Abstract
Two native species of the genus Cucujus show a wide geographic distribution in Europe, Cucujus cinnaberinus (Scopoli, 1763) and C. haematodes Erichson, 1845. Although data on the distribution and ecology of these rare and endangered species are increasing, there are few reports on their biology and behaviour, and some aspects of their feeding ecology remain problematic. Our aim was to study, for the first time, the cuticular chemical profiles of these two beetles to (i) investigate the presence of chemicals potentially involved in defence by pathogens and (ii) lay the foundation for understanding the role of their bright red colour. The analysis of the cuticular profile was performed in-vivo by solid phase microextraction coupled with gas chromatography-mass spectrometry. In the cuticular profiles of the two species we identified 24 compounds belonging to different classes of molecules, i.e. hydrocarbons, aldehydes, esters, n-alkyl morpholines, and a high number of organic acids. Qualitative differences in terms of both signal intensity and detected compounds were found between the two species. As reported in other insects, the remarkable array of avoidance substances suggests a strict relationship with the bright red colour of the adults, which probably acts as an aposematic or warning signal. European Cucujus species are probably well protected against enemies because some identified chemicals, particularly fatty acids, are related to an anti-predatory strategy to fight off predators that use their sense of smell to locate their prey. Other substances found on the cuticular layer of these beetles are probably involved in an antimicrobial and antifungal function, as demonstrated in other insects living in habitats that host many pathogens.
Introduction
The flat bark beetles of the genus Cucujus are an example of extreme adaptation to the under-bark microhabitat. Their extremely flat bodies allow them to crawl through the thin space that opens between the xylem and phloem of decaying dead trees. Adults and larvae share a broad, flat head with enlarged genae that provide an enhanced insertion for the muscles of a pair of robust mandibles. The 14 species described so far are defined as “saproxylic” by Speight (Citation1989) and are distributed in the Holarctic Region, but only one lives in North America. Nearly all are bright red but some, like C. mniszechi Grouvelle and C. nigripennis Lee and Satô, are dark blue or dark brown (Lee & Satô Citation2007). The European species C. cinnaberinus (Scopoli, 1763) is strictly protected and enclosed in Annexes II and IV of the EU Habitats Directive 92/43, with the aim of maintaining existing populations. It has been classified as “near threatened” in Europe by the International Union for Conservation of Nature (IUCN) organisation and “vulnerable” in Italy (Carpaneto et al. Citation2015). Recently, there have been reports on the role of Cucujus cinnaberinus and Clinidium canaliculatum as possible biodiversity indicators. In Southern Italy, they provide a simple and useful tool for periodic diversity monitoring in forest nature reserve networks (Mazzei et al. Citation2018).
Another related species, C. haematodes Erichson, 1845, is even more at risk (“endangered” in Carpaneto et al. Citation2015), and is currently very rare in Europe, apart from in Calabria.
Although there is plenty of data on the distribution and ecology of these cucujid beetles, there are very few reports on their biology and behaviour, and those that exist are contradictory. Adults of all European species share a bright red colour (). They live for several months under bark and are active outside for a relatively short period from April to July (Horák & Chobot Citation2009).
Under bark, C. cinnaberinus is associated with Silvanidae, Carabidae, red and protected Pyrochroidae (Pyrochroa coccinea, Nardi & Bologna Citation2000) and non-coleopteran taxa such as ants from the genus Lasius, mites, flies, centipedes and springtails (Horák et al. Citation2011a). Cucujus haematodes is also associated with Acarina and Formicidae, Carabidae, Histeridae and Chrysomelidae (Horák et al. Citation2011b). The majority of flying activities in C. cinnaberinus occur from mid-spring to mid-summer (e.g. Bussler Citation2002; Horák & Chobot Citation2009; Horák et al. Citation2010; Straka Citation2017). Schlaghamerský et al. (Citation2008) recorded flying activity using window traps in April and May only; Marczak (Citation2016) reported that the adults are much more active during the night than in the diurnal hours but, more recently, adults of C. cinnaberinus have been observed in copula in the morning on tree surfaces (Populus sp.) in the Danube floodplain forest (Lower Austria) (Straka Citation2017).
There is little data on the food preferences of either adults or larvae of these beetles, and that which is available is still unclear. Smith and Sears (Citation1982), examining the gut contents of the North American species Cucujus clavipes, reported predatory feeding habits of larvae. Ślipiński (Citation1982) believes that the Cucujus spp. adults are facultative predators. Lawrence (Citation1991) considers the larvae of Cucujus to be facultative predators after finding insect parts, but also plant and fungal material, in the dissected gut. Mamaev et al. (Citation1977) indicated Cucujus species are scavengers, only occasionally preying on larvae and pupae of other beetles. Zdeněk et al. (Citation2012) reported on the opportunistic omnivore behaviour of C. cinnaberinus. Other preliminary studies showed the cannibalistic and predator habits of C. cinnaberinus larvae under artificial conditions (Palm Citation1941; Mazzei et al. Citation2011; Bonacci et al. Citation2012). Cucujus haematodes is known to be a scavenger, feeding on pupae and larvae of other insects (Mamaev et al. Citation1977) and on other subcortical beetles (Burakowski et al. Citation1986). Necrophagous and predacious feeding behaviour of C. haematodes was reported by Nikitskiy et al. (Citation2008) and Horák and Nakládal (Citation2009).
In a few cases, adults of C. cinnaberinus and C. haematodes were observed to be motionless under bark (Palm Citation1941) and showed thanatosis when disturbed (Horák & Chobot Citation2009, T. Bonacci personal observations), a behaviour displayed by many insects when potential threat or danger occurs. No studies have reported other defence strategies used by these species against enemies. Larvae and adults of both species are closely associated with moribund or dead trees (Horák et al. Citation2010) with wood-inhabiting fungi and microbes.
This study addresses two fundamental questions: (i) Why do C. cinnaberinus and C. haematodes display aposematic colour patterns? (ii) Could these beetles have developed cuticular defences against pathogens inhabiting pine bark?
Although there is little doubt that bright colour is often an anti-predatory strategy (Joron Citation2003), why has conspicuous colour (aposematism) evolved in these species if they spend most of their life under bark? How can these species live in habitats full of micro-organisms, such as the bark of the endemic pine, Pinus laricio var. calabrica?
As regards aposematism in insects, Joron (Citation2003) raises some questions, e.g. whether aposematic colours are signals that help predators learn to better differentiate inedible from edible prey, or whether bright colours are more easily memorised and associated to bad taste by predators. According to Rowe (Citation2001), aposematism is simply the correlation between conspicuous signals, such as bright colour, and prey unprofitability. Many aposematic insects use chemicals as repellents for protection from enemies, generally synthesised in particular glands or acquired from food resources (Bowers Citation1992). The kind of defensive chemicals and the amount present in an unpalatable insect may determine its degree of unpalatability and its potential for protection from enemies (Howard et al. Citation1982).
The bright colour of European Cucujus spp. () was the primary factor that led us to make cuticular chemical investigations.
This peculiarity and other unknown aspects of their biology suggest that the species lend particular interest to defensive behaviour towards potential predators. Furthermore, it is known that cuticular defences include antimicrobial peptides produced in many cells and glands of insect bodies (Meister et al. Citation2000; Schmid-Hempel Citation2005). Polar compounds found on the cuticle of many species of arthropods are the main defence against fungi and bacteria (Gillespie & Kanost Citation1997; Turillazzi et al. Citation2006). Microbiological investigations have shown that cuticular polar extracts of Rhynchophorus ferrugineus (Coleoptera Dryophthoridae) inhibit the growth of some microorganisms (e.g. gram-positive bacteria Bacillus subtilis and B. thuringiensis) (Mazza et al. Citation2011). Polar chemicals identified on the cuticle layer of this invasive coleopteran species end the germination of Beauveria bassiana’s spores. Mycelial growth of fungal species has been prevented by caprylic and lauric acid, both identified in Liposcelis bostrychophila (Psocoptera: Liposcelidae) cuticular extracts (Lord & Howard Citation2004).
In the grain beetles of the genus Cryptolestes, formerly included in Cucujidae but now part of the related family Laemophloeidae, the only chemicals detected are aggregation pheromones named “cucujolides”, which are a mixture of macrocyclic lactones (Oehlschlager et al. Citation1987; Vanderwel & Oehlschlager Citation1987) that may include fatty acids as a precursor in their biosynthesis (Francke & Dettner Citation2005). Fatty acids are substances of fundamental importance in the defensive strategies of many insect species. They play a role as precursors in the biosynthesis of pheromones, waxes and eicosanoids, and as components of defensive secretions (Stanley-Samuelson et al. Citation1988).
Gas chromatography (GC) is a powerful separation technique, particularly when combined with the extreme selectivity and sensitivity of mass spectrometric detection (Guido et al. Citation2012). Indeed, mass spectrometry (MS) has long been used in the development of methods for investigation of biomolecules (Lo Feudo et al. Citation2011) and biomarkers, especially when it is applied as tandem mass spectrometry (Monteleone et al. Citation2012; Naccarato et al. Citation2013).
In this work, we describe, for the first time, the cuticle chemical compositions of C. cinnaberinus and C. haematodes, with the aim of providing more insight into the possible role that some chemicals could play in the biology of these red beetles. This purpose has been achieved using solid phase microextraction (SPME) as the analytical sampling approach. The use of SPME allows the simultaneous extraction and preconcentration of the analytes in a single step and without the use of organic solvents (Naccarato et al. Citation2014; Naccarato & Pawliszyn Citation2016). Furthermore, this microextraction technique allows the performance of in-vivo analysis of the cuticular profile of the beetle, thus providing a better assessment of the molecules produced by the investigated beetles, and is compatible with the release of protected beetles into their original habitat.
Material and methods
Taxonomy and biogeographic background
The genus Cucujus F. is distributed throughout the Holarctic region and highly concentrated in Asia, with many endemics especially in India, Nepal, Myanmar, China, Taiwan and Japan (Lee & Pütz Citation2008; Horák & Chobot Citation2009). Only two native species were known from Europe, C. cinnaberinus (Scopoli, 1763), and C. haematodes Erichson, 1845, but lately a third species, C. tulliae Bonacci et al. Citation2012, from Calabria has been described. C. cinnaberinus is a saproxylic beetle endemic to Europe, living in several countries including Spain, Eastern France, the Netherlands, Ukraine, Russia and Sweden. Its populations are more densely distributed in Eastern Europe, from Austria and Bavaria eastwards (Horák & Chobot Citation2009; Teunissen & Vendrig Citation2012; Fuchs et al. Citation2014). Moreover, in the last 10 years, a re-expansion of its habitat along riverine forests has often been reported (Hörren & Tolkiehn Citation2016). Although the beetle was thought to be extinct in Italy after 1960, in the last decade it has been found in Piedmont and on the Alburni mountains in the Campania Region, by Biscaccianti et al. (Citation2009), and, after 49 years of absence, in the Sila National Park in Calabria (Mazzei et al. Citation2011). Cucujus haematodes is a palearctic species spanning areas from Bavaria and Southern Italy to Japan; it comprises at least one well-differentiated subspecies, C. h. opacus Lewis, 1988, found in Japan and Taiwan, whereas the nominal form is known from Southern Italy (Calabria), Greece and Eastern Europe to the Primorskiy Region of Far Eastern Russia and China (Horák & Chobot Citation2009). Another form, C. h. caucasicus Motschulsky, 1845, is known from Armenia, Georgia, and Russia, although the status of this subspecies was considered more dubious (Horák & Chobot Citation2009), because Mamaev et al. (Citation1977), in a larval key, considered the pre-imaginal characters to be those of a separate species. In this respect, Bonacci et al. (Citation2012) have re-evaluated the status of this taxon and have finally considered C. h. caucasicus to be a distinct subspecies.
Cucujus sampling and rearing
Samples of C. cinnaberinus (N = 4) and C. haematodes (N = 5) larvae were collected by hand in Natura 2000 SAC (“Special Areas of Conservation”) forest sites of the Sila National Park (Calabria, Southern Italy) from May to July 2016, with careful inspections under the bark of fallen or standing dead Calabrian pine trees. The beetles were kept alive in the lab and stored in controlled conditions in a thermostatic chamber with natural substrate (dead wood and bark), at constant temperature (15°C), 12:12 L:D light rhythm, and constant adequate humidity values of the decaying wood and bark layer. The larvae were fed with small pieces of chopped veal and Tenebrio molitor until pupation, in accordance with Bonacci et al. (Citation2012). After chemical analysis in the laboratory, some of the emerged beetles were released into the sample sites.
Instrumentation
GC-MS analysis was performed using a TSQ Quantum GC (Thermo Fischer Scientific) system constituted by a triple quadrupole mass spectrometer (QqQ-MS) Quantum and a TRACE GC Ultra equipped with programmable temperature vaporiser (PTV) injector. Chromatographic separation of the analytes was performed using a Restek Rxi-5MS capillary column 30 m × 0.25 mm inner diameter, 0.25 µm film thickness (95% polydimethylsiloxane, 5% polydiphenylsiloxane). The GC oven temperature was initially held at 100°C for 3 min, then ramped at 16°C/min to 280°C and held at this temperature for 15 min. The carrier gas was helium (99.999%, purity) at 1 mL/min. The transfer line and ionisation source temperatures were set at 280 and 250°C, respectively. For SPME analysis, a Thermo PTV straight Liner 0.75 mm × 2.75 mm × 105 mm was used as GC inlet liner. The analyses were performed in splitless mode with the injector temperature set at 280°C. The MS was operated in electron ionisation (EI) and full scan modes (40–500 m/z as mass range). The filament emission current was set at 25 µA. Instrument control and data processing were performed using Xcalibur software, version 2.0.0 (Thermo Fisher Scientific). Experimental data were evaluated with Microsoft Excel software, USA.
Analytical procedure
The cuticular organic profile of adult beetles (C. cinnaberinus: N = 4 males; C. haematodes: N = 5 males) was investigated. All individuals were analysed 5 days after adult emergence, without feeding. The survey of cuticular compounds was performed in vivo by solid phase microextraction coupled with gas chromatography-mass spectrometry (SPME-GC-MS). The analysis was carried out using a polydimethylsiloxane 100 µm (PDMS) fibre according to the following procedure: the SPME fibre was exposed and gently rubbed against the body of the live insect for about 1 min. Afterwards, the fibre was withdrawn into the needle and then exposed into the injector port of the gas chromatograph. The detected compounds were identified by matching the EI+ spectra against the NIST 02 database (NIST/EPA/NIH Mass Spectral Library, version 2.0 and by comparison of measured retention indices (RIs) with data collections of Kovats RIs.
Results
Cuticular profiles
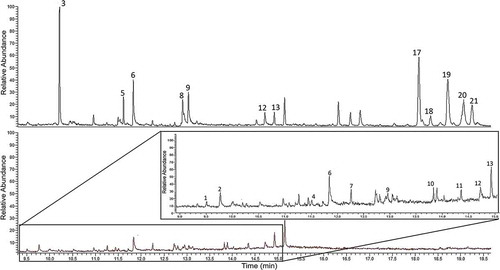
The compounds detected on the cuticle of the investigated Cucujus species are listed in .
Table I. Chemical compounds identified in adults of Cucujus haematodes and C. cinnaberinus (tR = retention time).
The cuticle of the beetles is mainly composed of hydrocarbons, organic acids and esters. Hydrocarbons are the major compounds; a total of 14 hydrocarbons were detected. There is a remarkable presence of organic acids on Cucujus spp. cuticle: n-hexadecanoic acid, oleic acid, octadecanoic acid and dehydroabietic acid. Seven compounds, namely tetradecanal; one unidentified ester; benzene, (1-methyldodecyl)-; octadecanal; two unidentified hydrocarbons; and cholesterol (), were only detected on the cuticular layer of C. cinnaberinus. In comparison, the C. haematodes cuticular profile is characterised by the greater presence of hydrocarbons with high molecular weight as well as the presence of atraric acid and heptadecanal ().
The compounds in common among the Cucujus species are three organic acids, namely n-hexadecanoic, oleic and octadecanoic acid, while the only sterol detected, cholesterol, occurs exclusively in C. cinnaberinus.
Discussion and conclusions
During recent decades, there has been a great increase in knowledge of the chemicals occurring on insect cuticle. In this research field, solid phase microextraction provides a significant contribution due to its features, such as the ability to perform the solvent-free extraction of analytes as well as the possibility to carry out in-vivo sampling. In this work, the combined use of SPME extraction and GC-MS analysis has been applied to investigate the chemical profile of living Cucujus individuals through non-invasive sampling. These bright red bark dwellers are characterised by cuticular profiles significantly different from those of other beetles (Bonacci et al. Citation2008). Indeed, in addition to the hydrocarbons also found in the cuticle layer of many other insect species (Howard et al. Citation1995), on the Cucujus cuticle we found a substantial number of organic acids. Fatty acids are involved in some important biochemical pathways of insects; indeed, they play a major role in several physiological functions and are used also as pheromones or defensive substances. For example, toxic alkaloids derived from the unsaturated fatty acid, and in particular oleic acid (Attygalle et al. Citation1994), were identified in coccinellid beetles. Oleic acid (C18H34O2) has been detected only in a few taxa, such as Formicidae, Termites and Chrysomelidae (Wilson et al. Citation1958). Eggs of the green dock beetle Gastrophysa cyanea contain oleic acid in amounts which were demonstrated to deter several species of ants from feeding (Howard et al. Citation1982). This fatty acid elicits corpse-carrying behaviour in several ants (Wilson et al. Citation1958; Haskins & Haskins Citation1974; Lanza et al. Citation1992) and foraging behaviour in other social contexts. Some coccinellid eggs are fortified with alkaloids derived from fatty acids. In addition, in Pogonomyrmex and other ant genera, fatty acids were found to act as a releaser of necrophoric behaviour (Haskins & Haskins Citation1974). Octadecanoic acid has also been detected in Graphosoma lineatum (Heteroptera, Pentatomidae) and is used by this bug as a defensive compound (Durak & Kalender Citation2009). The same chemical was identified in the defensive secretion of the polydesmoid millipede Pseudopolydesmus serratus (Conner et al. Citation1977; Makarov et al. Citation2012). Atraric acid has been extracted from a bark sample contaminated by Parmelia olivetorum and P. perlata (Bourgeois et al. Citation1999). Other authors have reported on the antimicrobial role of atraric acid in lichen species (Mitrović et al. Citation2011). The role that some of the hydrocarbons detected could play against microorganisms should not be left out. The surface extract of other insects living in habitats full of pathogens exhibited antimicrobial activity (Butera et al. Citation2009). Moreover, other authors have shown the action of some hydrocarbons detected in arthropods against entomopathogens (Banerjee & Dangar Citation1995). Recently, microbiological findings (Mazza et al. Citation2011) showed that polar substances detected in the red palm weevil are effective against microorganisms.
In summary, this study provides, for the first time, the qualitative chemical cuticular profiles of two endangered species of the genus Cucujus. The compounds detected show the profile of living Cucujus because the sampling was performed on the beetles in a non-invasive way. In these insects, among the molecules detected we found several fatty acids, which are reported and identified as defensive substances in other arthropods (Attygalle et al. Citation1994). In particular, oleic acid is reported to play a defensive or repellent role in many other insects. Our results do not explain the anti-predator or defence function of the chemicals found in these beetles but encourage us to conduct further research on many aspects of their biology and of their defensive mechanisms.
Indeed, the remarkable range of substances found in Cucujus suggests a close relationship with the bright red colour of the adults that concurrently acts as an aposematic signal for visual and olfactory predators. Considering tangible data published on the role of colour patterns in animals, we cannot disregard the suggestion that principles applicable to one group of animals, in all conspicuous colouration cases, may be valid also in the case of others (Longley Citation1917), like the red body pattern of Cucujus. Moreover, a literature overview of the gut contents of several insectivore taxa (reptiles, small mammals, birds, bats) shows that bright red beetles like cucuids, pyrochroids and lycids have never been recorded in their predation spectrum (Gaetano Aloise, Zoology Museum, University of Calabria, personal communication). The colour/odour combination probably plays an important role during the spring flying phase and in June and July, when the adults stay motionless on the outer bark side, waiting perhaps for sexual partners or copulating (Straka Citation2017). Our results support the theory that conspicuous colour and defence chemicals in these two beetles can produce a sufficient aposematic signal to limit attack by ambush and active predators (Matthews Citation1977). Animals protected by chemical defence are often conspicuously coloured (Alcock Citation1979), since unpalatability is frequently coupled with warning signals (aposematic colours and odours, Cott Citation1940; Tullberg et al. Citation2000). In this case, the fatty acids produced by the species investigated could act as predator/pathogen repellents. Preliminary food choice tests carried out in the laboratory by the authors showed that Cucujus larvae prefer Lasius (Hymenoptera, Formicidae) pupae and larvae versus other associated potential preys (unpublished data). It is known that many insect species acquire the chemicals by ex novo synthesis (autogenously) or exogenously by dietary sequestration of secondary compounds from food. Thus, the question arises: are flat bark beetles able to acquire their chemicals by preying on other associated insects? This issue should be addressed in future studies aimed at better understanding the complex mechanism of synthesis of the compounds found on the adult cuticle and the significance of habitat association with similar and protected species.
The presence of polar compounds with antimicrobial activity has already been detected on the epicuticular layer of arthropods (Kuhn-Nentwig Citation2003), including social insects (Hölldobler & Wilson Citation1990; Turillazzi et al. Citation2006; Mazza et al. Citation2011). Cucujus spp. live in association with many pathogens, entomo-pathogenic fungi and microbes related to under-bark habitat. Fatty acids and cuticular hydrocarbons with a possible antimicrobial function appear to be fundamental components of the defence biology of the red Cucujus beetles, and encourage further investigation.
Acknowledgements
The authors are particularly indebted to the Administration of the Sila National Park (President: Prof. Sonia Ferrari; past Director: Dr Michele Laudati) for providing collection permits, and the research fund: “Foreste Vetuste” phases II and III. Thanks go to the new Director, Dr Giuseppe Luzzi, for help in the field and financial assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alcock J. 1979. Animal behavior. An evolutionary approach. Sunderland, MA: Sixth Sinauer Associates, Inc. Publishers. 530 p.
- Attygalle AB, Blankespoor CL, Eisner T, Meinwald J. 1994. Biosynthesis of a defensive insect alkaloid: Epilachnene from oleic acid and serine. Proceeding of the National Academy of Sciences USA 91:12790–12793. DOI: 10.1073/pnas.91.26.12790.
- Banerjee A, Dangar TK. 1995. Pseudomonas aeruginosa a facultative pathogen of red palm weevil Rhynchophorus ferrugineus. World Journal of Microbiology & Biotechnology 11:618–620. DOI: 10.1007/BF00361002.
- Biscaccianti A, Audisio P, Monguzzi R. 2009. Aggiornamenti sulla distribuzione di Cucujus cinnaberinus e altri Cucujoidea (Coleoptera: Nitidulidae, Cucujidae, Laemophloeidae). Bollettino Associazione Romana di Entomologia 63:47–57.
- Bonacci T, Brandmayr P, Dalpozzo R, De Nino A, Massolo A, Tagarelli A, Zetto Brandmayr T. 2008. Odour and colour similarity in two species of gregarious carabid beetles (Coleoptera) from the Crati valley, southern Italy: A case of Müllerian mimicry? Entomological News 119:325–337. DOI: 10.3157/0013-872X-119.4.325.
- Bonacci T, Mazzei A, Horák J, Brandmayr P. 2012. Cucujus tulliae sp. n. – An endemic Mediterranean saproxylic beetle from genus Cucujus Fabricius, 1775 (Coleoptera, Cucujidae), and keys for identification of adults and larvae native to Europe. ZooKeys 212:63–79. DOI: 10.3897/zookeys.212.3254.
- Bourgeois G, Suire C, Vivas N, Benoist F, Vitry C. 1999. Atraric acid, a marker for epiphytic lichens in the wood used in cooperage: Identification and quantification by GC/MS/(MS). Analusis 27:281–283. DOI: 10.1051/analusis:1999100.
- Bowers MD. 1992. The evolution of unpalatability and the cost of chemical defense in insects. In: Roitberg BD, Isman MB, editors. Insect chemical ecology: An evolutionary approach. New York: Routledge Chapman and Hall. pp. 216–244.
- Burakowski B, Mroczkowski M, Stefańska S. 1986. Chrząszcze, Coleoptera, Cucujoidea, część 1, Katalog fauny Polski. Część XXIII, tom 12. Warszawa: PWN.
- Bussler H. 2002. Untersuchungen zur Faunistik und Ökologie von Cucujus cinnaberinus (Scop., 1793) in Bayern (Coleoptera: Cucujidae). Nachrichtenblatt der Bayerischen Entomologen 51:42–60.
- Butera G, Barrica G, Ferraro C, Alonzo G, Colazza S, Quatrini P. 2009. Analisi della comunità batterica intestinale di larve del punteruolo rosso. In: La ricerca scientifica sul punteruolo rosso e gli altri fitofagi delle palme in Sicilia. Vol. 1. Palermo: Regione Siciliana Assessorato Agricoltura e Foreste Dipartimento Interventi Infrastrutturali. pp. 143–146.
- Carpaneto GM, Baviera C, Biscaccianti AB, Brandmayr P, Mazzei A, Mason F, Battistoni A, Teofili C, Rondinini C, Fattorini S, Audisio P. 2015. A red list of Italian saproxylic beetles: Taxonomic overview, ecological features and conservation issues (Coleoptera). Fragmenta Entomologica 47:53–126. DOI: 10.4081/fe.2015.138.
- Conner WE, Jones TH, Eisner T, Meinwald J. 1977. Benzoyl cyanide in the defensive secretion of polydesmoid millipeds. Experientia 33:206–207. DOI: 10.1007/BF02124069.
- Cott HB. 1940. Adaptive coloration in animals. London: Oxford Press.
- Durak D, Kalender Y. 2009. Fine structure and chemical analysis of the metathoracic scent gland secretion in Graphosoma lineatum (Linnaeus, 1758) (Heteroptera, Pentatomidae). Comptes Rendus Biologies 332:34–42. DOI: 10.1016/j.crvi.2008.10.004.
- Francke W, Dettner K. 2005. Chemical signalling in beetles. Topics in Current Chemistry 240:85–166.
- Fuchs L, Callot H, Godinat G, Brustel H. 2014. Cucujus cinnaberinus (Scopoli, 1763), nouvelle espèce pour la faune de France (Coleoptera: Cucujidae). L’Entomologiste 70:213–221.
- Gillespie JP, Kanost MR. 1997. Biological mediators of insect immunity. Annual Review of Entomology 42:611–643. DOI: 10.1146/annurev.ento.42.1.611.
- Guido A, Vescogni A, Mastandrea A, Demasi F, Tosti F, Naccarato A, Tagarelli A, Russo F. 2012. Characterization of the micrites in the late miocene vermetid carbonate bioconstructions, Salento Peninsula, Italy: Record of a microbial/metazoan association. Sedimentary Geology 263–264:133–143. DOI: 10.1016/j.sedgeo.2011.10.005.
- Haskins CP, Haskins EF. 1974. Notes on necrophoric behavior in the archaic ant Myrmecia vindex (Formicidae: Myrmeciinae). Psyche: A Journal of Entomology 81:258–267. DOI: 10.1155/1974/80395.
- Hölldobler B, Wilson EO. 1990. The ants. Cambridge, MA: Belknap Press of Harvard University.
- Horák J, Chobot K. 2009. Worldwide distribution of saproxylic beetles of the genus Cucujus Fabricius, 1775 (Coleoptera: Cucujidae). In: Buse J, Alexander KNA, Assmann T, editors. Saproxylic beetles their role and diversity in European woodland and tree habitats. Sofia: Pensoft Publishers. pp. 189–206.
- Horák J, Chumanová E, Hilszczański J. 2011a. Saproxylic beetle thrives on the openness in management: A case study on the ecological requirements of Cucujus cinnaberinus from Central Europe. Insect Conservation and Diversity 5:403–413. DOI: 10.1111/icad.2012.5.issue-6.
- Horák J, Nakládal O. 2009. Beetles associated with trees and predation between them: Part III. Annotated checklist of beetles with predation potential. Lesnícky Časopis Forestry Journal 55:181–193.
- Horák J, Vávrová E, Chobot K. 2010. Habitat preferences influencing populations, distribution and conservation of the endangered saproxylic beetle Cucujus cinnaberinus (Coleoptera: Cucujidae) at the landscape level. European Journal of Entomology 107:81–88. DOI: 10.14411/eje.2010.011.
- Horák J, Zaitsev A, Vávrová E. 2011b. Ecological requirements of a rare saproxylic beetle Cucujus haematodes – The beetles’ stronghold on the edge of its distribution area. Insect Conservation and Diversity 4:81–88. DOI: 10.1111/icad.2011.4.issue-2.
- Hörren T, Tolkiehn J. 2016. Erster Nachweis von Cucujus cinnaberinus (Scopoli, 1763) in Schleswig-Holstein-eine FFH-Art erschließt sich Lebensräume in Norddeutschland (Coleoptera: Cucujidae). Entomologische Zeitschrift Schwanfeld 126:208–210.
- Howard DF, Blum MS, Jones TH, Phillips DW. 1982. Defensive adaptations of eggs and adults of Gastrophysa cyanea (Coleptera: Chrysomelidae). Journal of Chemical Ecology 8:453–462. DOI: 10.1007/BF00987793.
- Howard RW, Howard CD, Colquhoun S. 1995. Ontogenic and environmentally induced changes in cuticular hydrocarbons of Oryzaephilus surinamensis (Coleoptera: Cucujidae). Annals of the Entomological Society of America 88:485–495. DOI: 10.1093/aesa/88.4.485.
- Joron M. 2003. Mimicry. In: Cardé RT, Resh VH, editors. Encyclopedia of insects. New York: Academic Press. pp. 714–726.
- Kuhn-Nentwig L. 2003. Antimicrobial and cytolytic peptides of venomous arthropods. Cellular and Molecular Life Sciences 60:2651–2668. DOI: 10.1007/s00018-003-3106-8.
- Lanza J, Schmitt MA, Awad AB. 1992. Comparative chemistry of elaiosomes of three species of Trillium. Journal of Chemical Ecology 18:209–221. DOI: 10.1007/BF00993754.
- Lawrence JF. 1991. Cucujidae. In: Stehr FW, editor. Immature insects. Vol. 2. Dubuque, IA: Kendall/Hunt. pp. 463–488.
- Lee CF, Pütz A. 2008. A new species of Cucujus Fabricius, 1775 from China and key to the east–Palaearctic species of the genus (Coleoptera: Cucujidae). Entomologische Zeitschrift 118:211–213.
- Lee CF, Satô M. 2007. A review of the genus Cucujus Fabricius (Insecta; Cucujoidea; Cucujiidae) from Taiwan, Japan, and China, with description of two new species and the larvae of Cucujus mniszechi Grouvelle. Zoological Studies 46:311–321.
- Lo Feudo G, Macchione B, Naccarato A, Sindona G, Tagarelli A. 2011. The volatile fraction profiling of fresh tomatoes and triple concentrate tomato pastes as parameter for the determination of geographical origin. Food Research International 44:781–788. DOI: 10.1016/j.foodres.2011.01.017.
- Longley WH. 1917. Studies upon the biological significance of animal coloration. II. A revised working hypothesis of mimicry. The American Naturalist 51:257–285. DOI: 10.1086/279604.
- Lord JC, Howard RW. 2004. A proposal role for the cutucular fatty amides of Liposcelis bostrychophila (Pcoptera: Liposcelidae) in preventing adhesion of entomopaphogenic fungi with dry-conidia. Mycopathologia 158:211–217. DOI: 10.1023/B:MYCO.0000041837.29478.78.
- Makarov SE, Vujisić L, Ćurčić PM, Ilić BS, Tešević VV, Vajs VE, Vučković IM, Mitić BM, Lučić LR, Đorđević IŽ. 2012. Chemical defense in the cave-dwelling millipede Brachydesmus troglobius Daday, 1889 (Diplopoda, Polydesmidae). International Journal of Speleology 41:95–100. DOI: 10.5038/1827-806X.41.1.10.
- Mamaev IM, Krivosheina VA, Potockaia VA. 1977. Opredelitel lioinok, chišonych nesekomych entomofagov stolvych vrediteleiy. Vzdavatelistvo Nauka Moskva. 392 pp.
- Marczak D. 2016. Cucujus cinnaberinus in Kampinos National Park and comments regarding the specie’s monitoring. Studia i Materiały CEPL w Rogowie 18:142–152.
- Matthews EG. 1977. Signal-based frequency-dependent defense strategies and the evolution of mimicry. The American Naturalist 111:213–222. DOI: 10.1086/283156.
- Mazza G, Arizza V, Baracchi D, Barzanti GP, Benvenuti C, Francardi V, Frandi A, Gherardi F, Longo S, Manachini B, Perito B, Rumine P, Schillaci D, Turillazzi S, Cervo R. 2011. Antimicrobial activity of the red palm weevil Rhynchophorus ferrugineus. Bulletin of Insectology 64:33–41.
- Mazzei A, Bonacci T, Contarini E, Zetto T, Brandmayr P. 2011. Rediscovering the umbrella species candidate Cucujus cinnaberinus (Scopoli, 1763) in Southern Italy (Coleoptera Cucujidae), and notes on bionomy. Italian Journal of Zoology 78:264–270. DOI: 10.1080/11250003.2010.485210.
- Mazzei A, Bonacci T, Horák J, Brandmayr P. 2018. The role of topography, stand and habitat features for management and biodiversity of a prominent forest hotspot of the Mediterranean Basin: Saproxylic beetles as possible indicators. Forest Ecology and Management 410:66–75. DOI: 10.1016/j.foreco.2017.12.039.
- Meister M, Hetru C, Hoffmann JA. 2000. The antimicrobial host defense of Drosophila. In: Du Pasquier L, Litman GW, editors. Current topics in microbiology and immunology. Vol. 28. Berlin, Germany: Springer-Verlag. pp. 17–36.
- Mitrović T, Stamenković S, Cvetković V, Tošić S, Stanković M, Radojević I, Stefanović O, Čomić L, Đačić D, Ćurčić M, Marković S. 2011. Antioxidant, antimicrobial and antiproliferative activities of five lichen species. International Journal of Molecular Sciences 12:5428–5448. DOI: 10.3390/ijms12085428.
- Monteleone M, Naccarato A, Sindona G, Tagarelli A. 2012. A rapid and sensitive assay of perfluorocarboxylic acids in aqueous matrices by headspace solid phase microextraction-gas chromatography-triple quadrupole mass spectrometry. Journal of Chromatography A 1251:160–168. DOI: 10.1016/j.chroma.2012.06.033.
- Naccarato A, Gionfriddo E, Elliani R, Sindona G, Tagarelli A. 2014. A fast and simple solid phase microextraction coupled with gas chromatography-triple quadrupole mass spectrometry method for the assay of urinary markers of glutaric acidemias. Journal of Chromatography A 1372:253–259. DOI: 10.1016/j.chroma.2014.10.069.
- Naccarato A, Moretti S, Sindona G, Tagarelli A. 2013. Identification and assay of underivatized urinary acylcarnitines by paper spray tandem mass spectrometry. Analytical and Bioanalytical Chemistry 405:8267–8276. DOI: 10.1007/s00216-013-7232-3.
- Naccarato A, Pawliszyn J. 2016. Matrix compatible solid phase microextraction coating, a greener approach to sample preparation in vegetable matrices. Food Chemistry 206:67–73. DOI: 10.1016/j.foodchem.2016.03.036.
- Nardi G, Bologna MA. 2000. Cantharidin attraction in Pyrochroa (Coleoptera: Pyrochroidae). Entomological News 111:74–75.
- Nikitskiy NB, Bibin AR, Dolgin BB. 2008. Ksilofilniye zhestkokryliye (Coleoptera) Kavkazskogo gosudarstvennogo prirodnogo biosfernogo zapovednika i sopredelnykh territoriy. Syktyvkar, Russia: Rossiyskaya Akademiya Nauk.
- Oehlschlager AC, King GGS, Pierce Jr HD, Pierce AM, Slessor KN, Millar JG, Borden H. 1987. Chirality of macrolide pheromones of grain beetles in the genera Oryzaephilus and Cryptolestes and its implications for species specificity. Journal of Chemical Ecology 13:1543–1554. DOI: 10.1007/BF01012296.
- Palm T. 1941. Über die Entwicklung und Lebensweise einiger wenig bekannten Käfer-Arten im Urwaldgebiet am Fluss Dalälven (Schweden) II und III. Opuscula Entomologica 6:17–26.
- Rowe C. 2001. Warning signals and mimicry. Special issue of Evolutionary Ecology 1999, vol. 13: n.7/8. Dordrecht, The Netherlands: Kluwer.
- Schlaghamerský J, Manák V, Cechovský P. 2008. On the mass occurrence of two rare saproxylic beetles, Cucujus cinnaberinus (Cucujidae) and Dircaea australis (Melandryidae), in South Moravian floodplain forests. Revue d’Ecologie, la Terre et la Vie 63:107–113.
- Schmid-Hempel P. 2005. Evolutionary ecology of insect immune defenses. Annual Review of Entomology 50:529–551. DOI: 10.1146/annurev.ento.50.071803.130420.
- Ślipiński S. 1982. Klucze do oznaczania owadów Polski, Coleoptera-Cucujidae. Panstwowe wydawnictwo naukowe. Warszawa: Wroclaw. 35 p (in Polish).
- Smith DB, Sears MK. 1982. Mandibular structure and feeding habits of three morphologically similar coleopterous larvae: Cucujus clavipes (Cucujidae), Dendroides canadensis (Pyrochroidae), and Pytho depressus (Salpingidae). The Canadian Entomologist 114:173–175. DOI: 10.4039/Ent114173-2.
- Speight M. 1989. Saproxylic invertebrates and their conservation. Strasbourg: Council of Europe, French. 82 p.
- Stanley-Samuelson DW, Jurenka RA, Cripps C, Blomquist GJ, de Renobales M. 1988. Fatty acids in insects: Composition, metabolism, and biological significance. Archives of Insect Biochemistry and Physiology 9:1–33. DOI: 10.1002/(ISSN)1520-6327.
- Straka U. 2017. Beocachtungen zur Imaginalbiologie des Scharlachkäfers Cucujus cinnaberinus (Scopoli, 1763) (Coleoptera: Cuculiidae). Beiträge zur Entomofaunistik 18:109–116.
- Teunissen APJA, Vendrig GFP. 2012. Een Nederlandse populatie van de zeldzame en beschermde vermiljoenkever Cucujus cinnaberinus (Coleoptera: Cucujidae). Entomologische Berichten 72:218–221.
- Tullberg BS, Leimar O, Gamberale-Stille G. 2000. Did aggregation favour the initial evolution of warning coloration? A novel world revisited. Animal Behaviour 59:281–287. DOI: 10.1006/anbe.1999.1302.
- Turillazzi S, Mastrobuoni G, Dani FR, Moneti G, Pieraccini G, La Marca G, Bartolucci G, Perito B, Lambardi D, Cavallini V, Dapporto L. 2006. Dominulin A and B: Two new antibacterial peptides identified on the cuticle and in the venom of the social paper wasp Polistes dominulus using MALDI-TOF, MALDI-TOF/TOF, and ESI-Ion trap. Journal of the American Society for Mass Spectrometry 17:376–383. DOI: 10.1016/j.jasms.2005.11.017.
- Vanderwel D, Oehlschlager AC. 1987. Biosyntheis and endocrine regulation of pheromone production in Coleoptera. In: Prestwich GD, Blomquist D, editors. Pheromone biochemistry. New York: Academic Press. pp. 175–215.
- Wilson EO, Durlach NI, Roth LM. 1958. Chemical releasers of necrophoric behavior in ants. Psyche 65:10–114.
- Zdeněk B, Turcani M, Horak J. 2012. Sharing the same space: Foraging behaviour of saproxylic beetles in relation to dietary components of morphologically similar larvae. Ecological Entomology 37:117–123. DOI: 10.1111/j.1365-2311.2012.01343.x.