Abstract
The present paper presents a faunal study of Calanoida in both fresh and marine waters, considering both planktonic and benthic species, for the first time specifically devoted to a national territory (Italy). A total of 270 Calanoida species belonging to 34 families are found in Italy, including 34% of European freshwater species and 61% of Mediterranean marine species. Six species, all from coastal environments, are endemic. One freshwater species and three found in coastal brackish waters have been recognised as recently introduced (non-indigenous). Calanoida are abundant and always present in any marine plankton sample, while they are present in only half of the strictly freshwater bodies. The data collected confirm the general low level of adaptation of Calanoida to fresh water, notwithstanding the high adaptability of the single family Diaptomidae. Finally, due to the geographical position of Italy in the middle of the Mediterranean Sea, the Italian Calanoida fauna can be considered highly representative of both the Mediterranean Sea and the European continent.
History of studies
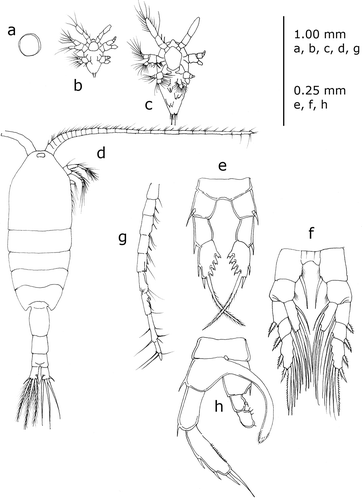
The history of studies of Calanoida in Italy begins with Prestandrea (Citation1833), who was the first to report a species (Cyclops marinus, now Euchaeta marina) in Italian waters, among a group of six species of marine Crustacea found in the Strait of Messina. He did not publish pictures of the species, but Claus (Citation1863) did, accepting the change of genus name to Euchaeta, proposed by Philippi (Citation1843), who dedicated the species to its first describer (E. prestandreae). Finally, Giesbrecht (Citation1893) confirmed this new genus name but correctly reintroduced the original species name (marina) given by Prestandrea (). In the meantime, Pavesi (Citation1877) reported Heterocope robusta (now H. saliens) in Lake Maggiore as the first Italian freshwater calanoid ().
Figure 1. Standard representation of Euchaeta marina (Prestandrea Citation1833), the first Calanoida species reported from Italian marine waters: (a) first nauplius; (b) metanauplius; (c) maxilliped; (d) extremity of antennulae – d1 female, d2 male; (e) fifth pair of thoracic legs, male; (f) furca – f1 female, f2 male; (g) adult male, lateral view; (h) adult female, lateral view. (Redrawn from different sources: (a) from Sazhina Citation1985; (b) from Bjornberg Citation1972; (c–g) from Giesbrecht Citation1892; (h) from Claus Citation1863).
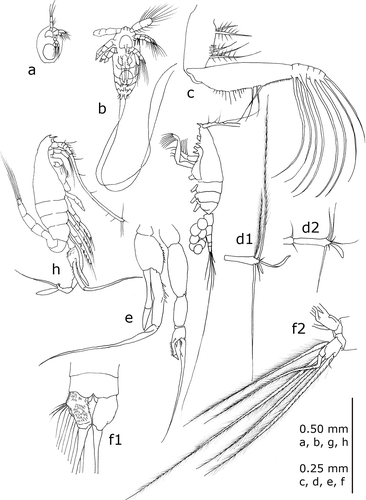
Figure 2. Standard representation of Heterocope saliens (Lilljeborg, 1862), the first Calanoida species reported from Italian fresh waters. (a) egg; (b) nauplius; (c) metanauplius; (d) adult female, dorsal view; (e) fifth pair of thoracic legs, female; (f) fourth pair of thoracic legs, female; (g) part of the right antennula, male; (h) fifth pair of thoracic legs, male. (Redrawn from different sources: (a–c) from Ravera Citation1953; (d, e, g, h) from Sars Citation1902; (f) from Dussart Citation1967).
The first attempt to describe and list Calanoida species present in Italian waters is found in the marvellously illustrated work by Giesbrecht (Citation1893 [“1892”]) on marine planktonic Copepoda (not only Calanoida) in the Gulf of Naples. That author presented 80 Calanoida species found in the Gulf of Naples and probably the whole of the Tyrrhenian Sea. He compared them with 135 other Calanoida species found in various seas all over the world, showing their morphology in 43 plates which are still considered masterpieces of naturalistic illustration.
Ninety years later, Carli and Crisafi (Citation1983) proposed a short guide for identifying the 20 most common Calanoida species in Italian lagoons and coastal lakes, as well as other copepods. Stella (Citation1984) published the first monograph dedicated entirely to Italian Calanoida (22 species) but referred exclusively to those of lakes and ponds without a direct connection with the sea.
An identification guide has been proposed for marine Calanoida in Italian waters (Mazzocchi Citation2006), but for practical reasons it contains detailed descriptions of only 61 common species belonging to 17 families, and only from the neritic zone. In the same period, a checklist of all Italian fauna was published, but it listed freshwater (Ruffo & Stock Citation2005) and marine species (Relini Citation2010) in different volumes, while the volume on marine species treated planktonic and hyperbenthic Calanoida separately (Mazzocchi & DiCapua Citation2010; Zagami Citation2010). Calanoida from inland coastal waters are traditionally considered marine species by Italian zoologists, but eminent non-Italian specialists consider them to lie within the dominion of inland waters, together with the strictly freshwater species (see Dussart Citation1989; Dussart & Defaye Citation2002). The aim of the present review is to go beyond this conflict by tackling Calanoida as a whole, not separating them by ecological compartment. Such a zoo-systematic approach aims to put the focus on the evolutionary diversification of the taxon, as well as to offer a useful tool for management purposes, by specifying the presence and distribution of the taxon within the political territory (land and sea) of Italy.
Finally, the present contribution on Calanoida is the first to refer to a political or jurisdictional territory without reference to vague and/or approximate geographical boundaries. This serves to provide environment managers with precise indications on species distribution.
Aims of the review
The traditional approach to studying nature, on an ecological basis (i.e. separating fresh and marine waters, or plankton from benthos communities), inevitably leads to the loss of important information concerning the evolutionary path (comparative morphology, physiology, life cycles, etc.) followed by each high-level taxon during adaptation to different situations under its own developmental constraints. In addition, the ecological approach has made it common practice for naturalists in one ecological compartment to completely ignore the work of specialists in the same taxon who are operating in another compartment. For example, Mauchline (Citation1998) dedicated a monograph of more than 710 pages to the general biology of Calanoida, considering only marine species, however, and disregarding this detail in the title. On the other hand, Stella (Citation1984) wrote a monograph on Italian Calanoida species in inland waters, completely disregarding coastal brackish lakes because they are in direct communication with the sea (thalassic).
The same can be said for the plankton–benthos dichotomy, which generally considers species separately according to their compartment (as in the case of planktonic and benthic Copepoda Harpacticoida).
The present review proposes a unifying approach for the study of a taxon with representatives in fresh, brackish, and marine waters, both in plankton and in benthos.
In terms of biogeographical studies of the marine habitat, the present review considers exclusively the political boundaries of present-day Italy, in contrast to what is commonly proposed in the literature. In a well-known volume on marine planktonic Copepoda, Rose (Citation1933) attributed all 333 Calanoida species from the Western Mediterranean and the European part of the Atlantic Ocean to French fauna. The identification guide for Calanoida in Spain by Vives and Shmeleva (Citation2007) considered the entire community of the Mediterranean Sea and the East Atlantic Ocean as potentially present (even if not actually found) in Spanish waters. In that case, more than 600 Calanoida species were proposed, excluding (as is the rule) those found in fresh waters. The book contained about 1000 pages, which is not easy for environmental consultants to manage at work. This traditional approach has actually impeded the creation of a guide for Calanoida identification referring precisely to a single country.
In contrast, it is argued here that a precise reference to a specific political territory (Italy in the present case) will be helpful for the purposes of conservation and management of the natural environment, which nowadays is requested and planned by national authorities.
Methods
Only modern Italian waters were considered, with no generalisations based on natural sea boundaries (generally not coinciding with political ones), and/or past political boundaries that set certain Italian territories lying within other countries (e.g. Veneto, Friuli), or certain other countries’ territories lying within Italy (e.g. Istria, Rhodes, Albania, Libya), during the past 200 years.
The present approach is proposed here for the first time. It posits a political Italy in place of a “physical” one (which includes San Marino, Corsica in France, Ticino in Switzerland, Istria in Slovenia/Croatia and Malta), in order to accurately report a situation useful for species management by the Italian government and local environment managers.
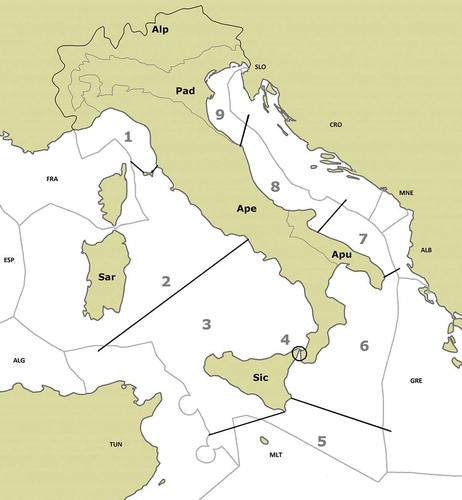
The political area considered includes both land governed by the Italian Republic (301,000 km2) and the sea within the limits of the exclusive economic zone of the Republic (587,000 km2) ().
Figure 3. Map of the Italian territory considered in the present review. Economic-political borders contain nine marine subareas (numbers 1–9) and six inland subareas. (1) Ligurian, (2) North Tyrrhenian and Sardinian, (3) South Tyrrhenian and Sicilian, (4) Messina Strait, (5) Central South Mediterranean, (6) Ionian, (7) South Adriatic, (8) Central Adriatic, (9) North Adriatic. (Alp) Alpine, (Pad) Po valley and Venetian plain, (Ape) Apennine, (Apu) Apulia, (Sic) Sicily, (Sar) Sardinia.
Useful information on geographical distribution, morphology and species nomenclature was derived from literature geographically related or indirectly referring to Italy and published since 1833. The most important reference for marine waters was the continuously updated website http://copepodes.obs-banyuls.fr/en/ (reported as Razouls et al. 2005–2018). Information from this site was checked on the basis of the available literature concerning exclusively Italian waters, since the subdivision of the Mediterranean Sea in Razouls et al. (2005–2018) does not overlap precisely with Italian marine boundaries. In detail, the geographical distributions of Razouls et al. (2005–2018) were compared mainly with those in Mazzocchi and DiCapua (Citation2010), whose geographical subdivision is adopted here. The species reported by Razouls et al. (2005–2018) but not present in the Mazzocchi and DiCapua (Citation2010) list were checked individually in order to precisely assign them to Italian marine waters. Species not reported by Razouls et al. (2005–2018) but present in previous papers (mostly in Kovalev & Shmeleva Citation1982) are indicated as doubtful, if the source is not given.
The classic morphological approach (the only one encompassing all species in a time span of 200 years) and accurate drawings of Calanoida in monographs and papers were used to provide maximum support for the identification of each species.
The polymorphism of each species (two sexes, three developmental stages, and different ages for each stage) was not considered by all describers. As a consequence, each species is identified based on a set of morphological details regarding only the adult stage.
Continental waters lying within Italian territory were divided into six geographical subareas recognised by Italian zoogeographers mostly on the basis of macrofauna distribution (Ruffo & Vigna Taglianti 2004). The distribution of Calanoida in these geographical subareas is based on the work of Ruffo and Stock (Citation2005), verifiable on the website http://www.faunaitalia.it/ckmap/index.html, and updated by checking the recent literature including a personal multiyear investigation in unexplored Italian inland waters (Belmonte et al. Citation2006; Alfonso et al. Citation2010, Citation2016; Alfonso & Belmonte Citation2011, Citation2013).
Names have been cross-checked for the correct scientific nomenclature and synonyms, which can also be viewed on the website http://www.marinespecies.org.
Results and discussion
A total of 282 Calanoida species belonging to 34 families (234 species from marine waters and 60 species from inland waters, with some present in both dominions) are listed (). Eleven species are reported as doubtful, however, because they are indicated in past lists without proper citation, and are not present in any bibliographical databases. One species, Pontellina elegans (Claus, 1892), has not been reported in the last 100 years, and should be considered extinct for Italian fauna. The final number, corrected on the basis of the preceding indications, is 270 species. In any case, it does not correspond to the most up-to-date websites (Razouls et al. 2005–2018 for the marine fauna; Ruffo & Stock Citation2005 for the freshwater fauna), mostly due to the different extension of the geographical area considered (for marine waters) and/or due to updating (in the case of inland waters).
Table I. List of 282 species of Italian fauna along with a brief indication of their geographical distribution. Terrestrial faunal territories: Alp = Alpine, Pad = Po Valley and Venetian Plain, Ape = Apennine, Apu = Apulia, Sar = Sardinia, Sic = Sicily. Numbers (1–9) indicate marine subareas: 1 = Liguria, 2 = North Tyrrhenian and Sardinia seas, 3 = South Tyrrhenian and North Sicilian seas, 4 = Messina Strait, 5 = Southern Central Mediterranean (south Sicily and Pelagie Islands), 6 = Ionian, 7 = South Adriatic, 8 = Central Adriatic, 9 = North Adriatic. An “x” indicates the presence of a species.Species in bold are Italian faunal endemics. Doubtful species are highlighted in grey. Underlining indicates species that are probably extinct.
shows a complete list of species arranged alphabetically by family. Six species (Acartia enzoi, Pseudocyclops costanzoi, P. faroensis, P. giussanii, Stephos cryptospinosus, S. marsalensis) are reported in the literature as being exclusive to Italy (endemics), and these are all found only in coastal brackish lakes. Italian scientists contributed descriptions of 11 new species among the 270 reported, but we can add to this number the 47 species described by Wilhelm Giesbrecht (born in Gdansk in 1854 and scientifically trained at Kiel University, Germany) because they occurred during his scientific work in an Italian institution (the Zoological Station, Naples, 1881–1913).
Marine waters
The updated website of Razouls et al. (2005–2018) reports a total of 382 Calanoida species found in the entire Ponto-Mediterranean Province and 287 species in areas 2–7, which include Italian seas ((the numerical labelling of marine areas by Razouls et al. (2005–2018), does not correspond to the subareas considered in the present work)). The present review, however, establishes that only 232 Calanoida species from the Razouls et al. (2005–2018) list are reported to be found in Italian marine waters, thus disproving any attempt to portray the species richness of a delimited section as coinciding with that of a wider, more inclusive geographical area.
Sixty-two species were added to the most recent official marine list by the Società Italiana di Biologia Marina (SIBM; Mazzocchi & DiCapua Citation2010; Zagami Citation2010) for Italian seas. In Italian seas, the Calanoida fauna is richer in subareas 2 and 3, i.e. the North and South Tyrrhenian (187 and 189 species respectively), and poorer in subarea 9, the North Adriatic Sea (71 species). A total of 48 species are very common (found in all nine marine subareas), and 33 are rare (present in only one subarea) (). Those species present in all nine marine subareas are not equally important or homogeneously distributed, however. For example, at least for the family Acartiidae, four species are reported to be found in all nine Italian marine subareas, but according to Belmonte and Potenza (Citation2001), of these, only Acartia clausi is abundant.
Although all seas are considered to have been sufficiently studied, and the marine area lying within Italian jurisdiction is about double the land surface area, no more than 500 collection sites (approximately 1 site for each 1170 km2) contributed data to the species list, with the greatest sampling effort in coastal areas and/or neritic waters (those with a depth of less than 200 m). Recent detailed monitoring of coastal zooplankton (Programma di monitoraggio dell’ambiente marino costiero 2001–2007) by the Italian Ministry of the Environment provided important support to knowledge of marine plankton because it collected samples from 81 transects (each transect made up of three stations) along the Italian coasts for a period of 6 years, taking about 5800 samples (Aguzzi Citation2008).
All the marine subareas are characterised by the constant presence of Calanoida species, which represent the majority of the zooplankton fauna in terms of population. Each marine site (and even each sample) sees the constant coexistence of many Calanoida species, although they are often co-generic. For example, of the 504 samples collected in over two years in the Taranto Sea system (Ionian Sea, subarea 6), 61 Calanoida species were reported (Belmonte et al. Citation2013), with an average of 10 species co-occurring in each sample. In the waters around Ponza Island (North Tyrrhenian Sea, subarea 2), 100 species were reported by Scotto di Carlo et al. (Citation1984), with a vertical subdivision along the water column of 70 samples collected at a single station on six dates. The depth of that water column (3000 m) was probably responsible for such a high species richness, as in the case of the 100 Calanoida species found in another extended study (96 samples collected on 12 dates at a depth range of 0–1000 m) in a single station in the Gulf of Naples (South Tyrrhenian Sea, subarea 3) by Hure and Scotto di Carlo (Citation1968).
Razouls et al. (2005–2018) report a total of 29 endemic species in the Mediterranean Sea, just six of which are found exclusively in Italian marine waters, with five new species recently described in coastal brackish water lakes in Sicily (Costanzo et al. Citation2000; Zagami et al. Citation2000, Citation2008; Baviera et al. Citation2007; Brugnano et al. Citation2010).
Three species have been reported as recently arrived (and probably indirectly introduced by human activities), namely Paracartia grani, Acartia tonsa and Pseudodiaptomus marinus (Gravili et al. Citation2010; De Olazabal & Tirelli Citation2011; Sabia et al. Citation2015), occasionally present in coastal areas, where they are probably exported by tidal currents from coastal brackish lakes.
Inland waters
Calanoida species have been reported in 942 inland sites in Italy (761 by Ruffo & Stock Citation2005; and additional sites by Belmonte et al. Citation2006; Marrone et al. Citation2006, Citation2011; Alfonso et al. Citation2010, Citation2011, Citation2016; Alfonso & Belmonte Citation2011; Fadda et al. Citation2011; Ariani et al. Citation2015; Seminara et al. Citation2016). However, this data set does not consider coastal brackish lakes that are in some way connected to the sea (thalassic lakes). If such brackish habitats are considered, the number of inland water sites hosting Calanoida species reaches 1000, double the number of marine sites.
In any case, the number of sampled water bodies is much higher because, unlike marine habitats, where the complete absence of Calanoida species in study sites (or even samples) has never been noted, many inland water bodies have been found to have no Calanoida species. For example, Marrone et al. (Citation2006) reported Calanoida species to be present in only 65 of 250 sites explored in Sicily, and Alfonso and Belmonte (Citation2011) found Calanoida in only 55 sites out of 121 sampled in southern Italy. Data from general investigations of inland water bodies show Calanoida species to be absent in about 60% of investigated sites (). Nobody, however, has identified a particular type of natural water body as selectively hostile to Calanoida.
Table II. Presence of Calanoida species in Italian freshwater bodies. The papers selected for comparison cover 29% of the known distribution of Calanoida, and are representative of all the geographical faunal subareas of inland Italian territory.
The sampling effort in Italian inland territory (with approximately 1 site for each 130 km2) has been, consequently, much higher than in Italian seas.
A total of 60 species are reported in inland waters, including brackish coastal ones, with 22 of these also reported in marine coastal waters. Arctodiaptomus alpinus is the most frequent species in Italian inland (athalassic) sites, being present in 24.7% of total inland sites hosting Calanoida species, but the species is restricted to the Alpine subarea (Alp). On the other hand, Metadiaptomus chevreuxi and Hemidiaptomus (Gigantodiaptomus) superbus are the rarest species (Marrone & Naselli-Flores Citation2005; Marrone et al. Citation2011), being present in only one site (a pond on Favignana Island, subarea Sic, and a temporary pond in the Ravenna pinewood, subarea Pad). In addition to these three species, 21 others have been reported from only one subarea.
No one species has been found in all six inland subareas. Diaptomus cianeus, Arctodiaptomus wierzejski, Mixodiaptomus kupelwieseri and Calanipeda aquaedulcis are the most widely distributed species, being reported for five subareas.
In Italian fresh waters, endemic species have never been reported. Five endemic species in inland (thalassic) waters have been reported in coastal brackish lakes in Sicily (already cited among species of marine waters).
At least four species have been reported to have recently arrived in Italy, namely Boeckella triarticulata in agricultural freshwater ponds in Pad, Ape and Apu (Alfonso & Belmonte Citation2008) and Paracartia grani, Acartia tonsa and Pseudodiaptomus marinus in coastal brackish lakes (Brugnano et al. Citation2010; Gravili et al. Citation2010).
Unlike in the marine domain, Calanoida species rarely coexist in the same athalassic water site on land. This absence of coexistence does not seem to depend on lake size. Indeed, Lake Garda (the largest Italian lake) hosts just one species (Salmaso & Naselli-Flores Citation1999), while the Goglia temporary pond (100 m × 40 m; in the Alta Murgia National Park, Apu) hosts five (Alfonso & Belmonte Citation2013), a condition equalled, in the Palaeartic domain, only by lake Bajkal (five species and one variety, according to Boxshall & Evstigneeva Citation2004).
Among the six inland subareas, Calanoida species were well represented in Apu (14 species in athalassic waters, plus 18 species in thalassic coastal waters) and less represented in the Alp subarea (10 species, strictly freshwater).
Although the presence of Calanoida seems to be a characteristic of large water areas like the sea, in inland water bodies, species richness is generally higher in small temporary ponds than in large stable lakes (Alfonso et al. Citation2016). In any case, co-existing species are never co-generics.
Conclusion
The present paper is the first dealing with the Calanoida of the whole set of water types and a whole political territory (land and sea). To obtain this result it was necessary to unify studies of marine and freshwater species, setting to one side the tradition by which ecology is the guiding principle in the fieldwork of naturalists.
In addition, the set of marine Calanoida presented here for Italy is limited to only political marine boundaries; consequently it is not perfectly comparable with that of other European countries (which refer to wider areas), although it could be compared with Mediterranean Calanoida as a whole. From this comparison it emerges that the Italian fauna includes 61% of Mediterranean Calanoida species.
Although only 40% of inland water bodies are characterised by the presence of Calanoida species, and although the Italian land surface area is smaller than that of the Italian seas, the number of inland sites useful for a study of Calanoida distribution exceeds the number of marine sites. Even in homogeneous geographical districts, single ponds are considered representative of different situations because they are physically isolated. In contrast, in the marine environment the trend is to consider neighbouring areas as potentially connected, and a fine grid of sampling sites as unnecessary. This approach is also valid for large lakes, which are considered single and uniform water bodies regardless of the number of sampling sites. The problem, however, is both an under-evaluation of environmental diversity due to the choice of excessively distant sample points, and an over-estimation of environmental diversity by considering ponds in the same plain to be separate, although they share the mode of species arrival by wind and rain (Càceres & Soluk Citation2002).
Calanoida in Italy represent a highly diversified zoological group inhabiting all types of water bodies (their absence is not related to any particular abiotic conditions). The geographical position of Italy seems to result in maximum habitat diversity, which ensures a high number of species from the Western Palaearctic biogeographical area (Marrone et al. Citation2017), and there is general agreement in attributing to Italy first place in Europe in terms of species richness (Ruffo & Vigna Taglianti 2004). In the case of Calanoida, Italy hosts 34% of European freshwater species, which is less than only Russia and Ukraine (Bledzki & Rybak Citation2016). In marine terms, Italian fauna is even more representative, with the number of species (233) corresponding to about 61% of the whole Mediterranean list.
The presence of Calanoida species in only 40% of freshwater sites clearly indicates either the limited adaptability of Calanoida to freshwater habitats or its still-young evolutionary history. The only exception is the high evolutionary success of Diaptomidae (a purely freshwater family), which is the richest family (510 species) in the entire Calanoida order. This last datum allowed Boxshall and Jaume (Citation2000) to propose a single colonisation wave of fresh waters from the marine environment and, consequently, a relatively short evolutionary history compared with that of Cyclopoida, which invaded the same habitats many millions of years before. Competition with Cyclopoida, which were already established in fresh waters at the time of Calanoida invasion, may be co-responsible for the limited occupation of Calanoida in water bodies. Future work could consider the quality of parental care (egg-carrying females among Diaptomidae) associated with the possibility of resting, as the possible reason for the evolutionary success of Cyclopoida and mainly of Diaptomidae among Calanoida.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Aguzzi L 2008. Studio del popolamento mesozooplanctonico marino costiero per la ricerca di nuovi indicatori ambientali. PhD thesis. University of Tuscia. 191 pp.
- Alfonso G, Beccarisi L, Pieri V, Frassanito A, Belmonte G. 2016. Using crustaceans to identify different pond types. A case study from the Alta Murgia National Park, Apulia (South-eastern Italy). Hydrobiologia 782:53–69. doi:10.1007/s10750-016-2669-y
- Alfonso G, Belmonte G. 2008. Expanding distribution of Boeckella triarticulata (Thomson, 1883) (Copepoda: Calanoida: Centropagidae) in Southern Italy. Aquatic Invasions 3:247–251. doi:10.3391/ai
- Alfonso G, Belmonte G. 2011. Calanoida (Crustacea Copepoda) from the inland waters of Apulia (south-eastern Italy). Journal of Limnology 70:57–68. doi:10.4081/jlimnol.2011.57
- Alfonso G, Belmonte G. 2013. Neolovenula alluaudi (Guerne & Richard, 1890) (Calanoida: Diaptomidae, Paradiaptominae): First record in Italy, and review of geographical distribution. Journal of Limnology 72:251–261. doi:10.4081/jlimnol.2013.e20
- Alfonso G, Belmonte G, Ernandez P, Zuccarello V. 2011. Stagni temporanei mediterranei in Puglia. Lecce: Grifo. pp. 144.
- Alfonso G, Belmonte G, Marrone F, Naselli-Flores L. 2010. Does lake age affect zooplankton diversity in Mediterranean lakes and reservoirs? A case study from southern Italy. Hydrobiologia 653:149–164. doi:10.1007/s10750-010-0350-4
- Andrei M, Gandolfi G. 1965. I Laghi di val Nure (Appennino piacentino). Bollettino Pesca Piscicoltura e Idrobiologia 41:61–142.
- Ariani AP, Wittmann KJ, Gaviria S. 2015. Novità faunistiche (Crustacea, Copepoda) da un ambiente salmastro del Golfo di Taranto. Thalassia Salentina 37:3–10.
- Baviera C, Crescenti N, Zagami G. 2007. Pseudocyclops costanzoi, a new species (Copepoda, Calanoida, Pseudocyclopidae) from the Mediterranean Sea, Faro Lake, Sicily. Crustaceana 80:569–576. doi:10.1163/156854007780765560
- Belmonte G, Alfonso G, Moscatello S. 2006. Copepod fauna (Calanoida and Cyclopoida) in small ponds of the Pollino National Park (South Italy), with notes on seasonality and biometry of species. Journal of Limnology 65:107–113. doi:10.4081/jlimnol.2006.107
- Belmonte G, Potenza D. 2001. Biogeography of the family Acartiidae (Copepoda) in the Ponto-Mediterranean Province. Hydrobiologia 453/454:171–176. doi:10.1023/A:1013192623131
- Belmonte G, Vaglio I, Rubino F, Alabiso G. 2013. Zooplankton composition along the confinement gradient of the Taranto Sea System (Ionian Sea, south-eastern Italy). Journal of Marine Systems 128:222–238. doi:10.1016/j.jmarsys.2013.05.007
- Bjornberg TKS. 1972. Developmental stages of some tropical and subtropical planktonic marine copepods. Studies on Marine Fauna of Curacao and Other Caribbean Islands 136:1–185.
- Bledzki LA, Rybak JI. 2016. Freshwater crustacean zooplankton of Europe. Switzerland: Springer International Publ. pp. 923.
- Boxshall JA, Evstigneeva TD. 2004. The evolution of species flocks of copepods in Lake Bajkal: A preliminary analysis. Advances in Limnology 44:235–245.
- Boxshall JA, Jaume D. 2000. Making waves: The repeated colonization of freshwater by copepod crustaceans. Advances in Ecological Research 31:61–79.
- Brugnano C, Celona A, Zagami G. 2010. A new species of Pseudocyclops (Copepoda: Calanoida) from Lake Faro (Central Mediterranean Sea). Vie et Milieu – Life and Environment 60:1–7.
- Càceres CE, Soluk DA. 2002. Blowing in the wind: A field test of overland dispersal and colonization by aquatic invertebrates. Oecologia 131:402–408. doi:10.1007/s00442-002-0897-5
- Carli A, Crisafi P 1983. Copepodi Lagunari. Guide per il riconoscimento delle specie animali delle acque lagunari e costiere italiane. AQ/I/230.CNR. 126 pp.
- Claus C. 1863. Die Frei Lebenden Copepoden mit besonderer berucksichtigung der Fauna Deutschlands, der Nordsee und des Mittelmeeres. Leipzig: Verlag. pp. 212–230.
- Costanzo G, Campolmi M, Zagami G. 2000. Stephos marsalensis, new species (Copepoda, Calanoida, Stephidae) from coastal waters of Sicily, Italy. Journal Plankton Research 22:2007–2014. doi:10.1093/plankt/22.10.2007
- De Olazabal A, Tirelli V. 2011. First record of the egg-carrying calanoid copepod Pseudodiaptomus marinus in the Adriatic Sea. Marine Biodiversity Records. doi:10.1017/S1755267211000935
- Dussart B. 1967. Les Copepodes des eaux continentals d’Europe occidentale. I: calanoides et Harpacticoides. Paris: Boubee & Cie. pp. 5000.
- Dussart B. 1989. Crustacés Copépodes Calanoϊdes des eaux intérieures africaines. Crustaceana, Supplement 15. Leiden: Brill. pp. 203.
- Dussart B, Defaye D. 2002. World directory of Crustacea Copepoda of inland waters. Leiden: Backhuys. pp. 276.
- Fadda A, Marková S, Kotlík P, Lugli A, Padedda P, Buscarinu P, Sechi N, Manca M. 2011. First record of planktonic crustaceans in Sardinian reservoirs. Biologia 66/5:856–865. doi:10.2478/s11756-011-0092-4
- Giesbrecht W. 1892. Sistematik und Faunistik der pelagischen Copepoden des Golfes von Neapel und der angrenzenden Meeres-Abschnitte. Fauna und Flora des Golfes von Neapel 19:831.
- Giesbrecht W. 1893. Mittheilungen über Copepoden 1-6. Mitteilungen aus der Zoologischen Station Zu Neapel 11:56–106, pls. 5–7.
- Gravili C, Belmonte G, Cecere E, Denitto F, Giangrande A, Guidetti P, Longo C, Mastrototero F, Moscatello S, Petrocelli A, Piraino S, Terlizzi A, Boero F. 2010. Non indigenous species along the Apulian coast, Italy. Chemistry and Ecology 26(supplement):121–142. doi:10.1080/02757541003627654
- Hure J, Scotto di Carlo B. 1968. Comparazione tra lo zooplankton del Golfo di Napoli e dell’Adriatico meridionale presso Dubrovnik. I. Copepoda. Pubblicazioni della Stazione Zoologica di Napoli 36:21–102.
- Kovalev AV, Shmeleva AA. 1982. Fauna of Copepoda in the Mediterranean. Ecologia Moria 8:82–87in Russian, with an English abstract.
- Marrone F, Alfonso G, Miserocchi D, Lo Brutto S. 2011. First record of Hemidiaptomus (Gigantodiaptomus) superbus (Schmeil, 1895) in Italy, with notes on distribution and conservation status (Copepoda, Calanoida, Diaptomidae). Journal of Limnology 70:149–155. doi:10.4081/jlimnol.2011.149
- Marrone F, Alfonso G, Naselli-Flores L, Stoch F. 2017. Diversity patterns and biogeography of Diaptomidae (Copepoda, Calanoida) in the Western Palearctic. Hydrobiologia 800:45–60. doi:10.1007/s10750-017-3216-1
- Marrone F, Castelli G, Barone R, Naselli-Flores L. 2006. Ecology and distribution of calanoid copepods in Sicilian inland waters (Italy). Verhandlungen International Verein Limnologie 29:2150–2156.
- Marrone F, Naselli-Flores L. 2005. First record of a representative of the subfamily Paradiaptominae (Copepoda Calanoida Diaptomidae) in Italy: Metadiaptomus chevreuxi (Guerne & Richard, 1894). Journal of Limnology 64:89–92. doi:10.4081/jlimnol.2005.89
- Mauchline J. 1998. The biology of calanoid copepods. Advances in Marine Biology 33. San Diego: Academic Press. pp. 710.
- Mazzocchi MG. 2006. Guida al riconoscimento del Plancton dei mari italiani. Vol II. Zooplankton neritico, Testo. Vol II Tavole (26-119). Roma: Ministero dell’Ambiente, ICRAM. pp. 53–152.
- Mazzocchi MG, DiCapua I. 2010. Planktonic copepods: Calanoida, Cyclopoida, Harpacticoida, Mormonilloida, Siphonostomatoida. Biologia Marina Mediterranea 17(suppl.1):420–431.
- Moroni A. 1966. I laghi di val Cedra (Appennino parmense). Bollettino Pesca Piscicoltura e Idrobiologia 21:163–266.
- Pavesi P. 1877. Intorno all’esistenza della fauna pelagica o d’alto lago anche in Italia. - Lettera del Prof. P. Pavesi dell’Università di Pavia al Dott. G. Cavanna. Bollettino Della Società Entomologica Italiana 9:293–296.
- Philippi A. 1843. Fernere Beobachtungen uber die Copepoden des Mittelmeeres. Archiv fur Naturgeschichte 9:54–71 pls. 3–4.
- Prestandrea N. 1833. Su di alcuni nuovi crustacei dei mari di Messina. Effemeridi, Scienze Letterarie per la Sicilia 6:3–14.
- Ravera O. 1953. Gli stadi di sviluppo dei copepodi pelagici del lago Maggiore. Memorie dell’Istituto Italiano di Idrobiologia 7:129–151.
- Razouls C, de Bovée F, Kouwenberg J, Desremaux N 2005-2018. Diversity and geographic distribution of marine planktonic copepods. Available: http://copepodes.obs-banyuls.fr/en. Accessed Jan 2018 09.
- Relini G. 2010. Check list della flora e della fauna dei mari italiani. Biologia Marina Mediterranea 17(suppl.1):898.
- Rose M. 1933. Copépodes Pélagiques. Faune de France 26:1–374.
- Ruffo S, Stock F. 2005. Check list e distribuzione della fauna italiana. Memorie del Museo Civico di Storia Naturale di Verona, 2° serie, Sez. Scienze Della Vita 16:1–307.
- Ruffo S, Vigna Taglianti A. 2004. An overall view of Italian wildlife. In:Argano R, Chemini C, La Posta S, Minelli A, Ruffo S, editors. Wildlife in Italy. Milano: Touring Editore. pp. 24–28.
- Sabia L, Zagami G, Mazzocchi MG, Zambianchi E, Uttieri M. 2015. Spreading factors of a globally invading coastal copepod. Mediterranean Marine Science 16:460–471. doi:10.12681/mms.1154
- Salmaso N, Naselli-Flores L. 1999. Studies on the zooplankton of the deep subalpine lake Garda. Journal of Limnology 58:66–76. doi:10.4081/jlimnol.1999.66
- Sars GO. 1902. Copepoda Calanoida, parts V, VI, Scolecithricidae, Stephidae, Tharybidae, Pseudocyclopiidae. An account of the crustacea of Norway, with short descriptions and figures of all the species. Bergen Museum 4:49–72.
- Sazhina LI. 1985. Naupliusi massovik vidiv pelagicheskik copepod mirovogo oceana. Kiev: Naukova Dumka. pp. 240.
- Scotto di Carlo B, Ianora A, Fresi E, Hure J. 1984. Vertical zonation patterns for Mediterranean copepods from the surface to 3000 m at a fixed station in the Tyrrhenian Sea. Journal of Plankton Research 6:1031–1056. doi:10.1093/plankt/6.6.1031
- Seminara M, Vagaggini D, Stoch F. 2016. A comparison of Cladocera and Copepoda as indicators of hydroperiod length in Mediterranean ponds. Hydrobiologia 782. doi:10.1007/s10750-016-2693-y
- Stella E. 1984. Crustacea. Copepoda: Calanoida (d’acqua dolce). Bologna: Calderini. pp. 101.
- Tonolli V, Tonolli L. 1951. Osservazioni sulla biologia ed ecologia di 170 popolamenti zooplanctonici di laghi italiani di alta quota. Memorie Istituto Italiano di Idrobiologia 6:53–136.
- Vives F, Shmeleva AA. 2007. Crustacea: copépodos marinos I, Calanoida. Fauna Ibérica. 29. Madrid: CSIC. pp. 1156.
- Zagami G. 2010. Copepoda Calanoida Iperbentonici. Biologia Marina Mediterranea 17(suppl. 1):432–434.
- Zagami G, Campolmi M, Costanzo G. 2000. A new species of Stephos T. Scott, 1892 (Copepoda, Calanoida) from coastal waters of Sicily. Journal Plankton Research 22:15–27. doi:10.1093/plankt/22.1.15
- Zagami G, Costanzo G, Brugnano C. 2008. Pseudocyclops giussanii (Copepoda, Calanoida, Pseudocyclopidae), a new species from lake Faro (Central Mediterranean Sea). Zoological Studies 47:605–613.
