Abstract
Polygynous mammals often have male-biased sexual size dimorphism due to male–male competition for mates. However, fecundity selection may enlarge female body size. The Mongolian gerbil Meriones unguiculatus (Milne-Edwards, 1867) is a social rodent of promiscuous mating. Male gerbils exhibit social dominance hierarchy, which may differentiate reproductive success among male gerbils with larger males having more reproductive opportunities. This study investigated the hypothesis that social dominance hierarchy would result in male-biased sexual size dimorphism in Mongolian gerbils. We also tested the prediction of fecundity selection, that litter size would be positively related to female body mass of Mongolian gerbils. Body mass, carcass weight and body length of male gerbils were greater than those of females. Therefore, male-biased sexual size dimorphism was supported in Mongolian gerbils. Although litter size of female gerbils increased with increasing carcass weight, ecological constraints (e.g. burrow living and increased energy expenditure during pregnancy) probably kept female body size smaller than male body size of the gerbil. Social interactions may mediate sexual and fecundity selections on the body size of social rodents.
Introduction
Large body size may provide mammals with advantages in competition for mates and resources (Blanckenhorn Citation2000; Bonnet et al. Citation2000). Sexual selection may enlarge the body of males to enhance their mating or reproductive success. The sexual selection hypothesis posits that male size enlargement by male–male competition would cause male-biased sexual size dimorphism (SSD) where males are larger than females in size (Hedrick & Temeles Citation1989; Andersson Citation1994). Additionally, if fecundity selection acts on females as a form of natural selection, female-biased or reverse SSD may arise, with females being larger than males. For instance, the fecundity advantage hypothesis predicts that larger body size may allow females to carry more embryos or foetuses, having large litter sizes and high reproductive success (Heske & Ostfeld Citation1990; Blanckenhorn Citation2000; Fokidis et al. Citation2007). To explain the degree of sexual size dimorphism in a species, the variety of selective factors that affects the sizes of males and females needs to be considered (Andersson Citation1994; Himuro & Fujisaki Citation2014). Therefore, sexual and fecundity selection may act on male and female body sizes in concert, influencing SSD (García‐Navas et al. Citation2015).
Social systems may mediate the effects of intra- and intersex competition on SSD (Heske & Ostfeld Citation1990; Johnson & Macdonald Citation2001; Schulte-Hostedde et al. Citation2004). For instance, male–male competition may increase from monogamy to promiscuity and to polygyny in mammals (Heske & Ostfeld Citation1990; Isaac Citation2006). Consequently, SSD is more pronounced in polygynous than in promiscuous species and is least pronounced in monogamous or polygyandrous species (Isaac Citation2006). Male-biased SSD is common in mammals because most mammals are polygynous (Isaac Citation2006; Lindenfors et al. Citation2007). Mating systems may be used to predict the SSD or monomorphism of mammals, particularly in medium- and large-sized mammals (Isaac Citation2005). Nevertheless, García‐Navas et al. (Citation2015) found that the effects of mating system on the degree of SSD was not significant in voles (Arvicolinae).
The Mongolian gerbil Meriones unguiculatus (Milne-Edwards, 1867) is a small social rodent in the Mongolian Plateau (Gulotta Citation1971). The gerbil lives in groups with 2–17 individuals year round (Agren et al. Citation1989; Wang et al. Citation2011), excavates complex underground burrow systems (Scheibler et al. Citation2006), reproduces communally with plural female breeders (Liu et al. Citation2009b) and caches food in autumn for the winter (Scheibler et al. Citation2006). Captive Mongolian gerbils exhibit a promiscuous mating pattern (Ågren Citation1990). Male gerbils in natural and semi-natural conditions exhibit social dominance hierarchy and combat competition among themselves over mates (Agren Citation1984). Furthermore, growing foetuses may enlarge the bodies of gestating females. It is feasible to predict that female gerbils may have smaller body sizes than males, allowing females to use burrows excavated by males. Such ecological constraints may contribute to SSD of small burrowing mammals. Therefore, the Mongolian gerbil is a model species for studies of the associations of SSD and mating systems or male–male competition in small burrowing mammals. This study aimed to test two predictions of sexual and fecundity selection on SSD. First, male gerbils would be larger in size than female gerbils owing to male–male competition. Second, litter size would be positively related to female body size owing to fecundity selection.
Methods
Data
This study reanalysed data from Zhou et al. (Citation1985). Data available from Zhou et al. (Citation1985) were collected during the late spring and early summer (May and June) of 1980. Mongolian gerbils were captured using snap traps in a grassland intermixed with agriculture in Shizhiwang Banner, Inner Mongolia (Zhou et al. Citation1985). The trapping site was about 140 ha in size. Captured individuals were sexed and weighed to the nearest 0.5 g. Body length was measured from nose to anus to the nearest 1 mm with a ruler. Males were classified as adults if they had scrotal testes, enlarged seminal vesicles, and grooves on the posterior surface of the first upper molar (Xia et al. Citation1982). Captured gerbils were dissected to measure carcass weight with internal body organs, such as lungs, hearts, intestines, kidneys and reproductive organs, being removed. The use of carcass weight can eliminate the possible confounding effects of different amounts of food in the stomach and intestines and different numbers and sizes of foetuses in pregnant females. The carcass was primarily made of fur, skin, muscle, skeleton and body fat. Zhou et al. (Citation1985) also determined the litter size of reproducing females.
Statistical analysis
The logarithmic transformation at the base of 10 (hereafter referred to as log10 transformation) and raw data of body mass, carcass weight and body length were used to compare body sizes between the sexes to detect SSD using a t test. A generalised linear model with a log link function (i.e. Poisson regression for foetus count) was used to test for positive relationships between female carcass weight and litter size at the original scale. A quadratic term was included to test for optimal litter size. All statistical tests were conducted at the significance level of 0.05.
Results
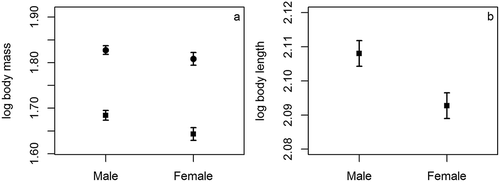
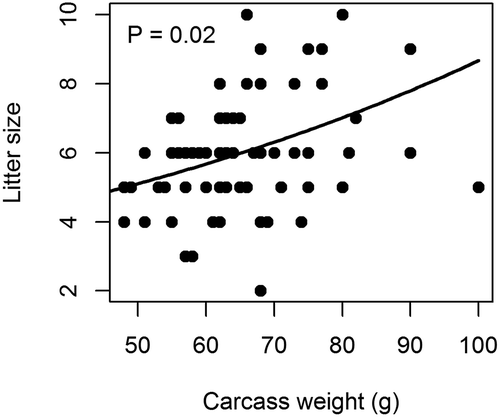
The sample size for the SSD test was 145 (61 males and 84 females). Average body length (female mean ± standard deviation (SD): 123.91 ± 4.99 mm; male mean ± SD: 128.31 ± 4.34) at the original scale differed significantly between males and females (t = −5.54, df = 143, P ≤ 0.01), and so did means of untransformed carcass weight (female: 44.46 ± 6.72; male: 48.57 ± 4.73; t = −4.10, df = 143, P ≤ 0.01). On the other hand, average body mass at the original scale (female: 65.02 ± 9.89; male: 67.48 ± 5.65) did not differ significantly between males and females (t = −1.74, df = 142, P = 0.08). Log10 transformed body weight (t = −2.1182, df = 143, P = 0.04), log10 transformed carcass weight (t = −4.4, df = 143, P ≤ 0.01; squares in Figure 1(a)), and log10 transformed body length (t = −5.6, df = 143, P ≤ 0.01; )) differed significantly between males and females. Differences in carcass weight were more pronounced than differences in whole body mass between males and females, with percentage differences between the two sexes being 3.63% in whole body mass but 8.47% in carcass weight. Litter size was positively related to female carcass weight (P = 0.02; ). A quadratic term of carcass weight was insignificant (P = 0.22).
Discussion
Sexual selection, fecundity selection and ecological constraints may all act on male and female body sizes and subsequently determine the SSD of mammals (Hedrick & Temeles Citation1989; Andersson Citation1994; Isaac Citation2006). Although the association of SSD and polygyny has been found to be common in mammals, exceptions have been reported in the literature, partially owing to resource and social competition (García‐Navas et al. Citation2015; Kappeler & Fichtel Citation2015). Likewise, territorial competition may make fish Tropheus moorii lose SSD (Odreitz & Sefc Citation2015). SSD is absent or low in many social mammals (Johnson & Macdonald Citation2001; Cooper et al. Citation2011; Kappeler & Fichtel Citation2015). In contrast, data in this study demonstrated the existence of SSD in Mongolian gerbils, a small social rodent, and provided support for positive relationships between litter size and female carcass weight. Body enlargement by male–male competition and ecological constraints to female body size resulting from burrowing living may evolve in concert in social rodents of multi-male breeding systems and social dominance hierarchy.
Male size enlargement by sexual selection may bestow advantages in dominance over conspecifics, mate competition, and territorial defense to enhance both male survival and reproductive success (Schulte-Hostedde Citation2007). Male–male competition may be correlated with differences in social dominance among males in polygynous species, which further skew reproductive opportunities to one or a few dominant males (Fairbairn Citation2013). SSD was found in all three metrics of body size of Mongolian gerbils: body mass, carcass weight and body length (). Nevertheless, relative sexual difference in carcass weight (8.47%) was more than double those of body mass (3.63%) and body length (3.43%). Thus, foetuses carried by pregnant females may mask or reduce differences in body mass between males and females. Ostfeld and Heske (Citation1993) suggest that body length may be more appropriate than body mass to detect SSD in small mammals. This study found that carcass weight may be a sensitive metric of body size for studies of mammalian SSD. The evidence of SSD in carcass weight and body mass indicates the existence of male–male competition and variance in reproductive success among male Mongolian gerbils.
Field observations of the mating systems of Mongolian gerbils are limited, but suggest promiscuous mating systems. Females were found mating with more than one male groupmate and with extra-group males (Agren et al. Citation1989). Although male–male competition decreases from polygynous to promiscuous mammals, Agren et al. (Citation1989) demonstrated male–male competition and the dominance of larger males over smaller males of Mongolian gerbils. Male Mongolian gerbils compete for females by combat rather than by scramble competition. The largest reproductively active males defend territories by chasing subordinates or strangers and marking the territory borders (Agren et al. Citation1989). Subordinate male groupmates may lose reproductive opportunities, becoming helpers at the nest (Clark & Galef Citation2000; Liu et al. Citation2009a). Therefore, variation in reproductive success among males resulting from the dominance hierarchy of social groups may lead to male-biased SSD of Mongolian gerbils.
Positive relationships between litter size and body size suggest that fecundity selection may act on female body size of Mongolian gerbils. It is plausible that enlargement of female bodies may have fitness costs in burrowing rodents. First, small bodies may allow females to have access to small burrows, especially during pregnancy (Gliwicz Citation1988). Mongolian gerbils live in complex underground burrow systems with a central den being connected to food storage chambers with long, narrow tunnels (Scheibler et al. Citation2006). Burrow living may constrain females from enlarging their body sizes to the extent of male body enlargement. Second, smaller body size requires a lower absolute amount of energy for life maintenance and may allow females to allocate more energy to reproduction (Moors Citation1980). Nevertheless, the benefit to reproductive energy allocation may be less notable in the gerbil than in large- or medium-sized mammals. Ecological and habitat conditions have been shown to influence SSD in mammals (Isaac Citation2006; García‐Navas et al. Citation2015) and amphibians (Liao & Chen Citation2012). Therefore, ecological constraints of burrow living may keep the female body size of the gerbil smaller than that of the male gerbil. Group living, communal breeding and plural female breeders within a social group of Mongolian gerbils may lead to intensified male–male competition when estrous female groupmates are available.
Acknowledgements
The author is grateful to Professors Q. Zhou and W. Zhong for providing the data used in this work. Three anonymous reviewers made helpful, constructive comments on this manuscript. The author also thanks the field technicians who assisted in data collection. This study was supported financially by the Mississippi State University Forest Wildlife Research Center.
References
- Agren G. 1984. Pair formation in the Mongolian gerbil. Animal Behaviour 32:528–535. DOI:10.1016/S0003-3472(84)80291-2.
- Ågren G. 1990. Sperm competition, pregnancy initiation and litter size: Influence of the amount of copulatory behaviour in Mongolian gerbils, Meriones unguiculatus . Animal Behaviour 40:417–427. DOI:10.1016/S0003-3472(05)80521-4.
- Agren G , Zhou Q , Zhong W . 1989. Ecology and social behavior of Mongolian gerbils, Meriones unguiculatus, at xilinhot, Inner Mongolia, China. Animal Behaviour 37:11–27. DOI:10.1016/0003-3472(89)90002-X.
- Andersson M . 1994. Sexual selection. Princeton, NJ: Princeton University Press.
- Blanckenhorn WU . 2000. The evolution of body size: What keeps organisms small? Quarterly Review of Biology 75:385–407. DOI:10.1086/393620.
- Bonnet X , Naulleau G , Shine R , Lourdais O . 2000. Reproductive versus ecological advantages to larger body size in female snakes, Vipera aspis . Oikos 89:509–518. DOI:10.1034/j.1600-0706.2000.890310.x.
- Clark MM , Galef BG . 2000. Why some male Mongolian gerbils may help at the nest: Testosterone, asexuality and alloparenting. Animal Behaviour 59:801–806. DOI:10.1006/anbe.1999.1365.
- Cooper JD , Waser PM , Hellgren EC , Gabor TM , DeWoody JA . 2011. Is sexual monomorphism a predictor of polygynandry? Evidence from a social mammal, the collared peccary. Behavioral Ecology and Sociobiology 65:775–785. DOI:10.1007/s00265-010-1081-2.
- Fairbairn DJ . 2013. Odd couples: extraordinary differences between the sexes in the animal kingdom. Princeton, NJ: Princeton University Press.
- Fokidis HB , Risch TS , Glenn TC . 2007. Reproductive and resource benefits to large female body size in a mammal with female-biased sexual size dimorphism. Animal Behaviour 73:479–488. DOI:10.1016/j.anbehav.2006.08.010.
- García‐Navas V , Bonnet T , Bonal R , Postma E . 2015. The role of fecundity and sexual selection in the evolution of size and sexual size dimorphism in New World and Old World voles (Rodentia: Arvicolinae). Oikos 125:1260–1260.
- Gliwicz J . 1988. Sexual dimorphism in small mustelids: Body diameter limitation. Oikos 53:411–414. DOI:10.2307/3565544.
- Gulotta EF . 1971. Meriones unguiculatus . Mammalian Species 3:1–5. DOI:10.2307/3503988.
- Hedrick AV , Temeles EJ . 1989. The evolution of sexual dimorphism in animals: Hypotheses and tests. Trends in Ecology & Evolution 4:136–138. DOI:10.1016/0169-5347(89)90212-7.
- Heske EJ , Ostfeld RS . 1990. Sexual dimorphism in size, relative size of testes, and mating systems in North American voles. Journal of Mammalogy 71:510–519. DOI:10.2307/1381789.
- Himuro C , Fujisaki K . 2014. Evolutionary causes of male-biased sexual size dimorphism from a female perspective in the seed bug togo hemipterus (Heteroptera: Lygaeidae). Applied Entomology and Zoology 49:347–352. DOI:10.1007/s13355-014-0258-y.
- Isaac JL . 2005. Potential causes and life‐history consequences of sexual size dimorphism in mammals. Mammal Review 35:101–115. DOI:10.1111/mam.2005.35.issue-1.
- Isaac JL . 2006. Sexual dimorphism in a marsupial: Seasonal and lifetime differences in sex-specific mass. Australian Journal of Zoology 54:45–50. DOI:10.1071/ZO05060.
- Johnson DD , Macdonald DW . 2001. Why are group-living badgers (Meles meles) sexually dimorphic? Journal of Zoology 255:199–204. DOI:10.1017/S0952836901001273.
- Kappeler PM , Fichtel C . 2015. Eco-evo-devo of the lemur syndrome: Did adaptive behavioral plasticity get canalized in a large primate radiation? Frontiers in Zoology 12:S15. DOI:10.1186/1742-9994-12-S1-S15.
- Liao WB , Chen W . 2012. Inverse Rensch’s rule in a frog with female-biased sexual size dimorphism. Naturwissenschaften 99:427–431. DOI:10.1007/s00114-012-0913-5.
- Lindenfors P , Gittleman JL , Jones KE . 2007. Sexual size dimorphism in mammals. In: Fairbairn DJ , Blanckenhorn WU , Székely T , editors. Sex, size and gender roles: evolutionary studies of sexual size dimorphism. Oxford, UK: The University of Oxford Press. pp. 16–26.
- Liu W , Wang G , Wan XR , Zhong WQ . 2009a. Effects of supplemental food on the social organization of Mongolian gerbils during the breeding season. Journal of Zoology 278:249–257. DOI:10.1111/j.1469-7998.2009.00574.x.
- Liu W , Wang GM , Wang YN , Zhong WQ , Wan XR . 2009b. Population ecology of wild Mongolian gerbils Meriones unguiculatus . Journal of Mammalogy 90:832–840. DOI:10.1644/08-MAMM-A-265.1.
- Moors PJ . 1980. Sexual dimorphism in the body size of mustelids (Carnivora): The roles of food habits and breeding systems. Oikos 34:147–158. DOI:10.2307/3544175.
- Odreitz U , Sefc KM . 2015. Territorial competition and the evolutionary loss of sexual size dimorphism. Behavioral Ecology and Sociobiology 69:593–601. DOI:10.1007/s00265-014-1870-0.
- Ostfeld RS , Heske EJ . 1993. Sexual dimorphism and mating systems in voles. Journal of Mammalogy 74:230–233. DOI:10.2307/1381925.
- Scheibler E , Liu W , Weinandy R , Gattermann R . 2006. Burrow systems of the Mongolian gerbil (Meriones unguiculatus Milne Edwards, 1867). Mammalian Biology 71:178–182. DOI:10.1016/j.mambio.2005.11.007.
- Schulte-Hostedde AI , Millar JS , Gibbs HL . 2004. Sexual selection and mating patterns in a mammal with female-biased sexual size dimorphism. Behavioral Ecology 15:351–356. DOI:10.1093/beheco/arh021.
- Schulte-Hostedde AI . 2007. Sexual size dimorphism in rodents. In: Sherman PW , Wolff JO , editors. Rodent societies: an ecological and evolutionary perspective. Chicago, IL: The University of Chicago Press. pp. 115–128.
- Wang Y , Liu W , Wang G , Zhong W , Wan X . 2011. Genetic consequences of group living in Mongolian gerbils. Journal of Heredity 102:554–561. DOI:10.1093/jhered/esr069.
- Xia W , Liao C , Zhong W , Sun C . 1982. Population dynamics and regulation of Mongolian gerbils in agricultural areas north of the Yin Mountains. Acta Theriologica Sinica 2:51–69.
- Zhou Q , Zhong W , Sun C . 1985. Comparison of population characteristics of Meriones unguiculatus adapting farmland and grassland. Acta Theriologica Sinica 5:25–33.