 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
One hundred and six specimens of the Por’s goatfish, Upeneus pori Ben-Tuvia & Golani (1989), a Lessepsian species native to the Western Indian Ocean, were collected from bottom trawlers’ catches from 2012 to 2016 on the deep shelf off the southern coast of Lampedusa Island (Strait of Sicily, Central Mediterranean Sea). Since it first appeared in Iskenderun Bay (Turkey) in 1950, the Por’s goatfish has quickly spread in Levantine Sea waters to become a commercial species used by local fisheries, while continuing its range expansion along the south-eastern coasts of the Mediterranean. Because the Strait of Sicily currently represents the westernmost sector of this species distribution area, it might serve as a stepping-stone for this species’ expansion in the western basin of the Mediterranean. Supporting this hypothesis was our finding of specimens with post-spawning gonads. The pattern of westward expansion by the Por’s goatfish in the Mediterranean Sea and its settlement in the Strait of Sicily are discussed in relation to the warming trend over the last 30 years.
Introduction
The biodiversity of the Mediterranean Sea has changed considerably over the past two decades, mainly due to the increasing occurrence of non-indigenous species (NIS) introduced both naturally (i.e. via Suez Canal and the Strait of Gibraltar) and via human activities, such as marine shipping (e.g. ballast waters, fouling) and aquaculture (Galil et al. Citation2017; Scannella et al. Citation2017). However, the arrival of exotic species in the Mediterranean Sea is a dynamic ongoing process, in that the total number of NIS doubled (223%) between 1970 and 2015, with the greatest increase recorded in the last 25 years (Galil et al. Citation2016).
Recently, Zenetos et al. (Citation2017) listed a total of 821 NIS in the region, of which 613 were defined as established. Fishes are an important component of this NIS list and almost half of them are Lessepsian species (i.e. Erythrean non-indigenous species: ENIS) that arrived from the Red Sea through the Suez Canal (Deidun et al. Citation2015). Most of these ENIS fish species remain largely confined to waters of the Levantine Sea (Quignard & Tomasini Citation2000; Mavruk & Avsar Citation2007), the Mediterranean area with the highest water temperature and salinity. Lately, however, increasingly more fish species are showing a trend of westward geographical expansion alongside the ongoing warming of the Mediterranean Basin (Skliris et al. Citation2012). Clear examples of this movement are Stephanolepis diaspros Fraser-Brunner, 1940 (Deidun et al. Citation2015), Siganus luridus Rüppell, 1828 (Azzurro & Andaloro Citation2004), Lagocephalus sceleratus Gmelin, 1789 (Azzurro et al. Citation2014), Acanthopagrus bifasciatus Forsskål, 1775 (Ben Souissi et al. Citation2014), Etrumeus teres DeKay, 1842 (Falautano et al. Citation2006), and Fistularia commersonii Rüppell, 1838 (Azzurro et al. Citation2013). This last species represents the most successful example of fish colonisation in the Mediterranean, expanding its range to the whole basin in just 7 years (Azzurro et al. Citation2013; Vitale et al. Citation2016).
Among the Lessepsian fish, the Por’s goatfish (Upeneus pori) is native to the Western Indian Ocean, where its distribution ranges from the Red Sea to Oman, Madagascar and South Africa (Ben-Tuvia & Golani Citation1989). In the Mediterranean Sea this fish was first reported from Iskenderun Bay (Turkey) by Kosswig (Citation1950) as Upenoides (= Upeneus) tragula. After this initial detection, U. pori was recorded off the coast of Israel (Golani Citation1994), Egypt (El-Sayed Citation1994), Lebanon (George & Athanassiou Citation1996) and Libya (El-Drawany Citation2013; Sergiwa et al. Citation2017). The species also appeared along the Tunisian coasts Gulf of Gabes and Bizerte Lagoon (Ben Souissi et al. Citation2005; Azzouz et al. Citation2010) where only two specimens were recorded, and, more recently, in the Aegean Sea (Filiz Citation2012; Stamouli & Dogrammatzi Citation2016).
Information on the biological traits of Por’s goatfish in the Levantine Sea are provided by Taskavak and Bilecenoglu (Citation2001), Cicek et al. (Citation2002, Citation2006), Ismen (Citation2006), Cicek and Avsar (Citation2011), Ok (Citation2012) and Edelist (Citation2014). Recently, the biology of this species along the coasts of Libya was also studied (El-Drawany Citation2013; Sergiwa et al. Citation2017).
In the present study, we report the first biological information on U. pori occurring in the Strait of Sicily (General Fisheries Commission for the Mediterranean (GFCM) - Geographical Sub-Area (GSA) 13), currently the westernmost Mediterranean area where the species is confirmed as established. The biometrics, meristics, measurement size distribution, sex ratio, length–weight relationship (LWR), and length at age were described and compared with those studies carried out in the Central-Eastern Mediterranean Sea. The westward expansion of U. pori from the Levantine Sea as related to the ongoing warming trend of Mediterranean waters is also discussed, as well as the potential for this species’ future expansion in the western basin.
Materials and methods
A total of 106 specimens of U. pori were collected from commercial trawlers that targeted the striped red mullet (Mullus surmuletus Linnaeus, 1758) off the south coast of Lampedusa Island in the period 2012–2016 ().
Figure 1. Updated map showing location of Upeneus pori first records in the Mediterranean basin reported according to the catch date. 1: 1950 (Kosswig Citation1950); 2: 1953 (Ben-Tuvia Citation1953); 3: 1954 (George & Athanassiou Citation1996); 4: 1984–1986 (Golani Citation1994); 5: 1991–1994 (Torcu & Mater Citation2000); 6: 1994 (El-Sayed Citation1994); 7: 1994 (Ben-Abdallah et al. Citation2005); 8: 1999–2000 (Cicek et al. Citation2002); 9: 2000–2001 (Öğretmen et al. Citation2016); 10: 2003 (Ben Souissi et al. Citation2005); 11: 2003 (Corsini et al. Citation2005); 12: 2005–2006 (Shakman Citation2007); 13: (Tzomos Citation2007); 14: 2007–2008 (El-Drawany Citation2013); 15: 2009 (Filiz Citation2012); 16: 2010 (Azzouz et al. Citation2010); 17: 2010–2012 (Bazairi et al. Citation2013); 18: 2011–2012 (Ramadan & El-Halfawy Citation2014); 19: 2015 (Stamouli & Dogrammatzi Citation2016); +: present study.
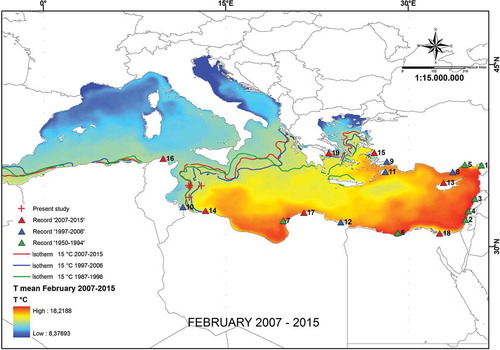
Specifically, in 2012, 2014, 2015 and 2016 the numbers caught were respectively two, nine, 31 and 64 individuals. All the specimens were fished in autumn (November and December) from 50–70 m depth, identified and compared to the meristics and morphological characters provided by Ben-Tuvia and Golani (Citation1989) and Ben Souissi et al. (Citation2005) (; ).
Table I. Biometric comparisons of Upeneus pori among Mediterranean authors, expressed as percentage of standard length (%SL), together with meristic counts (*Ben Souissi et al. Citation2005, **Ben-Tuvia & Golani Citation1989). Moreover, mean, standard deviation (SD), and minimum and maximum values recorded in the present study are provided. NA: Not Available.
Each specimen was sexed, measured (total length: TL) to the nearest 0.1 cm, and weighed to the nearest 0.1 g. Maturity stage was determined by visual examination of the gonads according to a 7-stage gonadic scale. In particular, juveniles (immature) were classified within the first two stages while adults were classified by the third, fourth and fifth stages in the maturing, spawning phase and by the sixth and seventh stages in the post-spawning/resting phase (International bottom trawl survey in the Mediterranean (MEDITS) scale, Anonymous Citation2016).
The LWR for U. pori was calculated using the equation (Sparre et al. Citation1989), where W is wet body weight (g) and TL is total length (cm), with the estimated a and b parameters then compared with those reported for other Mediterranean areas (). The condition factor (K) was calculated according to Bagenal and Tesch (Citation1978) as:
Table II. Synopsis of the available information on sex ratio, length–weight relationship (LWR), and LWR type of Upeneus pori (A+ positive allometric growth; A- negative allometric growth; I isometric growth) in different Mediterranean areas.
Otoliths (sagittae) were extracted from each specimen and read as a whole under a stereomicroscope at 9× magnification with reflected light.
These readings were taken at least 3 times by two trained readers. When a mismatch in this count occurred, then the same two readers together re-analysed the otolith under a two-seat stereomicroscope. Since the specimens were collected only during autumn months, it was not possible to verify the periodicity of marks laid monthly following the classical marginal increment analysis (MIA; Campana Citation2001). Similar to Mullus barbatus Linnaeus, 1758, the first translucent ring was considered the demersal check laid down during bottom settlement after the pelagic life stage (Sieli et al. Citation2011). Annuli (i.e. winter rings) were identified as those translucent rings clearly marked all the way around the otolith. Assuming that one annulus is laid per year, a specimen’s age was estimated by counting the number of annuli. To explore the relationship between the Por’s goatfish’s expansion in the central–south Mediterranean and the ongoing warming trend, we analysed the temporal pattern in the position of the 15°C isotherm for February and the species occurrence. According to Bianchi (Citation2007), the 15°C isotherm is the main barrier to expansion of warm-affinity species from the eastern Mediterranean towards the western basin. Sea surface temperature (SST) data were obtained from E.U. Copernicus Marine Service Information (Simoncelli et al. Citation2014) and averaged over three decennial periods: 1987–1996, 1997–2006 and 2007–2015.
Results
Upeneus pori specimens showed an elongated body. The snout is rounded. The chin has two short, thin barbels. The body is reddish-brown with darker hues above and lighter hues below. The upper lobe of caudal fin has 4–7 oblique crossbars; the lower lobe has 4–7 crossbars occupying less than half of the outer side of the lobe, followed by an almost solid brown space, while the inner edge of the lobe is crossed by 3–4 oblique bars (; ).
The catch sample was composed of U. pori specimens between 12.2 and 16.2 cm TL, with the females (12.2 and 16.2 cm TL) significantly longer (Kruskal–Wallis test: P < 0.01) than the males (12.6–15.4 cm TL; ).
Figure 3. Length frequency distribution (LFD) of Upeneus pori recorded off Lampedusa Island (Strait of Sicily).
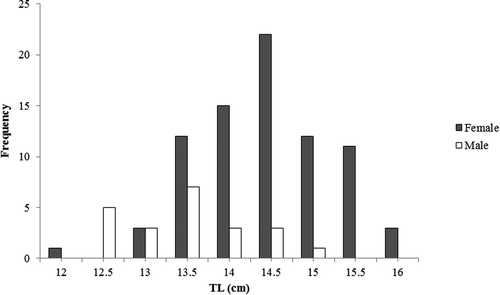
Females accounted for approximately 78% of the total sample. Examination of the female gonads showed that 49 specimens (mean ± standard deviation: 14.3 ± 0.8 cm TL) had a translucent ovary that was approximately half as long as the body cavity, but with no visible eggs, thus indicating either an immature stage or a recovering phase. A total of 22 specimens (15.0 ± 0.5 cm TL) were maturing, with eggs visible within a translucent ovaric tunica two-thirds the length of their body cavity. Just four specimens (15.0 ± 0.9 cm TL) were mature, having an ovary with conspicuous superficial blood vessels and ripe eggs, while another four (15.3 ± 0.6 cm TL) were spent, featuring flaccid ovaric walls that contained remnants of disintegrated eggs.
Observation of the male gonads revealed 18 specimens (13.6 ± 0.8 cm TL) that were maturing or in a recovering phase (symmetrical testes, approximately half the body cavity length), two specimens (13.0 and 14.5 cm TLs) that were mature (whitish to creamy testes, approximately two-thirds the body cavity length), and two (13.5 and 14.9 cm TLs) that were spent (bloodshot and flabby testes, shrunken to approximately half of body cavity length).
Since the length–weight plot showed no sex difference, and given the reduced number of males in the sample, the LWR was analysed by pooling the two sexes ().
Figure 4. Weight at length (grey diamonds) and length weight relationship (LWR, continuous curve) of Upeneus pori recorded off Lampedusa island (Strait of Sicily). Available LWRs of the species from other areas of the Mediterranean are also reported (dotted curves).
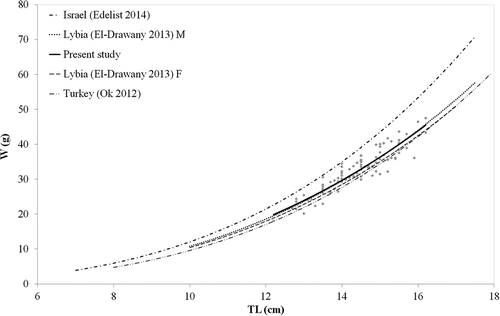
This relationship took the form of W = 0.0132TL2.923 (r2 = 0.862), which corresponded to negative allometric growth (t-test: P < 0.01) and the lowest b parameter for U. pori in the Mediterranean. The average value of condition factor (K) for this species was 1.34. The main results for the sex ratio and LWR of the U. pori collected in the present study, and a comparison with those from the literature, are summarised in .
According to Tuset et al. (Citation2012), the otolith shape is elliptic and the ventral margin crenate. The anterior region is peaked while the posterior region is oblique. Otolith inspection indicated the occurrence of a translucent zone along the margin, suggesting that fish were in a period of slow growth. The ring pattern featured well-defined translucent rings appearing all around the otoliths, which might indicate a seasonal growth pattern ().
Figure 5. Otoliths (sagittae) of a Upeneus pori female, 15.2 cm TL, caught off Lampedusa Island (magnification 70×).
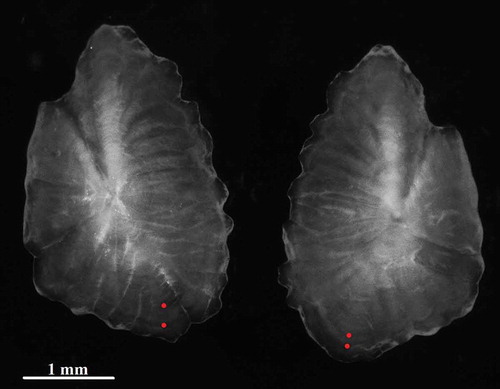
The specimens’ estimated ages ranged between 1 and 5 years old for a female of 15.9 cm. Age class 2+ predominated in both sexes, accounting for approximately 54 and 58% of the females and males, respectively ().
Table III. Mean, standard deviation, minimum and maximum length at age (total length, TL; all in mm) of the Upeneus pori caught off Lampedusa Island (Strait of Sicily). NA: Not Available.
The SST data indicated that the 15°C February isotherm in the Aegean and Eastern Ionian Sea (Eastern Mediterranean Sea) has gradually moved northward over the last 30 years. In the Strait of Sicily (Central Mediterranean Sea), the 15°C February isotherm oscillated during the same period but without a specific directional pattern ().
Discussion
After first being recorded off the Mediterranean Turkish coast, U. pori spread and established in Levantine waters, off Lebanon and Israel, during the 1950s. Between the middle 1980s and the middle 1990s, the species expanded westward along the northern coast of Africa, reaching the Gulf of Sirte in 1994, the southern Aegean Sea in the second half of the 2000s, and more recently the Strait of Sicily. But its north-east expansion along the Turkish coast has been slower, with U. pori reaching the areas adjacent to Rhodes Island only in the early-middle 2000s (e.g. Corsini et al. Citation2005) ().
Our results showed that the Por’s goatfish is well established on the East Tunisian continental shelf, with its population size likely increasing. In the early 2000s just two specimens were recorded in Bahiret El Biban (Ben Souissi et al. Citation2005) and the Bizerte lagoons (Azzouz et al. Citation2010), but in 2016 this reached 64 specimens collected off Lampedusa Island in a single trawl fishing trip. All these findings should be probably interpreted as the natural expansion of the Libyan population described in 2007–2008 by El-Drawany (Citation2013).
Morphometric and meristic data of U. pori specimens collected off Lampedusa Island and relative length proportions generally corresponded to those reported by Ben Souissi et al. (Citation2005) and Ben-Tuvia and Golani (Citation1989), with exception of caudal fin length, base anal fin width and first dorsal fin height ().
The modal total lengths – 14.5 cm TL for females and 13.5 cm TL for males – of the specimens collected in this study were higher than the corresponding sizes reported from Turkey (Ismen Citation2006), at 13.0 and 12.0 cm TL. Furthermore, Akar and Gündoğdu (Citation2013) and Cicek and Avsar (Citation2011) reported the modal length as 9.0 cm TL, also from Turkey. Recently, in the Gulf of Suez, Sabrah et al. (Citation2017) collected specimens that were 9.0 to 16 cm long, with a modal length of 15 and 10 cm TL, respectively, for the females and males. The maximum length recorded in the present study was 3.0 cm shorter than maximum length reported in the Mediterranean (19.2 cm TL) – by Ok (Citation2012) in Turkey – and in its native area (Randall Citation1995). Such differences may be explained by the fact that the samples included in this study were collected during commercial trawling that targeted M. surmuletus always at the same depth (from 50 to 70 m) and in one season (autumn). As such, our sampling likely represented only a fraction of the existing population.
The specimens collected off Lampedusa Island showed a negative allometric growth pattern (b = 2.92), which is the lowest yet recognised in the Mediterranean (see ). However, when comparing the LWR for the pooled-sex sample with those from the available literature, our curve looked quite similar to these other ones, with the exception of those specimens caught off Israel (Edelist Citation2014). However, unlike the available data for other Mediterranean regions, the sex ratio of our sample was skewed towards females (), a discrepancy that is likely explained by the restricted depth range in the Strait of Sicily from which our specimens were captured. Since the largest specimens of Mullidae, usually females, occur on the deepest marine bottoms (Warburton & Blaber Citation1992; Machias & Labropoulou Citation2002), the unbalanced sex ratio in our study is not that surprising.
Spawning data for the Por’s goatfish collected off Lampedusa Island indicated a spawning period that extended into autumn. Most of the studies carried out in the Mediterranean limit this species’ spawning season to the period from April to September (Golani Citation1994), with its spawning peak varying longitudinally from April (Turkey: Cicek et al. Citation2002; Ismen Citation2006; Ok Citation2012) to May (Egypt: Ramadan & El-Halfawy Citation2014) and June (Libya: El-Drawany Citation2013). Our results agree with Mediterranean records for the Gulf of Suez, for which the spawning period extends from May to September (Sabrah et al. Citation2017).
A preliminary analysis of the age structure of the specimens sampled off Lampedusa Island indicated a predominance (approx. 55%) of age class 2 or higher, similar to that found off the Turkish coasts by Ismen (Citation2006). Due to the small number of specimens we collected it was not possible to estimate a von Bertalanffy growth curve. Nonetheless, superimposing our length-at-age estimates with the available von Bertalanffy curves suggests that our specimens in age class 1+ were apparently larger than those from other Mediterranean areas, while our specimens in age classes 2+ and 3+ overlapped with the curves reported by Ok (Citation2012) from Turkey and by El-Drawany (Citation2013) from Lybia ().
Figure 6. Length-at-age of Upeneus pori recorded off Lampedusa Island (Strait of Sicily). Available von Bertanlanffy growth curves of the species from other areas of the Mediterranean are also reported (dotted curves).
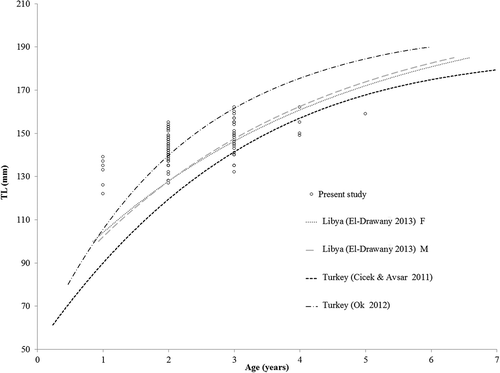
Although based on a few individuals, our lengths at age classes 4 and 5 seem shorter than those reported in the literature for other Mediterranean areas. According to El-Drawany (Citation2013). U. pori attained more than 51% of its maximum size during the first year of life, after which its annual growth rate ranged between 1.4 and 3.7 cm. Similarly, the Por’s goatfish in Turkey attained approximately 48% of its calculated maximum size during the first year of life, after which its annual growth rate decreased to between 1 and 3 cm (Ismen Citation2006).
We believe that this preliminary information on the biological traits of U. pori on the continental shelf off Lampedusa Island is relevant for monitoring this species’ spread in the Mediterranean Sea. The Lampedusa population of Por’s goatfish represents the westernmost occurrence of the species; hence, it can potentially serve for its further expansion into the central and western Mediterranean basin via the Strait of Sicily. The abundance of the species in this area, now documented by > 100 specimens caught by trawling, likely indicates the occurrence of an established population, one that is probably positively affected by the ongoing phase of increasing temperature and salinity in the Mediterranean waters (Adloff et al. Citation2015). The rise in sea surface temperature over the last 20 years has led to a progressive, although non-linear, westward shift of the 15°C isotherm. This isotherm is regarded as one of the main abiotic barriers for constraining the expansion of warm-affinity species from the eastern to the western Mediterranean basin (Bianchi Citation2007). This applies to the Por’s goatfish, as its distribution range seems at the moment limited to those areas with a winter SST over 15°C. It is clear, however, that other environmental factors could play an important role in the future expansion of this species. For example, to investigate the reason behind the fast colonisation of the whole Mediterranean by Fistularia commersonii, Azzurro et al. (Citation2013) used a habitat suitability model and found a significant effect of both chlorophyll a and salinity.
Warming and climate change have been shown to favour both the entry and the dispersal of tropical species in the Mediterranean Sea (Astraldi et al. Citation1995; Ben Rais Lasram et al. Citation2008; Raitsos et al. Citation2010; Skliris et al. Citation2012), some of which have already reached its northernmost sectors (Dulčić et al. Citation2007; Daniel et al. Citation2009; Lipej et al. Citation2009; Puce et al. Citation2009). Indeed, the fast global warming has inevitably led to a progressive vanishing of the thermal differences among surface waters across the basin. This process is likely to produce a progressive north-west shift of the water temperature and salinity barriers limiting non-native species distributions in Mediterranean. This should lead to an increased expansion of the “warm” aquatic biota and the replacement of “cold species”, with as-yet uncertain impacts on biodiversity and ecosystem services. Por’s goatfish, similarly to what has already occurred off the coast of the Middle East (Ismen Citation2006; De Meo et al. Citation2017), has become a new fishery resource for local fishing fleets. However, any economic benefits produced will depend on how this species impacts the indigenous species, in particular its potential competitors, namely the autochthonous goatfish M. barbatus and M. surmuletus.
Geolocation information
Geolocation information about the finding of U. pori caught between 2012 and 2016 in the Strait of Sicily.
Acknowledgements
This study has been conducted using E.U. Copernicus Marine Service Information. Furthermore, all specimens were caught within the module of monitoring commercial catch (CampBiol) of the European Data Collection Framework (DCF).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adloff F, Somot S, Sevault F, Jordà G, Aznar R, Déqué M, Herrmann M, Marcos M, Dubois C, Padorno E, Alvarez-Fanjul E, Gomis D. 2015. Mediterranean Sea response to climate change in an ensemble of twenty first century scenarios. Climate Dynamics 45:2775–2802. DOI:10.1007/s00382-015-2507-3.
- Akar M, Gündoğdu S. 2013. A comparison of weighted least square estimation and ordinary least square estimation for analysing weight-length relationship of Por’s Goatfish (Upeneus pori Ben-Tuvia & Golani, 1989) in Iskenderun bay. Journal of Applied Biological Sciences 7:46–50.
- Anonymous. 2016. MEDITS-handbook. Version n. 8. MEDITS Working Group, p. 177.
- Astraldi M, Bianchi CN, Gasparini GP, Morri C. 1995. Climatic fluctuations, current variability and marine species distribution-a case-study in the Ligurian Sea (North-West Mediterranean). Oceanologica Acta 18:139–149.
- Azzouz K, Mansour S, Boumaiza M. 2010. Occurrence of the Por’s goatfish Upeneus pori (Osteichthyes: Mullidae) in the Lagoon of Bizerte (Northern Tunisia, Central Mediterranean). Annales Series Historia Naturalis 2:29–32.
- Azzurro E, Andaloro F. 2004. A new settled population of the lessepsian migrant Siganus luridus (Pisces: Siganidae) in Linosa Island-Sicily Strait. Journal of the Marine Biological Association of the UK 84:819–821. DOI:10.1017/s0025315404009993h.
- Azzurro E, Castriota L, Falautano M, Giardina F, Andaloro F. 2014. The silver-cheeked toadfish Lagocephalus sceleratus (Gmelin, 1789) reaches Italian waters. Journal of Applied Ichthyology 30:1050–1052. DOI:10.1111/jai.12471.
- Azzurro E, Soto S, Garofalo G, Maynou F. 2013. Fistularia commersonii in the Mediterranean Sea: Invasion history and distribution modeling based on presence-only records. Biological Invasions 15:977–990. DOI:10.1007/s10530-012-0344-4.
- Bagenal TB, Tesch FW. 1978. Age and growth. In: Bagenal TB, editor. Methods for the assessment of fish production in fresh waters. Oxford: Blackwell Scientific Publication. pp. 101–136.
- Bazairi H, Sghaier YR, Benamer I, Langar H, Pergent G, Bouras E, Verlaque M, Soussi JB, Zenetos A. 2013. Alien marine species of Libya: First inventory and new records in El-Kouf National Park (Cyrenaica) and the neighbouring areas. Mediterranean Marine Science 14:451. DOI:10.12681/Mms.555.
- Ben Rais Lasram F, Tomasini J, Guilhaumon F, Romdhane M, Do Chi T, Mouillot D. 2008. Ecological correlates of dispersal success of Lessepsian fishes. Marine Ecology Progress Series 363:273–286. DOI:10.3354/meps07474.
- Ben Souissi J, Mejri H, Zauali J, Capapé C. 2005. On the occurrence of the Por’s goatfish, Upeneus pori (Mullidae) in southern Tunisia (central Mediterranean). Cybium 29:410–412.
- Ben Souissi J, Rifi M, Ghanem R, Boughedir W, Capapé C, Azzurro E. 2014. First record of the two bar sea bream Acanthopagrus bifasciatus (Teleostei: Sparidae) in the Mediterranean Sea. Mediterranean Marine Science 15:437–439. DOI:10.12681/mms.774.
- Ben-Abdallah AR, Alturky A, Fituri A. 2005. Records of exotic fishes in the Libyan coast. Libyan Journal of Marine Science 10:1–8.
- Ben-Tuvia A. 1953. Mediterranean fishes of Israel. Bulletin of the Sea Fisheries Research Station, Haifa 8:1–14.
- Ben-Tuvia A, Golani D. 1989. A new species of goatfish Mullidae of the genus Upeneus from the Red Sea and the eastern Mediterranean. Israel Journal of Zoology 36:103112.
- Bianchi CN. 2007. Biodiversity issues for the forthcoming tropical Mediterranean Sea. Hydrobiologia 580:7–21. DOI:10.1007/s10750-006-0469-5.
- Campana S. 2001. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. Journal of Fish Biology 59:197–242. DOI:10.1006/jfbi.2001.1668.
- Cicek E, Avsar D. 2011. Age, growth and mortality of Upeneus pori Ben-Tuvia and Golani, 1989 off the Karatas Coasts of Iskenderun Bay. Journal of Animal and Veterinary Advances 10:878–882. DOI:10.3923/javaa.2011.878.882.
- Cicek E, Avsar D, Yeldan H, Ozutok M 2002. Population characteristics of the Por’s goatfish, Upeneus pori (Ben-Tuvia & Golani, 1989). In: Inhabiting in Babadillimanı Bight Northeastern Mediterranean-Turkey, Workshop on Lessepsian Migration, 20–21 July 2002, Gokceada, Turkey. pp. 92–99.
- Cicek E, Avsar D, Yeldan H, Ozutok M. 2006. Length-weight relationships for 31 teleost fishes caught by bottom trawl net in the Babadillimani Bight (northeastern Mediterranean). Journal of Applied Ichthyology 22:290–292. DOI:10.1111/j.1439-0426.2006.00755.x.
- Corsini M, Margies P, Kondilatos G, Economidis PS. 2005. Lessepsian migration of fishes to the Aegean Sea: First record of Tylerius spinosissimus (Tetraodontidae) from the Mediterranean, and six more fish records from Rhodes. Cybium 29:347–354.
- Daniel B, Piro S, Charbonnel E, Francour P, Letourneur Y. 2009. Lessepsian rabbitfish Siganus luridus reached the French Mediterranean coasts. Cybium 33:163–164.
- De Meo I, Miglietta C, Mutlu E, Deval MC, Balaban C, Olguner MT. 2017. Ecological distribution of demersal fish species in space and time on the shelf of Antalya Gulf, Turkey. Marine Biodiversity DOI:10.1007/s12526-017-0739-7.
- Deidun A, Fenech-Farrugia A, Castriota L, Falautano M, Azzurro E, Andaloro F. 2015. First record of the silver-cheeked toadfish Lagocephalus sceleratus (Gmelin, 1789) from Malta. BioInvasions Records 4:139–142. DOI:10.3391/bir.2015.4.2.11.
- Dulčić J, Scordella G, Guidetti P. 2007. On the record of the Lessepsian migrant Fistularia commersonii (Rüppell, 1835) from the Adriatic Sea. Journal of Applied Ichthyology 24:101–102. DOI:10.1111/j.1439-0426.2007.01022.x.
- Edelist D. 2014. New length-weight relationships and Lmax values for fishes from the Southeastern Mediterranean Sea. Journal of Applied Ichthyology 30:521–526. DOI:10.1111/j.1439-0426.2012.02060.x.
- El-Drawany MA. 2013. Some biological aspects of the Por’s goatfish, (Family: Mullidae) from Tripoli Cost of Libya. The Egyptian Journal of Aquatic Research 39:261–266. DOI:10.1016/j.ejar.2013.11.003.
- El-Sayed RS. 1994. Check-list of Egyptian Mediterranean fishes. Alexandria, Egypt: Institute of Oceanography and Fisheries. pp. 77, ix.
- Falautano M, Castriota L, Andaloro F, Faltas S. 2006. First record of Etrumeus teres (Clupeidae) in the central Mediterranean Sea. Cybium 30:287–288.
- Filiz H. 2012. Northernmost occurrence of Upeneus pori (Mullidae) in the Aegean Sea. Mediterranean Marine Science 13/1:162–174. DOI:10.12681/mms.33.
- Galil BS, Marchini A, Occhipinti-Ambrogi A. 2016. East is east and West is west? Management of marine bioinvasions in the Mediterranean Sea. Estuarine, Coastal and Shelf Science DOI:10.1016/j.ecss.2015.12.021.
- Galil BS, Marchini A, Occhipinti-Ambrogi A, Ojaveer H. 2017. The enlargement of the Suez Canal: Erythraean introductions and management challenges. Management of Biological Invasions 8:141–152. DOI:10.3391/mbi.2017.8.2.02.
- George CJ, Athanassiou VA. 1996. Observations on Upeneus asymmetricus (Lachner, 1954) in St. George bay, Lebanon. Annali Del Museo Civico Di Storia Naturale Di Genova 76:68–74.
- Golani D. 1994. Niche separation between colonizing and indigenous goatfishes (Mullidae) of the Mediterranean coast of Israel. Journal of Fish Biology 45:503–513. DOI:10.1111/j.1095-8649.1994.tb01332.x.
- Ismen A. 2006. Growth and reproduction of Por’s goatfish (Upeneus pori, Ben-Tuvia and Golani, 1989) in Iskenderun Bay, the Eastern Mediterranean. Turkish Journal Zoology 30:91–98.
- Kosswig C. 1950. Erythräische Fische im Mittelmeer und an der Grenze der Ägais. Syllegomena Biologica. Festschrift Kleinschmidt. Leipzig: Akademie Verlag. pp. 203–212.
- Lipej L, Mavrič B, Orlando Bonaca M. 2009. Recent changes in the Adriatic fish fauna: Experiences from Slovenia. Varstvo Narave 22:91–96.
- Machias A, Labropoulou M. 2002. Intra-specific variation in resource use by Red Mullet, Mullus barbatus. Estuarine, Coastal and Shelf Science 55:565–578. DOI:10.1006/ecss.2001.0924.
- Mavruk S, Avsar D. 2007. Non-native fishes in the Mediterranean from the Red Sea, by way of the Suez Canal. Reviews in Fish Biology and Fisheries 18:251–262. DOI:10.1007/s11160-007-9073-7.
- Öğretmen F, Yılmaz F, Koç HT. 2016. An investigation on fishes of Gökova bay (Southern Aegean Sea). Balıkesir Üniversitesi Fen Bilimleri Enstitüsü Dergisi 7:19–36.
- Ok M 2012. Evaluation of the demersal fish assemblages of the Northeastern Levant Sea. PhD thesis, Middle East Technical University, Turkey. 227 pp.
- Puce S, Bavestrello G, Di Camillo CG, Boero F. 2009. Long-term changes in hydroid (Cnidaria, Hydrozoa) assemblages. Effect of Mediterranean Warming? Marine Ecology 30:313–326. DOI:10.1111/j.1439-0485.2009.00283.x.
- Quignard JP, Tomasini JA. 2000. Mediterranean fish biodiversity. Biologia Marina Mediterranea 7:1–66.
- Raitsos DE, Beaugrand G, Georgopoulos D, Zenetos A, Pancucci-Papadopoulou AM, Theocharis A, Papathanassiou E. 2010. Global climate change amplifies the entry of tropical species into the eastern Mediterranean Sea. Limnology and Oceanography 55:1478–1484. DOI:10.4319/lo.2010.55.4.1478.
- Ramadan AM, El-Halfawy MM. 2014. Ovarian maturation and spawning season of Por’s goatfish Upeneus pori (Mullidae) from Mediterranean Sea. Egyptian Journal of Ichthyology 54:905–912. DOI:10.1134/s0032945214100154.
- Randall JE. 1995. Coastal fishes of Oman. Honolulu, Hawaii: University of Hawaii Press. pp. 439.
- Sabrah MM, Heneish RA, Alwany ME, Ahmad MI. 2017. Sexual maturity, spawning activity, sex ratio and fecundity of two Mullidae species dwelling the Gulf of Suez, Red Sea. The Egyptian Journal of Aquatic Research 43:83–91. DOI:10.1016/j.ejar.2016.04.007.
- Scannella D, Falsone F, Geraci ML, Froglia C, Fiorentino F, Giusto GB, Zava B, Insacco G, Colloca F. 2017. Westward expansion of the Northern brown shrimp Penaeus aztecus Ives, 1891 (Crustacea, Penaeidae) in the Mediterranean Sea: First records in the Strait of Sicily. Bio Invasion Record 6:67–72. DOI:10.3391/bir.2017.6.1.11.
- Sergiwa SSS, Ali RAS, Elsameea ZMA, Sayed SM, Elmor MAA. 2017. Food and feeding habits of the Por’s goatfish Upeneus pori (Ben-Tuvia and Golani, 1989), Mullidae, in Ain El-Ghazala Lagoon, Eastern Libya Mediterranean Sea. European-American Journal 3:33–50.
- Shakman EA. 2007. Distribution and characterization of Lessepsian migrant fishes along the coast of Libya. Acta Ichthyologica Et Piscatoria 37:7–15. DOI:10.3750/aip2007.37.1.02.
- Sieli G, Badalucco C, Di Stefano G, Rizzo P, D’Anna G, Fiorentino F. 2011. Biology of red mullet, Mullus barbatus (L. 1758), in the Gulf of Castellammare (NW Sicily, Mediterranean Sea) subject to a trawling ban. Journal of Applied Ichthyology 27:1218–1225. DOI:10.1111/j.1439-0426.2011.01784.x.
- Simoncelli S, Fratianni C, Pinardi N, Grandi A, Drudi M, Oddo P, Dobricic S. 2014. “Mediterranean Sea physical reanalysis (MEDREA 1987-2015) (Version 1)”. [Data set]. E.U. Copernicus Marine Service Information DOI:10.25423/medsea_reanalysis_phys_006_004.
- Skliris N, Sofianos S, Gkanasos A, Mantziafou A, Vervatis V, Axaopoulos P, Lascaratos A. 2012. Decadal scale variability of sea surface temperature in the Mediterranean Sea in relation to atmospheric variability. Ocean Dynamics 62:13–30. DOI:10.1007/s10236-011-0493-5.
- Sparre P, Ursin E, Venema SC 1989. Introduction to tropical fish stock assessment. Part 1. Manual FAO Fisheries Techinical Paper 306.1. p. 337.
- Stamouli C, Dogrammatzi A. 2016. First record of the Lessepsian fish Upeneus pori Ben-Tuvia & Golani, 1989 in Saronikos Gulf. Mediterranean Marine Science 17:230–252. DOI:10.12681/mms.1684.
- Taskavak E, Bilecenoglu M. 2001. Length–Weight relationships for 18 Lessepsian (Red Sea) immigrant fish species from the eastern Mediterranean coast of Turkey. Journal of the Marine Biological Association of the UK 81:895. DOI:10.1017/s0025315401004805.
- Torcu H, Mater S. 2000. Lessepsian fishes spreading along the coasts of the Mediterranean and the Southern Aegean Sea of Turkey. Turkish Journal of Zoology 24:139–148.
- Tuset VM, Azzurro E, Lombarte A. 2012. Identification of Lessepsian fish species using the sagittal otolith. Scientia Marina 76:289–299. DOI:10.3989/scimar.03420.18e.
- Tzomos TH 2007. Investigation of the progress of the lessepsian immigratory current, in respect of Mollusca and Pisces. Masters thesis. Thessaloniki, Greece: Aristoteleio University of Thessaloniki.
- Vitale S, Arculeo M, Vaz A, Giusto GB, Gancitano S, Ragonese S. 2016. Otolith-based age and growth of the Lessepsian species Fistularia commersonii (Osteichtyes: Fistulariidae) in South of Sicily (Central Mediterranean Sea). Italian Journal of Zoology 83:490–496. DOI:10.1080/11250003.2016.1223759.
- Warburton K, Blaber SJM. 1992. Patterns of recruitment and resource use in a shallow-water fish assemblage in Moreton Bay, Queensland. Marine Ecology Progress Series 90:113–126. DOI:10.3354/meps090113.
- Zenetos A, Çinar ME, Crocetta F, Golani D, Rosso A, Servello G, Shenkar N, Turon X, Verlaque M. 2017. Uncertainties and validation of alien species catalogues: The Mediterranean as an example. Estuarine, Coastal and Shelf Science 191:171–187. DOI:10.1016/j.ecss.2017.03.031.

