Abstract
The aim of this work is to estimate the relationship between developmental mode and flight style, body mass and wing morphology of birds. We revealed high evolutionary correlation between developmental mode and flight style of birds. Different developmental modes, as well as flight styles, repeatedly appeared in birds’ evolution. Precocial birds are associated with continuous-flapping flight. Small altricial birds mostly use passerine-type flight. Soaring birds are large and have an intermediate developmental mode. Developmental strategies and flight styles correlate with differences in body mass and traits of wing morphology (wingspan, wing area, humerus, ulna, manus, and primary feather lengths). Nevertheless, by comparing results of phylogenetic and standard discriminant function analyses, we reveal that phylogeny strongly affects the morphology of wing traits and body mass in birds. When using phylogenetic t-tests, we did not find an association between relative length of wing elements and different developmental mode and flight style groups, except altricial birds with flapping and gliding flight style (Apodidae, Hirundinidae).
Introduction
Developmental strategies and flight styles of birds are usually studied as separate topics. Traditionally, developmental modes are considered in association with anatomical and physiological traits of the neonates, nest attendance, feeding behavior or parent–chick relationship (Portmann Citation1935; Nice Citation1962; Ricklefs Citation1983; O’Connor Citation1984; Starck Citation1993; Starck & Ricklefs Citation1998). Nevertheless, the type of bird development was taken into account when discussing evolution, origin and phylogeny of birds and used to be an important diagnostic feature in their classification (i.e. Verheyen Citation1961). Carey and Adams (Citation2001) suggested that the evolution of traits associated with parental care preadapted protobirds for the evolution of flight. These traits are feathers (for solar and heat shields), beaks (for nest building and feeding young), forelimbs (for protection of nest peripheries) and nest sequestration behavior (for maximal safety of young). Especially noteworthy is the article of Dial (Citation2003) where the author proposed to consider comprehensively the relationship between developmental modes and other features of birds’ biology. Dial (Citation2003) emphasized that nesting strategies, flight styles, hatchling and adult body size, developmental altricial–precocial trajectories, and origin of flight were interrelated and interdependent phenomena. Unfortunately, his work did not contain quantitative data to support this hypothesis. In this article, we test for the relationship between developmental mode and flight style. In other words, are these traits independent in birds’ evolution? If this relationship is supported by our findings, it means that birds with a certain developmental mode use a limited range of flight styles, or typical flight styles.
The second issue that we examine in this article is how wing morphology and body mass are reflected in flight styles that are used by birds with different developmental strategies. Flight styles and flight characteristics are associated with body mass. Flight styles are determined on the basis of wing-beat frequencies that are calculated using body mass, wing morphology, and some environmental variables (Pennycuick Citation1996; Bruderer et al. Citation2010). Considering the differences between birds along the altricial–precocial spectrum in flight styles, we expect that there are also differences in body mass and wing morphology. Numerous articles deal with the flight style of birds (Gladkov Citation1949; Savile Citation1957; Rayner Citation1988; Pennycuick Citation1996, Citation2001; Warrick et al. Citation1998; Alerstam et al. Citation2007; Bruderer et al. Citation2010) and its correlation with wing morphology (Rayner Citation1988; Nudds et al. Citation2007; Simons Citation2010; Wang et al. Citation2011). However, we found no articles in which interconnection between flight styles and developmental modes of birds was studied.
We aim to analyze the relationship between developmental modes, flight styles, body mass, and wing morphology of birds, based on quantitative data and using standard and phylogenetic comparative methods. A combination of standard and phylogenetic methods, in our opinion, is helpful for a comprehensive approach. Standard statistical methods can reveal whether there are differences in wing morphology. Phylogenetic comparative methods can detect whether these differences are caused by flight styles and developmental modes or by the influence of phylogeny.
Materials and methods
Measurements of humerus (hu), ulna (ul), manus (mn), and primary feather (fprim) were taken from Wang et al. (Citation2011) and supplemented with our own measurements of wing elements of birds from the collection of the Museum of Animal Anatomy (National University of Life and Environmental Sciences of Ukraine) (32 species). The length of primary feathers was considered because it strongly affects wingspan. Total arm (ta) length was estimated as a sum of hu, ul and mn. Data on body mass, wingspan and wing area were taken from Bruderer et al. (Citation2010). Since the data on wing morphology were taken from different sources, a complete set of variables was not available for all species. So, we used a “wingspan” data set (wingspan, wing area and body mass) that was available for 149 species, and a “wing elements” data set (humerus, ulna, manus, primary feather and body mass) that was available for 182 (developmental-mode groups) or 179 (flight-style groups) species.
Flight styles were indicated as defined by Bruderer et al. (Citation2010). Bruderer et al. distinguished four basic flight styles based on wing-beat frequency data measured by radar, complemented by video and cinematic recordings: continuous flapping (CF), flapping and gliding (FG), flapping and soaring (FS), and passerine-type flight (PT). FG comprises relatively long flapping phases and comparably long gliding phases with fully stretched wings. Birds with FS flight use extended flapping phases only occasionally, mainly to reduce the descent rate or maintain flight level during gliding phases. PT flight comprises non-flapping phases with closed wings or partially flexed (arrow-like) wings and with short bursts of flapping and brief glides with stretched wings.
Developmental mode was determined using the simplified classification of Nice (Citation1962): precocial (according to Nice (Citation1962); precocial 1–4), intermediate (semi-precocial and semi-altricial 1–2) and altricial (altricial).
In total, we gathered data for 261 species, which represented 157 genera of birds. We used 5000 trees from the database at http://birdtree.org to make a phylogenetic reconstruction (Jetz et al. Citation2012). The Hackett All Species subset was chosen (Hackett et al. Citation2008). A maximum clade credibility tree was produced with TreeAnnotator (Drummond et al. Citation2012). In total, phylogenetic relationships for 257 species were established.
Correlation between categorical variables (flight style and developmental mode) was evaluated with the help of stochastic character mapping (Huelsenbeck et al. Citation2003). Thus, we took into account phylogenetic relationship between species of birds. We chose D and dij statistics to describe the evolutionary association between traits (Huelsenbeck et al. Citation2003), as the portion of time when each pair of trait categories were associated together. D measures overall disagreement between observed and expected models of evolution; the further D is from 0, the less likely is independent evolution. The nature of association can be seen from dij. When dij is negative (or positive) then states i and j are found together less (or more) frequently than expected for independence. First, we made 1000 stochastic realizations for each trait. Second, we simulated the independent evolution of flight style and developmental mode (also 1000 realizations) with transition rates estimated at the first step. The best-fit (according to Akaike information criterion, AIC) transition model for the evolution of developmental mode was all-rates-different transition rates; flight style evolution was best described with an equal rates model. Third, we estimated D and dij for the observed and expected evolution. Then, significance values were calculated by comparing posterior distributions of the observed and simulated D and dij (Huelsenbeck et al. Citation2003). All these calculations were made with the help of R package phytools v. 0.5–52 (Revell Citation2012).
The phylogenetic signal for continuous traits was evaluated using Pagel’s λ (Pagel Citation1999), implemented in the function phylosig of package phytools (Revell Citation2012).
All continuous variables were log10-transformed. For multivariate analysis we also used (body mass)1/3 and (wing area)1/2 in order to linearize the relationship. Due to the latter transformation, contributions of traits were at the same scale. The effect of body mass, developmental mode and flight style on the other traits was evaluated with phylogenetic generalized linear models, implemented in the function pgls, package caper v. 0.5.2 (Orme et al. Citation2013). The optimal model was chosen according to Akaike information criterion (AICc) differences (Δi = AICci – AICcmin) (Burnham & Anderson Citation2002).
Phylogenetic multivariate analysis of variance (MANOVA) with Wilks’s Λ summarizing statistic, implemented in the function aov.phylo of R package geiger (Harmon et al. Citation2008), was applied to check for multivariate differences among measured variables between developmental-mode and flight-style groups. Significance was evaluated on 1000 simulations under a Brownian-motion model of evolution. Then, phylogenetic flexible discriminant analysis (pFDA) was used (Schmitz & Motani Citation2011), which is an analog of linear discriminant analysis. Equal prior probabilities were set to construct a confusion matrix of misclassifications. We also conducted standard linear discriminant analysis with the function lda in R package MASS (Venables & Ripley Citation2002). We used two sets of measurements: log10-transfomed wing elements and body mass (wing elements data set), and log10-transformed measurements of wingspan, wing area and body mass (wingspan data set). Body mass was included in both models to account for its effect.
Raw unstandardized coefficients of discriminant functions were used to evaluate the contribution of the traits to discrimination. Structure coefficients (i.e. Pearson’s r correlation between log10-transfomed measurements and canonical variates) were used to explain discriminant functions’ morphospace.
The relative length of wing elements was calculated as a ratio of hu, ul, mn or fprim to ta. We also analyzed the ratio of hu to ul lengths, the brachial index (BI). Standard and phylogenetic pairwise t-tests were used to estimate morphological differences between groups. They were implemented in the R functions pairwise.t.test of package stats (R Core Team Citation2014) and phylANOVA of the package phytools (Revell Citation2012).
We used false discovery rate (FDR) correction for multiple testing (Benjaminini & Hochberg Citation1995); the corrected significance level is reported in the article as pFDR. All statistical computations were conducted with R software (version 3.1.2, R Core Team Citation2014).
Results
Flight styles and developmental modes of birds
Basic summary statistics (number of species, mean and standard deviation) of traits for different groups of birds are given in . Raw data are presented in Supplementary Tables S1 and S2.
Table I. Basic statistic estimates of developmental mode – flight style groups: n = number of species in the group; mean = mean value; SD = standard deviation; CF = continuous flapping; FG = flapping and gliding; PT = passerine type; FS = flapping and soaring flight styles; hu = humerus; ul = ulna; mn = manus; fprim = primary feather; ta = total arm length.
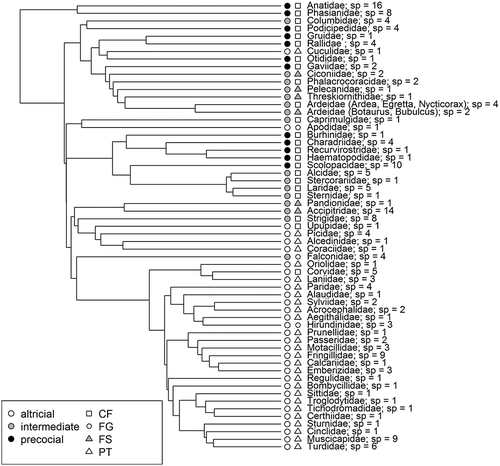
The distribution of developmental modes and flight styles onto the phylogeny of Hackett et al. (Citation2008) is mapped in . The figure shows that precocial, altricial and intermediate developmental modes repeatedly appeared in the evolution of birds. Precocity is a characteristic trait for Galliformes and Anseriformes, as well as for Gruiformes, Podicipediformes, Gaviiformes and, partly, Charadriiformes (suborder Charadrii). Altricial developmental mode appeared in Apodiformes, Upupiformes, Coraciiformes, Piciformes, Psittaciformes, Cuculiformes and Passeriformes. Similar flight styles also evolved several times. CF flight style is the most widely represented among orders: it developed in Anseriformes, Galliformes, Gruiformes, Gaviiformes, Podicipediformes, Strigiformes, Columbiformes, Caprimulgiformes, Upupiformes and part of Pelecaniformes, Ciconiiformes, Charadriiformes and Passeriformes. PT flight style appeared in Piciformes, Passeriformes, Cuculiformes and Coraciiformes. Birds with large body mass among Pelecaniformes, Falconiformes and Ciconiiformes have the FS flight style. FG flight evolved in Apodiformes, and partly Falconiformes and Passeriformes.
We found a high degree of association (D = 0.88, p < 0.001) of evolutionary histories of flight style and developmental mode of birds (). This correlation is mainly due to the association between being altricial and PT (dij = 0.19, p < 0.001), being precocial and CF (dij = 0.15, p < 0.001), and mutual exclusion of altricial and CF (dij = −0.14, p = 0.001), and precocial and PT (dij = −0.10, p < 0.001) pairs of states. The same trend can be observed in living birds. Precocial birds are only associated with continuous flapping flight (). Altricial birds mostly use passerine-type flight (81% of the studied altricial birds). Although recent intermediate birds use mainly continuous flapping (49%), flapping and soaring flight (38%), and less often flapping and gliding (13%) styles, evolutionary modeling supported only weak association of intermediate developmental mode and FS (dij = 0.09, p = 0.003), and mutual exclusion of intermediate birds and PT (dij = −0.09, p = 0.001); CF and FG style were associated with an intermediate developmental mode by chance (p > 0.09).
Table II. Association of evolutionary histories of flight style and developmental mode of birds: D = overall measure of evolutionary association; dij = measure of association for specific pairs of developmental mode and flight style; p = significance value of estimates; CF = continuous flapping; FG = flapping and gliding; FS = flapping and soaring; PT = passerine type flight styles.
Figure 1. Phylogenetic hypothesis of relationships amongst studied birds (Hackett et al. Citation2008) showing modes of development and flight styles: sp = number of the studied species; CF = continuous flapping; FG = flapping and gliding; FS = flapping and soaring; PT = passerine type flight styles.
Phylogenetic signal
Phylogeny highly influenced traits of wing morphology and body mass. We found that Pagel’s λ significantly (p << 0.001) differed from zero for all traits and was larger than 0.97 for all traits. Thus, closely related birds tend to be similar, and species values should not be considered statistically independent. Further, we accounted for phylogeny by implementing a special set of phylogenetic comparative methods.
Effect of body mass
Phylogenetic generalized least squares (PGLS) results showed that body mass was an important explanatory variable (); hence, body mass was included in both data sets to account for its effect. Best-fit PGLS models included developmental-mode or flight-style variables. Manus length was best described with body mass and either flight or developmental mode; the difference in the AICc values of the models was less than 2 (Burnham & Anderson Citation2002). Primary feather length was associated with body mass and flight style. Humerus, ulna length and wing area were best fitted with body mass coupled with developmental mode (). The length of humerus, ulna, wingspan and wing area were highly correlated with body mass according to multiple R2 (). Only about 60% of the variance of the length of manus or primary feathers was associated with body mass ().
Table III. The effect of body mass estimated with the analysis of best-fit PGLS models with the AICc differences (Δ) and multiple R2: dev.mode = developmental mode, flight = flight style; hu = humerus; ul = ulna; mn = manus; fprim = primary feather length. All models were significant (pFDR < 0.03).
Traits that best discriminate developmental modes
Centroids of developmental modes were well separated with the wingspan data set (phylogenetic MANOVA: Wilks’s Λ = 0.200, p = 0.001) and the wing elements data set (MANOVA: Wilks’s Λ = 0.147, p = 0.002). Nevertheless, the discrimination power of the phylogenetic discriminant function analysis was rather weak. Phylogenetic discriminant function analysis scores and coefficients are given in Supplementary Tables S3 and S4. The developmental mode was correctly predicted for 51.0% species of birds using the wingspan data set and for 53.3% with the wing elements data set ().
The first pFDA axis was associated with 74% of the variation of the wingspan data set, and 64% of the wing elements data set. Wing area and body mass contributed at similar levels to the discrimination process (); Supplementary Table S4). Within the wing elements data set, humerus and ulna length and body mass mostly influenced the discrimination between developmental modes. At the same time, fprim and mn showed a weak contribution to the discrimination (); Supplementary Table S4).
Figure 2. Phylogenetic discriminant analysis of developmental mode and flight style groups by wingspan (plots (a) and (c)) and wing elements (plots (b) and (d)) data sets. Convex hulls, which connect the most distant points of each group, are shown. Other group points were omitted from the plot. The length of arrows represents the contribution of the traits to discrimination. altr. = altricial; interm. = intermediate; prec. = precocial developmental modes; CF = continuous flapping; FG = flapping and gliding; FS = flapping and soaring; PT = passerine type flight styles; hu = humerus; ul = ulna; mn = manus; fprim = primary feather length.
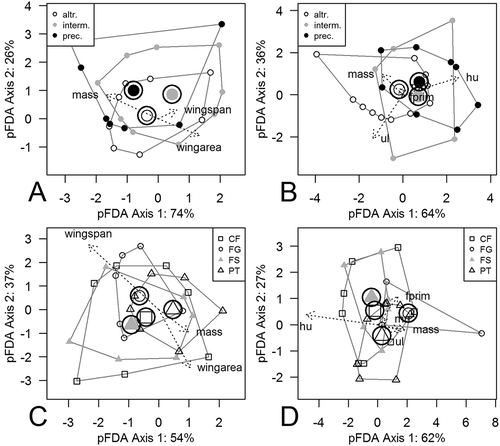
The major axis of variation was moderately or slightly correlated with the traits in the wingspan data set: wing area (correlation, r = 0.538), wingspan (r = 0.497) and body mass (r = 0.336). It was pFDA Axis 2 which had a strong correlation with these traits: wing area (r = 0.718), wingspan (r = 0.744) and body mass (r = 0.839). So, Axis 2 could be associated with the overall size of birds when within-developmental mode variation was taken into account.
Major variation of the wing elements data set was associated with the overall size: all wing traits were positively correlated with pFDA Axis 1 (hu = 0.708, ul = 0.667, mass = 0.667, mn = 0.627, fprim = 0.554). Axis 2 of the pFDA of wing elements data set was slightly associated with smaller ul (r = −0.302).
Discrimination with standard linear discriminant function analysis was much better (see ): more than 79% of developmental modes were correctly classified.
Traits that best discriminate flight styles
Flight-style groups were also well separated by phylogenetic MANOVA (wingspan data set: Wilks’s Λ = 0.175, p = 0.002; wing elements data set: Wilks’s Λ = 0.170, p = 0.001). However, phylogenetic discriminant function analysis had weak power. Flight-style groups were predicted in 46% of the wingspan data set and 53% of the wing elements data set. None of the groups occupied a distinct region in the morphospace of discriminant functions ((c) and (d)). Standard, not phylogenetic, discriminant function analysis of flight styles was correct in 75% (wingspan data set) and 82% (wing elements data set) of cases. That was very close to what Wang et al. (Citation2011) reported for wing element traits.
The variation of traits within flight styles was described with three pFDA axes. Axis 1 was associated with 62% and 54% of the variation in the wingspan and wing elements data sets, respectively. Axis 2 explained 27% and 37%, respectively ((c) and (d)).
In our analyses, traits that mostly contribute to discrimination were wingspan and wing area ((c)), and hu ((d)). Body mass played a weaker role in the discrimination of flight styles (Supplementary Table S4). The first and second axes of pFDA of the wingspan data set were mostly moderately correlated with the traits wingspan (Axis 1 r = −0.709; Axis 2 r = −0.406), wing area (Axis 1 r = −0.667; Axis 2 r = −0.495) and body mass (Axis 1 r = −0.605; Axis 2 r = −0.507). The first major axis of variation of the wing elements analysis was slightly correlated with hu (r = −0.427), ul (r = −365), and body mass (r = −0.347). Axis 2 of the wing elements analysis was responsible for the size variation; all traits strongly correlated with it (fprim = 0.884, mn = 0.812, ul = 0.808, mass = 0.767, hu = 0.764).
The outlier on the plot of discriminant function analysis of flight styles with the wing elements data set was the common swift, Apus apus ((d), see the point at x = 7.1, y = −0.3). It is altricial and FG, and has the smallest relative humerus length among the studied birds.
Relative lengths of wing elements
BI was different between developmental modes (standard t-test, pFDR < 0.001). It increased from altricial and intermediate to precocial birds (). Phylogenetic t-test showed significant differences between precocial – altricial (pFDR = 0.018) and precocial – intermediate (pFDR = 0.032). The largest BI was observed in only one of 15 studied bird families with intermediate developmental mode, Alcidae (mean = 1.27), and in many precocials – Gaviidae (1.24), Rallidae (1.19), Anatidae (1.15), Phasianidae (1.11) and Podicipedidae (1.09). Within flight styles, brachial index differed in CF from other flight styles only in a standard t-test (pFDR < 0.001). When phylogeny was taken into account, none of the flight styles differed by BI (phylogenetic t-test, pFDR > 0.120).
Although relative lengths of wing bones and primary feather to ta overlap highly for most of the groups (), we observed high morphological differences in mean group estimates by standard t-test for many groups (Supplementary Table S5). But when phylogeny is taken into account, the only trait that significantly differed was ul/ta (Supplementary Table S6, phylogenetic t-test, pFDR = 0.036): precocials had a smaller relative length of ulna than did birds with an intermediate developmental mode (). All other pairs of developmental modes did not differ by relative length of wing elements (pFDR > 0.169).
Figure 3. Standard discriminant analysis of developmental mode and flight style groups by wingspan (plots (a) and (c)) and wing elements (plots (b) and (d)) data sets. Convex hulls, which connect the most distant points of each group, are shown. Other group points were omitted from the plot. The length of arrows represents the contribution of the traits to discrimination. altr. = altricial; interm. = intermediate; prec. = precocial developmental modes; CF = continuous flapping; FG = flapping and gliding; FS = flapping and soaring; PT = passerine type flight styles; hu = humerus; ul = ulna; mn = manus; fprim = primary feather length.
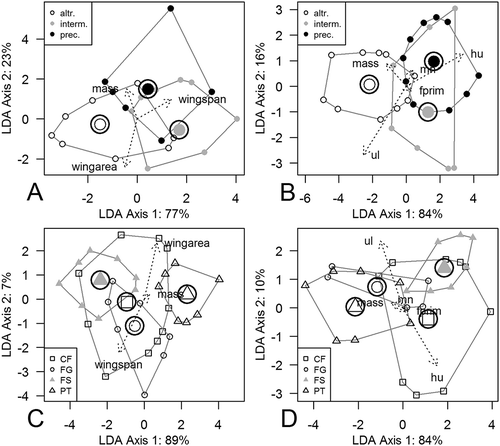
Figure 4. Cleveland’s dot chart of the relative length of wing elements. Each point represents a species. Developmental modes and flight styles are marked with different types of points. altr = altricial; interm = intermediate; prec= precocial developmental modes; CF = continuous flapping; FG = flapping and gliding; FS = flapping and soaring; PT = passerine type flight styles; hu = humerus; ul = ulna; mn = manus; fprim = primary feather; ta = total arm length.
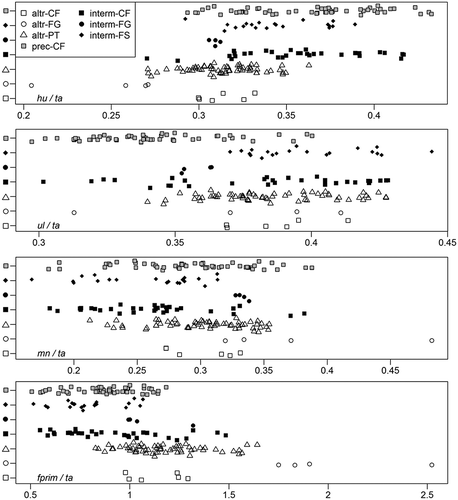
From the phylogenetic point of view, flight styles were also mostly similar by pairwise comparison of the relative length of wing elements (Supplementary Table S6; ). The FG group had significantly smaller relative length of hu when compared to CF birds (Supplementary Table S6, pFDR = 0.024), and significantly larger relative length of fprim when compared to CF and FS groups (pFDR = 0.024). There are only eight FG bird species in our wing elements data set. Four of them are altricial (swift, martins and swallow), and four others are intermediate (falcons). In spite of the small sample size, altricial – FG birds significantly differed from all other birds in the relative length of primary feathers (phylogenetic t-test, Supplementary Table S6, pFDR < 0.016). They had longer fprim. Additionally, altricial – FG birds had a relatively shorter humerus than altricial – PT (pFDR = 0.024), precocial – CF (pFDR = 0.012), and intermediate – CF (pFDR = 0.012) and – FS (pFDR = 0.024).
Discussion
Relationship between development modes and flight styles
The results of our study support the hypothesis of an association between flight style and developmental mode of birds. Transitions in different developmental strategies repeatedly occurred in the evolution of birds and were mediated by changes in overall body size. Body mass was the important variable in discriminating developmental modes of birds (Supplementary Table S4).
The precocial developmental mode, which is considered evolutionarily older (Elzanowski Citation1981; Starck & Ricklefs Citation1998; Zhou & Zhang Citation2004), is associated with the CF flight style. This flight style is typical for birds of medium and large body size. In addition to precocials, similar body size was widely represented among birds with the intermediate developmental mode. However, large birds with intermediate developmental mode (for example, Accipitridae, Pandionidae) use the FS flight style, in contrast to large precocial birds (Otidae) that fly using the CF style. Different flight styles in birds with approximately similar body mass are associated with their habitat, lifestyle and feeding strategy (Shestakova Citation1953). For instance, eagles spend a lot of time in the air, tracking down prey (Flint et al. Citation1967). The most important condition for this type of foraging is the low energy cost for flight maintenance, which corresponds to the FS flight style, with infrequent flapping and using ascending thermal air currents during flight (Shestakova Citation1953). We believe that the evolutionary transition from precociality to altriciality was associated with a wide range of changes in ecological features of birds. Increasing altriciality provided a greater degree of care for the offspring, including feeding chicks, and the possibility of expanding the diversity of lifestyles, adult birds’ feeding strategies and the use of different flight styles.
Evolution to the fully altricial developmental mode was associated with both a decrease in body mass and a transition to other flight styles, mainly PT, which is functionally more advantageous to birds with small body mass (Bruderer et al. Citation2010). The overall decrease in the adult size of altricials may be associated with a shift in habitat from relatively simple, open environments to the complex three-dimensional structure of tree canopies (Dyke & Kaiser Citation2010), increasingly sophisticated nest-building and enhanced nestling-care (Dial Citation2003; Dyke & Kaiser Citation2010). Some of the altricials developed CF flight (Corvidae and Upupidae), but they, as well as other CF birds, are characterized by a greater overall size of wing and body mass.
Based on stochastic evolutionary modeling, transitions from CF or PT flight styles to the FG type were secondary. The FG flight style appeared independently in altricial Apodidae and Hirundinidae, and semialtricial Falconidae. All these birds are agile open-area foragers and able to fly at high speeds (Poznanin Citation1978).
Ecological factors, phylogeny and wing morphology
The answer to the question regarding the impact of ecological factors (flight style and developmental mode) on wing morphology is not so obvious. The developmental modes, as well as flight styles, were discriminated by the values of hu and ul lengths that were both strongly correlated with body mass (). The wingspan and wing area were also correlated with body mass (). However, developmental modes were better discriminated with body mass and flight styles were better discriminated with wingspan (Supplementary Table S4).
Precocials differ from altricials in BI. BI is correlated with phylogeny (Nudds et al. Citation2004). Early-branching Neornithines, including Paleognathes and Galloanseres (“Eoaves”), are characterized by a BI greater than 1, whereas most of the “Neoaves” exhibit a BI of less than 1 (Nudds et al. Citation2004). BI is also associated with wing kinematics (Nudds et al. Citation2007) and is close to unity or larger in diving “Neoaves”, like Gaviidae, Podicipedidae (Nudds et al. Citation2007), Alcidae and Rallidae (Supplementary Table S1). We noted that precocial birds had the largest mean values of the BI whereas altricials had the smallest BI; that can be explained by the influence of phylogeny and wing kinematics. Many precocials are members of “Eoaves” while all altricials are “Neoaves”. An aquatic lifestyle with diving behavior is more typical for birds with precocial or intermediate developmental modes.
Nevertheless, using comparative phylogenetic methods, we revealed that phylogeny highly influenced traits of wing morphology. Phylogenetic discriminant function analysis gave half as many correct predictions for developmental modes and flight styles as standard discriminant function analysis. The cause of this phenomenon is probably that phylogenetically related bird species are ecologically close. Birds within a family, and often within an order, have the same developmental mode and flight style (). On the other hand, the same developmental mode can be observed in species that differ in flight style, as mentioned above. Obscuring of clear ecological adaptation of analyzed wing traits can also be caused by the fact that flight style is more associated with wing shape than with the relative length of skeletal elements (Gladkov Citation1949).
When using phylogenetic t-tests, we did not find an association between relative length of wing elements and different developmental-mode and flight-style groups, except altricial with FG birds. The length of humerus and primary feathers were the most important traits in the discrimination of FG birds ((d)). Nevertheless, wing element proportions changed significantly only in small altricial FG birds; they are characterized by a short humerus and long primary feathers. Falcons followed the same trend (), but they did not statistically differ from the other groups within intermediate or other developmental modes after applying phylogenetic t-test. Thus, we assume that the evolutionary shift from CF or PT to high-speed FG flight style was associated with changes in wing shape only in small, altricial birds (swallows, martins, swifts). The above-mentioned features of wing morphology contributed to the adaptation to long and high-speed flight in these birds. Karhu (Citation1992) noted that the shortening humerus in swifts made the wing lighter and, therefore, increased the effect of reducing the moment of inertia. Strong elongation of primary feathers contributed to the overall lengthening of the wing that reduces induced drag and, consequently, decreases required power and energy costs during the flight (Karhu Citation1992). Lengthening of the wing due to the primary feathers is especially advantageous since it occurs with a minimal increase in body mass. In addition, long primary feathers allow for a narrower wing apex and decrease the depth of the wing profile, thus reducing total drag (Karhu Citation1992).
It should be noted that features of flight styles and proportions of wing elements in birds with different developmental modes require further investigation. This is due to the somewhat artificial separation of developmental modes and flight styles and the necessity for more detailed analysis of the anatomical structure of the wing. The group of intermediate birds analyzed in this article is very extensive and includes species with a wide range of body mass and flight styles. We assume that a more detailed analysis within this group will reveal new relationships. Bird species with the continuous flapping flight style can vary greatly in flight characteristics. For example, representatives of Galliformes and Corvidae have a continuous flapping flight style, although they differ significantly in flight traits. Gladkov (Citation1947, Citation1949) showed that representatives of Galliformes have a special type of flapping flight, which was characterized by noisy takeoff and rapid but short flight; representatives of Corvidae fly by steadily flapping wings.
We conclude that evolution within the altricial–precocial spectrum is associated not only with the coevolution of reproductive strategy, including egg composition (Deeming Citation2007) and energetics (Sibly et al. Citation2012), clutch mass (Birchard et al. Citation2013), features of embryonic development (Starck & Ricklefs Citation1998; Kovtun et al. Citation2008), and many aspects of nestling anatomy, physiology and behavior (Portmann Citation1935; Nice Citation1962; Ricklefs Citation1983; Starck Citation1993; Starck & Ricklefs Citation1998), but also with changes in body size and specific flight styles.
Supplementary Material
Download PDF (304.2 KB)Acknowledgements
The authors thank X. Wang and F. Liechti for submitted data that were used in the analysis, and O. P. Melnik for permission to work with collections. We thank I. Dzeverin, M. F. Kovtun and I. A. Bogdanovich for valuable comments and discussion.
Supplemental data
Supplemental data for this article can be accessed here: https://doi.org/10.1080/24750263.2017.1329359.
References
- Alerstam T, Rosén M, Bäckman J, Ericson PGP, Hellgren O, Sheldon B. 2007. Flight speeds among bird species: Allometric and phylogenetic effects. PLoS Biology 5:1656–1662. DOI:10.1371/journal.pbio.0050197.
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B 57:289–300.
- Birchard GF, Ruta M, Deeming DC. 2013. Evolution of parental incubation behaviour in dinosaurs cannot be inferred from clutch mass in birds. Biology Letters 9:20130036. DOI:10.1098/rsbl.2013.0036.
- Bruderer B, Dieter P, Boldt A, Liechti F. 2010. Wing-beat characteristics of birds recorded with tracking radar and cine camera. Ibis 152:272–291. DOI:10.1111/j.1474-919X.2010.01014.x.
- Burnham KP, Anderson DR. 2002. Model selection and inference: A practical information-theoretic approach. New York, NY: Springer Verlag.
- Carey JR, Adams J. 2001. The preadaptive role of parental care in the evolution of avian flight. Archaeopteryx 19:97–108.
- Deeming DC. 2007. Allometry of mass and composition in bird eggs: Effects of phylogeny and hatchling maturity. Avian and Poultry Biology Reviews 18:71–86. DOI:10.3184/147020607X289022.
- Dial KP. 2003. Evolution of avian locomotion: Correlates of flight style, locomotor modules, nesting biology, body size, development, and the origin of flapping flight. The Auk 120:941–952. DOI:10.1642/0004-8038(2003)120[0941:EOALCO]2.0.CO;2.
- Drummond AJA, Suchard MAM, Xie DD, Rambaut AA. 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Molecular Biology and Evolution 29:1969–1973. DOI:10.1093/molbev/mss075.
- Dyke GJ, Kaiser GW. 2010. Cracking a developmental constraint: Egg size and bird evolution. Records of the Australian Museum 62:207–216. DOI:10.3853/j.0067-1975.62.2010.1547.
- Elzanowski A. 1981. Embryonic bird skeletons from the late Cretaceous of Mongolia. Acta Palaeontologica Polonica 42:147–176.
- Flint VE, Boeme RL, Kostin YV, Kuznetsov AA. 1967. Birds of the USSR. Moscow: Mysl. (In Russian).
- Gladkov NA. 1947. Connection between the anatomical structure of the wing and character of the flight of birds. Proceedings of the USSR Academy of Sciences (Biology Series) 1:139–155. (in Russian).
- Gladkov NA. 1949. Biological principles of bird flight. Moscow: Izdatelstvo MOIP. (in Russian).
- Hackett SJ, Kimball RT, Reddy S, Bowie RCK, Braun EL, Braun MJ, Chojnowski JL, Cox WA, Han K-L, Harshman J, Huddleston CJ, Marks BD, Miglia KJ, Moore WS, Sheldon FH, Steadman DW, Witt CC, Yuri T. 2008. A phylogenomic study of birds reveals their evolutionary history. Science 320:1763–1768. DOI:10.1126/science.1157704.
- Harmon LJ, Weir JT, Brock CD, Glor RE, Challenger W. 2008. GEIGER: Investigating evolutionary radiations. Bioinformatics 24:129–131. DOI:10.1093/bioinformatics/btm538.
- Huelsenbeck JP, Nielsen R, Bollback JP. 2003. Stochastic mapping of morphological characters. Systematic Biology 52:131–158. DOI:10.1080/10635150390192780.
- Jetz WW, Thomas GHG, Joy JBJ, Hartmann KK, Mooers AOA. 2012. The global diversity of birds in space and time. Nature 491:444–448. DOI:10.1038/nature11631.
- Karhu AA. 1992. Phylogeny of order Apodiformes. Thesis abstract for a candidate degree in biological sciences, speciality 04.00.09. Moscow. (in Russian). Available: http://dlib.rsl.ru/viewer/01000131241#?page=15. Accessed June 2017 20.
- Kovtun MF, Shatkovska OV, Shatkovsky Y. 2008. Formation of the skull and the skeleton of limbs of birds in embryogenesis. Kiev: Naukova dumka. (in Ukrainian).
- Nice MM. 1962. Development of behavior in precocial birds. Transactions of the Linnean Society (NY) 8:1–211.
- Nudds RL, Dyke GJ, Rayner JMV. 2007. Avian brachial index and wing kinematics: Putting movement back into bones. Journal of Zoology 272:218–226. DOI:10.1111/jzo.2007.272.issue-2.
- Nudds RL, Dyke J, Rayner JMV. 2004. Forelimb proportions and the evolutionary radiation of Neornithes. Proceedings of the Royal Society B: Biological Sciences 271:S324–327. DOI:10.1098/rsbl.2004.0167.
- O’Connor RJ. 1984. The growth and development of birds. Chichester: Wiley.
- Orme D, Freckleton R, Thomas G, Petzoldt T, Fritz S, Isaac N, Pearse W. 2013. caper: Comparative Analyses of Phylogenetics and evolution in R. R package version 0.5.2. Available: https://CRAN.R-project.org/package=caper. Accessed June 2017 20.
- Pagel M. 1999. Inferring the historical patterns of biological evolution. Nature 401:877–884. DOI:10.1038/44766.
- Pennycuick CJ. 1996. Wingbeat frequency of birds in steady cruising flight: New data and improved predictions. Journal of Experimental Biology 199:1613–1618.
- Pennycuick CJ. 2001. Speeds and wingbeat frequencies of migrating birds compared with calculated benchmarks. Journal of Experimental Biology 204:3283–3294.
- Portmann A. 1935. Die Ontogenese der Vögel als Evolutionsproblem. Acta Biotheor 1:59–90. DOI:10.1007/BF02324297.
- Poznanin LP. 1978. Ecological aspects of evolution of birds. Moscow: Nauka. (in Russian).
- R Core Team. 2014. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available: http://www.R-project.org/. Accessed June 2017 20.
- Rayner JMV. 1988. Form and function in avian flight. Current Ornithology 5:1–66.
- Revell LJ. 2012. Phytools: An R package for phylogenetic comparative biology (and other things). Methods in Ecology and Evolution 3:217–223. DOI:10.1111/j.2041-210X.2011.00169.x.
- Ricklefs RE. 1983. Avian postnatal development. In: Farner DS, King JR, Parkes KC, editors. Avian biology. New York: Academic Press. pp. 1–83.
- Savile DBO. 1957. Adaptive evolution in the avian wing. Evolution 11:212–224. DOI:10.1111/j.1558-5646.1957.tb02889.x.
- Schmitz L, Motani R. 2011. Nocturnality in dinosaurs inferred from scleral ring and orbit morphology. Science 332:705–708. DOI:10.1126/science.1200043.
- Shestakova GS. 1953. Morpho-biological analysis as a method of studying the flight of birds. Trudy instituta morfologii zhivotnykh im. A. N. Severtsova 9:3–13.
- Sibly RM, Witt CC, Wright NA, Venditti C, Jetze W, Brown JH. 2012. Energetics, lifestyle, and reproduction in birds. Proceedings of the National Academy of Sciences 109:10937–10941. DOI:10.1073/pnas.1206512109.
- Simons ELR. 2010. Forelimb skeletal morphology and flight mode evolution in pelecaniform birds. Zoology 113:39–46. DOI:10.1016/j.zool.2009.05.002.
- Starck JM. 1993. Evolution of avian ontogenies. Current Ornithology 10:275–366.
- Starck JM, Ricklefs RE. 1998. Avian growth and development: Evolution within the Altricial Precocial Spectrum. New York: Oxford University Press.
- Venables WN, Ripley BD. 2002. Modern applied statistics with S. 4th ed. New York: Springer.
- Verheyen R. 1961. A new classification for the non-passerine birds of the world. Bulletin de l’Institut Royal des Sciences Naturelles de Belgique 38:1–36.
- Wang X, McGowan AJ, Dyke GJ. 2011. Avian wing proportions and flight styles: First step towards predicting the flight modes of Mesozoic birds. PLoS ONE 6:e28672. DOI:10.1371/journal.pone.0028672.
- Warrick DR, Dial KP, Biewener AA. 1998. Asymmetrical force production in the slow maneuvering flight of birds. Auk 115:916–928. DOI:10.2307/4089510.
- Zhou Z, Zhang F. 2004. A precocial avian embryo from the lower Cretaceous of China. Science 306:653. DOI:10.1126/science.1100000.
