Abstract
The aim of the study is to present a complete and updated fish inventory of inland waters of the Emilia-Romagna region, Northern Italy, and to highlight the presence of fully exotic fish communities. Overall, based on 208 sampling locations, the observed fish fauna consisted of 45 species, 22 native and 23 exotics. A significant element of the inventory is the identification of xenodiversity hotspots (spatially clustered sites, one lowland and one upland region), where a complete substitution of native species by exotic species was observed (in total seven sites in the lowland and two sites in the upland with no native species presence). These xenodiversity hotspots were found to host specific combinations of exotic species, which may be able to constitute balanced exotic communities. The hotspots of the lowland region are located in the northeast lowland part of the territory, hosting exotic species combinations mainly composed by wels catfish (Silurus glanis Linnaeus, 1758, a large predator), common carp (Cyprinus carpio Linnaeus, 1758, a large benthivore), crucian carp (Carassius spp., a small-bodied generalist) and other less dominant exotic species. The hotspots in the upland region were located in the southwest part of the territory and were dominated by only one exotic species (rainbow trout, Oncorhynchus mykiss (Walbaum, 1792)). A difference between these xenodiversity hotspots is that in the lowland the introductions were mostly unintentional and are not continued, while in the upland the introduction of rainbow trout is intentional and currently carried out by local fishermen.
Introduction
The increase in exotic fish species invasions is alarming, creating an important threat for freshwater ecosystems (Leprieur et al. Citation2008). Exotic species can promote habitat deterioration/alteration such as decline of aquatic vegetation, increase of turbidity and nutrients release due to sediment resuspension, increase of phytoplankton blooms and eutrophication, genetic alterations within populations, spreading of pathogens and parasites, competition with, and predation of, native species (Dibble & Kovalenko Citation2009; Leunda Citation2010; Ribeiro & Leunda Citation2012; Castaldelli et al. Citation2013). Among European nations, one of those most impacted by exotic species invasions is Italy (see e.g. Bianco & Ketmaier Citation2001; Bianco Citation2014), with the Po River basin being one of the most invaded areas. The severity of the invasions has already reached critical limits, especially in the lowland areas near the estuary, where at least 10 native fish species faced local extinction while many exotic ones showed a population explosion during the period 1991–2009 (Castaldelli et al. Citation2013).
The first aim of this study is to present a complete and updated inland water fish inventory of the Emilia-Romagna region in Northern Italy. The Emilia-Romagna region belongs to the southern side of the Padanian-Venetian ichthyogeographic district (see Bianco Citation1995) and hosts a number of native fish species of great conservational interest (e.g. twaite shad Alosa fallax (Lacépède, 1803), Italian barbel Barbus plebejus Bonaparte, 1839, or Italian nase Chondrostoma soetta Bonaparte, 1840) according to the EU Habitat Directive (92/43/EEC). Arising from the inventory, a significant element is the identification of xenodiversity hotspots, where there was a complete substitution of native species by exotic species (Castaldelli et al. Citation2013). These xenodiversity hotspots were found to host specific combinations of exotic species, which were able to constitute fully exotic communities. A discussion on the structure and attributes of these communities was the second aim of this study. A fish inventory including specific information on the distribution of exotic species could be relevant for managers, as it would be most useful to prioritize concrete conservation actions for native biodiversity.
Materials and methods
The study area is located in Northern Italy and it is defined by the administrative boundaries of the Emilia-Romagna Region with total coverage of 22,446 km2 (). It is naturally bound north and south by the Po River and the Apennine Mountains, respectively. The study region has a Mediterranean continental climate. Altogether, data from 208 river monitoring sites were analyzed in this study (), covering a wide range of inland water habitats at different altitudinal zones. The samplings were performed in natural rivers (e.g. Po, Trebbia, Taro, Secchia, Panaro, Reno, Lamone, Fiumi Uniti, Bevano, Marecchia, etc.) and in large artificial irrigation canals which are mainly located in the lowlands (e.g. Po di Volano, Po di Primaro, Canal Bianco, Canale Circondariale, etc.).
Figure 1. Study area (data source: http://gadm.org), hydrographic network of main rivers and streams (data source: http://www.eea.europa.eu/data-and-maps/data/european-river-catchments-1) and location of sampling sites.
Fish data were collected from sampling stations that were homogeneously positioned in 64 waterways of the region (), away from recreationally managed sites, and with their section width ranging from 8 to 350 m (the maximum value corresponds to the Po River). The samplings were conducted during the warm season (from April to September) of the period 1998–2004 as part of the institutional regional monitoring program for the compilation of the official Fish Inventory of the Emilia-Romagna Region (Pascale et al. Citation2004, Citation2006; Castaldelli & Rossi Citation2008).
Fish sampling was performed by electrofishing, adapting the standard guidelines to the particular conditions of waterway typologies (Backiel & Welcomme Citation1980; Reynolds Citation1983). Electrofishing was performed thoroughly with a direct current at 400–600 V and 4–5 A (Reynolds Citation1983; Godinho & Ferreira Citation2000) once during daylight, in an upstream zigzag direction by wading, when depth was less than 1 m, and by boat in deeper waters. The transect lengths were equivalent to 10 times the river width, ensuring that the range of present macrohabitats of each site was fully surveyed (Hankin & Reeves Citation1988; Godinho & Ferreira Citation2000). The duration of sampling was therefore quite variable, ranging from half an hour to more than 2 hours, as in the case of the Po River. Electrofishing is considered the best quantitative method for fish sampling in shallow waters, up to a maximum of 1 m (Zalewski & Cowx Citation1990) but its efficacy may be low in deeper waters, with high conductivity, or in the presence of big and mobile specimens. Such special conditions occurred in almost all the lower stretches of rivers and in the large canals of the lowlands. For this reason, electrofishing in these sites was verified by sampling using a standard set of nets, composed and operated as follows: three sinking trammel nets (50 m long and 1.80 m high), composed of two external panels with knot-to-knot mesh size of 70 mm and an internal one of 300 mm, and three sinking gill nets (50 m long and 1.80 m high) with knot-to-knot mesh sizes of 40, 20 and 10 mm, respectively. The presence of young-of-the-year specimens was assessed by using 2 × 2 m drop nets with a 5-mm mesh size in parallel with the other nets. Fishing with nets was performed immediately after electrofishing sessions, with the support of professional fishermen, and the duration was approximately 1 hour for the trammel nets and half an hour for the gill nets, in order to avoid mortality or damage of captured specimens. Fish species were identified according to Kottelat and Freyhof (Citation2007) and Bianco (Citation2014) and attributed either native or exotic status, relative to the Padanian-Venetian ichthyo-geographical district. Sampling sites were then grouped according to the presence/absence of native and exotic fish species.
Results
Overall, the observed fish fauna from the sampling sites consisted of 45 species, 22 native and 23 exotic, belonging to 12 families (). Since a profound discussion on the Salmo trutta complex has been undertaken after the surveys, it was not possible to resolve whether the sampled individuals of this complex were native or exotic. Regarding Gobioninae, Gobio gobio (Linnaeus, 1758) should be added among the aliens due to its invasions in all waters of the Padano-Venetian district. Gobio benacensis (Pollini, 1816) is placed among the natives with a question mark as its presence was not certainly detected.
Table I. Observed fish species in the freshwater systems of the Emilia Romagna region. The reported status of each species refers to the Emilia Romagna region (within the Padanian hydrographical district).
In a total of nine sampling sites, only exotic species were present. These xenodiversity hotspots were distributed in two main groups (one with seven sites in the lowlands, and one with two sites at higher elevation) which are shown in . summarizes the exotic species communities found in these sites.
Table II. Exotic species present in the nine positions belonging to the xenodiversity hotspots where native species were absent ().
Figure 2. Altitude (data source: https://lta.cr.usgs.gov/GTOPO30) and separation of sampling sites based on the presence/absence of native and exotic species.
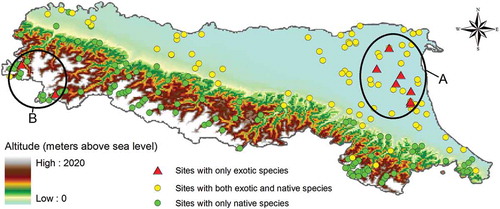
Overall, it was possible to identify three different groups of sampling sites, based on the presence/absence of native and exotic species:
A group of nine sites populated solely with exotic species, mostly located in the lowlands. These xenodiversity hotspots showed different combinations of 14 out of the 23 exotic species detected in the region (the missing ones were European barbel Barbus barbus (Linnaeus, 1758), roach Rutilus rutilus (Linnaeus, 1758), asp Leuciscus aspius (Linnaeus, 1758), channel catfish Ictalurus punctatus (Rafinesque, 1820), largemouth black bass Micropterus salmoides (Lacépède, 1803) and pond loach Misgurnus anguillicaudatus (Cantor, 1842) (see ).
A group of 92 sites hosting both native and exotic species, located from the lowlands to the foothills. These sites showed different combinations of native and exotic species, where all 23 exotic species were present, while from the 22 native species only three were missing (Italian nase, bullhead Cottus gobio Linnaeus, 1758, Eurasian minnow Phoxinus phoxinus (Linnaeus, 1758)).
A group of 107 sites where only native species were present, located mostly at higher altitudes. These sites showed different combinations of 16 out of 22 native species (the missing ones were twaite shad, tench Tinca tinca (Linnaeus, 1758), Italian rudd Scardinius hesperidicus Bonaparte, 1845, Southern pike Esox cisalpinus Bianco & Delmastro, 2011, thinlip grey mullet Liza ramada (Risso, 1827), flathead grey mullet Mugil cephalus Linnaeus, 1758, and European flounder Platichthys flesus (Linnaeus, 1758), the latter three typical of salt water but often found in inland waters.
Discussion
The existence of xenodiversity hotspots indicates that exotic fish species might be able to constitute fully exotic communities. These xenodiversity hotspots are surrounded by sites where the presence of native species is extremely low, at least in the lowlands. As fish surveys employed redundant sampling methods, it is unlikely that native species were not detected accurately. It is clear that other anthropogenic drivers (e.g. hydrologic alteration, habitat degradation) are also at play in the area and could favor exotic species, at least in the lowlands (Castaldelli et al. Citation2013). Therefore, it is likely that these xenodiversity hotspots could arise from species interactions occurring in altered environmental conditions. Unfortunately, because there are no long-term records of the fish communities for all these sites, it is impossible to completely disentangle the exact mechanism of interaction with native fishes.
The presence of fish xenodiversity hotspots is particularly alarming, because there have been few documented cases of non-isolated freshwater systems with high interconnectivity where native fish communities have been completely substituted by exotics. It is well known that exotic species can cause the displacement of natives, by outcompeting natives for spatial and trophic niches (Mooney & Cleland Citation2001); however, reports that prove complete multiple local fish extinctions as a result of exotic fish species invasions are relatively scarce, and sometimes questionable for reasons explained in Gurevitch and Padilla (Citation2004). The case of direct predation by brown trout (Salmo trutta Linnaeus, 1758) causing extinction of galaxiids fishes in New Zealand is one of the very few examples of these (Townsend Citation1996), while the case of Nile perch (Lates niloticus (Linnaeus, 1758)) in Lake Victoria is more controversial (e.g. Kitchell et al. Citation1997; Witte et al. Citation2000). Predatory interactions can only explain a limited part of the native species decline found in our study area, as the exotic fish communities comprise several species with a wide spectrum of eco-functional traits. The analysis of these traits, and how the combination of the specific exotic species may lead to fully exotic populations, should be the subject of future investigations.
The original native communities composed mostly by ciprinids such as Italian bleak Alburnus arborella (Bonaparte, 1841), Italian rudd, and exocids such as southern pike were locally extinct within the lowlands xenodiversity hotspots (), where exotic communities were mainly composed by wels catfish Silurus glanis (a large predator), common carp Cyprinus carpio (a large benthivore) and crucian carp Carassius spp. (a small-bodied generalist). These constitute the backbone of the fish communities in most xenodiversity hotspots. Biogeographical origin could partly explain this composition: the main exotic species in these sites (e.g. common bream Abramis brama (Linnaeus, 1758) and wels catfish) come from the same area, the Danube River, where they likely co-evolved and developed mechanisms of niche partitioning and coexistence (Castaldelli et al. Citation2013). The large size attained by some of these species (e.g. common carp or grass carp Ctenopharyngodon idella (Valenciennes, 1844)) and the deep body of others (e.g. common bream, crucian carp) could also partly explain their coexistence with predators. A notable exception to Danube River species are the pumpkinseed Lepomis gibbosus (Linnaeus, 1758), a North American centrarchid, and the black bullhead Ameiurus melas (Rafinesque, 1820), a North American ictalurid, which are both exotic species introduced over a century ago (Bianco Citation1998). These species seem able to constitute small populations within these sites, probably due to their well-known trophic and ecologic flexibility (Wainwright et al. Citation1991).
The xenodiversity hotspots in the lowlands of our study area could provide a valuable example of invasional meltdown in fish communities. Invasional meltdown is the mutual facilitation of invasion by different species (Simberloff & Von Holle Citation1999). Albeit not a new hypothesis, it still remains controversial (Simberloff Citation2006) and very few examples of it are known from fish communities (e.g. the opposite effect found in Britton et al. Citation2010). The ecosystem engineering capabilities of some of these exotic species could explain why these communities thrive. Common and crucian carp, for example, have been known to increase water turbidity and reduce macrophytes through their feeding actions (e.g. Richardson et al. Citation1995; Bonneau & Scarnecchia Citation2015). While their invasion in Western Europe was completed long ago, they are capable of continued effects on the environment. Furthermore, grass carp have been shown to be established in the area (Milardi et al. Citation2015) and feed directly on macrophytes, increasing the positive feedback on turbidity. Increased water turbidity can favor predators such as wels catfish or pike-perch, which are particularly adapted for predation in turbid waters, over native predators such as southern pike, which largely rely on sight and do not have special adaptations. Moreover, the interactions between exotic and native species are likely magnified by hydrologic alteration, as already hypothesized by Castaldelli et al. (Citation2013). Native fish are mostly riverine-adapted species, contrary to exotics which are more lacustrine in origin; therefore, the natives survive in streams or torrents on hills or sub-mountain zones, which should be considered sanctuaries for their survival, as previously discussed by Bianco and Ketmaier (Citation2001). Ultimately, further studies are needed to confirm whether our study area shows clear signs of invasional meltdown.
The xenodiversity hotspots at higher elevations () were located in the upper reaches of the Taro River and in a smaller stream (Rio Castello) feeding into the Trebbia River. These sites did not show significant habitat degradation or hydrologic alteration; however, the community was not composed by native trout and gobies or cottids, but rather solely composed by rainbow trout (Oncorhynchus mykiss). This species was previously reported to establish in different areas of Italy (Stanković et al. Citation2015), even if many more populations are known but not yet reported (Milardi, unpublished data), but its interactions with native fish and invertebrates are still largely unexplored. Candiotto et al. (Citation2011) hypothesized that rainbow trout could colonize mainly river stretches where no other fish were present. Our data suggest that in the Taro River and the Trebbia River, a population of rainbow trout can exist well within systems where other natives are present both up- and downstream (suggesting also that it could occasionally effectively displace native species, at least locally). Even though surveyed sites are far from sites where recreational stocking occurs, it is probable that stocking of rainbow trout for recreational fisheries could strongly contribute to the distribution pattern of this species in sites at higher altitudes.
Acknowledgements
The authors would like to thank Dr. D. Barchi (Director), Dr. R. Finco, Dr. G. Collina and Dr. R. Spiga of the Fisheries Bureau of the Emilia-Romagna Region for providing the Fish Inventories data in the context of a long-term research collaboration.
References
- Backiel T, Welcomme RL. 1980. Guidelines for sampling fish in inland waters. Rome, Italy: FAO.
- Bianco PG. 1995. Mediterranean endemic freshwater fishes of Italy. Biological Conservation 72:159–170. DOI:10.1016/0006-3207(94)00078-5.
- Bianco PG. 2014. An update on the status of native and exotic freshwater fishes of Italy. Journal of Applied Ichthyology 30:62–77. DOI:10.1111/jai.2014.30.issue-1.
- Bianco PG. 1998. Freshwater fish transfers in Italy: History, local changes in fish fauna and a prediction on the future of native populations. In: Cowx IG, editor. Stocking and introductions of fishes. Fishing New Book. Oxford: Blackwell Science. pp. 165–197.
- Bianco PG, Ketmaier V. 2001. Anthropogenic changes in the freshwater fish fauna of Italy, with reference to the central region and Barbus graellsii, a newly established alien species of Iberian origin. Journal of Fish Biology 59(Suppl. A):190–208. DOI:10.1111/jfb.2001.59.issue-sa.
- Bonneau JL, Scarnecchia DL. 2015. Response of benthic macroinvertebrates to carp (Cyprinus carpio) biomanipulation in three tributaries of a eutrophic, Great Plains reservoir, USA. Transactions of the Kansas Academy of Science 118:13–26. DOI:10.1660/062.118.0103.
- Britton JR, Harper DM, Oyugi DO, Grey J. 2010. The introduced Micropterus salmoides in an equatorial lake: A paradoxical loser in an invasion meltdown scenario? Biological Invasions 12:3439–3448. DOI:10.1007/s10530-010-9742-7.
- Candiotto A, Bo T, Fenoglio S. 2011. Biological and ecological data on an established rainbow trout (Oncorhynchus mykiss) population in an Italian stream. Fundamental and Applied Limnology 179:67–76. DOI:10.1127/1863-9135/2011/0179-0067.
- Castaldelli G, Pluchinotta A, Milardi M, Lanzoni M, Giari L, Rossi R, Fano EA. 2013. Introduction of exotic fish species and decline of native species in the lower Po basin, north-eastern Italy. Aquatic Conservation: Marine and Freshwater Ecosystems 23:405–417. DOI:10.1002/aqc.v23.3.
- Castaldelli G, Rossi R. 2008. Emilia-Romagna fish inventory, A and B zones. Emilia-Romagna region. Bologna, ITA: Greentime.
- Dibble ED, Kovalenko K. 2009. Ecological impact of grass carp: A review of the available data. Journal of Aquatic Plant Management 47:1–15.
- Godinho FN, Ferreira MT. 2000. Composition of endemic fish assemblages in relation to exotic species and river regulation in a temperate stream. Biological Invasions 2:231–244. DOI:10.1023/A:1010022123669.
- Gurevitch J, Padilla DK. 2004. Are invasive species a major cause of extinctions? Trends in Ecology & Evolution 19:470–474. DOI:10.1016/j.tree.2004.07.005.
- Hankin DG, Reeves GH. 1988. Estimating total fish abundance and total habitat area in small streams based on visual estimation methods. Canadian Journal of Fisheries and Aquatic Sciences 45:834–844. DOI:10.1139/f88-101.
- Kitchell JF, Schindler DE, Ogutu-Ohwayo R, Reinthal PN. 1997. The Nile perch in Lake Victoria: Interactions between predation and fisheries. Ecological Applications 7:653–664. DOI:10.1890/1051-0761(1997)007[0653:TNPILV]2.0.CO;2.
- Kottelat M, Freyhof J. 2007. Handbook of European freshwater fishes. BerlIn: Kottelat, Cornol and Freyhof. pp. 646.
- Leprieur F, Beauchard O, Blanchet S, Oberdorff T, Brosse S. 2008. Fish invasions in the world’s river systems: When natural processes are blurred by human activities. PloS Biology 6:404–410.
- Leunda PM. 2010. Impacts of non-native fishes on Iberian freshwater ichthyofauna: Current knowledge and gaps. Aquatic Invasions 5:239–262. DOI:10.3391/ai.
- Milardi M, Lanzoni M, Kiljunen M, Torniainen J, Castaldelli G. 2015. Natural recruitment contributes to high densities of grass carp Ctenopharyngodon idella (Valenciennes, 1844) in Western Europe. Aquatic Invasions 10:439–448. DOI:10.3391/ai.
- Mooney HA, Cleland EE. 2001. The evolutionary impact of invasive species. Proceedings of the National Academy of Sciences 98:5446–5451.
- Pascale M, Perosino G, Piccinini A. 2004. Carta Ittica dell’Emilia-Romagna Zona D. Regione Emilia-Romagna Ed., Crest, Torino. pp. 160.
- Pascale M, Perosino G, Piccinini A. 2006. Carta Ittica dell’Emilia Romagna Zona C. Regione Emilia-Romagna Ed., Crest, Torino. pp. 160.
- Reynolds J. 1983. Electrofishing. In: Nielsen L, Johnson D, editors. Fisheries techniques. Bethesda, MD: American Fisheries Society. pp. 147–163.
- Ribeiro F, Leunda PM. 2012. Non-native fish impacts on Mediterranean freshwater ecosystems: Current knowledge and research needs. Fisheries Management and Ecology 19:142–156. DOI:10.1111/j.1365-2400.2011.00842.x.
- Richardson MJ, Whoriskey FG, Roy LH. 1995. Turbidity generation and biological impacts of an exotic fish Carassius auratus, introduced into shallow seasonally anoxic ponds. Journal of Fish Biology 47:576–585.
- Simberloff D. 2006. Invasional meltdown 6 years later: Important phenomenon, unfortunate metaphor, or both? Ecology Letters 9:912–919. DOI:10.1111/ele.2006.9.issue-8.
- Simberloff D, Von Holle B. 1999. Positive interactions of nonindigenous species: Invasional meltdown? Biological Invasions 1:21–32. DOI:10.1023/A:1010086329619.
- Stanković D, Crivelli AJ, Snoj A. 2015. Rainbow trout in Europe: Introduction, naturalization, and impacts. Reviews in Fisheries Science & Aquaculture 23:39–71. DOI:10.1080/23308249.2015.1024825.
- Townsend CR. 1996. Invasion biology and ecological impacts of brown trout Salmo trutta in New Zealand. Biological Conservation 78:13–22. DOI:10.1016/0006-3207(96)00014-6.
- Wainwright PC, Osenberg CW, Mittelbach GG. 1991. Trophic polymorphism in the pumpkinseed sunfish (Lepomis gibbosus Linnaeus): Effects of environment on ontogeny. Functional Ecology 5:40–55. DOI:10.2307/2389554.
- Witte F, Msuku BS, Wanink JH, Seehausen O, Katunzi EFB, Goudswaard PC, Goldschmidt T. 2000. Recovery of cichlid species in Lake Victoria: An examination of factors leading to differential extinction. Reviews in Fish Biology and Fisheries 10:233–241. DOI:10.1023/A:1016677515930.
- Zalewski M, Cowx IG. 1990. Factors affecting the efficiency of electrofishing. In: Cowx IG, Lamarque P, editors. Fishing with electricity. London: Fishing News Books. pp. 89–111.
