Abstract
In the present review, we outline the relationship between starvation and taste-aversive learning (conditioned taste aversion: CTA) in the pond snail Lymnaea stagnalis and introduce the “necessity knows no law” concept. When snails were food-deprived for a short period, the snails learned and formed memory of CTA well, whereas when snails were food-deprived for a prolonged period, the snails appeared not to learn CTA or form long-term memory (LTM) of it. However, in severely food-deprived snails (i.e. snails that were food-deprived for a prolonged period), memory was found to indeed form but was overpowered by the effect of severe food deprivation. That is, snails are partially restricted in the “necessity knows no law” concept. Moreover, this CTA-LTM was context dependent and was observed only when the snails were in a context similar to that in which the training occurred. In addition, when insulin was injected into the severely food-deprived snails, they started to exhibit learning and memory. That is, insulin rescued the snails’ “hidden” ability of memory retrieval. In addition to these topics in snails, we survey the literature on starvation and learning obtained in other animals for general discussion. We hope that this review will stimulate further detailed studies of motivation in invertebrates.
Introduction
W. G. Quinn described that “an ideal subject for studying the cellular and molecular bases of learning should, probably, have ten large nerve cells, ten genes, a generation time of 1 week, and the ability to play a cello and recite Shakespeare” (quoted in Dudai Citation1989, p. 49). Although it is hard to find animals that can play a cello and recite Shakespeare, molluscan gastropods, such as Aplysia, Hermissenda, Limax, Lymnaea and so forth, have been widely recognized as useful animals with which to study the molecular and cellular mechanisms underlying learning and memory (Kandel & Tauc Citation1965; Gelperin Citation1975; Alkon Citation1976; Kemenes & Benjamin Citation1989; Ito et al. 1994, 2013; Lukowiak et al. Citation1996; Kobayashi et al. Citation1998; Kojima et al. Citation1998; Fujie et al. Citation2005; Martens et al. Citation2007). For example, after the central pattern generator of feeding behavior was discovered in the pond snail Lymnaea stagnalis in 1979 (Benjamin & Rose Citation1979; Rose & Benjamin Citation1979), many attempts to clarify the cellular and molecular mechanisms underlying taste-appetitive and -aversive learnings have been carried out (Benjamin et al. Citation2000).
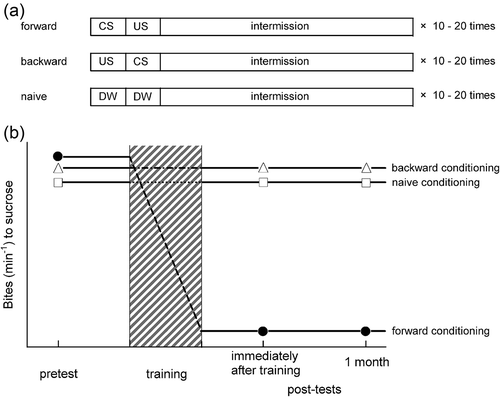
Our research group has particularly studied one kind of taste-aversive learning, called conditioned taste aversion (CTA), in Lymnaea (Yamanaka et al. Citation1999; Wagatsuma et al. Citation2004; Kita et al. Citation2011; Otsuka et al. Citation2013; Sunada et al. Citation2017a). Innately, sweet food attracts various kinds of animals, as it is one of the sources that can be converted into energy in the body. Likewise in snails, a sucrose solution evokes the feeding response (i.e. the number of bites in response to sucrose), and thus a sucrose solution can be used as a conditioned stimulus (CS) in our conditioning paradigm (Sadamoto et al. Citation2000; Sugai et al. Citation2006). On the other hand, an aversive stimulus (e.g. a potassium chloride [KCl] solution or an electric shock) induces a withdrawal response in snails. A withdrawal response is a very important behavior for snails, and so when the snail body withdraws into the shell, the feeding response is also stopped. Thus, an aversive stimulus inducing a withdrawal response can be used as an unconditioned stimulus (US) in our paradigm (Nakamura et al. Citation1999; Ito et al. Citation2015b; Takigami et al. Citation2016). In the CTA paradigm, the CS is paired with the US. After repeated temporally contingent presentations of the CS and US, the CS no longer elicits a feeding response (i.e. an unconditioned response), and this aversive conditioning persists for at least a month as long-term memory (LTM) (Kojima et al. Citation2001; Ito et al. Citation2012) (). Further, whether or not CTA and its consolidated LTM will form depends on the snail’s internal state (i.e. starvation or satiety), and indeed the members of the Ito laboratory have uncovered some important phenomena regarding this issue (Mita et al. Citation2014a; Yamagishi et al. Citation2015).
In the present review, we discuss the relationship between starvation and CTA performance in snails. We outline the following points: (1) One-day food-deprived snails can learn CTA and form its LTM well. (2) However, the 5-day food-deprived snails indeed form CTA-LTM but do not exhibit its memory phenotype. Here we introduce the “necessity knows no law” concept for such extremely hungry snails, because they cannot restrain themselves from eating the food they must not eat. (3) The CTA-LTM in snails is controlled by insulin working in the central nervous system (CNS). For example, the poor scores for CTA in the 5-day food-deprived snails are improved by an injection of insulin. We also introduce the changes in dopamine and octopamine contents in the CNS during food deprivation. (4) Finally, we survey the literature on the relationship between starvation and learning in vertebrates and invertebrates for general discussion.
Figure 1. Training paradigm for conditioned taste aversion (CTA) and learning performance. (a) Three types of trainings. Forward: a conditioned stimulus (CS, a sucrose solution) is paired with an unconditioned stimulus (US), a KCl solution or an electric shock. After the application of the US, an intermission is needed. This forward training is CTA training. Backward: the US is paired with the CS, and after the application of the CS, the same intermission as for forward training is needed. The duration of the intermission was set, as this backward conditioning does not bring about learning or memory. That is, the backward conditioning is a control procedure. Naïve: both the CS and the US are replaced by the application of distilled water (DW). This is also a control procedure. (b) Schematic presentations of the changes in the number of feeding responses (bites/min) in response to the sucrose CS before and after training. At the pretest, there are no significant differences in the feeding responses among the three cohorts (i.e. forward, backward and naïve snails). After the training, the feeding response to sucrose in forward snails is suppressed, and this suppression remains for at least 1 month. The scale of the y-axis is arbitrary (the expression of the y-axis is similar in the other figures).
Starvation and learning performance: Yerkes–Dodson/Hebb law
Empirically, we have so far noticed that modestly food-deprived snails are better capable of taste-aversion learning (i.e. CTA) and forming LTM than satiated snails, whereas severely food-deprived snails cannot form CTA-LTM (Sugai et al. Citation2007). Thus, snails are usually trained with a conditioning paradigm after 1-day (i.e. modest) food deprivation (Hatakeyama et al. Citation2006) ()). These 1-day food-deprived snails are called “Day 1 snails”. These learning and memory-forming abilities can be explained by an inverted-U law in which there is an optimal level of arousal for learning (Yerkes & Dodson Citation1908; Hebb Citation1955; Ito et al. Citation2015b). Stress alters learning and memory formation. Yerkes and Dodson (Citation1908) stated that “an easily acquired habit may be readily formed under strong stimulation, whereas a difficult habit may be acquired only under relatively weak stimulation” (Yerkes & Dodson Citation1908). Hebb (Citation1955) discussed stress and memory using the words of inverted-U law (Hebb Citation1955). That is, 1-day food deprivation (hereafter Day 1) is an optimal level of stress that results in the best memory, whereas 5-day food deprivation (hereafter Day 5) is a stress that results in poorer memory formation and/or its recall.
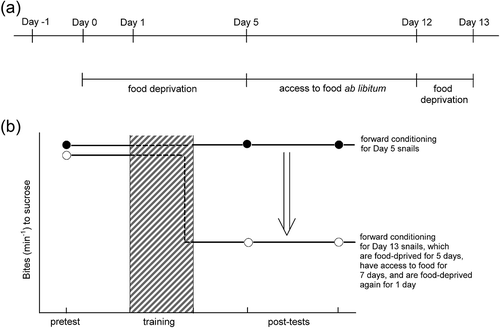
Figure 2. The “necessity knows no law” concept in food-deprived snails. (a) Definition of food-deprived snails. The day on which the snails start food deprivation is Day 0. Day −1 snails have access to food ad libitum. Day 1 snails are the modestly food-deprived snails, which were food-deprived for only 1 day. Day 5 snails are the severely food-deprived snails. To test the hypothesis that Day 5 snails would indeed learn conditioned taste aversion (CTA) and form its long-term memory (LTM), the snails were given access to food ad libitum for 7 days following the 5-day food deprivation and CTA training, and then they were food-deprived for 1 day. Finally, they were tested by an application of the sucrose CS (i.e. Day 13 snails), resulting in the exhibition of CTA-LTM. (b) Schematic presentations of the differences in the number of feeding responses (bites/min) in response to the sucrose CS between Day 5 snails and Day 13 snails. As mentioned above, Day 13 snails recall their “hidden” memory by a context of “starvation” (i.e. the final 1-day food deprivation). That is, the feeding response to sucrose in Day 13 snails is suppressed. This figure shows that the memory phenotype is established on Day 12, and thus a suppression of bites occurs just before the first post-test.
Necessity knows no law concept in severely food-deprived snails
The memory phenotype in severely food-deprived snails was carefully studied. We considered that severely food-deprived snails might in fact learn CTA and form LTM, but that the severe deprivation would block their performance that acts on their memory and thus snails would be expected to exhibit “necessity knows no law” behavior (Ito et al. Citation2015a). The concept of “necessity knows no laws” has been considered an English proverb since the 1550s (see the Oxford English Dictionary). It basically means that “during a famine a very honest person may break the law to feed their children”, or “if one is desperate one may have to do illegal things”. Thus, if snails are fed until 1 day before CTA training (i.e. 1-day food deprivation) as usual, they will adhere to the rule and not eat (i.e. CTA). However, if snails are 5-day food-deprived (i.e. being desperate), they will break the rule and eat (Ito et al. Citation2015a). In other words, Day 5 snails were expected to learn CTA but not to exhibit its memory phenotype.
To examine this hypothesis, Day 5 snails were trained under various conditions. One condition included the idea that food deprivation produces a very specific behavioral state, which was considered to be the context (Palmer & Kristan Citation2011; D’yakonova Citation2014). In this case, the context was the state associated with food deprivation (Ito et al. Citation2015a). To be specific, Day 5 snails were trained with the CTA training paradigm (i.e. pairings of 10 mM sucrose CS and 3-s electric shock US). After 20 pairings of the CS and US in a single day, the snails were given access to food for 7 days right after the training. When these snails were tested at this point, they did not exhibit CTA memory. However, this 7-day period was followed with a 1-day food deprivation period, and then the snails were tested again (i.e. an application of the CS; )). The snails exhibited CTA memory, which was the suppression of the feeding response to the CS ()). It was concluded that the memory was indeed formed but that the severe food deprivation rendered the snails unable to act on that memory. That is, the memory retrieval and the memory performance are differentiated even in snails. The severely food-deprived snails have the ability of memory retrieval, but they do not exhibit the memory performance (i.e. the suppression of feeding response to the sucrose CS) without a motive (i.e. a modest starvation).
Moreover, this CTA-LTM was judged to be context dependent and was observed only when the snails were in a context similar to that in which the training occurred. That is, when the memory test was conducted right after the 7 days of food access and without depriving the snails for 1 day before the test, the snails did not exhibit CTA memory. Therefore, the phenomenon is considered to be “a consequence of conflict resolution”, and so the snails are indeed subject to the “necessity knows no law” concept (Ito et al. Citation2015a).
Here the possible biological functions of the “necessity knows no law” concept in snails are discussed. Generally, animals avoid poisonous substances to keep their own safety. That is, food preference is a kind of self-defense mechanism. CTA allows snails to learn that sucrose signals the delivery of shock, and to withdraw from it even despite the fact that it is a substance that they innately like and consume. Snails that are severely food-deprived (i.e. Day 5) must eat something to survive, even if that something has become a reliable predictor of an aversive event such as an electric shock. The fact that the memory phenotype was not exhibited in Day 5 snails has a biological meaning that has to do with the “necessity knows no law” concept (Ito et al. Citation2015a).
Rescue of poor learning in severely food-deprived snails by insulin
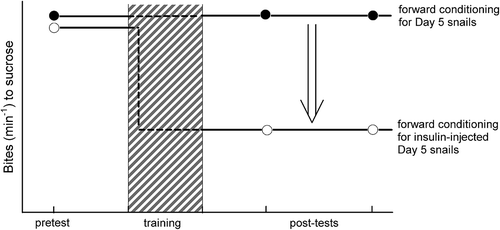
A snail’s behavior is partially restricted in the “necessity knows no law” concept. On the other hand, our research group found that insulin plays an important role in the consolidation of LTM (Azami et al. Citation2006; Hatakeyama et al. Citation2013; Murakami et al. Citation2013a; Kojima et al. Citation2015). The experiments were designed to inject 100 nM insulin into severely food-deprived snails (i.e. Day 5 snails), which were poor learners for CTA (Mita et al. Citation2014b). Then these snails were trained with the CTA paradigm using a sucrose solution and electric shock. As expected, CTA-LTM was formed (), whereas the control experiments (i.e. vehicle injection) failed to form CTA-LTM. That is, insulin rescued the ability of “hidden” learning and memory. On the other hand, when an insulin receptor antibody was injected into modestly food-deprived snails (i.e. Day 1 snails), which readily learned CTA, they did not form a memory (Murakami et al. Citation2013b). It was concluded that, in snails, insulin plays a key role in learning and memory in starved states. Further, we would like to point out that intranasal insulin is used for the prevention or the symptomatic treatment of Alzheimer’s disease in humans (Ribe & Lovestone Citation2016).
Figure 3. Improvement of the poor performance of conditioned taste aversion (CTA) in severely food-deprived snails by insulin injection. The injection of insulin before CTA training rescued CTA ability in Day 5 snails. Here, schematic presentations of insulin’s effects are depicted. As can be seen in comparison with , the memory phenotype is thought to appear earlier in the training session because of insulin’s effect.
However, this effect of insulin on learning and memory varies in other animals. For example, in Drosophila, flies show aversive taste learning in a food-deprivation state induced for 9–16 hours before the conditioning, corresponding very well to the state of our Day 1 snails. This was thought to be due to the downregulation of the insulin signaling pathway and the upregulation of the signaling pathway of cAMP-regulated transcriptional coactivator (CRTC) (Altarejos & Montminy Citation2011; Hirano et al. Citation2013). Hirano and Saitoe (Citation2013) explained this mechanism as follows (Hirano & Saitoe Citation2013). Blocking the activity of cyclic-AMP response element binding protein (CREB) in the memory center, mushroom bodies, impairs this fasting-LTM. CREB is well known to be required for LTM, and CREB requires co-activators, one of which is CRTC to activate transcription. The fasting-LTM in flies is dependent on CRTC. On the other hand, CRTC is regulated by insulin signaling. During fasting, insulin signaling is suppressed, which in turn activates CRTC. Hirano and Saitoe concluded that reduced insulin signaling is essential for CRTC-dependent fasting-LTM formation (Hirano & Saitoe Citation2013). On the other hand, when facing severe food deprivation, flies abolish the formation of aversive LTM (Plaçais & Preat Citation2013). The correspondence between our Day 5 snails and very severely food-deprived flies is still unsolved.
Effects of starvation on general learning and memory in mammals – two important regions in the mammalian brain: the hippocampus and the amygdala
There is a limited number of studies that explore the relationship between starvation and CTA in rats, for example the studies about anorexia nervosa (Liang et al. Citation2011). Liang et al. (Citation2011) showed that experience with anorexia nervosa-like behaviors results in an acquired aversion to a preferred food sooner and a longer retention of the negative food associations. This study remained at the behavioral level. Thus, we should expand our discussion to the relationship between starvation and general learning and memory in mammals. In studies using mammals, two specific brain regions, the hippocampus and the amygdala, are noted.
In mammals, several brain regions are well known to play important roles in learning and memory (Izquierdo et al. Citation2016; Schwabe Citation2017), and interestingly some functions of those brain regions are regulated by starvation. For example, the hippocampus is known to be affected by hunger signals, and thus it is thought to regulate food intake (Davidson & Jarrard Citation1993). Further, food-restricted rats showed higher long-term potentiation at hippocampal CA1 excitatory synapses compared with rats fed ad libitum, and these food-restricted rats showed a reinforcement of long-term spatial memory (Talani et al. Citation2016). These effects of food restriction on learning and memory may involve changes in the expression of cannabinoid type-1 receptors in the hippocampus. The hippocampal cannabinoid system is thought to control cognition, behavior and some diseases like epilepsy (Lupica et al. Citation2017). Indeed, the involvement of cannabinoid receptors in learning and memory has also been shown to be important in snails (Sunada et al. Citation2017b).
The relationship between a kind of associative learning, fear conditioning and starvation has also been examined in mammals. The main brain region controlling fear conditioning is the amygdala in mammals. Recent studies revealed that short-term food deprivation before fear conditioning impaired long-term fear memory, whereas food deprivation before fear extinction facilitated extinction learning (Verma et al. Citation2016). Another research group clarified that food deprivation enhanced fear extinction and elucidated the relationship between deprivation and extinction (Huang et al. Citation2016). Using pharmacological techniques, Huang’s group further explored the features of fear extinction and long-term depression at the lateral amygdala synapses.
These two main regions in the mammalian brain, the hippocampus and the amygdala, change their functions as a result of starvation. In invertebrates like Lymnaea, used in the present study, as well as in vertebrates, the mechanisms underlying learning and memory are affected by starvation, telling us that the internal state of experimental animals is an important factor for brain function.
Effects of monoamine level on CTA-LTM in snails
In both vertebrates and invertebrates, dopamine functions as a reward system in the brain (Mizunami et al. Citation2015; Bourgeois et al. Citation2016). Some psychological studies paid attention to the difference in the dopamine system between food-satiated and food-deprived states (Nader et al. Citation1997). Recently, the effects of dopamine/octopamine level on CTA-LTM were examined at various starvation states in Lymnaea (Aonuma et al. 2016, 2017). Modestly food-deprived (Day 1) snails were the best learners and had significantly lower dopamine/octopamine levels compared to snails under the other conditions. In contrast, severely food-deprived (Day 5) snails showed poor performance and had higher dopamine/octopamine levels. That is, severe starvation increased the dopamine/octopamine levels in the Lymnaea CNS. In particular, dopamine functions as a reward transmitter as described above, and thus CTA in the severely deprived snails was thought to be mitigated by a high dopamine level.
Conclusion
Starvation causes a change in learning performance in CTA of snails. As a result, snails eat what they should not eat, even though they know they should not eat it. The reason that they “know” it is easily understandable by the fact that the “hidden” memory was recalled in a context-dependent manner. On the other hand, insulin is a de novo target molecule for studies of learning and memory. Insulin plays an important role in improvement of deficits of learning and memory-forming abilities in snails.
Additional information
Funding
References
- Alkon DL. 1976. Neural modification by paired sensory stimuli. Journal of General Physiology 68:341–358. DOI:10.1085/jgp.68.3.341.
- Altarejos JY , Montminy M. 2011. CREB and the CRTC co-activators: Sensors for hormonal and metabolic signals. Nature Reviews Molecular Cellular Biology 12:141–151. DOI:10.1038/nrm3072.
- Aonuma H , Kaneda M , Hatakeyama D , Watanabe T , Lukowiak K , Ito E . 2016. Relationship between the grades of a learned aversive-feeding response and the dopamine contents in Lymnaea . Biology Open 5:1869–1873. DOI:10.1242/bio.021634.
- Aonuma H , Kaneda M , Hatakeyama D , Watanabe T , Lukowiak K , Ito E . 2017. Weak involvement of octopamine in aversive taste learning in a snail. Neurobiology of Learning and Memory 141:189–198. DOI:10.1016/j.nlm.2017.04.010.
- Azami S , Wagatsuma A , Sadamoto H , Hatakeyama D , Usami T , Fujie M , Koyanagi R , Azumi K , Fujito Y , Lukowiak K , Ito E . 2006. Altered gene activity correlated with long-term memory formation of conditioned taste aversion in Lymnaea . Journal of Neuroscience Research 84:1610–1620. DOI:10.1002/jnr.21045.
- Benjamin PR , Rose RM . 1979. Central generation of bursting in the feeding system of the snail, Lymnaea stagnalis . Journal of Experimental Biology 80:93–118.
- Benjamin PR , Staras K , Kemenes G . 2000. A systems approach to the cellular analysis of associative learning in the pond snail Lymnaea . Learning and Memory 7:124–131. DOI:10.1101/lm.7.3.124.
- Bourgeois A , Chelazzi L , Vuilleumier P . 2016. How motivation and reward learning modulate selective attention. Progress in Brain Research 229:325–342. DOI:10.1016/bs.pbr.2016.06.004.
- D’yakonova VE . 2014. Neurotransmitter mechanisms of context-dependent behavior. Neuroscience and Behavioral Physiology 44:256–267. DOI:10.1007/s11055-014-9905-6.
- Davidson TL , Jarrard LE . 1993. A role for hippocampus in the utilization of hunger signals. Behavioral and Neural Biology 59:167–171. DOI:10.1016/0163-1047(93)90925-8.
- Dudai Y . 1989. The neurobiology of memory: Concepts, findings, trends. Oxford, UK: Oxford University Press.
- Fujie S , Yamamoto T , Murakami J , Hatakeyama D , Shiga H , Suzuki N , Ito E . 2005. Nitric oxide synthase and soluble guanylyl cyclase underlying the modulation of electrical oscillations in a central olfactory organ. Journal of Neurobiology 62:14–30. DOI:10.1002/(ISSN)1097-4695.
- Gelperin A . 1975. Rapid food-aversion learning by a terrestrial mollusk. Science 189:567–570. DOI:10.1126/science.1145215.
- Hatakeyama D , Okuta A , Otsuka E , Lukowiak K , Ito E . 2013. Consolidation of long-term memory by insulin in Lymnaea is not brought about by changing the number of insulin receptors. Communicative and Integrative Biology 6:e23955. DOI:10.4161/cib.23955.
- Hatakeyama D , Sadamoto H , Watanabe T , Wagatsuma A , Kobayashi S , Fujito Y , Yamashita M , Sakakibara M , Kemenes G , Ito E . 2006. Requirement of new protein synthesis of a transcription factor for memory consolidation: Paradoxical changes in mRNA and protein levels of C/EBP. Journal of Molecular Biology 356:569–577. DOI:10.1016/j.jmb.2005.12.009.
- Hebb DO . 1955. Drives and the C.N.S. (conceptual nervous system). Psychological Review 62:243–254. DOI:10.1037/h0041823.
- Hirano Y , Masuda T , Naganos S , Matsuno M , Ueno K , Miyashita T , Horiuchi J , Saitoe M . 2013. Fasting launches CRTC to facilitate long-term memory formation in Drosophila . Science 339:443–446. DOI:10.1126/science.1227170.
- Hirano Y , Saitoe M . 2013. Hunger and memory; CRTC coordinates long-term memory with the physiological state, hunger. Communicative and Integrative Biology 6:e25152. DOI:10.4161/cib.25152.
- Huang CC , Chou D , Yeh CM , Hsu KS . 2016. Acute food deprivation enhances fear extinction but inhibits long-term depression in the lateral amygdala via ghrelin signaling. Neuropharmacology 101:36–45. DOI:10.1016/j.neuropharm.2015.09.018.
- Ito E , Kojima S , Lukowiak K , Sakakibara M . 2013. From likes to dislikes: Conditioned taste aversion in the pond snail Lymnaea stagnalis . Canadian Journal of Zoology 91:405–412. DOI:10.1139/cjz-2012-0292.
- Ito E , Oka K , Collin C , Schreurs BG , Sakakibara M , Alkon DL . 1994. Intracellular calcium signals are enhanced for days after Pavlovian conditioning. Journal of Neurochemistry 62:1337–1344. DOI:10.1046/j.1471-4159.1994.62041337.x.
- Ito E , Otsuka E , Hama N , Aonuma H , Okada R , Hatakeyama D , Fujito Y , Kobayashi S . 2012. Memory trace in feeding neural circuitry underlying conditioned taste aversion in Lymnaea . PLoS ONE 7:e43151. DOI:10.1371/journal.pone.0043151.
- Ito E , Yamagishi M , Hatakeyama D , Watanabe T , Fujito Y , Dyakonova V , Lukowiak K . 2015a. Memory block: A consequence of conflict resolution. Journal of Experimental Biology 218:1699–1704. DOI:10.1242/jeb.120329.
- Ito E , Yamagishi M , Takigami S , Sakakibara M , Fujito Y , Lukowiak K . 2015b. The Yerkes-Dodson law and appropriate stimuli for conditioned taste aversion in Lymnaea . Journal of Experimental Biology 218:336–339. DOI:10.1242/jeb.113266.
- Izquierdo I , Furini CR , Myskiw JC . 2016. Fear memory. Physiological Reviews 96:695–750. DOI:10.1152/physrev.00018.2015.
- Kandel ER , Tauc L . 1965. Heterosynaptic facilitation in neurones of the abdominal ganglion of Aplysia depilans . Journal of Physiology 181:1–27. DOI:10.1113/jphysiol.1965.sp007742.
- Kemenes G , Benjamin PR . 1989. Appetitive learning in snails shows characteristics of conditioning in vertebrates. Brain Research 489:163–166. DOI:10.1016/0006-8993(89)90019-X.
- Kita S , Hashiba R , Ueki S , Kimoto Y , Abe Y , Gotoda Y , Suzuki R , Uraki E , Nara N , Kanazawa A , Hatakeyama D , Kawai R , Fujito Y , Lukowiak K , Ito E . 2011. Does conditioned taste aversion learning in the pond snail Lymnaea stagnalis produce conditioned fear? Biological Bulletin 220:71–81. DOI:10.1086/BBLv220n1p71.
- Kobayashi S , Kojima S , Yamanaka M , Sadamoto H , Nakamura H , Fujito Y , Kawai R , Sakakibara M , Ito E . 1998. Operant conditioning of escape behavior in the pond snail, Lymnaea stagnalis . Zoological Science 15:683–690. DOI:10.2108/zsj.15.683.
- Kojima S , Hosono T , Fujito Y , Ito E . 2001. Optical detection of neuromodulatory effects of conditioned taste aversion in the pond snail Lymnaea stagnalis . Journal of Neurobiology 49:118–128. DOI:10.1002/neu.1069.
- Kojima S , Kobayashi S , Yamanaka M , Sadamoto H , Nakamura H , Fujito Y , Kawai R , Sakakibara M , Ito E . 1998. Sensory preconditioning for feeding response in the pond snail, Lymnaea stagnalis . Brain Research 808:113–115. DOI:10.1016/S0006-8993(98)00823-3.
- Kojima S , Sunada H , Mita K , Sakakibara M , Lukowiak K , Ito E . 2015. Function of insulin in snail brain in associative learning. Journal of Comparative Physiology A 201:969–981. DOI:10.1007/s00359-015-1032-5.
- Liang NC , Bello NT , Moran TH . 2011. Experience with activity based anorexia enhances conditioned taste aversion learning in rats. Physiology & Behavior 102:51–57. DOI:10.1016/j.physbeh.2010.10.004.
- Lukowiak K , Ringseis E , Spencer G , Wildering W , Syed N . 1996. Operant conditioning of aerial respiratory behaviour in Lymnaea stagnalis . Journal of Experimental Biology 199:683–691.
- Lupica CR , Hu Y , Devinsky O , Hoffman AF . 2017. Cannabinoids as hippocampal network administrators. Neuropharmacology. pii:S0028-3908(17)30140-5. DOI:10.1016/j.neuropharm.2017.04.003.
- Martens K , Amarell M , Parvez K , Hittel K , De Caigny P , Ito E , Lukowiak K . 2007. One-trial conditioning of aerial respiratory behaviour in Lymnaea stagnalis . Neurobiology of Learning and Memory 88:232–242. DOI:10.1016/j.nlm.2007.04.009.
- Mita K , Okuta A , Okada R , Hatakeyama D , Otsuka E , Yamagishi M , Morikawa M , Naganuma Y , Fujito Y , Dyakonova V , Lukowiak K , Ito E . 2014a. What are the elements of motivation for acquisition of conditioned taste aversion? Neurobiology of Learning and Memory 107:1–12. DOI:10.1016/j.nlm.2013.10.013.
- Mita K , Yamagishi M , Fujito Y , Lukowiak K , Ito E . 2014b. An increase in insulin is important for the acquisition conditioned taste aversion in Lymnaea . Neurobiology of Learning and Memory 116:132–138. DOI:10.1016/j.nlm.2014.10.006.
- Mizunami M , Hamanaka Y , Nishino H . 2015. Toward elucidating diversity of neural mechanisms underlying insect learning. Zoological Letters 1:8. DOI:10.1186/s40851-014-0008-6.
- Murakami J , Okada R , Fujito Y , Sakakibara M , Lukowiak K , Ito E . 2013a. Paired pulse ratio analysis of insulin-induced synaptic plasticity in the snail brain. Journal of Experimental Biology 216:1771–1773. DOI:10.1242/jeb.083469.
- Murakami J , Okada R , Sadamoto H , Kobayashi S , Mita K , Sakamoto Y , Yamagishi M , Hatakeyama D , Otsuka E , Okuta A , Sunada H , Takigami S , Sakakibara M , Fujito Y , Awaji M , Moriyama S , Lukowiak K , Ito E . 2013b. Involvement of insulin-like peptide in long-term synaptic plasticity and long-term memory of the pond snail Lymnaea stagnalis . Journal of Neuroscience 33:371–383. DOI:10.1523/JNEUROSCI.0679-12.
- Nader K , Bechara A , van der Kooy D . 1997. Neurobiological constraints on behavioral models of motivation. Annual Review of Psychology 48:85–114. DOI:10.1146/annurev.psych.48.1.85.
- Nakamura H , Kojima S , Kobayashi S , Ito I , Fujito Y , Suzuki H , Ito E . 1999. Physiological characterization of lip and tentacle nerves in Lymnaea stagnalis . Neuroscience Research 33:291–298. DOI:10.1016/S0168-0102(99)00020-6.
- Otsuka E , Matsunaga M , Okada R , Yamagishi M , Okuta A , Lukowiak K , Ito E . 2013. Increase in cyclic AMP concentration in a cerebral giant interneuron mimics part of a memory trace for conditioned taste aversion of the pond snail. Biophysics 9:161–166. DOI:10.2142/biophysics.9.161.
- Palmer CR , Kristan Jr. WB . 2011. Contextual modulation of behavioral choice. Current Opinion in Neurobiology 21:520–526. DOI:10.1016/j.conb.2011.05.003.
- Plaçais PY , Preat T . 2013. To favor survival under food shortage, the brain disables costly memory. Science 339:440–442. DOI:10.1126/science.1226018.
- Ribe EM , Lovestone S . 2016. Insulin signalling in Alzheimer’s disease and diabetes: From epidemiology to molecular links. Journal of Internal Medicine 280:430–442. DOI:10.1111/joim.12534.
- Rose RM , Benjamin PR . 1979. The relationship of the central motor pattern to the feeding cycle of Lymnaea stagnalis . Journal of Experimental Biology 80:137–163.
- Sadamoto H , Yamanaka M , Hatakeyama D , Nakamura H , Kojima S , Yamashita M , Ito E . 2000. Developmental study of anatomical substrate for conditioned taste aversion in Lymnaea stagnalis . Zoological Science 17:141–148. DOI:10.2108/zsj.17.141.
- Schwabe L . 2017. Memory under stress: From single systems to network changes. European Journal of Neuroscience 45:478–489. DOI:10.1111/ejn.13478.
- Sugai R , Azami S , Shiga H , Watanabe T , Sadamoto H , Kobayashi S , Hatakeyama D , Fujito Y , Lukowiak K , Ito E . 2007. One-trial conditioned taste aversion in Lymnaea: Good and poor performers in long-term memory acquisition. Journal of Experimental Biology 210:1225–1237. DOI:10.1242/jeb.02735.
- Sugai R , Shiga H , Azami S , Watanabe T , Sadamoto H , Fujito Y , Lukowiak K , Ito E . 2006. Taste discrimination in conditioned taste aversion of the pond snail Lymnaea stagnalis . Journal of Experimental Biology 209:826–833. DOI:10.1242/jeb.02069.
- Sunada H , Lukowiak K , Ito E . 2017a. Cerebral giant cells are necessary for both the formation and recall of memory of conditioned taste aversion in Lymnaea . Zoological Science 34:72–80. DOI:10.2108/zs160152.
- Sunada H , Watanabe T , Hatakeyama D , Lee S , Forest J , Sakakibara M , Ito E , Lukowiak K . 2017b. Pharmacological effects of cannabinoids on learning and memory in Lymnaea . Journal of Experimental Biology. DOI:10.1242/jeb.159038
- Takigami S , Sunada H , Lukowiak K , Ito E , Sakakibara M . 2016. An automated learning apparatus for classical conditioning of Lymnaea stagnalis . Journal of Neuroscience Methods 259:115–121. DOI:10.1016/j.jneumeth.2015.10.008.
- Talani G , Licheri V , Biggio F , Locci V , Mostallino MC , Secci PP , Melis V , Dazzi L , Carta G , Banni S , Biggio G , Sanna E . 2016. Enhanced glutamatergic synaptic plasticity in the hippocampal CA1 field of food-restricted rats: Involvement of CB1 receptors. Neuropsychopharmacology 41:1308–1318. DOI:10.1038/npp.2015.280.
- Verma D , Wood J , Lach G , Herzog H , Sperk G Tasan R. 2016. Hunger promotes fear extinction by activation of an amygdala microcircuit. Neuropsychopharmacology 41:431–439. DOI:10.1038/npp.2015.163.
- Wagatsuma A , Sugai R , Chono K , Azami S , Hatakeyama D , Sadamoto H , Ito E . 2004. The early snail acquires the learning. Comparison of scores for conditioned taste aversion between morning and afternoon. Acta Biologica Hungarica 55:149–155. DOI:10.1556/ABiol.55.2004.1-4.18.
- Yamagishi M , Watanabe T , Hatakeyama D , Ito E . 2015. Effects of serotonin on the heartbeat of pond snails in a hunger state. Biophysics 11:1–5. DOI:10.2142/biophysics.11.1.
- Yamanaka M , Sadamoto H , Hatakeyama D , Nakamura H , Kojima S , Kimura T , Yamashita M , Urano A , Ito E . 1999. Developmental changes in conditioned taste aversion in Lymnaea stagnalis . Zoological Science 16:9–16. DOI:10.2108/zsj.16.9.
- Yerkes RM , Dodson JD . 1908. The relation of strength of stimulus to rapidity of habit-formation. Journal of Comparative Neurology and Psychology 18:459–482. DOI:10.1002/cne.920180503.
