Abstract
Aggregations of sea pens are important soft-bottom communities providing a three-dimensional complexity from which several associated species can benefit. The red sea pen Pennatula rubra is one of the Mediterranean coastal field-forming sea pens able to establish dense aggregations on the sandy/muddy bottoms of the infra- and circumlittoral zones. This species was first described at the end of the 17th century, but since then little information has been published about its biology, ecology and biogeography. Even less is known about its behaviour, its reactions after disturbance and its possible escape strategies. Several species of pennatulaceans can withdraw partially or completely into the sediment, usually in a fast (i.e. a few seconds) process of polyp closure and expulsion of part of the water contained within the colony. The present study reports and discusses the withdrawal behaviour of P. rubra after disturbance. This behaviour has never been documented before in this species. It proved to be a slow process requiring between 210 and 340 seconds (3 min 30 sec to 5 min 40 sec) for the complete withdrawal. Moreover, a soft bioluminescence was observed in two undisturbed colonies in the study area, while two other colonies were found to be out of the sediment, inflating themselves with seawater and getting carried by currents as a sort of dispersal behaviour.
Introduction
Sea pens are a specialised and morphologically distinct group of octocorals (Class Anthozoa, Subclass Octocorallia) adapted to live on soft sediments thanks to an anchoring muscular peduncle (Bayer Citation1956; Williams Citation2011). These animals are often found in moderately high-energy environments and may form locally dense aggregations, known as facies or fields (Pérès & Picard Citation1964; Gage & Tyler Citation1991) and belonging to the so-called animal forests (Rossi Citation2013; Rossi et al. Citation2017). Examples of sea pens forming fields (Aguilar et al. Citation2009; Mastrototaro et al. Citation2013; Kenchington et al. Citation2014; Porporato et al. Citation2014; Chimienti et al. Citation2015), structuring flat, low-relief soft-bottom habitats and acting as nurseries for fish (Pirtle Citation2005; Baillon et al. Citation2012) highlight their role in benthic ecosystems (Ruiz-Pico et al. Citation2017). Pennatulacean fields alter water current flow, retaining nutrients near the seafloor (Birkeland Citation1974; Tissot et al. Citation2006; Kenchington et al. Citation2011), and provide refuge for planktonic and benthic invertebrates (Birkeland Citation1974; Porporato et al. Citation2011, Citation2012) which may be preyed on by fish (Krieger Citation1993). Williams (Citation1999) provided extensive bibliographic sources regarding pennatulacean biology, ecology and behaviour.
The red sea pen Pennatula rubra (Ellis, 1761) is an emblematic pennatulacean species in the Mediterranean Sea. This species was mentioned already by Linnaeus (Citation1758) who distinguished it from the congeneric Pennatula phosphorea Linnaeus, Citation1758. He wrote: “Penna (Rubra) pinnis falciformibus, tentaculis in pinnarum facie concava densissime dispositis” about P. rubra and “Penna (Rosea) pinnis falciformibus, tentaculis in pinnarum facie concava laxe dispositis” about P. phosphorea, referring to the number of autozooids on the polyp leafs. Bohadsch (Citation1761) observed some colonies collected by fishermen off Naples and reiterated the concept of “polyps placed in one row within half a line of one another” for P. phosphorea and “placed in a double row and as near as they can be together” for this red sea pen, suggesting that this latter could be a different species, which he called Penna rubra following Linnaeus (Citation1758). The work of Bohadsch (Citation1761) was subsequently suppressed by the International Commission on Zoological Nomenclature for nomenclatural purposes (Williams Citation1999). Ellis (Citation1763) gave a copy of Bohadsch’s figures and descriptions, analysed a colony from the Mediterranean Sea and re-established the name Pennatula rubra. In a letter to the Honorable Coote Molesworth, Ellis (Citation1763) underlined the orientation of the autozooids in P. rubra and its differences from P. phosphorea, as recently shown by Chimienti et al. (Citation2015). Ellis (Citation1763) also assumed that P. rubra can move in the water using its polyp leaves as fins. However, no particular behaviour of this species has been documented in its natural environment to date and no information about its defence/escape strategies is known (e.g. movement, withdrawal, bioluminescence). Light bioluminescence has been observed after electric stimulation of P. rubra in an aquarium (Panceri Citation1871; Titschack Citation1966), but no evidence of its bioluminescent behaviour in nature has been published thus far.
In the present study, withdrawal behaviour of P. rubra observed in the field after disturbance is described and discussed for the first time. Pennatulacean withdrawal behaviour already fascinated Charles R. Darwin, in Patagonia, who described the rather striking ability of an intertidal species of the genus Virgularia to quickly withdraw itself into the seabed when disturbed (Darwin Citation1845). In fact, several species of sea pens can withdraw partially or completely into the sediment by the closure of the polyps and the expulsion of part of the water contained within the colony (Hoare & Wilson Citation1977). Withdrawal behaviour has been well documented in laboratory studies of several different sea pen species (e.g. Mori Citation1960; Pavans de Ceccatty et al. Citation1963; Pavans de Ceccatty & Buisson Citation1965; Imafuku Citation1976, Citation1980; Hoare & Wilson Citation1977; Dickinson Citation1978), while the only in situ observations of this behaviour were reported by Birkeland (Citation1974) for Ptilosarcus gurneyi (Gray, 1860), Langton et al. (Citation1990) for Pennatula aculeata Danielssen, 1860 and Ambroso et al. (Citation2013) for Virgularia mirabilis (Müller, 1776). In particular, withdrawal behaviour was only observed in 12% of the colonies of P. aculeata observed by Langton et al. (Citation1990), but without any appreciable disturbance. However, the reasons for withdrawal behaviour in pennatulaceans are not well understood. For some species it has been explained by light conditions and metabolic factors, as in Cavernularia obesa and Veretillum cynomorium (Mori Citation1960; Buisson Citation1964; Imafuku Citation1976, Citation1980) or tidal-based rhythms, as in V. mirabilis (Wilson Citation1975). Predation and disturbance are usually mentioned as possible causes, even if no evidence is reported in the literature.
Materials and methods
The field of P. rubra here studied is located off Punta Alice (southern Italy) in the Ionian Sea (39°35.13ʹN, 16°52.16ʹE), between 60 and 70 m depth (Chimienti et al. Citation2015). A survey was carried out during November 2015 using the remotely operated vehicle (ROV) Pollux III, during the cruise Marine Strategy 2015, onboard the R/V Minerva Uno. The ROV was equipped with a low-definition Charge Coupled Device (CCD) video camera for navigation and a high-definition video camera (Sony HDR-HC7, 2304 × 1296 pixels) for the detailed observation of the colonies. The ROV also hosted a depth sensor, a compass, a grabber arm and three laser beams providing a 20-cm scale for dimensional measurements. The ROV survey was performed during the daytime, from UTC 9:48 a.m. to 12:19 p.m., with 2 hours and 30 minutes of video recording on the seabed at a constant depth of 64–65 m.
Ten different colonies of P. rubra were randomly selected for the behavioral observations after a mechanical disturbance. Each colony was gently touched every 15 seconds with the ROV arm, or just by opening and closing the arm’s fingers in the proximity of the colony. The three fingers on the arm were completely covered with cotton in order to have a softer touch and to not damage the polyps. The time of complete withdrawal was measured for each colony, and the values of the mean and standard deviation were calculated. The ROV’s headlights were periodically turned off before and during the disturbance in order to observe eventual bioluminescent behaviour of P. rubra. More observations were randomly carried out within the P. rubra field to assess whether there is any bioluminescence in undisturbed colonies or any particular behaviour. Moreover, the associated megafauna observed was identified at the lowest possible taxonomic level.
Results
All the colonies of P. rubra disturbed with the ROV arm started to withdraw into the substratum. This behaviour was performed with the backward and forward bending of the colony, one or several times, for the expulsion of the seawater through the siphonozooids. Afterwards, when the colony was already partially withdrawn and considerably smaller than before, it returned to the erect position and withdrew completely into the hole (). Contracted colonies can be more than three times smaller than extended ones, thus fitting completely in their holes after water expulsion. Five main phases of the withdrawal behaviour were distinguished: (1) erect colony, not disturbed; (2) backward bending; (3) forward bending; (4) erect and contracted colony; (5) colony withdrawn. This proved to be a quite slow process, taking between 210 and 340 seconds (3 min 30 sec–5 min 40 sec) for the complete withdrawal, with a mean time of 290 ± 47 seconds. Figure 2 shows the sequence of a colony which took 256 seconds (4 min 16 sec) to completely withdraw into the sediment.
Two of the 10 colonies disturbed became inaccessible to the ROV arm during one of the forward bending phases and it was not possible to disturb them again. In these two cases the withdrawal behaviour stopped when the disturbance ended and the colonies remained bent forward and only partially withdrawn.
None of the disturbed colonies showed a bioluminescent behaviour during the disturbance and the withdrawal, whereas light bioluminescence was observed in two undisturbed colonies (). Some detailed observations of other undisturbed colonies highlighted the presence of eggs at the base of the polyp leaves of several colonies of this broadcast-spawning species (.
Figure 1. Withdrawal behaviour of Pennatula rubra. (a) Still photographs of the different moments of the withdrawal, and (b) schematic representation of the five main phases distinguished: (1) erected colony, not disturbed; (2) backward bending; (3) forward bending; (4) erected and contracted colony; (5) colony withdrawn. Scale bars = 2 cm.
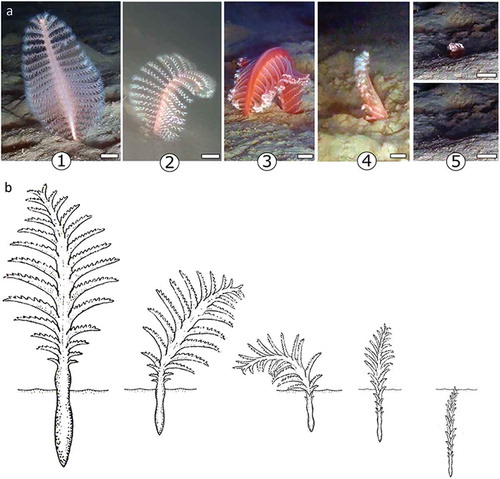
Figure 2. Example of the withdrawal behaviour of a colony of Pennatula rubra. In 256 seconds, the colony passes from (a) the erect position, when not disturbed, to (b) the backward bending, (c) the forward bending, (d) the erected and contracted colony, partially withdrawn, and (e) the entire colony withdrawal into the hole until (f) the complete retraction of the colony under the seabed. White arrows indicate the colony (a–d) and the hole (e–f). Scale bars = 5 cm.

Figure 3. Behaviour of Pennatula rubra, and anthropic impacts. (a) Light bioluminescence (white arrows); (b) eggs (white arrows) at the base of the polyp leaves of a colony; (c) sequence of a current-driven movement of a colony out of the mud; (d) trawl mark (white arrows) observed within the field of P. rubra. Scale bars: a, c = 2 cm; b = 1 cm; d = 5 cm.
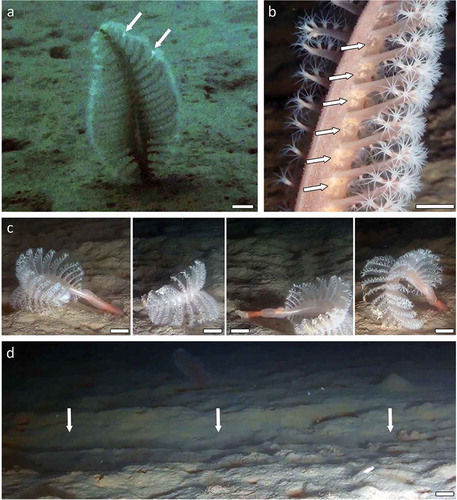
Two colonies of P. rubra were found out of the mud, inflating themselves with seawater and getting carried by currents (.
In terms of the associated megafauna, a few specimens of Polychaeta belonging to the genus Sabella and the fishes Cepola macrophthalma (Linnaeus, Citation1758), Lesueurigobius suerii (Risso, 1810) and Serranus hepatus (Linnaeus, Citation1758) as well as some unidentified Gobidae were observed in the area nearby (). In particular, S. hepatus usually showed a hiding behaviour behind the colonies of P. rubra. Gobidae and C. macropthalma often took shelter in the holes of the bioturbated mud, the latter by entering vertically (). The burrowing ability of the red band-fish C. macropthalma has been studied in the North Atlantic by Atkinson and Pullin (Citation1996).
Figure 4. Associated megafauna. (a) Tube of Sabella sp.; (b–c) Serranus hepatus; (d) Cepola macrophthalma; (e) Lesueurigobius suerii; (f) unidentified Gobidae. Scale bars = 2 cm.
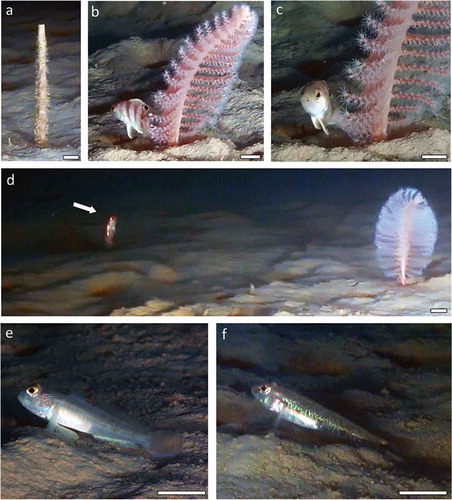
Several marks of commercial trawl fishing were also observed (, and four trawling fishery boats were working around the area during the survey.
Discussion
The present study confirms that P. rubra is a pivoting species able to completely withdraw into sediments, but not able to actively move on the seabed as hypothesised by Ellis (Citation1763). Withdrawal was found to be the only reaction to disturbance, and it can be complete after a prolonged disturbance or not complete if the disturbance ends before the slow time needed to expel water and withdraw. This slow process is different from the fast one observed in other species, such as V. mirabilis, which are able to withdraw in a few seconds (Ambroso et al. Citation2013). This could be due to the fact that Virgularia species, despite occurring as deep as the bathyal zone, can settle in very shallow environments under tidal influence, thus developing a fast withdrawal response. On the contrary, no Pennatula species living in such shallow waters are known to date.
Two healthy and completely extended colonies were observed out of the mud (, but it was not possible to assess whether their dislodgement was accidental (e.g. due to trawling impacts in the area or to the ROV survey) or natural (e.g. due to predation or as an escape strategy). We hypothesise that this current-carried behaviour may represent the only possible displacement strategy for P. rubra and that in this way a colony can slowly and randomly move from one place to another. This possible dispersal behaviour of detachment from the substratum and conveyance by benthic currents to a different geographical area has also been surmised for the so-called rockpens (sea pens that attach to rocky surfaces), by Williams and Alderslade (Citation2011).
Pennatula rubra showed a slight, rare bioluminescence during daytime (, while it was not possible to observe this behaviour during night time because ROV night dives were not allowed. Before our observation, the bioluminescent behaviour of P. rubra had only been observed by Panceri (Citation1871) and by Titschack (Citation1966) in an aquarium, in total darkness and under experimental conditions (i.e. under electric stimulation in a controlled environment).
Despite sea pen fields representing important habitats for associated species (Ruiz-Pico et al. Citation2017), the ROV survey revealed only a few demersal fish species (). The function of P. rubra colonies as shelter for small fishes such as S. hepatus was frequently observed in the area. Further targeted studies are likely to reveal an associated community, which could benefit from the presence of P. rubra fields.
Geolocation information
The study area is located off Punta Alice, southern Italy, in the Ionian Sea: 39°35.13ʹN, 16°52.16ʹE.
Acknowledgements
Captain, crew and scientific staff of the Marine Strategy cruise, and the two ROV pilots Alessio Cesari and Ciro Di Criscienzo, are warmly acknowledged for their efficient and skillful cooperation at sea. This paper is Ismar-Bologna scientific contribution no. 1953.
Additional information
Funding
References
- Aguilar R, Torriente A, García S. 2009. Propuesta de Áreas Marinas de Importancia Ecológica. Zona galaico-cantábrica. Oceana-Fundación Biodiversidad. 252 pp.
- Ambroso S, Dominguez-Carrió C, Grinyó J, López-González PJ, Gili J-M, Purroy A, Requena S, Madurell T. 2013. In situ observations on withdrawal behaviour of the sea pen Virgularia mirabilis. Marine Biodiversity 43:257–258. DOI: 10.1007/s12526-013-0172-5.
- Atkinson RJA, Pullin RSV. 1996. Observations on the burrows and burrowing behaviour of the Red Band-Fish, Cepola rubescens L. Marine Ecology 17:23–40. DOI: 10.1111/j.1439-0485.1996.tb00487.x.
- Baillon S, Hamel J-F, Wareham VE, Mercier A. 2012. Deep cold-water corals as nurseries for fish larvae. Frontiers in Ecology and the Environment 10:351–356. DOI: 10.1890/120022.
- Bayer FM. 1956. Octocorallia. In: Moore RC, editor. Treatise on invertebrate paleontology. Part F. Coelenterata. Lawrence: Geological Society of America and the University of Kansas Press. pp. 166–231.
- Birkeland C. 1974. Interactions between a sea pen and seven of its predators. Ecological Monographs 44:211–232. DOI: 10.2307/1942312.
- Bohadsch JB. 1761. De quibusdam animalibus marinis, eorumque proprietabibus, orbi litterario vel nondum vel minus notis, liber cum nonnullis tabulis aeri incisis, ab auctore super vivis animalibus delineatis. Dresdae: apud G.C. Walther. 194 pp.
- Buisson B. 1964. Données physiologiques sur l’intégration et la polarité dans les colonies de Veretillum cynomorium Pall. (Cnidaria, Pennatulacea). Comptes rendus hebdomadaires des séances de l’Académie des sciences, Paris 259:3361–3362.
- Chimienti G, Maiorano P, Mastrototaro F. 2015. Pennatula rubra facies in the Ionian Sea (Central Mediterranean). Biologia Marina Mediterranea 22:76–80.
- Darwin CR. 1845. Journal of researches into the natural history and geology of the countries visited during the voyage of H.M.S. Beagle round the world. London: John Murray. 519 pp.
- Dickinson P. 1978. Conduction systems controlling expansion-contraction behavior in the sea pen Ptilosarcus gurneyi. Marine Behaviour and Physiology 5:163–183. DOI: 10.1080/10236247809378532.
- Ellis J. 1763. An account of the sea pen, or Pennatula phosphorea of Linnaeus; likewise a description of a new species of sea pen, found on the coast of South-Carolina, with observations on sea-pens in general. In a letter to the honourable Coote Molesworth, Esq; M.D. and F.R.S. from John Ellis, esq; F.R.S. and member of the Royal Academy of Upsal. Philosophical Transactions of the Royal Society of London 53:419–435.
- Gage JS, Tyler PA. 1991. Deep-sea biology - a natural history of organisms at the deep-sea floor. Cambridge: Cambridge University Press. 504 pp.
- Hoare R, Wilson EH. 1977. Observations on the ecology of the pennatulid Virgularia mirablis in Holyhead Harbor. In: Keegan BF, Ceidligh PO, Boaden PJS, editors. Biology of benthic organisms. Oxford: Pergamon. pp. 329–337.
- Imafuku M. 1976. On the mechanism of the activity rhythm of the sea-pen, Cavernularia obesa Valenciennes. Publications of the Seto Marine Biological Laboratory 23:1–17. DOI: 10.5134/175927.
- Imafuku M. 1980. Activity rhythm of the sea pen Cavernularia obesa Valenciennes under temperature and light cycles. Publications of the Seto Marine Biological Laboratory 25:119–130. DOI: 10.5134/175989.
- Kenchington E, Murillo FJ, Cogswell A, Lirette C. 2011. Development of encounter protocols and assessment of significant adverse impact by bottom trawling for sponge grounds and sea pen fields in the NAFO Regulatory Area, no. 6005. NAFO SCR Doc 11/75. 53 pp.
- Kenchington E, Murillo FJ, Lirette C, Sacau M, Koen-Alonso M, Kenny A, Ollerhead N, Wareham V, Beazley L. 2014. Kernel density surface modelling as a means to identify significant concentrations of vulnerable marine ecosystem indicators. PLoS ONE 9:e109365. DOI: 10.1371/journal.pone.0109365.
- Krieger KJ. 1993. Distribution and abundance of rockfish determined from a submersible and by bottom trawling. Fishery Bulletin 91:87–96.
- Langton RW, Langton EW, Theroux RB, Uzmann JR. 1990. Distribution, behavior and abundance of sea pens, Pennatula aculeata, in the Gulf of Maine. Marine Biology 107:463–469. DOI: 10.1007/BF01313430.
- Linnaeus C. 1758. Systema naturae. Editio decima, reformata 1:1–824. Holmiae.
- Mastrototaro F, Maiorano P, Vertino A, Battista D, Indennidate A, Savini A, Tursi A, D’Onghia G. 2013. A facies of Kophobelemnon (Cnidaria, Octocorallia) from Santa Maria di Leuca coral province (Mediterranean Sea). Marine Ecology 34:313–320. DOI: 10.1111/maec.12017.
- Mori S. 1960. Influence of environmental and physiological factors on the daily rhythmic activity of a sea pen. Cold Spring Harbor Symposia on Quantitative Biology 25:333–344. DOI: 10.1101/SQB.1960.025.01.034.
- Panceri P. 1871. Gli organi luminosi e la luce delle pennatule. In: Atti della Regia Accademia delle Scienze Fisiche e Matematiche. Vol. V. Napoli: Stamperia del Fibreno. 46 pp.
- Pavans de Ceccatty M, Buisson B. 1965. Reciprocal behavior of the rachis and peduncle in colonies of Veretillum cynomorium. Pall American Zoologist 5:531–535. DOI: 10.1093/icb/5.3.531.
- Pavans de Ceccatty M, Buisson B, Garquoil YM. 1963. Rhythmes naturels et réactions motrices chez Alcyonium digitatum Linn. et Veretillum cynomorium Pall. Comptes Rendus des Séances de la Société de Biologie et de ses filiales 157:616–618.
- Pérès JM, Picard J. 1964. Nouveau manuel de bionomie benthique de la Méditerranée. Recueil des Travaux de la Station Marine d’Endoume 31:1–37.
- Pirtle JL. 2005. Habitat-based assessment of structure-forming megafaunal invertebrates and fishes on Cordell Bank, California. Ph.D. thesis, University of Washington. 74 pp.
- Porporato EMD, De Domenico F, Mangano MC, Rinelli P, Spanò N. 2012. Ebalia nux (Decapoda, Brachyura) found among the leaves of Pteroeides spinosum (Anthozoa, Octocorallia). Crustaceana 85:125–128. DOI: 10.1163/156854012X623539.
- Porporato EMD, De Domenico F, Mangano MC, Spanò N. 2011. Macropodia longirostris and Latreillia elegans (Decapoda, Brachyura) climbing on Mediterranean Pennatulidae (Anthozoa, Octocorallia): A preliminary note. Crustaceana 84:1777–1780. DOI: 10.1163/156854011X612884.
- Porporato EMD, Mangano MC, De Domenico F, Giacobbe S, Spanò N. 2014. First observation of Pteroeides spinosum (Anthozoa: Octocorallia) fields in a Sicilian coastal zone (Central Mediterranean Sea). Marine Biodiversity 44:589–592. DOI: 10.1007/s12526-014-0212-9.
- Rossi S. 2013. The destruction of the ‘animal forests’ in the oceans: Towards an oversimplification of the benthic ecosystems. Ocean & Coastal Management 84:77–85. DOI: 10.1016/j.ocecoaman.2013.07.004.
- Rossi S, Bramanti L, Gori A, Orejas C. 2017. An overview of the animal forests of the world. In: Rossi S, editor. Marine animal forests. The Netherlands: Springer International Publishing AG 2017. pp. 1–26. DOI: 10.1007/978-3-319-17001-5_1-1.
- Ruiz-Pico S, Serrano A, Punzón A, Altuna A, Fernández-Zapico O, Velasco F. 2017. Sea pen (Pennatulacea) aggregations on the northern Spanish shelf: Distribution and faunal assemblages. Scientia Marina 81:1–11. DOI: 10.3989/scimar.04359.06A.
- Tissot BN, Yoklavich MM, Love MS, York K, Amend M. 2006. Benthic invertebrates that form habitat structures on deep banks off southern California, with special reference to deep sea corals. Fisheries Bulletin 104:167–181.
- Titschack H. 1966. Über die Lumineszenz und ihre Lokalisation bei Seefedern. Zoologischer Anzeiger 29:120–131.
- Williams GC. 1999. Index Pennatulacea - annotated bibliography and indexes of the sea pens of the world 1469–1999. Proceedings of the California Academy of Sciences 51:19–103.
- Williams GC. 2011. The global diversity of sea pens (Cnidaria: Octocorallia: Pennatulacea). PLoS ONE 6:e22747. DOI: 10.1371/journal.pone.0022747.
- Williams GC, Alderslade P. 2011. Three new species of pennatulacean octocorals with the ability to attach to rocky substrata (Cnidaria: Anthozoa: Pennatulacea). Zootaxa 3001:33–48.
- Wilson EW. 1975 Some aspects of the biology of Virgularia mirabilis O.F. Müller (Octocorallia, Pennatulaceae). MSc thesis, University of Wales, Bangor.
