Abstract
The present study reports the spread of the cirratulids Chaetozone corona Berkeley & Berkeley, 1941 and Chaetozone carpenteri McIntosh, 1911 in the Western Central Adriatic Sea, off the coasts of Pescara (Italy). The two species were collected between 2014 and 2016 from soft bottom stations (at depths from 16.5 to 130 m) where the environment was more or less disturbed due to fishing activities. One specimen of C. corona was found also off the coast of Calafuria (Livorno, Italy), representing the first record of this species in the Tyrrhenian Sea. Chaetozone carpenteri could be a native species present in the Mediterranean for a long time but rarely recorded because of taxonomic confusion. Chaetozone corona was already known from the eastern Mediterranean Sea (except from the Adriatic Sea), where it is considered an established alien species. Our results extend the geographic range of these two cirratulid species, providing some information on their ecology and habitat preference. We also suggest a likely vector of spread of C. corona from the easternmost part of the Mediterranean towards the study area. The finding of reproducing specimens of C. corona and C. carpenteri supports the hypothesis that these two species have found a suitable habitat in the Western Central Adriatic Sea, and there will become well established. Although nothing suggests that C. corona would be invasive, it may, however, compete with native species. These findings also seem particularly relevant in order to improve the knowledge of Mediterranean biodiversity.
Introduction
Historically, bitentaculate specimens of Chaetozone having posterior spines arranged into cinctures on posterior segments were referred globally to Chaetozone setosa Malmgren, the type species originally described from Spitsbergen, in the Arctic north of Norway. Recently, the elucidation of new characters among species of Chaetozone from North America and elsewhere, and the redescription of C. setosa provided by Chambers (Citation2000), led to descriptions of numerous new species and the identification of distinct species groups (Blake Citation1996, Citation2006; Chambers & Woodham Citation2003; Doner & Blake Citation2006; Chambers et al. Citation2007). With the realisation that the genus Chaetozone contains numerous species, many of which have gone unrecognised, the greater majority of the older records of C. setosa from worldwide locations are now believed to refer to other taxa (Blake Citation2015). Blake (Citation2015) provided an updated overview, as well as a review of characters important in the taxonomy of the genus Chaetozone. This author also suggested that C. setosa is limited to Arctic and subarctic areas around Spitsbergen and other areas of northern Europe.
To date, in the Italian inventories of polychaetes the genus Chaetozone is still represented by only three species (Castelli et al. Citation2008) – Chaetozone caputesocis Saint-Joseph, 1894, Chaetozone gibber Woodham & Chambers, 1994 and the type species C. setosa, Malmgren, 1867 – together with the closely related Caulleriella zetlandica McIntosh, Citation1911. Subsequent to the recent redescription provided by Woodham and Chambers (Citation1994a), this species should be referred to the genus Chaetozone, as it was in the original description provided by McIntosh (Citation1911) (Blake Citation1996). Chambers et al. (Citation2011) described a similar state of art in the Chaetozone species inventory for the Mediterranean Sea, at least until recently. Yet other bitentaculate cirratulids had already been recorded from the Mediterranean coasts over the years (McIntosh Citation1911; Simboura Citation1996; Arvanitidis Citation2000; Simboura & Nicolaidou Citation2001; Simboura & Zenetos Citation2002; Çinar et al. Citation2004, 2006; Zaâbi et al. Citation2009), which had often been identified as Chaetozone sp., and which in turn may encompass more than one species. Most records of the new Chaetozone species from the Mediterranean were previously referred to the type species, C. setosa Malmgren, 1867, or to C. gibber, in technical reports and published papers (Çinar & Ergen Citation2007; Simboura et al. Citation2010; Chambers et al. Citation2011). The problem of confusion among Chaetozone was exacerbated by the lack of original generic diagnoses for this genus (Woodham & Chambers Citation1994b). Chaetozone setosa is currently recognised to be a species complex (Chambers & Woodham Citation2003), and according to Blake (Citation2015) C. setosa, Chaetozone carpenteri McIntosh, Citation1911 and Chaetozone corona Berkeley & Berkeley, Citation1941 belong to the same species group (i.e. the C. setosa group), characterised by species with an enlarged lobe or crest overlying the peristomium.
Recently, during sampling programmes off the coasts of Italy (Procida Island and Sardinia, Punta Tramontana; Tyrrhenian Sea) and Croatia (Rovinj, Adriatic Sea), bitentaculate cirratulids were found in most samples and, after a painstaking examination, Chambers et al. (Citation2011) identified several specimens of Chaetozone as C. carpenteri. These authors (Chambers et al. Citation2011) also suggested that many specimens previously detected from the Mediterranean might have been misidentified. Similarly, some specimens reported as C. setosa or Chaetozone sp. from the east (Izmir Bay, Aegean Sea) and central Mediterranean Sea (Zakynthos Island, Ionian Sea) were re-examined and identified as C. corona (Çinar & Ergen Citation2007; Simboura et al. Citation2010).
Chaetozone carpenteri has been rarely reported from the Mediterranean Sea; its first description dates back to a few specimens collected from the Atlantic coasts of Spain and the coast of Algiers (Algeria, western Mediterranean Sea), in 1870 (McIntosh Citation1911). The specimen from Algiers was the only one reported from the Mediterranean until the recent record of specimens from the eastern Adriatic and the Tyrrhenian Sea reported by Chambers et al. (Citation2011) and then by Mikac (Citation2015). Recently, further specimens have also been reported from the Sea of Marmara (eastern Mediterranean Sea), by Çinar et al. (Citation2014). Chaetozone corona was originally reported from the Pacific Ocean (Berkeley & Berkeley Citation1941; Hartman Citation1961; Blake Citation1996) and is currently recorded in the eastern Mediterranean, specifically only in the Levantine Sea, the Greek Ionian Sea, the Aegean Sea and the Sea of Marmara (Çinar & Ergen Citation2007; Simboura et al. Citation2010; Çinar et al. Citation2011, 2012, 2014; Çinar & Dagli Citation2013; Çinar & Bakir Citation2014). Both the species C. corona and C. carpenteri have been recently re-described, by Çinar and Ergen (Citation2007) and Chambers et al. (Citation2011), respectively; their presence in the Mediterranean Sea has been recognised only in the last decade.
In this study, we report the first occurrence of C. corona in the Adriatic Sea, as well as in the Tyrrhenian Sea, and point out the presence of C. carpenteri off the Adriatic coasts of Italy, thus extending the geographic range of these two species within the Mediterranean Sea. We also give some morphological details as well as ecological notes about these two species, in order to improve the knowledge of their ecology. We discuss the hypothesis of an introduction of C. corona to the Italian coasts as well as the potential vectors of its introduction and spread through the Mediterranean Sea.
Materials and methods
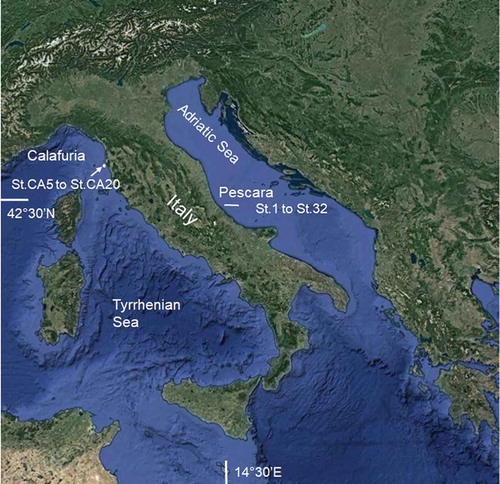
Specimens of Chaetozone corona and Chaetozone carpenteri were collected in the Central Adriatic Sea, during several monitoring surveys of benthic macroinvetebrates, carried out at 32 stations located along a 140-km-long, east–west transect in Italian territorial waters from the town of Pescara to the island of Pianosa (). Sampling campaigns were carried out in two different seasons (i.e. winter and summer), from 2014 to 2016. Thirty-two soft-bottom stations were sampled, in duplicate from St. 1 to St. 22 and in triplicate from St. 23 to St. 32. Sediment samples were collected with a Van Veen grab (area: 0.1 m2) and sieved onboard through a 1-mm mesh; material retained on the sieve was fixed in 8% buffered formaldehyde solution. In the laboratory, samples were sieved through a 0.5-mm mesh, and macroinvertebrates were stained with Rose Bengal to facilitate sorting and identification to the species level.
Only one individual of C. corona was collected from a hard-bottom sampling station, offshore from the Calafuria resort (closed to the town of Livorno) in the Tyrrhenian Sea (). In this study area, three stations (5, 10 and 20 m) were sampled in triplicate along a depth transect. At each station, fouling organisms on hard surfaces were scraped by divers within 25 × 25 cm quadrats.
Specimens pertaining to the genus Chaetozone were examined using a stereomicroscope and a Nikon Eclipse (E200) compound microscope equipped with a digital camera to achieve images. Measurements of length and width were detected by the photo analysis program Nis-Elements D (v. 2.30).
Several specimens were dehydrated and goldcoated for scanning electron microscope (SEM) study following the standard procedure described in Munari (Citation2014).
The material examined was deposited at the Laboratory of Marine Benthos Ecology collection of the University of Ferrara.
Sediment characteristics, depth, total organic carbon (TOC) concentrations in sediment and water parameters are summarised for Adriatic and Tyrrhenian stations from which specimens of C. corona and C. carpenteri were collected ().
Table I. Details, water parameters at the bottom, TOC (total organic carbon) concentrations in sediment and sediment granulometric composition of the stations where specimens of Chaetozone corona Berkeley & Berkeley, Citation1941 and C. carpenteri McIntosh, Citation1911 were recorded in the Central Adriatic Sea and Tyrrhenian Sea. n.e. = not examined.
Measurements of TOC concentrations in sediment were carried out only in summer 2015. TOC analyses were performed following Cicero and Di Girolamo (Citation2001).
Results
Sampling stations in the Adriatic Sea were characterised by muddy (silty clay–silty sand) sediments. In the present study, TOC concentrations varied from 0.10% (St. 21) to 0.93% (St. 19) at stations off Pescara coasts (Central Adriatic Sea).
Taxonomic accounts
Class POLYCHAETA Grube, 1850
Order TEREBELLIDA sensu Rouse & Fauchald, 1997
Family CIRRATULIDAE Carus, 1863
Genus Chaetozone Malmgren, 1867
Chaetozone corona Berkeley & Berkeley, Citation1941
Chaetozone spinosa corona Berkeley & Berkeley Citation1941: 45–46.
Chaetozone corona: Hartman Citation1960: 125, 1961: 109–110, 1969: 235, figs 1–3; Blake Citation1996: 285–287, fig. 8.6; Çinar & Ergen Citation2007: 342–344, figs 2–4; Le Garrec et al. Citation2016: 2, fig. 2.
Material examined
Central Adriatic Sea, off the coast of the town of Pescara. Winter 2014: St. 7, one specimen, St. 8, two specimens; winter 2015: St. 22, one specimen; winter 2016: St. 7, one specimen. Central Tyrrhenian Sea, off the shoreline of Calafuria resort, close to Livorno town, summer 2016: St. CA20, one specimen.
Description
Maximum body length (complete specimen) 14.2 mm for 52 chaetigers, width 1.16 mm. Other specimens were incomplete (19–43 chaetigers) ranging from 0.9 to 1.5 mm wide across the widest part of the body (and from 4.8 to 14.8 mm in length).
Body thickened, becoming slightly compressed posteriorly. Prostomium directed anteriorly, triangular in shape, with blunt tip; with a pair of black eyes laterally. Peristomium dorsally inflated with one large anterior and two shorter posterior rings, extending posteriorly as a median ridge or crest to the anterior side of chaetiger 1. Dorsal tentacles attached between peristomium and chaetiger 1. Branchiae arising immediately posterior and slightly medial to dorsal tentacles (). Subsequent branchiae emerging from the posterior edge of parapodia. Most of the branchiae along the body length were lost. Parapodia dorsally elevated, with parapodial lamellae bearing fascicles of noto- and neurochaetae. Notopodia on chaetiger 1 bearing 7–10 capillary chaetae (330 µm long). Neuropodia on chaetiger 1 with 3–4 (up to seven) capillary chaetae and 2–3 pale yellow, slightly curved spines; up to four spines at chaetiger 1 in larger specimens (e.g. specimen not complete from St. 7 of winter 2016: 4.81 mm long, 0.95 mm wide, for 19 chaetigers). Capillary chaetae increasing in length up to chaetiger 13, reaching up to 1480 µm long, then gradually shortening towards the posterior end. Capillary chaetae of posterior parapodia thinner and smooth, without fibrils. Spine on notopodia first present from chaetiger 6, in only some specimens from chaetiger 5 (eg. specimen from St. 7); in the specimen from the Tyrrhenian Sea notopodial spines from chaetiger 8. Spines increasing in size and darkening towards the posterior end. Notopodia and neuropodia of chaetiger 10 with 4–7 capillaries and 2–3 spines (up to four neuropodial spines in the Tyrrhenian specimen). In the middle part of the body (chaetiger 30), 4–6 notopodial and 4–5 neuropodial capillary chaetae; around four notopodial and five neuropodial spines (b)). In the posterior part of the body (chaetiger 41), notopodia and neuropodia with 4–6 spines (up to eight neuropodial spines in some larger specimens). The number of capillary chaetae was reduced to 3–4 in the middle and the end part of the body. Noto- and neuropodial spines together form partial cinctures in posterior segments, accompanied by thin capillaries (c)).
Figure 2. Chaetozone corona. (a) Dorsal view, anterior end, with dorsal tentacle (dt) and branchiae (br). (b) Dorsal view of the notopodia showing spines and capillary chaetae, middle part of the body. (c) Posterior part of the body and pygidium, dorso-lateral view. Scale bars: a = 100 µm; b = 30 µm; c = 20 µm.
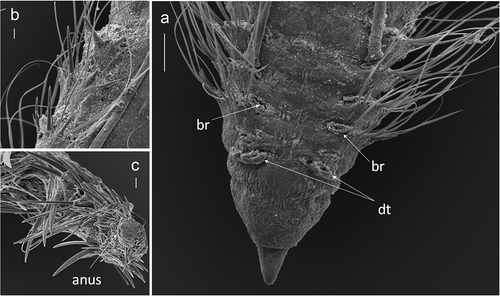
Chaetal arrangements vary among specimens and among body regions: for example, one capillary alternating with one spine in the middle parapodia, but one capillary between two or three spines in posterior parapodia (c)). Pygidium (c)) with blunt tip; anus in dorsal position.
Larger specimens with body pinkish-coloured due to dense eggs in coelomatic cavity; diameter ± standard error (SE) = 109.8 ± 0.6 mm, range: 87.1–133.8 mm (n = 20). Other specimens without gametes cream to pale brownish in colour.
Remarks
Morphological characters of the Adriatic and Tyrrhenian specimens of C. corona were similar to those of the original (Berkeley & Berkeley Citation1941) and subsequent descriptions (Hartman Citation1969; Blake Citation1996; Çinar & Ergen Citation2007). Çinar and Ergen (Citation2007) suggested that the presence of fibrils on one edge of anterior capillary chaetae could be an artefact resulting from damage of capillaries. Similarly, in our specimens fibrils were evident in most of the anterior chaetae; thus, probably resulting from damage of capillaries.
Çinar and Ergen (Citation2007), as well as Le Garrec et al. (Citation2016), detected black irregular speckles on dorsal, lateral and ventral sides of the peristomium, and on the ventral side (or laterally) of chaetiger 1 (rarely chaetiger 2). Conversely, black spots were very difficult for us to detect, as they had almost disappeared in all our specimens. However, the examined specimens have been preserved in formalin for years, and we believe that the characteristic colour may rapidly disappear if the conservation status of individuals is not optimal. On the contrary, we clearly detected the presence of black speckles in specimens of C. carpenteri (see below).
Also, our specimens differed from those described by Çinar and Ergen (Citation2007) in the number of capillary chaetae and spines throughout the body: e.g. 14 capillary notochaetae and 12 in neurochaete on chaetiger 1 according to Çinar and Ergen (Citation2007), and 7–10 capillary notochaetae and 4–7 capillary neurochaetae in the anterior region of specimens from the Italian coasts. Notwithstanding, the number of capillary chaetae and spines observed on anterior parapodia in our specimens was comparable to that found in specimens described by Le Garrec et al. (Citation2016), and to that of specimens from the Pacific as reported by Hartman (Citation1961) and Blake (Citation1996). In any case, these differences may be mainly due to the natural variability among specimens. In fact, Çinar and Ergen (Citation2007), stated that numbers of capillary chaetae and spines vary among specimens, as also do chaetal arrangements. Moreover, as suggested for C. carpenteri by Chambers et al. (Citation2011), the exact number of spines may depend on how one measures and interprets a spine, as many chaetae are damaged.
The body size of individuals among different populations of C. corona are similar around the world: 13–14 mm long, in complete specimens with 60 chaetigers in southern California (Blake Citation1996); 18–25 mm long with 50–60 chaetigers in southern California (Hartman Citation1969); 19.5 mm with 62 chaetigers in Aegean Sea (Çinar & Ergen Citation2007); 11–16 mm with 50–55 chaetigers in the Loire estuary (Le Garrec et al. Citation2016); and 14.2 mm long, in a complete specimen with 52 chaetigers, of the Adriatic population (this study).
Although for a long time C. corona was confused with C. setosa it differs from the latter and other species belonging to the genus Chaetozone in having the neuropodial acicular spines from chaetiger 1 and a pair of black eyes, besides the distinctive shape and inflation of the dorsum of the peristomium. In fact, the most common Mediterranean Chaetozone, i.e. the C. setosa complex, is characterised by the absence of eyes and the first apparence of neuropodial spines on chaetiger 40. Chaetozone corona is also readily distinguished from the other Chaetozone species with eyes reported from the Mediterranean, such as Chaetozone gibber and Chaetozone caputesocis, in that the former shows neuropodial spines from chaetiger 90 and the latter show them from chaetiger 10. Chaetozone corona is also distinguished from Chaetozone zetlandica (recently redescribed by Woodham & Chambers Citation1994a) since the latter has heavy spines occurring only in posterior neuropodia, and chaetae of five types with awl-like spinous setae in the notopodia. It also differs from the recently redescribed C. carpenteri, which holds noto- and neuropodial spines from chaetiger 6.
Ecology and distribution
Chaetozone corona was first reported from the eastern Pacific (off southern California, western Mexico and the Gulf of California) to 119 m depth from mixed sediments (Blake Citation1996), and from the western Atlantic Ocean (off Brazil) (Omena & Creed Citation2004) in a community associated with Halodule wrightii Ascherson, 1868 in intertidal water (1–3 m). Çinar and Ergen (Citation2007) reported some specimens from the Aegean Sea (Izmir Bay) on sandy mud and Posidonia oceanica (Linnaeus) Delile, 1813, between 2.5 and 50 m depth. Simboura et al. (Citation2010) reported C. corona specimens, dated back to the 1980s, from the Ionian Sea (Zakynthos Island) on sand at 5 m depth. This species was also reported from other areas of the Greek coasts and islands (e.g. Elefsis Bay, Saronikos Gulf, the Kyklades and Crete) on sandy, muddy or mixed sediments, as well as biogenic detritus down to 90 m depth (Simboura Citation1996; Simboura et al. Citation2010). More recently C. corona was reported from the Turkish coasts to 100 m in depth (Çinar et al. Citation2011, 2012, 2014; Çinar & Dagli Citation2013; Zenetos et al. Citation2017). In particular, this species was found from samples collected between 2006 and 2010 at depths from 26 to 66 m in the Sea of Marmara (Çinar et al. Citation2011); Çinar et al. (Citation2012) recorded C. corona in samples collected in 2009 in Mersin Bay (Levantine Sea), at 21 and 72 m. Çinar and Dagli (Citation2013) in 2011 recorded some individuals from bare soft-bottom stations and from a Posidonia oceanica meadow, between 5 and 68 m, in the northern Aegean Sea. Finally, Le Garrec et al. (Citation2016) extended its distribution to the Atlantic coasts of France in infra-littoral muddy to sandy sediments, and occasionally in maerl beds. In this study, we found individuals mainly in silt and clayey silt (), in sediments with sand contents varying from 0.9 to 67.8%, silt contents from 39.3 to 99.2% and, finally, clay contents from 0.5 to 59.7%.
Because of taxonomic confusion, knowledge about the density of C. corona is still very poor. Data from Izmir Bay indicatd a variation of density from 10 ind. m−2 in winter to 70 ind. m−2 in spring (Çinar & Ergen Citation2007). The density of C. corona in the central Adriatic Sea (off Pescara coasts) varied from 5 ind. m−2 (at St. 7, St. 14 and St. 18 in winter 2016; at St. 22 in summer 2016; at St. 12, St. 16 and St. 22 in winter 2015; at St. 7 in winter 2014) to 10 ind. m−2 (at St. 11 in winter 2016; at St. 14 in summer 2016; and at St. 8 in winter 2014), between 19.5 and 100.5 m depth (). Chaetozone corona accounted for 0.5% (at St. 12, 27.5 m depth, in winter 2015) and 3.7% (at St. 22, 100.5 m depth, in winter 2016), respectively, of the total abundance of the benthic community.
In the Aegean Sea, C. corona was found in semi-polluted to polluted environments, but it was absent from highly polluted stations (Çinar & Ergen Citation2007; Simboura et al. Citation2010). In the western Central Adriatic Sea, C. corona was recorded from slightly disturbed sediments, whose ecological classification was based on the community composition and structure, through AZTI Marine Biotix Index (AMBI) (Borja et al. Citation2000) and Multivariate - AZTI Marine Biotic Index (M-AMBI) (Muxika et al. Citation2007) indices. Indeed, the benthic community of the sampling stations showed the presence of opportunistic species (e.g. Prionospio fallax Söderström, 1920 and Chaetozone sp.) together with pollution-sensitive ones (e.g. Oestergrenia digitata (Montagu, 1815), Nucula sulcata Bronn, 1831, Aricidea (Acmira) assimilis Tebble, 1959 and Brissopsis lyrifera (Forbes, 1841)). Similarly, in the Tyrrhenian Sea, we found C. corona in a slightly disturbed station, numerically dominated by sensitive organisms.
The species seems to exhibit a tolerant but not opportunistic feature, in accordance with the AMBI (Borja et al. Citation2000) and TUrkish Benthic Index (TUBI) (Çinar et al. Citation2015) classification indices, both of which assign this species to the pollution-tolerant group. However, further studies are needed to confirm this judgement.
On hard bottom, C. corona was recorded among encrusting algae, and biogenic constructions consisting of calcareous animals and plants mixed with debris and cemented by algae and other limestone-producing organisms. The community at the sampling station was characterised by syllid polychates and bivalves typical of hard bottoms, such as Striarca lactea (Linnaeus, 1758), Gregariella Monterosato, 1883 spp. and the endolithic Lithophaga lithophaga (Linnaeus, 1758).
Pathways of dispersal and status
Çinar and Ergen (Citation2007) hypothesised that C. corona may have been introduced from southern California into the East Mediterranean basin through ballast waters. Similarly, Le Garrec et al. (Citation2016) suggested that C. corona may have been introduced by way of commercial shipping to the Atlantic coasts of Europe (Loire Estuary) and then would have been transported by currents towards the north-west along the coast of Brittany. These authors also tend to rule out aquaculture as a mode of introduction of this species to French Atlantic coasts. Apart from the opening of the Suez Canal, the two main vectors of introduction of marine alien species in the Mediterranean are shipping, combining ballast water and hull fouling, and aquaculture (Galil Citation2000; Gollasch Citation2006). The Adriatic Sea is the site of intense shellfish farming along the Italian coast, and finfish farming along the Croatian coast. To our knowledge, C. corona has not been recorded in any shellfish farming areas, such as those of the northern Adriatic that have experienced several introductions of alien species (Bertasi Citation2016; Munari et al. Citation2016). Accordingly, we tend to exclude that aquaculture activities may have played a role in the spreading dynamics of C. corona to the Adriatic and Tyrrhenian seas. The Adriatic Sea is also subjected to heavy marine traffic from merchant ships, supplier vessels for offshore activities (e.g. gas platforms), ferry boats, trawl-fishing vessels and recreational boats ( http://www.marinetraffic.com ). Therefore, C. corona may have been introduced from the East Mediterranean basin to the central Adriatic Sea through commercial shipping, and then in the same way to the Tyrrhenian. Also, marine currents may have had a role in the current distribution and spread of C. corona. Indeed, two main currents dominate the Adriatic circulation: the West Adriatic Current flowing towards the south-east along the western coast, and the East Adriatic Current flowing north-east along the eastern coast. In addition, two main cyclonic gyres occur, one in the northern part and the other in the south. Le Garrec et al. (Citation2016) also hypothesised that the presence of thermic barriers, residual currents and nocturnal migration of adults in the water column could explain the distribution pattern of C. corona. Therefore, we believe that all these pathways might together have contributed to the dispersal of C. corona in the Mediterranean, and might have limited the progression of the species into the northern Adriatic Sea. A molecular analysis including specimens from different parts of the Mediterranean and the world would be needed to confirm such speculations.
The species was initially reported as cryptogenic in the Mediterranean Sea (Çinar & Ergen Citation2007), and also hypothesised to be a “rare” cryptogenic species along the Atlantic coasts of France (Le Garrec et al. Citation2016). More recently, the species has been considered an established alien in the eastern Mediterranean Sea (i.e. Sea of Marmara, Aegean Se, and Levantine Sea; Çinar & Bakir Citation2014; Zenetos et al. Citation2017).
Chaetozone carpenteri McIntosh, Citation1911
Chaetozone carpenteri McIntosh Citation1911: 166, pl. 6, fig. 5c–e; Chambers et al. Citation2011: 45, .
Material examined
Central Adriatic Sea, off the coast of Pescara, winter 2014: St. 5, one specimen, St. 12, three specimens; summer 2015: St. 14, four specimens, St. 16, four specimens; winter 2015: St. 15, five specimens, St. 17, two specimens, St. 22, two specimens; summer 2016: St. 22, one specimen; winter 2016: St. 14, one specimen.
Description
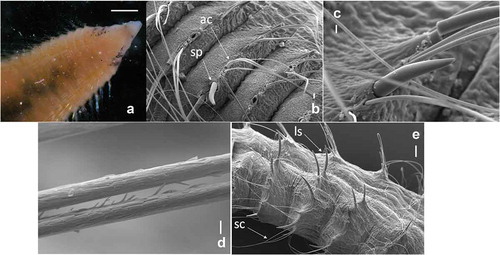
Maximum body length (complete specimen) 25.6 mm for 64 chaetigers and 2.3 mm width (from St. 5, 16.5 m depth, winter 2014). Other specimens were incomplete (29–53 chaetigers) ranging from 0.7 to 1.9 mm wide across the widest part of the body. Body surface smooth, iridescent and a little wider between chaetigers 12 and 22 in some specimens. The anterior dorsal surface is slightly rounded, and the ventral surface flattened with a longitudinal groove. Posterior segments are concertina-like, typical of the genus Chaetozone. The prostomium is conical with a pair of eyes not well defined; without nuchal groves. Peristomium achaetous, with a dorsal raised area posterior to the eyes; a ventral mouth and a pair of grooved palps (only palp bases present) originating from the dorsal surface posterior to the raised area. In some specimens we detected black irregular speckles on lateral and ventral sides of the peristomium and chaetiger 1 ().
Branchiae present from chaetiger 1 on the dorsal surface of the outer edge of the chaetae, between the parapodial folds (most of the branchiae are lost). Parapodia all biramous. Chaetae arranged in fan-shaped rows. There are four types of chaetae: awl-shaped capillaries ( )), large spines (b,), long spines and short capillaries ( )). The awl-shaped capillary chaetae are longer in the middle body region and present in numbers of 5–8 in anterior neuropodia, up to 11 in larger specimens (e.g. complete specimen from St. 14 of summer 2015: 23.4 mm long and 1.9 mm wide for 58 chaetigers) and 9–16 in anterior notopodia. They are constricted about the level of the skin and yellow coloured. The tip flattens out more in the shorter and less in the long forms, and tapers to a long, hair-like, curved extremity. Chaetae of anterior chaetigers with fine fibrils in some specimens (d)). Very large spines appear from chaetigers 7–9 in numbers of 2–3, both in neuro- and notopodia. Spines become longer from approximately chaetiger 20, increasing in noto- and neuropodia from 2–3 to 5–6 in posterior segments (beyond chaetiger 40). In posterior chaetigers capillary setae become shorter, thinner and fewer than in the anterior part of the body. Pygidium is a small, flat, rounded lobe.
Figure 3. Occurrence of Chaetozone corona Berkeley & Berkeley, Citation1941 and C. carpenteri McIntosh, Citation1911 in the Central Adriatic Sea: densities (average individuals m−2) ± standard error.
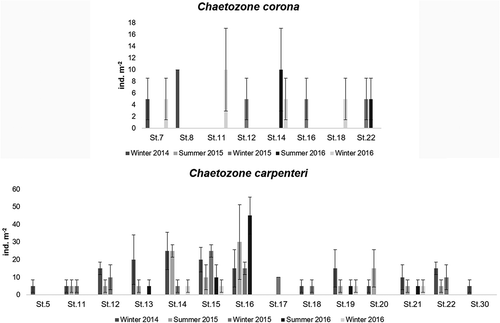
Figure 4. Chaetozone carpenteri. (a) Dorsal view of prostomium showing eyes and black speckles, anterior end. (b) Lateral view of awl-shaped capillary (ac) chaetae and large spines (sp), anterior end. (c) Detail of large spine. (d) Detail of damaged chaetae of first chaetigers. (e) Lateral view of long spines (ls) and short capillaries (sc), posterior end. Scale bars: a = 0.5 mm; b = 20 µm; c = 10 µm; d = 2 µm; ee = 100 µm.
Larger specimens had mature oocytes in the coelomatic cavity; diameter ± SE = 99.7 ± 0.6 mm, range = 79.6–135.2 mm (n = 31).
Remarks
Our specimens from the Central Adriatic Sea agreed closely with the original (McIntosh Citation1911) and subsequent descriptions (Chambers et al. Citation2011), but there were some differences worth mentioning.
Chambers et al. (Citation2011) found spines arising from chaetiger 7–8 in specimens from Cape Finisterre Atlantic coast of Spain), and from around chaetiger 6–9 in specimens from the Northern Adriatic and the Tyrrhenian Sea. This is in agreement with the characteristic of specimens from the Central Adriatic, in most of which spines were clearly visible from chaetiger 9. Conversely, according to McIntosh (Citation1911) spines arose from chaetiger 10. Also, the number of spines (i.e. 2–3 notopodial and neuropodial spines) in the anterior chaetigers was similar to that reported by Chambers et al. (Citation2011). In the original description, McIntosh (Citation1911) did not mention the number of spines in anterior chaetigers, but in the figure chaetiger 10 is illustrated with four spines in noto- and neuropodia; however, in this specimen the bristles were all broken, making their number unreliable. The number of spines from approximately chaetiger 20 to the posterior part of the body was similar in our specimens and those described by Chambers et al. (Citation2011) and by McIntosh (Citation1911): up to 4–6 long spines in posterior chaetigers of specimens from the Central Adriatic, up to 5–6 in those from Croatia and the Tyrrhenian Sea, and up to four (in chaetiger 40) in those from stations of the Porcupine Expedition of 1870. Number of capillaries differed from the description provided by Chambers et al. (Citation2011). On chaetiger 1 we found 10–16 notopodial capillaries in complete specimens of 18.2–23.4 mm long and 1.7–1.9 mm wide, with 58–60 chaetigers (from St. 14, at 46 m depth) and 9–10 notopodial capillaries in less wide specimens (e.g. incomplete specimen 6.9 mm long, 1.5 mm wide with 30 chaetigers, from St. 22, at 100.5 m depth). Numbers of notopodial and neuropodial capillary chaetae might vary in order of individual size, as reported for specimens of C. corona (Çinar & Ergen Citation2007). Differently from us, Chambers et al. (Citation2011) found 8–10 notopodial and 4–6 neuropodial capillary chaetae in anterior parapodia (maximum length of specimens 23 mm with 60 chaetigers; 2 mm wide).
We found a slight enlargement of the body between chaetigers 10 and 20, while Chambers et al. (Citation2011) found a constant width along the body. In some specimens we detected black, irregular speckles on lateral and ventral sides of the prostomium, peristomium and chaetiger 1, according to the original description by McIntosh (Citation1911) (a)).
The arrangements of spines and capillaries, together with the presence/absence of eyes, and number of segments, are useful characters for identification of Chaetozone species (Chambers et al. Citation2011). Chaetozone carpenteri can be quickly distinguished from the other Chaetozone species from the Mediterranean (C. setosa, C. gibber, C. zetlandica and C. corona) by the first appearance of neuropodial and notopodial spines from chaetiger 7–9, the presence of two or three types of spines, respectively, and two types of capillary chaetae, in addition to the black speckles on the prostomium, peristomium and chaetiger 1, according to McIntosh (Citation1911) and Chambers et al. (Citation2011).
Chambers et al. (Citation2011) and McIntosh (Citation1911) did not mention the presence of serration or minute fibrils that we found in our specimens; however, these fibrils may have resulted from damage of capillaries, as Çinar and Ergen (Citation2007) suggested for specimens of C. corona.
Ecology and distribution
Chaetozone carpenteri was first reported by McIntosh (Citation1911) from three Porcupine Expedition stations: on the coast of Algiers (Bono Bay, 45 m depth), and the Atlantic coast of Spain (off Cape Guardia and Cape Finisterre). Recently, it was recorded by Chambers et al. (Citation2011) off Italian and Croatian coasts. In the Northern Adriatic Sea it was found off the coast of Rovinj between 31 and 37 m depth, in detritic bottoms (silty sand); in the Tyrrhenian Sea it was found in a sandy bottom (silty sand, north-west Sardinia at 42 m depth); and in the Gulf of Naples it was found in sandy mud as well in mud, between 80 and 98 m depth (Chambers et al. Citation2011). Afterwards (between 2012 and 2013) it was reported from the Croatian coasts of the Central Adriatic Sea, both in the sandy mud of the Split Harbour at 20 m depth, and in the gravelly mud of the River Krka estuary (Mikac Citation2015). The species has also been found on the coasts of Turkey in the Sea of Marmara in soft bottom (including phanerogams), between 11 and 100 m depth (Çinar et al. Citation2014).
In the western Central Adriatic Sea we found this species mainly in silt and clayey silt (), in sediments with sand contents varying from 0.3 to 17.7%, silt contents from 36.2 to 99.8% and clay contents from 4 to 61.7%. It was absent in sediments with high sand contents.
In previous works, information about the density of C. carpenteri was not given. Off the coast of Pescara (central Adriatic Sea) the density of C. carpenteri varied from 5 ind. m−2 (at St. 14, St. 15, St. 20 and St. 21 in winter 2016; at St. 13, St. 19 and St. 21 in summer 2016; at St. 11, St. 14, and St. 18 in winter 2015; at St. 5, St. 11, St. 18, St. 20, and St. 30 in winter 2014) to 60 ind. m−2 (at St. 16 in summer 2015), between 16.5 and 130 m depth. Chaetozone carpenteri accounted for 0.2% (at St. 5, 16.5 m, in winter 2014) and 13% (at St. 16, 70 m, in summer 2015, and at St. 22, 100.5 m, in winter 2014) of the total abundance of the benthic community ().
In this study, C. carpenteri inhabits muddy sediments as does C. corona, with which it coexists in some of them (). In the sampling stations we recorded very low TOC concentrations, but these sediments (and thus the benthic communities) are subjected to high physical disturbance due to fishing activities. Considering the high density at which C. carpenteri was recorded with respect to C. corona, it seems to exhibit an opportunistic feature, in accordance with the AMBI classification index (Borja et al. Citation2000). However, although C. setosa and C. gibber have been found from semi-polluted sediments and thus reported as pollution indicators (Ergen Citation1992; Zenetos et al. Citation1994; Simboura et al. Citation1995; Borja et al. Citation2000; Simboura & Zenetos Citation2002; Solis-Weiss et al. Citation2004), knowledge about C. carpenteri populations is still too poor to affirm that it has the same role.
This is the first study to report the presence of reproductive specimens of C. carpenteri in the Mediterranean.
Conclusive remarks
Detailed morphological investigations of bitentaculate cirratulids suggest that numerous local, endemic species with defined habitat preferences, depth ranges and geographic distributions are present among materials previously assigned to a single species (Blake Citation1996, Citation2006). Thanks to the enhanced level of taxonomic awareness, which led to the re-examination of specimens previously considered unidentified or assigned to a higher level of taxonomic classification, and of those incorrectly identified as Chaetozone setosa, the number of fully identified species of the genus Chaetozone is currently rising in the Mediterranean Sea. The species with greatest morphological similarity to Chaetozone corona is Chaetozone carpenteri, known from the Mediterranean. A detailed comparison of the main morphological characters of the eight valid species of Chaetozone recorded in European waters was provided by Le Garrec et al. (Citation2016).
This study reports the first record of C. corona from the Italian coast of the Adriatic and Tyrrhenian seas. Taking into account the relatively poor information available on the benthic biocoenosis of the Central Adriatic Sea, it is not possible to state precisely how many years C. corona has been present in the Western Adriatic, as well as in the Tyrrhenian Sea, as it has never been recorded before from Italian coasts.
We found C. corona and C. carpenteri coexisting in soft bottom habitats of the Central Adriatic Sea. These two species showed wide distribution in sandy, muddy or mixed sediments at a wide depth range in sites disturbed and undisturbed ( and ) by fishing activities. From the coast to offshore, benthic assemblages are exposed to a heavy and prolonged history of exploitation, and are subjected to chronic and intensive effects of bottom trawling and fishing, with habitat degradation which in turn homogenised and simplified the benthic assemblages themselves (Bastari et al. Citation2017). In the five sampling periods of this study (from winter 2014 to winter 2016), C. corona was present at low density (5–10 ind. m−2), between 19.5 and 100.5 m depth, whereas C. carpenteri showed a wide distribution, as it was present at a large number of stations, between 27.5 and 130 m depth, at a density between 5 and 60 ind. m−2.
In accordance with the observations of Le Garrec et al. (Citation2016) for the Atlantic coasts, our findings support the hypothesis that C. corona is a non-invasive species, at least in the study area (Central Adriatic Sea). Nevertheless, on the basis of our knowledge, we may hypothesise a possible competition with the native surface detritivores. Indeed, Chaetozone species are surficial modifiers (Queirós et al. Citation2013), and competition among deposit-feeding species living at the sediment/water interface has been observed for a long time (Eagle & Hardiman Citation1977).
Findings of this study extend the range of distribution within the Mediterranean Sea of two Chaetozone species, also providing information on the ecological preferences of these species in terms of habitat.
The repeated observations of the two species from 2014, and the finding of reproducing specimens of both, suggest that C. corona and C. carpenteri have found a suitable habitat in the western (Central) Adriatic Sea, although at a low density as recorded in this study. Also, our results suggest that their populations may already be, or may become in the immediate future, self-sustaining and well established in the Adriatic Sea, as already happened at least in part of the Eastern Mediterranean (Çinar & Bakir Citation2014; Zenetos et al. Citation2017).
Considering its distribution in close proximity to intense ship routes, we would also venture the hypothesis that C. corona was introduced and then spread in the Adriatic Sea through ballast waters, and that its current distribution is limited by water currents and the availability of muddy to sandy sediments. Of particular relevance is our record of a specimen of C. corona from a hard bottom of the Tyrrhenian Sea. This finding represents an advancement of the knowledge of potential suitable habitats for this species.
As also recommended by other authors (Chambers & Woodham Citation2003; Çinar & Ergen Citation2007; Simboura et al. Citation2010; Chambers et al. Citation2011; Le Garrec et al. Citation2016), we believe that re-examination of Chaetozone specimens from laboratories or museum collections could determine whether the established alien C. corona and the native C. carpenteri occur elsewhere in the Mediterranean, beyond the spatial and temporal distribution given in this study. We also emphasise the need to collect new specimens in order to conduct genetic investigations that could allow us to define the history of introduction and spread of C. corona, as well as its relationship with the Mediterranean, and morphologically similar, C. carpenteri. To date, much remains unknown on the ecology and distribution of these two species in the Western Mediterranean Sea, as well as on their role in the benthic communities. Further efforts are needed to assess the geographic range of rare native species, and the geographic spread and potential invasiveness of alien ones.
Competing interests
The authors declare that they have neither competing interests nor potential conflict of interest.
Acknowledgements
We are very grateful to Mr Patrizio Fontana and Mr Albino Moretti for their help with the sampling. We extend our sincere gratitude to all crew members of the vessel Kiya for their support at sea.
We thank Terna S.p.A. which allowed to use samples collected within the framework of the environmental monitoring programme “MONITA”, following the installation of the HVDC 500 kV cc Italy–Montenegro submarine connection.
We would also like to thank the two anonymous reviewers.
References
- Arvanitidis C. 2000. Polychaete fauna of the Aegean Sea: Inventory and new information. Bulletin of Marine Science 66:73–96.
- Bastari A , Beccacece J , Ferretti F , Micheli F , Cerrano C. 2017. Local ecological knowledge indicates temporal trends of benthic invertebrates species of the Adriatic Sea. Frontiers in Marine Science 4:157. DOI:10.3389/fmars.2017.00157.
- Berkeley E , Berkeley C . 1941. On a collection of Polychaeta from southern California. Bulletin of the Southern California Academy of Sciences 40:16–60.
- Bertasi F . 2016. The occurrence of the alien species Polydora cornuta Bosc, 1802 (Polychaeta: Spionidae) in North Adriatic lagoons: An overlooked presence. Italian Journal of Zoology 83:77–88. DOI:10.1080/11250003.2016.1140839.
- Blake JA . 1996. Family Cirratulidae Ryckholdt, 1851. Including a revision of the genera and species from the Eastern North Pacific. In: Blake JA , Hilbig B , Scott PH , editors. Taxonomic atlas of the benthic fauna of the Santa Maria Basin and the Western Santa Barbara Channel. Volume 6: the Annelida Part 3. Polychaeta: Orbiniidae to Cossuridae. Santa Barbara, CA: Santa Barbara Museum of Natural History. pp. 263–384.
- Blake JA . 2006. New species and records of deepwater Cirratulidae (Polychaeta) from off Northern California. Scientia Marina 70(S3):45–57. DOI:10.3989/scimar.2006.70s3.
- Blake JA . 2015. New species of Chaetozone and Tharyx (Polychaeta: Cirratulidae) from the Alaskan and Canadian Arctic and the Northeastern Pacific, including a description of the lectotype of Chaetozone setosa Malmgren from Spitsbergen in the Norwegian Arctic. Zootaxa 3919:501–552. DOI:10.11646/zootaxa.3919.3.5.
- Borja A , Franco J , Pérez V . 2000. A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Marine Pollution Bulletin 40:1100–1114. DOI:10.1016/S0025-326X(00)00061-8.
- Castelli A , Bianchi CN , Cantone G , Çinar ME , Gambi MC , Giangrande A , Sareri DI , Lanera P , Licciano M , Musco L . 2008. Annelida Polychaeta. Checklist della Flora e della Fauna dei mari italiani (parte I). Biologia Marina Mediterranea 15:323–373.
- Chambers S , Dominguez-Tejo EL , Mair JM , Mitchell LA , Woodham A . 2007. The distribution of three eyeless Chaetozone species (Cirratulidae: Polychaeta) in the north-east Atlantic. Journal of the Marine Biological Association of the Uk 87:1111–1114. DOI:10.1017/S0025315407057724.
- Chambers S , Lanera P , Mikac B . 2011. Chaetozone carpenteri McIntosh, 1911 from the Mediterranean Sea and records of other bi-tentaculate Cirratulids. Italian Journal of Zoology 78:41–48. DOI:10.1080/11250003.2011.580565.
- Chambers SJ . 2000. A redescription of Chaetozone setosa Malmgren, 1867 including a definition of the genus, and a description of a new species of Chaetozone (Poychaeta: Cirratulidae) from the northeast Atlantic. Bulletin of Marine Science 67:587–596.
- Chambers SJ , Woodham A . 2003. A new species of Chaetozone (Polychaeta: Cirratulidae) from deep water in the northeast Atlantic, with comments on the diversity of the genus in cold northern waters. Hydrobiologia 496:41–48.
- Cicero AM , Di Girolamo I , editors. 2001. Metodologie analitiche di riferimento. Programma di monitoraggio per il controllo dell’ambiente marino costiero (triennio 2001-2003). ICRAM©ICRAM. Roma: Ministero dell’ambiente della Tutela del Territorio.
- Çinar ME , Bakir K . 2014. ALien Biotic IndEX (ALEX) – A new index for assessing impacts of alien species on benthic communities. Marine Pollution Bulletin 87:171–179. DOI:10.1016/j.marpolbul.2014.07.061.
- Çinar ME , Bakır K , Öztürk B , Katağan T , Dağlı E , Açık S , Doğan A , Bakır BB . 2015. TUBI (TUrkish Benthic Index): A new biotic index for assessing impacts of organic pollution on benthic communities. Journal of Black Sea/Mediterranean Environment 21:135–168.
- Çinar ME , Dagli E . 2013. Polychaetes (Annelida: Polychaeta) from the Aegean and Levantine coasts of Turkey, with descriptions of two new species. Journal of Natural History 47:911–947. DOI:10.1080/00222933.2012.752543.
- Çinar ME , Dagli E , Açik S . 2011. Annelids (Polychaeta and Oligochaeta) from the Sea of Marmara, with descriptions of five new species. Journal of Natural History 45:2105–2143. DOI:10.1080/00222933.2011.582966.
- Çinar ME , Dağli E , Kurt Şahin G . 2014. Checklist of Annelida from the coasts of Turkey. Turkish Journal of Zoology 38:734–764. DOI:10.3906/zoo-1405-72.
- Çinar ME , Ergen Z . 2007. The presence of Chaetozone corona (Polychaeta: Cirratulidae) in the Mediterranean Sea: An alien or a native species?. Cahiers De Biologie Marine 48:339–346.
- Çinar ME , Ergen Z , Dagli E . 2004. New records of polychaetes from the Turkish Aegean coast. Rapport Commission Internationale Pour l’Exploration Scientifique De La Mer Méditerranèe 37:508.
- Çinar ME , Katagan T , Öztürk B , Dagli E , Açik S , Bitlis B , Bakir K , Dogan A . 2012. Spatio-temporal distributions of zoobenthos in Mersin Bay (Levantine Sea, eastern Mediterranean) and the importance of alien species in benthic communities. Marine Biology Research 8:954–968. DOI:10.1080/17451000.2012.706305.
- Çinar ME , Katagan T , Öztürk B , Egemen Ö , Ergen Z , Kocatas A , Önen M , Kirkim F , Bakir K , Kurt G , Dagli E , Kaymakçi A , Açik S , Dogan A , Özcan T . 2006. Temporal changes of soft-bottom zoobenthic communities in and around Alsancak Harbor (Izmir Bay, Aegean Sea), with special attention to the autecology of exotic species. Marine Ecology 27:229–246. DOI:10.1111/mae.2006.27.issue-3.
- Doner SA , Blake JA . 2006. New species of Cirratulidae (Polychaeta) from the northeastern United States. Scientia Marina 70(S3):65–73. DOI:10.3989/scimar.2006.70s3.
- Eagle RA , Hardiman PA . 1977. Some observations on the relative abundance of species in a benthic community. In: Keegan BF , Boaden PJS , Ceidigh PO , editors. Biology of Benthic Organisms: 11th European Symposium on Marine Biology, Galway, October 1976. pp. 197–208.
- Ergen Z . 1992. The latest status of Polychaeta in the soft substrate of Izmir Bay. Rapport Commission Internationale Pour l’Exploration Scientifique De La Mer Méditerranèe 33:36.
- Galil BS . 2000. A sea under siege – alien species in the Mediterranean. Biological Invasions 2:177–186. DOI:10.1023/A:1010057010476.
- Gollasch S . 2006. Overview on introduced aquatic species in European navigational and adjacent waters. Helgoland Marine Research 60:84–89. DOI:10.1007/s10152-006-0022-y.
- Hartman O . 1960. Systematic account of some marine invertebrate animals from the deep basins of southern Califorina. Allan Hancock Pacific Expeditions 22:69–215.
- Hartman O . 1961. Polychaetous anellids from California. Allan Hancock Pacific Expeditions 25:1–226.
- Hartman O . 1969. Atlas of the Sedentariate Polychaetous Annelids from California. Los Angeles, CA: Allan Hancock Foundation, Univeristy of Southern California. pp. 1–812.
- Le Garrec V , Grall J , Chevalier C , Guyonnet B , Jourde J , Lavesque N , Bonifácio P , Blake JA . 2016. Chaetozone corona (Polychaeta, Cirratulidae) in the Bay of Biscay: A new alien species for the North-east Atlantic waters?. Journal of the Marine Biological Association of the United Kingdom 97:433–445.
- McIntosh WC . 1911. Notes from the Gatty Marine Laboratory, St Andrews. No. XXXII. Annals and Magazine of Natural History 8:145–173.
- Mikac B . 2015. A sea of worms: Polychaete checklist of the Adriatic Sea. Zootaxa 3943:1–172. DOI:10.11646/zootaxa.3943.1.
- Munari C . 2014. A new species of Cerapopsis (Amphipoda: Corophiidea: Kamakidae) from the Strait of Messina, central Mediterranean Sea. Italian Journal of Zoology 81:78–91. DOI:10.1080/11250003.2013.857730.
- Munari C , Bocchi N , Mistri M . 2016. Grandidierella japonica (Amphipoda: Aoridae): A non-indigenous species in a Po delta lagoon of the northern Adriatic (Mediterranean Sea). Marine Biodiversity Records 9:12. DOI:10.1186/s41200-016-0018-5.
- Muxika I , Borja Á , Bald J . 2007. Using historical data, expert judgement and multivariate analysis in assessing reference conditions and benthic ecological status, according to the European Water Framework Directive. Marine Pollution Bulletin 55:16–29. DOI:10.1016/j.marpolbul.2006.05.025.
- Omena E , Creed JC . 2004. Polychaete fauna of seagrass beds (Halodule wrightii Ascherson) along the coast of Rio de Janeiro (Southeast Brazil). Marine Ecology 25:273–288. DOI:10.1111/mae.2004.25.issue-4.
- Queirós AM , Birchenough SNR , Bremner J , Godbold JA , Ruth E , Parker RE , Romero-Ramirez A , Reiss H , Solan M , Somerfield PJ , Van Colen C , Van Hoey G , Widdicombe S . 2013. A bioturbation classification of European marine infaunal invertebrates. Ecology and Evolution 3958–3985. DOI:10.1002/ece3.769.
- Simboura N . 1996. Marine macrobenthic polychaetes of Greece: taxonomy, ecology, zoogeography. PhD Thesis, University of Athens. pp. 241.
- Simboura N , Kurt Sahin GK , Panagoulia A , Katsiaras N . 2010. Four new alien species on the coasts of Greece (Eastern Mediterranean). Mediterranean Marine Science 11:341–352. DOI:10.12681/mms.81.
- Simboura N , Nicolaidou A . 2001. The Polychaetes (Annelida, Polychaeta) of Greece: Checklisht, distribution and ecological characteristics. Monographs on Marine Sciences n. 4. Athens: National Centre for Marine Research.
- Simboura N , Zenetos A . 2002. Benthic indicators to use in ecological quality classification of Mediterranean soft bottom marine ecosystems, including a new biotic index. Mediterranean Marine Science 3:77–111. DOI:10.12681/mms.249.
- Simboura N , Zenetos A , Panayotidis P , Makra A . 1995. Changes in benthic community structure along an environmental pollution gradient. Marine Pollution Bulletin 30:470–474. DOI:10.1016/0025-326X(95)00237-H.
- Solis-Weiss V , Aleffi F , Bettoso N , Rossin P , Orel G , Fonda-Umani S . 2004. Effects of industrial and urban pollution on the benthic macrofauna in the Bay of Muggia (industrial port of Trieste, Italy). Science of the Total Environment 328:247–263. DOI:10.1016/j.scitotenv.2004.01.027.
- Woodham A , Chambers S . 1994a. A new species of Chaetozone (Polychaeta, Cirratulidae) from Europe, with a re-description of Caulleriella zetlandica (McIntosh). Mémoires Du Muséum National D’histoire Naturelle 162:307–316.
- Woodham A , Chambers S . 1994b. Some taxonomic problems of bi-tentaculate cirratulids. Polychaetae Research 16:14–15.
- Zaâbi S , Gillet P , Afli A , Boumaiza M . 2009. Biodiversity of polychaetous annelids from the peninsula of Cap Bon, northeast coast of Tunisia. Zoosymposia 2:587–600.
- Zenetos A , Çinar ME , Crocetta F , Golani D , Rosso A , Servello G , Shenkar N , Turon X , Verlaque M . 2017. Uncertainties and validation of alien species catalogues: The Mediterranean as an example. Estuarine, Coastal and Shelf Science 191:171–187. DOI:10.1016/j.ecss.2017.03.031.
- Zenetos A , Simboura N , Panayotidis P . 1994. Effects of sewage on the distribution of benthic fauna in the Saronikos Gulf (Greece). Final Reports on Research Projects Dealing with the Effects of Pollutants on Marine Organisms and Communities 80:37–92.