age classes (6+–8+; ).
Discussion
Since it was first observed in 1998, the European barbel has invaded many middle and lower reaches of the Tiber River catchment, and it is still expanding its range. As hypothesised for other non-native species in the study area, such as the topmouth gudgeon (Carosi et al. Citation2016), the European barbel spreads following a “stepping stone and diffusion” dispersal model (Simon et al. Citation2011). In particular, it has been assumed that the species was introduced over a short time period at three different sites (downstream reaches of the Paglia and Nestore rivers and middle reaches of the Tiber River; Mearelli et al. Citation2000; Lorenzoni et al. Citation2004, Citation2007), from which it has spread using the natural connections of the hydrographic system, thanks also to the great vagility that distinguishes this species (Britton & Pegg Citation2011). An upper-stream dispersal directionality of this species was observed in many cases in its distribution trends over time, demonstrating its ability to overcome barriers. This is even more concerning as the headwater creeks often represent “refuge areas” for fish assemblages that are composed exclusively of endemic rheophilous cyprinid species, i.e. Tiber barbel, Etruscan chub and Apennine roach. These areas are very important in the conservation of fish biodiversity and should therefore be the focus for the establishment of more extensive protected zones (Lorenzoni et al. Citation2006b; Carosi et al. Citation2015).
Increasing densities of the European barbel populations over time characterised the Paglia River basin, where the species has been present for a longer period and therefore has had more time to naturalise, reproduce and disperse; the age-based demographic trend analysis highlighted in this case the presence of well-structured populations in all three census periods. In all of the basins the current population structure revealed the prevalence of young-of-the-year and juveniles, typical characteristics of expanding populations. In two sampling sites in the middle course of the Tiber River and in one site in the downstream reaches of the Chiascio River, the non-native species has totally replaced the Tiber barbel, causing its local extinction. In many other sites in the lowland areas, where the two congeneric species coexist, such as the downstream reaches of the Paglia and the Nestore rivers, more abundant populations of the European barbel than the Tiber barbel were observed. The presence of the European barbel was not detected in the Nera River basin, most likely due to unsuitable ecological characteristics for cyprinid species and to the presence of many insurmountable barriers preventing the colonisation of fishes from the Tiber River.
The Tiber barbel, the Padanian barbel and the European barbel showed similar ecological preferences, as demonstrated by the overlap between the unimodal bell-shaped curves resulting from the CQO analysis, although some differences can be highlighted. According to the distribution patterns of the fish species along the longitudinal gradient of the rivers, the European barbel was located in the stream reaches occurring farther downstream than the Tiber barbel and the Padanian barbel, where its presence was associated with other non-native species, such as the topmouth gudgeon, the goldfish, the roach Rutilus rutilus (L., 1758) and the bitterling Rhodeus sericeus (Pallas, 1776), and low environmental quality. A previous study carried out in Mediterranean streams showed that in the lower watershed areas, the introduction of non-native species and poor water quality have resulted in a progressive deterioration of the fish assemblage’s integrity (Vila-Gispert et al. Citation2002). In the study area the Tiber barbel occurred in the middle stream reaches, remaining excluded both from the upper reaches, where the fish assemblage was composed almost exclusively by the brown trout, Salmo trutta complex, and from the downstream reaches, where water pollution and the presence of many exotic species (Lorenzoni et al. Citation2006b; Carosi et al. Citation2015, Citation2016) have made the environment unsuitable for the Tiber barbel. Even though the Tiber barbel showed a larger habitat use than the European barbel, these results highlight the ability of the European barbel to adapt to low environmental quality, although the Tiber River is not its natural habitat.
The assessment of habitat use changes suggests that in the downstream stretches the presence of the European barbel has led to the gradual replacement of the native species because of interspecific competition phenomena; this shift upstream of the Tiber barbel was demonstrated by the change occurring over time in the ecological niche of the species, currently characterised by smaller mean values of watershed area and distance from the source, while the altitude was higher than in the past. The contrasting results related to the increased temperature over time, together with the decrease in the flow rate, can be attributed to the global climate changes that have occurred in recent decades, which have led to an exacerbation of the torrential characteristics of the rivers.
According to the literature (Baras & Philippart Citation1999; Britton et al. Citation2013), the results of the present study support the hypothesis that in the Tiber River basin the water temperature is an important factor that affects the distribution of the barbel species and influences various aspects of their life cycle, such as reproduction and growth. As observed by Britton et al. (Citation2010) for some alien species introduced to England, the global climate changes resulting in a progressive increase in water temperature may advantage the alien species, increasing their invasiveness. With increasing temperature it is, therefore, possible to consolidate the hypothesis of a further expansion of the European barbel towards the mountain stream reaches in the future.
In the risk assessment for the Tiber barbel, it is also necessary to consider the hydrologic characteristics of the rivers that host the species; Mediterranean water courses, especially in the upstream reaches, are characterised by periods of drought that, in addition to reduced habitat and food availability, can often lead to an exacerbation of water pollution and can cause the isolation of fishes with different thermal tolerances (Mejìa-Mojica et al. 2015). In fact, the reduction of summer water flows can cause the fragmentation of the river continuity (Lorenzoni et al. Citation2014) and a resulting impediment to: (i) the movement of fish along the longitudinal gradient, and (ii) the achievement of thermal optimum for aquatic species. The effects will be more serious if the presence of other anthropogenic impacts, such as water abstraction and non-implementation of the Minimum Ecological Flow rules, are added (Palmer et al. Citation2009; Hermoso & Clavero Citation2011). It is known that the fragmentation of native fish populations and their reduced abundance exacerbate the impact of competition with alien species and increase the risk of extinction (Vila-Gispert et al. Citation2002; Didham et al. Citation2007). Moreover, alterations in the flow regime can favour the invasion of exotic species that more easily adapt to the modified hydrologic characteristics of the rivers, to the disadvantage of native species adapted to the local natural variability in flows (Dudgeon et al. Citation2006). Other aspects to be taken into account in the risk assessment are restocking activities and the use of live bait in sport fishing, which can increase the spread of exotic species (Elvira & Almodovar Citation2001; Arlinghaus et al. Citation2002; Cowx & Gerdeaux Citation2004); restocking programmes in particular are unnecessary practices for B. tyberinus which, like many other species belonging to the cyprinid family, is characterised by high fertility and a high ability to recolonise an environment after the removal of disturbance factors.
As can be expected for a recently established invader species outside its natural range, the estimated maximum age of 10+ was lower than the maximum age of 15+ reported in the literature for the European barbel by Kottelat and Freyhof (Citation2007), while Britton et al. (Citation2013) reported that in some rivers of the UK the older specimens of this species showed an age of 21 years. In addition, the maximum size recorded in the present study (TL = 62.5 cm) did not reach the maximum total length of 120.0 cm recorded for the species (Bianco Citation1998).
The comparison between the theoretical growth curves calculated for the European barbel and the Tiber barbel confirmed a better performance of growth for the alien species, reflecting its invasiveness, its capacity to more rapidly reach a larger maximum size, and the ability of its females to produce a greater number of eggs.
Our results confirmed the effectiveness of the use of body condition in the assessment of possible negative impacts of the European barbel on the Tiber barbel. Previous studies have shown that the European barbel constitutes a threat to the native Tiber barbel because of competition, hybridisation and genetic introgression between the two congeneric species (Buonerba et al. Citation2015). Carosi et al. (Citation2006) found that in the Tiber River basin, the condition of the Tiber barbel analysed by means of the Fulton condition index (Fulton Citation1911) was lower where the European barbel was present, indicating the possibility that negative competition occurs between the two species. Additionally, Giannetto et al. (Citation2012), through the analysis of relative weight, reported that in the Tiber River basin the presence of the European barbel was associated with a decrease in the condition of the Tiber barbel. In the present study, the results of the estimation of the relative weight for the Tiber barbel suggest a negative impact of European barbel presence on the status of the native populations, with evident effects on the upper age classes. Further investigations focusing on the diet overlap between the two species and on their habitat preferences could support the hypothesis that in the younger age classes there may exist some diversifications in terms of feeding or microhabitats use that would make less evident the negative role of the competitor.
In conclusion, it is possible to hypothesise that the European barbel currently represents a serious threat to the endemic Tiber barbel, considering the following possible impacts: (i) faster growth and higher fecundity of the non-native species compared to the native species; (ii) the likely presence of competitive interactions for food and habitat between the two species due to their similar ecological preferences, as also highlighted by the estimation of body condition; and (iii) further range expansion of the European barbel as a result of the high displacement capacity of the species and of the likely future increases in water temperatures.
Future studies are needed to investigate the mechanisms underlying the invasiveness of this alien species. However, the information obtained in the present study constitutes a premise to underpin some conservation strategies aimed to: (i) preserve freshwater ecosystems, for example by maintaining minimum vital flows and the natural local variability in flows (ecological flow), given the torrential regime of the analysed rivers, as well as through the improvement of the water quality of the polluted rivers; (ii) preserve native fish biodiversity, through the use of native bait fish and the prohibition of restocking programmes in the “refuge areas”; and (iii) control invasive species such as European barbel, through the early detection of introductions and the immediate activation of eradication programmes.
Acknowledgements
The authors wish to thank all of the people who joined in the field activities during the project. They also thank the anonymous reviewers for helpful comments on the original version of the manuscript.
Additional information
Funding
References
- Anderson MJ, Walsh DCI. 2013. PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecological Monograph 83:557–574. DOI:10.1890/12-2010.1.
- Anderson RO, Neumann RM. 1996. Length, weight and associated structural indices. In: Murphy BR, Willis DW, editors. Fisheries techniques. Bethesda: American Fisheries Society. pp. 447–483.
- Angeli V, Bicchi A, Carosi A, Pedicillo G, Spigonardi MP, Lorenzoni M. 2010. Calculation of standard weight (Ws) for the main fish species in the Tiber basin. Studi Trentini Scienze Naturali 87:141–143.
- Arlinghaus R, Mehner T, Cowx IG. 2002. Reconciling traditional inland fisheries management and sustainability in industrialized countries, with emphasis on Europe. Fish and Fisheries 3:261–316. DOI:10.1046/j.1467-2979.2002.00102.x.
- Bagenal TB. 1978. Fish production in fresh waters. Oxford: Blackwell.
- Baras E, Philippart JC. 1999. Adaptive and evolutionary significance of a reproductive thermal threshold in Barbus barbus. Journal of Fish Biology 55:354–375. DOI:10.1111/jfb.1999.55.issue-2.
- Bianco PG. 1993. L’ittiofauna continentale dell’Appennino umbro-marchigiano, barriera semipermeabile allo scambio di componenti primarie tra gli opposti versanti dell’Italia Centrale. Biogeographia 17:427–485.
- Bianco PG. 1995. A revision of the Italian Barbus species (Cypriniformes: Cyprinidae). Ichtiological Exploration of Freshwaters 6:305–324.
- Bianco PG. 1998. Diversity of Barbinae fishes in southern Europe with description of a new genus and a new species (Cyprinidae). Italian Journal of Zoology 65:125–136. DOI:10.1080/11250009809386804.
- Bianco PG. 2003. Barbus plebejus Bonaparte, 1839. In: Banarescu PM, Bogutskaya NG, editors. The freshwater fish of Europe, Vol 5, Cyprinidae 2, Part II. Wiebelsheim (D): AULA-Verlag. pp. 1–77.
- Bianco PG, Ketmaier V. 2001. Anthropogenic changes in the freshwater fish fauna of Italy, with reference to the central region and Barbus graellsii, a newly established alien species of Iberian origin. Journal of Fish Biology 59(Supplement A):190–208. DOI:10.1111/j.1095-8649.2001.tb01386.x.
- Blackwell BG, Brown ML, Willis DW. 2000. Relative weight (Wr) status and current use in fisheries assessment and management. Reviews in Fisheries Science 8:1–44. DOI:10.1080/10641260091129161.
- Britton JR, Cowx IG, Peirson G. 2004. Sources of error in the ageing of stocked cyprinids. Fisheries Management and Ecology 11:415–417. DOI:10.1111/fme.2004.11.issue-6.
- Britton JR, Cucherousset J, Davies GD, Godard M, Copp GH. 2010. Non-native fishes and climate change: Predicting species responses to warming temperatures in a temperate region. Freshwater Biology 55:1130–1141. DOI:10.1111/fwb.2010.55.issue-5.
- Britton JR, Davies GD, Pegg J. 2013. Spatial variation in the somatic growth rates of European barbel Barbus barbus: A UK perspective. Ecology of Freshwater Fish 22:21–29. DOI:10.1111/eff.2012.22.issue-1.
- Britton JR, Pegg J. 2011. Ecology of European barbel Barbus barbus: Implications for river, fishery, and conservation management. Reviews in Fisheries Science 19:321–330. DOI:10.1080/10641262.2011.599886.
- Buonerba L, Zaccara S, Delmastro GB, Lorenzoni M, Salzburger W, Gante HF. 2015. Intrinsic and extrinsic factors act at different spatial and temporal scales to shape population structure, distribution and speciation in Italian Barbus (Osteichthyes: Cyprinidae). Molecular Phylogenetics and Evolution 89:115–129. DOI:10.1016/j.ympev.2015.03.024.
- Burnham KP, Anderson DR. 1998. Model selection and inference: An information-theoretic approach. New York: Springer-Verlag.
- Carosi A, Ghetti L, Forconi A, Lorenzoni M. 2015. Fish community of the river Tiber basin (Umbria-Italy): Temporal changes and possible threats to native biodiversity. Knowledge and Management of Aquatic Ecosystem 416(22):16.
- Carosi A, Ghetti L, Lorenzoni M. 2016. Status of Pseudorasbora parva in the Tiber river basin (Umbria, central Italy) 20 years after its introduction. Knowledge and Management of Aquatic Ecosystem 417(22):11.
- Carosi A, Ghetti L, Lorenzoni M. 2017. Invasive Carassius spp. in the Tiber River basin (Umbria, Central Italy): Population status and possible interactions with native fish species. Cybium 41:11–23.
- Carosi A, Pedicillo G, Bicchi A, Angeli V, Lorenzoni M, Ghetti L. 2006. Distribution and abundance of Barbus barbus (Linnaeus, 1758) in the Tiber river basin in Umbria (Central Italy). Quaderni ETP 34:241–249.
- Clavero M, Blanco-Garrido F, Prenda J. 2004. Fish fauna in Iberian Mediterranean river basins: Biodiversity, introduced species and damming impacts. Aquatic Conservation 4:75–585.
- Cowx IG, Gerdeaux D. 2004. The effects of fisheries management practises on freshwater ecosystems. Fisheries Management and Ecology 11:145–151. DOI:10.1111/fme.2004.11.issue-3-4.
- Crivelli AJ. 1995. Are fish introductions a threat to endemic freshwater fishes in the northern Mediterranean region? Biological Conservation 72:311–319. DOI:10.1016/0006-3207(94)00092-5.
- Crivelli AJ. 2006. Squalius lucumonis. The IUCN Red List of Threatened Species 2006: E.T60828A12415631. Available: http://dx.doi.org/10.2305/IUCN.UK.2006.RLTS.T60828A12415631.en. Accessed May 2017 06.
- Didham RK, Tylianakis JM, Gemmell NJ, Rand TA, Ewers RM. 2007. Interactive effects of habitat modification and species invasion on native species decline. Trends in Ecology & Evolution 22:489–496. DOI:10.1016/j.tree.2007.07.001.
- Dudgeon D, Arthington AH, Gessner MO, Kawabata ZI, Knowler DJ, Lévêque C, Naiman RJ, Prieur-Richard AH, Soto D, Stiassny MLJ, Sullivan CA. 2006. Freshwater biodiversity: Importance, threats, status and conservation challenges. Biological Reviews 81:163–182. DOI:10.1017/S1464793105006950.
- Elvira B, Almodovar A. 2001. Freshwater fish introductions in Spain: Facts and figures at the beginning of the 21st century. Journal of Fish Biology 59(A):323–331. DOI:10.1111/j.1095-8649.2001.tb01393.x.
- Freyhof J. 2013. Barbus tyberinus. The IUCN Red List of Threatened Species 2013: E.T2591A9459724. Available: http://dx.doi.org/10.2305/IUCN.UK.2011-1.RLTS.T2591A9459724.en. Accessed May 2017 06.
- Fulton TW. 1911. The sovereignty of the sea: an historical account of the claims of England to the dominion of the British seas, and of the evolution of the territorial waters. Edinburgh: Blackwood W & Sons.
- Gago J, Anastácio P, Gkenas C, Banha F, Ribeiro F. 2016. Spatial distribution patterns of the non-native European catfish, Silurus glanis, from multiple online sources – a case study for the River Tagus (Iberian Peninsula). Fisheries Management and Ecology 23:503–509. DOI:10.1111/fme.12189.
- Gaygusuz Ö, Emiroğlu Ö, Tarkan AS, Aydin H, Top Dorak Z, Karakuş U, Başkurt S. 2013. Assessing the potential impact of nonnative fish on native fish by relative condition. Turkish Journal of Zoology 37:84–91.
- Geiger MF, Schreiner C, Delmastro GB, Herder F. 2016. Combining geometric morphometrics with molecular genetics to investigate a putative hybrid complex: A case study with barbels Barbus spp. (Teleostei: Cyprinidae). Journal of Fish Biology 88:1038–1055. DOI:10.1111/jfb.2016.88.issue-3.
- Ghetti PF. 1986. I macroinvertebrati nell’analisi di qualità dei corsi d’acqua. Trento: Bertelli.
- Giannetto D, Carosi A, Franchi E, Ghetti L, Pedicillo G, Pompei L, Lorenzoni M. 2012. Assessing the impact of non-native freshwater fishes on native species using relative weight. Knowledge and Management of Aquatic Ecosystems 404:03. DOI:10.1051/kmae/2011081.
- Gozlan RE, Andreou D, Asaeda T, Beyer K, Bouhadad R, Burnard D, Caiola N, Cakic P, Djikanovic V, Esmaeili HR, Falka I, Golicher D, Harka A, Jeney G, Kováč V, Musil J, Nocita A, Povz M, Poulet N, Virbickas T, Wolter C, Tarkan AS, Tricarico E, Trichkova T, Verreycken H, Witkowski A, Zhang C, Zweimueller I, Britton JR. 2010. Pan-continental invasion of Pseudorasbora parva: Towards a better understanding of freshwater fish invasions. Fish and Fisheries 11:315–340. DOI:10.1111/j.1467-2979.2010.00361.x.
- Hermoso V, Clavero M. 2011. Threatening processes and conservation management of endemic freshwater fish in the Mediterranean basin: A review. Marine and Freshwater Research 62:244–254. DOI:10.1071/MF09300.
- Huet M. 1949. Aperçu des relations de la pente et des populations piscicoles des eaux courantes. Schweizerische Zeitschrift Fur Hydrologie-Swiss Journal of Hydrology 2:332–351.
- Innal D, Erk’akan F. 2006. Effects of exotic and translocated fish species in the inland waters of Turkey. Reviews in Fish Biology and Fisheries 16:39–50. DOI:10.1007/s11160-006-9005-y.
- Ketmaier V, Finamore F, Largiadèr C, Milone M, Bianco PG. 2009. Phylogeography of bleaks Alburnus spp. (Cyprinidae) in Italy, based on cytochrome b data. Journal of Fish Biology 75:997–1017. DOI:10.1111/j.1095-8649.2009.02357.x.
- Kottelat M, Freyhof J. 2007. Handbook of European freshwater fishes. Cornol: Kottelat.
- Lorenzoni M, Barocco R, Carosi A, Giannetto D, Pompei L. 2014. The fish fauna of the Apennine streams related to changes in the regime of wet depositions. Biologia Ambientale 28:67–73.
- Lorenzoni M, Carosi A, Angeli V, Bicchi A, Pedicillo G, Viali P. 2006a. Individuazione e riconoscimento dei barbi autoctoni nel bacino del fiume Paglia. Terni: Arti Grafiche Iezzi.
- Lorenzoni M, Carosi A, Pedicillo G, Pompei L, Rocchini M. 2011. Reproductive properties of the chub Squalius squalus (Bonaparte, 1837) in the Assino Creek (Umbria, Italy). Knowledge and Management of Aquatic Ecosystems 403:09. DOI:10.1051/kmae/2011069.
- Lorenzoni M, Corboli M, Grillo E, Pedicillo G, Carosi A, Viali P, Ghetti L, Baldini G, Zeetti A, Natali M, Dolciami R, Biscaro Parrini A, Mezzetti A, Mossone M, Andreani M, Burchia A, Cassieri S, De Luca M, Quondam LS, Uzzoli C, Di Brizio M. 2004. Carta Ittica Regionale. Bacino del F. Nestore. Perugia: Centro Stampa Regione Umbria.
- Lorenzoni M, Mearelli M, Ghetti L. 2006b. Native and exotic fish species in the Tiber river watershed (Umbria – Italy) and their relationship to the longitudinal gradient. Bulletin français de la pêche et de la pisciculture 382:19–44. DOI:10.1051/kmae:2006005.
- Lorenzoni M, Pedicillo G, Carosi A, Tardiolo D, Viali P, Baldini G, Ghetti L, Zeetti A, Natali M, Biscaro Parrini A, Dolciami R, Mezzetti A, Burchia A, Di Brizio M, Lancioni T, Uzzoli C. 2007. Carta Ittica Regionale. Bacino del F. Tevere. Perugia: Centro Stampa Regione Umbria.
- Mangiafico S. 2017. Rcompanion: Functions to support extension education program evaluation. R package version 1.4.0. Available: https://CRAN.R-project.org/package=rcompanion. Accessed Feb 2017 1.
- Marr MS, Olden JD, Leprieur F, Arismendi I, Caleta M, Morgan DL, Nocita A, Šanda R, Serhan Tarkan A, García-Berthou E. 2013. A global assessment of freshwater fish introductions in mediterranean-climate regions. Hydrobiologia 719:317–329. DOI:10.1007/s10750-013-1486-9.
- Mearelli M, La Porta G, Lorenzoni M, Maio G. 2000. Conoscenza delle popolazioni ittiche per la definizione metodologica delle portate di minimo vitale nel bacino idrografico del fiume Tevere: Studio ittiofaunistico dell’alto corso del Tevere e dei suoi affluenti. Tevere 14/16:7–52.
- Mejía-Mojica H, Contreras Mac-Beath T, Ruiz-Campos G. 2015. Relationship between environmental and geographic factors and the distribution of exotic fishes in tributaries of the balsas river basin, Mexico. Environmental Biology of Fishes 98:611–621. DOI:10.1007/s10641-014-0298-8.
- Meraner A, Venturi A, Ficetola GF, Rossi S, Candiotto A, Gandolfi A. 2013. Massive invasion of exotic Barbus barbus and introgressive hybridization with endemic Barbus plebejus in Northern Italy: Where, how and why? Molecular Ecology 22:5295–5312. DOI:10.1111/mec.12470.
- Moran PAP. 1951. A mathematical theory of animal trapping. Biometrika 38:307–311. DOI:10.1093/biomet/38.3-4.307.
- Myers N, Mittermeie RA, Mittermeier CG, Da Fonseca GAB, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403:853–858. DOI:10.1038/35002501.
- Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Szoecs E, Wagner H. 2017. The Vegan package. Community ecology package. The Comprehensive R Archive Network. Nairobi, Kenya: World Agroforestry Centre.
- Palmer MA, Lettenmaier DP, LeRoy Poff N, Postel SL, Richter B, Warner R. 2009. Climate change and river ecosystems: Protection and adaptation options. Environmental Management 44:1053–1068. DOI:10.1007/s00267-009-9329-1.
- Pauly D, Munro JL. 1984. Once more on comparison of growth in fish and invertebrates. ICLARM Fishbyte 1:21–22.
- Pompei L, Carosi A, Pedicillo G, Rocchini E, Lorenzoni M. 2011. Age and growth analysis of the chub, Squalius squalus (Bonaparte, 1837), in the Assino Creek (Umbria, Italy). Knowledge and Management of Aquatic Ecosystems 400:09. DOI:10.1051/kmae/2011011.
- Przybylski M, Boroñ A, Krik A. 2004. Growth of barbel, Barbus barbus (L.) in the upper Warta River, Odra River system. Ecohydrology and Hydrobiology 4:183–190.
- R Core Team. 2016. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available: https://www.R-project.org/. Accessed Feb 2017 1.
- Ribeiro F, Leunda PM. 2012. Non-native fish impacts on Mediterranean freshwater ecosystems: Current knowledge and research needs. Fisheries Management and Ecology 19:142–156. DOI:10.1111/j.1365-2400.2011.00842.x.
- Ricker WE. 1975. Computation and interpretation of biological statistics of fish populations. Journal of the Fisheries Research Board of Canada 191:1–382.
- Rondinini C, Battistoni A, Peronace V, Teofili C. 2013. Lista Rossa IUCN dei Vertebrati Italiani. Roma: Comitato Italiano IUCN e Ministero dell’Ambiente e del Mare.
- Simon A, Britton R, Gozlan RE, van Oosterhout C, Volckaert FAM, Hänfling B. 2011. Invasive cyprinid fish in Europe originate from the single introduction of an admixed source population followed by long-distance dispersal. Plos ONE e18560. DOI:10.1371/journal.pone.0018560.
- Smith TA, Kraft CE. 2005. Stream fish assemblages in relation to landscape position and local habitat variables. Transactions of the American Fisheries Society 134:430–440. DOI:10.1577/T03-051.1.
- Sokal RR, Rohlf FL. 1987. Introduction to biostatistics. New York: Freeman.
- Vadas RL, Orth DJ. 2000. Habitat use of fish communities in a Virginia stream system. Environmental Biology of Fishes 59:253–269. DOI:10.1023/A:1007613701843.
- Vila-Gispert A, García-Berthou E, Moreno-Amich R. 2002. Fish zonation in a Mediterranean stream: Effects of human disturbances. Aquatic Sciences 64:163–170. DOI:10.1007/s00027-002-8064-y.
- Vilizzi L, Stakenas S, Copp GH. 2012. Use of constrained additive and quadratic ordination in fish habitat studies: An application to introduced pumpkinseed (Lepomis gibbosus) and native brown trout (Salmo trutta) in an English stream. Fundamental and Applied Limnology 180:69–75. DOI:10.1127/1863-9135/2012/0277.
- Vitule JRS, Arruda Freire C, Simberloff D. 2009. Introduction of non-native freshwater fish can certainly be bad. Fish and Fisheries 10:98–108. DOI:10.1111/j.1467-2979.2008.00312.x.
- von Bertalanffy L. 1938. A quantitative theory of organic growth. Human Biology 10:181–243.
- Wheeler B, Torchiano M. 2016. lmPerm: Permutation tests for linear models. R package version 2.1.0. Available: https://CRAN.R-pro-ject.org/package=lmPerm. Accessed Feb 2017 1.
- Yee T. 2004. A new technique for maximum-likelihood canonical gaussian ordination. Ecological Monographs 74:685–701. DOI:10.1890/03-0078.
- Yee T. 2006. Constrained additive ordination. Ecology 87:203–213. DOI:10.1890/05-0283.
- Zerunian S. 2002. Condannati all’estinzione? Bologna: Edagricole.
- Zippin C. 1956. An evaluation of the removal method of estimating animal populations. Biometrics 12:163–189. DOI:10.2307/3001759.