Abstract
Several individuals belonging to a new species of the genus Chromadorita (Nematoda: Adenophorea) were collected in a cold-seep area in the Gulf of Guinea during two French cruises: BIOZÄIRE 2 (2001) and BIOZAÏRE 3 (2003–2004) on board the R/V L’Atalante. In this area, rich chemosynthetic benthic communities have been discovered at 3150 m depth in the large pockmark field named Regab. Chromadorita regabi sp. nov. was found among the eggs in ovigerous specimens of the shrimp Alvinocaris muricola. The combination of long size (1500–2200 µm), nine strong preanal papillae and relatively small dorsal tooth with weak musculature distinguishes this species from all known congeneric ones. An identification key to all known species of Chromadorita is provided.
http://zoobank.org/urn:lsid:zoobank.org:pub:68B32253-D4B4-45EB-B4F8-4F7B015E6FCC
Introduction
Nematodes are the most numerous multicellular animals on Earth (Heip et al. Citation1985) and they are also among the most abundant marine and deep-sea taxa (Lambshead et al. Citation2003). They usually comprise 70–90% of the meiobenthos from bottom sediments (Mokievsky et al. Citation2007). According to Appeltans et al. (Citation2012) there are 11,900 known free-living nematode species (12% of the total 61,400 species estimated); at present, the nematodes represent 6900 of the 11,900.
Deep-sea bottoms comprise about 91% of the seabed, but the known nematode species have been described from within a bottom area of less than 100 m2. Accordingly, deep-sea nematode diversity, ecology and distribution are greatly understudied (Miljutin et al. Citation2010), especially for nematodes associated with chemosynthetic environments (i.e. seeps and vents) (Vanreusel et al. Citation2010; Tchesunov Citation2015).
Chemosynthetic environments, such as hydrothermal vents and cold seeps, differ from adjacent deep-sea areas in terms of physical (e.g. high temperature), chemical (e.g. high sulphide concentration, low oxygen levels) and biological conditions (Van Gaever et al. Citation2009; Bezerra et al. Citation2013). Their primary metabolic energy derives from chemical processes instead of depending on settling phytodetrital matter from the euphotic zone (Van Dover et al. Citation2002). Since they were discovered in 1977 (Dinet et al. Citation1988), many studies have been conducted on their microbioma (Boetius & Suess Citation2004; Orcutt et al. Citation2008) and macro- and megafauna (Segonzac Citation1992; Tyler et al. Citation2003; Desbruyères et al. Citation2006; Sarrazin et al. Citation2015), but much less work has been done on the smaller (< 1 mm) benthic component, the meiofauna (Vanreusel et al. Citation2010; Sandulli et al. Citation2015; Tchesunov Citation2015).
Chemosynthetic habitats are inhabited by typical meiofauna characterised by a low density and diversity (Van Gaever et al. Citation2006; Vanreusel et al. Citation2009; Gollner et al. Citation2010). In chemosynthetic habitats nematodes are usually the dominant taxon, followed by copepods in vents and foraminiferans in cold seeps (Zeppilli et al. Citation2017). In the Atlantic, vent meiofauna represents up to 50% of the total faunal diversity and it is characterised by generalist nematodes and endemic copepods (Sarrazin et al. Citation2015). Studies carried out along the East Pacific Rise (e.g. Zekely et al. Citation2006; Copley et al. Citation2007; Gollner et al. Citation2010) reported low diversity values for nematodes inhabiting vent areas, and a dominance of single species or a few species belonging to the well-known genera Thalassomonhystera, Geomonhystera, Anticoma and Chromadorita. At deep-sea seeps worldwide, meiofauna biodiversity, and particularly that of nematodes, is reduced (Levin Citation2005; Van Gaever et al. Citation2009; Lampadariou et al. Citation2013). A single nematode species of Halomonhystera dominated sediments around a mud volcano area in the Arctic Ocean (Van Gaever et al. Citation2006), whereas seep sediments in the Gulf of Guinea were dominated by only two species of nematodes: Sabatiera mortenseni and Desmodora sp. (Van Gaever et al. Citation2009). Conversely, in the Mediterranean Sea seep areas, a high nematode diversity without dominance of any genera was reported (Zeppilli et al. Citation2011, Citation2012).
Apparently, nematodes of deep-sea vents and seeps have not developed any obvious adaptations, but they must tolerate sulphidic or anoxic conditions (Vanreusel et al. Citation2010; Bezerra et al. Citation2013). For instance, the species Oncholaimus campylocercoides, inhabiting vents, can produce sulphur droplets, whereas other species of the genus Oncholaimus show epibiotic association with microorganisms, and members of the genus were found in high concentrations around the most active sites at the deep-sea Lucky Strike hydrothermal vent (Mid-Atlantic Ridge, MAR) (Tchesunov Citation2015; Zeppilli et al. Citation2017). Nematodes of the genus Halomonhystera are found to be ovoviviparous, and this method of reproduction has been considered an adaptation to thrive in these extreme environments (Van Gaever et al. Citation2006; Zeppilli et al. Citation2015). Nematodes living in seep habitats seem to have developed special physical characteristics, including a longer and thinner body shape, which are favourable for life in thiobiotic conditions (Bezerra et al. Citation2013; Lampadariou et al. Citation2013). Increased body length is considered an adaptation to sulphidic conditions in that nematodes can easily cover the distance between anoxic, sulphidic and oxic, sulphide-free sediments (Soetaert et al. Citation2002; Schratzberger et al. Citation2004), whilst the expansion of the body surface facilitates the access to oxygen for respiration (Jensen Citation1987). Bezerra et al. (Citation2013) recently described a new genus with two new species, from low-activity seep areas, belonging to the family Ethmolaimidae (superfamily Chromadoroidea). Members of this family are regularly found in association with chemosynthetic ecosystems and in the thiobios; they can inhabit deep sediment layers with low oxygen and high sulphide concentrations (Shirayama & Ohta Citation1990; Zeppilli & Danovaro Citation2009).
Contrary to many macro-invertebrates from deep seeps (Dubilier et al. Citation2008), hitherto no known nematode species show evidence of symbionts. Nematodes with endo- and ectosymbiotic chemosynthetic bacteria do exist, but are mainly restricted to shallow waters (Dubilier et al. Citation2008; Vanreusel et al. Citation2010), while most seep and vent nematodes are classified as deposit feeders, based on their small buccal cavity and absence of teeth, except for chromadorids and desmodorids with teeth in their buccal cavity (Dinet et al. Citation1988; Vanreusel et al. Citation2010). Actually, predators have so far never been found to be abundant in deep-sea seeps or vents, although they are a common part of the nematode community in many other ecosystems, including shallow-water vents, such as the Oncholaimus species in Mediterranean shallow vents (Vanreusel et al. Citation2010). Despite the fact that there is no clear evidence of endosymbionts in seep nematodes, it is possible that they could benefit from free-living chemoautotrophic bacteria as a food source (Van Gaever et al. Citation2009).
Non-parasitic associations between nematodes and aquatic multicellular animals have been known since 1834, when the marine non-parasitic nematode Odontobius ceti (De Vauzème, Citation1834) was found on baleen plates (De Vauzème Citation1834), while associations with unicellular organisms (e.g. suctorian ciliates) have been seldom reported (Fernandez-Leborans et al. Citation2017).
At the beginning of the 20th century, descriptions of associations of different kinds (e.g. epibiosis, parasitism, commensalism) between meiobenthic organisms (i.e. nematodes and copepods) and larger invertebrates became evident from the literature (Petter Citation1987).
Dinet and co-authors (Citation1988) investigated the meiofauna inhabiting the hydrothermal vents of the East Pacific Rise and Explore Ridge and reported the presence of meiobenthic copepods associated with macro- and megabenthic organisms (e.g. Vestimentifera; Humes & Dojiri Citation1980). The nature of this association could be parasitic or commensal.
Some nematode species, known to be free-living species, were reported as parasites of shrimps and fishes (Overstreet Citation1973; Justin et al. Citation2002). The genus Leptolaimus, for instance, has been recognised as a facultatively commensal nematode in the gills of both brown and white penaeid shrimp from ponds. It is not clear how shrimps acquire the nematodes or how long the roundworms remain in their hosts.
Monhysterid nematodes have been identified as typically commensalistic and most often occur within the mouthparts and gills of marine and freshwater Crustacea, and in the American oyster Crassostrea virginica (Meyers et al. Citation1985; Holovachov et al. Citation2011). Lorenzen (Citation1986a) reported the presence of the free-living nematode Odontobius ceti (Monhysteridae) from the baleen plates of whales.
Within the family Chromadoridae, Chromadorida majae was found in the gill-chamber and amongst the eggs of the decapod Maja squinado inhabiting the Mediterranean Sea (Wieser Citation1968). Lorenzen (Citation1986b) reported the presence of Chromadorida ceratoserolis amongst eggs in the marsupium of the marine benthic isopod Ceratoserolis trilobitoides from Antarctic waters at depths ranging from 233 to 728 m. In total, 15 species of adenophorean nematodes are known to live epibiotically on marine, freshwater and terrestrial crustaceans all belonging to the Peracarida or Decapoda (Lorenzen Citation1986b). In a recent work, Holovachov and co-authors (Citation2011) described a new species of non-parasitic Chromadoridae living epibiotically associated with the deep-sea gastropod Skenea profunda (North East Atlantic, 2830 m depth). Similarly, Chromadorina bioculata and C. leuckarti were found associated with zebra mussel (attached to the mantel; Karatayev et al. Citation2003; Mastitsky et al. Citation2008).
The present paper describes a new species of the genus Chromadorita (Nematoda, Adenophorea) occurring among the eggs of the alvinocaridid ovigerous shrimp Alvinocaris muricola (Williams Citation1988), collected in a cold-seep area in the Gulf of Guinea. In this area, rich chemosynthetic communities have been discovered at a depth of 3150 m in a large pockmark field (Olu et al. Citation2009). This active area, named Regab, is characterised by high methane concentrations supporting a massive bacterial production (Andersen et al. Citation2004). The Regab site, where A. muricola has been observed forming high-density populations (Ramirez-Llodra & Segonzac Citation2006), is characterised by a community dominated by bivalves including large mytilids such as Bathymodiolus aff. boomerang, vesicomyid clams Laubiericoncha chuni and Christineconcha regab, and vestimentiferans, such as Escarpia southwardae (Olu-Le Roy et al. Citation2007; Marcon et al. Citation2014 and references therein). The shrimps live as epibionts over the mussel or the clam beds, or among the vestimentiferans, or even over the sediment (Komai & Segonzac Citation2005). Alvinocaris muricola (Carida, Alvinocarididae) is one of the eight shrimp species described to date from chemosynthetic communities associated with hydrothermal vents, brine or cold seeps (Komai & Segonzac Citation2005), and it is a scavenger on the mussel bed fauna (Ramirez-Llodra & Segonzac Citation2006).
The meiofaunal abundance within the Regab seep varied between 20 ind. 10 cm–2 and 873 ind. 10 cm–2 (Van Gaever et al. Citation2009). The meiobenthos was characterised by small-scale (i.e. metres) patchiness, low species richness and the dominance of Desmodora sp. and Sabatieria mortenseni, a cosmopolitan nematode indicator of anoxic to suboxic sediments. The large size, high individual body weight and dominance of these species at the cold-seep site resulted in a significantly higher nematode biomass compared to the surrounding sites (Sibuet & Vangriesheim Citation2009; Van Gaever et al. Citation2009). The increased nematode biomass and subsurface distribution maxima suggest that meiobenthic community at Regab is based on chemosynthesis.
Materials and methods
Nematode samples studied here – from nine non-gravid females, three gravid females and six males, for a total of 18 nematodes – were extracted from the eggs of several ovigerous female Alvinocaris muricola shrimps found in a cold-seep area (Regab), Gulf of Guinea, west equatorial African margin, off Angola, at 3150 m depth, 8 km north of the Congo channel (). Sampling activities were conducted during two French cruises: BIOZÄIRE 2 (November–December 2001; Sibuet Citation2001) and BIOZAÏRE 3 (December 2003–January 2004; Khripounoff Citation2003) on board the French R/V L’Atalante, by means of a slurp gun (suction apparatus) mounted on the ROV Victor 6000, and through beam trawling.
Figure 1. Location of the Regab pockmark along the Congo–Angola margin and the approximate outline of the pockmark (insert map left corner; grey rectangle represents the area explored by ROV Victor 6000). Modified from Marcon et al. (Citation2014).
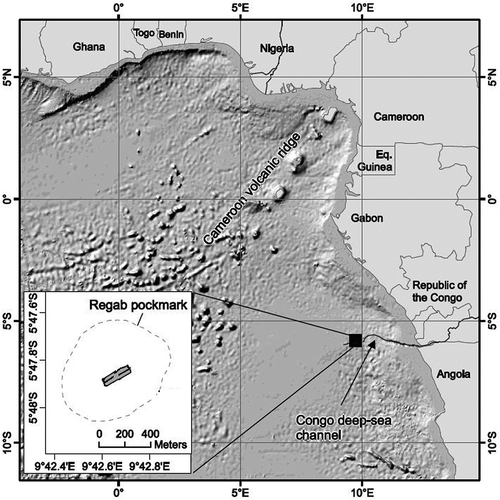
In this cold-seep area of 800 m in diameter, a 15–20 m deep pockmark is formed by the association of several individual pockmarks, resulting in a large area characterised by seepages of methane, gas hydrates and carbonate crusts, and covered with a dense chemosynthetic community (Van Gaever et al. Citation2009). Faunal assemblages are patchily distributed, dominated either by the bivalves Bathymodiolus aff. boomerang and Calyptogena sp., or by siboglinid tubeworms Escarpia southwardae (Andersen et al. Citation2004; Marcon et al. Citation2014).
Specimens of the decapod Alvinocaris muricola, collected by a slurp gun (BIOZAÏRE 2, PL 146–09, slurp gun 1, Regab site, 05°47.80’S, 09°42.60ʹE, −3151 m) and by a beam trawl (BIOZAÏRE 3, beam trawl CP 20, 4 km south-west of Regab site, 05°46.89’S, 09°44.66ʹW, −3113 m) were fixed on board in formalin and preserved in ethanol. From the broods of three ovigerous A. muricola females (BIOZAÏRE 2) a total of 15 nematodes were extracted, while three more nematodes, one of which was found with the anterior part in an egg, were extracted from one ovigerous females (BIOZAÏRE 3).
Nematodes were mounted on glass slides and identified with a Leica DLMS compound microscope (1000× magnification), using a pictorial key to nematode genera (Warwick et al. Citation1998) and the NeMys database (http://www.marinespecies.org). Species description was made from glycerol slides using interferential contrast microscopy, and drawings were made with a camera lucida. Images of the specimens were taken with an Axio Apotome-digital camera and processed with ZEN imaging software. All measurements are in µm and all curved structures were measured along the arc. Slides with the type specimens of the new species are deposited in the collection of the Muséum national d’Histoire naturelle, Paris (MNHN).
Abbreviations used:
| a, b, c | = | ratios of de Man (Citation1880); |
| a | = | body length divided by maximum body diameter; |
| b | = | body length divided by pharyngeal length; |
| c | = | body length divided by tail length; |
| V% | = | ratio [distance from anterior end to vulva]/body length, in %; |
| cbd | = | corresponding body diameter; |
| mbd | = | maximum body diameter; |
| L | = | length; |
| W | = | width; |
| H | = | height; |
| Supplements | = | preanal papillae. |
Systematics
Phylum Nematoda
Class Adenophorea
Subclass Chromadoria Pearse, 1942
Order Chromadorida Chitwood, 1933
Suborder Chromadorina Filipjev, Citation1929
Superfamily Chromadoroidea Filipjev, Citation1917
Family Chomadoridae Filipjev, Citation1917
Subfamily Hypodontolaimina de Coninck, Citation1965
Genus Chromadorita Filipjev, Citation1922
Chromadorita regabi sp. nov.
(–)
Figure 2. (a) Chromadorita regabi sp. nov. Drawings of the male (left) habitus and female (right) habitus. (b) Chromadorita regabi sp. nov. Drawings of the male anterior part (left), the copulatory apparatus (centre) and the nine preanal supplements (right). Scale bars: A = 100 μm; B = 100 μm, 20 μm and 10 μm (from left to right).
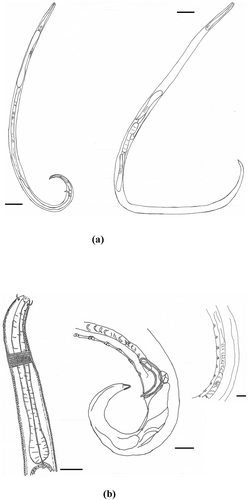
Figure 3. Chromadorita regabi sp. nov. Light micrographs of (a) male head; (b) female head; (c) posterior region of male; (d) cuticular ornamentation in the middle of the body region of the male; and (e) posterior region of female. Scale bars: 100 µm.
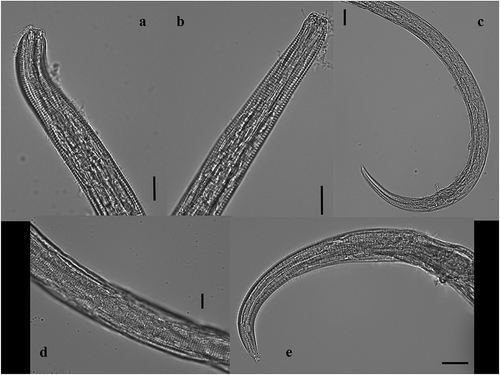
Figure 4. Chromadorita regabi sp. nov. Light micrographs of (a) the nine cup-shaped preanal papillae from the male (red arrows indicating the 1st and the 9th papillae); (b) spicule and apophysis; (c) reflexed ovaries, vulva and eggs from the female; and (d) detail of the ovary. Scale bars: A, C, D = 100 µm; B = 50 µm.
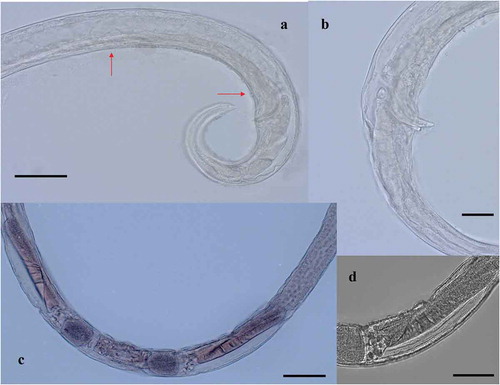
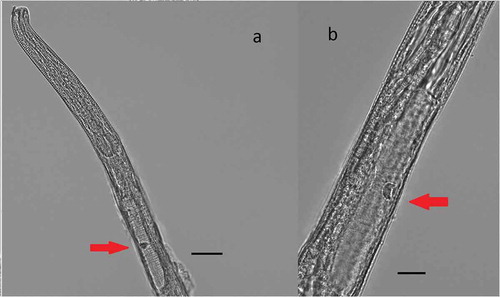
Figure 5. Chromadorita regabi sp. nov. Light micrographs of the female. (a) Anterior region showing pharynx with posterior bulbus and ventral gland (red arrow). (b) Detail of the ventral gland (red arrow) behind the pharynx, on the ventral side of the gut. Scale bars: A = 20 µm; B = 50 µm.
Type species
Chromadorita demaniana Filipjev, Citation1922. The genus Chromadorita presents a homogeneous cuticle ornamentation. A slightly more pronounced punctuation may be present at the level of the lateral field. One dorsal hollow tooth and one or two ventrosublateral teeth present; rarely one indistinct dorsal tooth only. Tiny denticles may be also present. Pharynx may be swollen anteriorly; single posterior bulb. Precloacal supplements may be present or absent.
Type material
Collection number MNHN-BN507 (PL 146–09): three males – one as holotype, formalin fixed, mounted on slide in glycerin and collected from the eggs of ovigerous female shrimps Alvinocaris muricola collected at Regab cold seep (Gulf of Guinea, west equatorial African margin). Two males – paratypes; two gravid females – paratypes; five non-gravid females – paratypes. Collection number MNHN-BN508 (PL 147–10): one gravid female – paratype; collection number MNHN-BN509 (Bioz 2): two males – paratypes; one gravid female – paratype; two non-gravid females – paratypes. Collection number MNHN-BN510 (Bioz 3): one non-gravid female – paratype; one male – paratype. The paratypes are from the same site as the holotype, from different eggs of ovigerous female shrimps Alvinocaris muricola.
Etymology
The species name refers to the locality of collection, Regab cold seep.
Measurements
See .
Table I. Morphometrics of Chromadorita regabi sp. nov. Data presented as minimum–maximum values. All measurements in µm. HT: holotype; -: non-gravid females; +: gravid females.
Taxonomic accounts
Male
Long nematode, 1822 µm in length, and 37 µm in width. Cuticle homogeneous over total body length, with transverse rows of rod-like punctuations. No lateral differentiations. No somatic setae. Head tapering towards anterior end. Four 8 µm long cephalic setae. No amphid visible. Small buccal cavity with one dorsal hollow tooth and two smaller subventral teeth. Pharynx with posterior bulbus. No strong musculature around dorsal tooth. Ventral gland reaching behind the pharynx, on the ventral side of the gut. One testis present, at the right side of the gut. Two spicules, 62 µm long, strongly curved and cuticularised. Simple needle-like apophysis. Nine strong cup-shaped preanal papillae present, first one at 24 µm from anus, others separated from each other by 9 to 12 µm. Each papilla measures 5 µm at its base and is 3 µm high.
Female
Body length can exceed that of the male (maximum body length 2370 µm). Two reflexed ovaries; the anterior one right and the posterior one left of the gut. Vulva about 8 µm long and weakly cuticularised.
This species is assigned to the genus Chromadorita because of the homogeneous cuticle, without lateral differentiation, and the larger hollow dorsal tooth. All other species belonging to this genus and described so far are smaller in size or have a different number of preanal papillae. Only C. ceratoserolis described from the marsupium of the Antarctic isopod Ceratosolis trilobitoides is larger in total body length than the newly described species. All other Chromadorita species are much smaller. Chromadorita leuckartii, C. mucrodonta and C. tenuis exceed 1000 µm in body length (to a maximum 1460 µm for C. tenuis) but they all have a different number of preanal papillae. The combination of its long size, the presence of nine strong preanal papillae, and the relatively small dorsal tooth with weak musculature helps distinguish this species from all the others.
C. hyalocephala presents between eight and 10 preanal papillae; C. brachypharynx and schuurmanstekhoveni are characterised by the presence of nine preanal papillae. Nevertheless, all these genera are < 1100 µm in body length and the preanal papillae are small.
Conclusive remarks
Several “external” nematodes (so-called epibionts) have been reported on crustaceans. These nematodes can be considered free-living epibionts with limited impact on host survival (Longshaw Citation2011). Lorenzen (Citation1986b) gives an overview of nematode species found in association with crustaceans. These epibionts are mainly associated with gill chambers of Decapoda. He refers to 10 different species of different taxonomic origin, belonging to the families Chromadoridae, Monhysteridae and Leptolaimidae. Some species (four) are associated with Amphipoda, Isopoda and even Mysidacea, but the association is only specified in one case.
The species Chromodorita ceratoserolis was found between eggs, specifically in the marsupium of the isopod Ceratoserolis trilobitoides (Lorenzen Citation1986b). This species not only exhibits the same kind of association with a crustacean as C. regabi sp. nov., but also represents the most closely related species, belonging to the same genus. Two other Chromadoridae species, Chromadorina astacicola and Chromadorina maja respectively, were found in the gill chambers of decapods, although C. maja has also been recorded from the marsupium (Lorenzen Citation1986b). The free-living nematode Chromadorina bioculata was reported in high abundances as an endosymbiont of zebra mussel from fresh waters (Karatayev et al. Citation2003; Mastitsky et al. Citation2008). Nevertheless, this association appeared to be non-obligate for the nematode and it was suggested that C. bioculata uses the bivalve as shelter and for food (Karatayev et al. Citation2003). Holovachov et al. (Citation2011) reported a similar association between the free-living nematode Endeolophos skeneae (Chromadoridae) and the deep-sea gastropod Skenea profunda. As for C. bioculata, the authors suggested that E. skeneae finds food and shelter from the gastropod, but this association remains a non-obligate one for the nematode.
Apart from studies mentioned above, the most common and documented associations of commensalism between free-living nematodes and aquatic and semi-aquatic multicellular animals involved the marine Monhysterida. Amongst studies conducted in chemosynthetic environments all over the world (e.g. Van Gaever et al. Citation2009; Hauquier et al. Citation2011; Pape et al. Citation2011; Setoguchi et al. Citation2014; Tchesunov Citation2015), the presence of chromadorids has been rarely or not reported. Instead, monhysterids are found to be deep-sea generalists known to dominate extreme environments such as hydrothermal vents and seeps, and Halomonhystera is a cosmopolitan genus that has been recovered from various marine sediments, including sulphidic sediments (Hauquier et al. Citation2011).
In a very few studies, one conducted along vents in the East Pacific Rise (Copley et al. Citation2007), one in a deep-sea cold seep in Japan (Shirayama & Ohta Citation1990) and one in a North Sea pockmark area (Dando et al. Citation1991), specimens of the genus Chromadorita were reported to be quite abundant (as the second or third most abundant group) in the sediments. Zekely et al. (Citation2006) reported the presence of two different, but not identified, species of Chromadorita from MAR and East Pacific Rise vent areas.
Considering other epibiotic worms on crustaceans, nemerteans were reported as obligate symbionts on decapod crustaceans, needing a host during almost their whole life cycle (Kuris & Wickham Citation1987). Similarly, Shields and Segonzac (Citation2007) reported symbiotic nemertean worms infesting several species of crabs from deep-sea hydrothermal vents in the Pacific Ocean. Juveniles and regressed adults of nemerteans were exclusively reported, leading to the hypothesis that these worms can develop only by eating eggs of their hosts (Shields & Segonzac Citation2007). A new species of Polychaeta from shallow waters of Indo-West Pacific, Polydora robi, was found by Williams (Citation2000) to be an active predator of host hermit crab embryos and newly fertilised eggs, and to cause negative effects on the hosts (Williams Citation2001, Citation2002).
The fact that no juveniles of C. regabi were reported from our samples leads us to suppose that the relationship between C. regabi and its host is not obligate for the developing of the nematode.
Lorenzen (Citation1986b) maintained that no information was available on the food source of C. ceratoserolis. During sample collection, one specimen of C. regabi was found with the anterior part of its body inside an embryo of a female A. muricola, suggesting a predatory action (see also Komai & Segonzac Citation2005; Ramirez-Llodra & Segonzac Citation2006). The authors hypothesise that the presence of dark embryos, showing disrupted development compared to normal embryos, was due to the presence of the nematodes. The ability of C. regabi to pierce and suck the egg content is in agreement with the feeding behaviour of chromadorids in which they pierce and suck out the contents of hard-shelled cells (Vanreusel et al. Citation2010).
Kuris and Wickham (Citation1987) conducted a study on the nemertean predation effect on crustacean eggs. The authors showed that nemerteans punctured the egg’s membrane, sucking the contents out and causing high egg mortality at different stages of development for several commercial crustacean species. Nematodes, together with flatworms, protozoans and some small polychaetes, were found in old polychaete egg masses (up to 10 days after deposition; Martin et al. Citation2000) feeding on the fertilised eggs. We hypothesise that C. regabi may predate on the shrimp eggs during its life cycle as free-living nematode. The eggs of A. muricola may represent one of the food sources “easily” accessible for the nematodes due to the high density of the shrimp at Regab site (Ramirez-Llodra & Segonzac Citation2006). We should also consider that the large caloric content of eggs and embryos indicates that the benefit of this predation is considerable for the nematode. However, a possible negative effect exerted by the nematode on the fecundity of the shrimp may be hypothesised, as previously documented for other epibiotic worms on crustacean eggs (Kuris & Wickham Citation1987; Williams Citation2001, Citation2002), but further studies are needed to address this issue.
Identification key to all known species of Chromadorita
The present identification key is based on 30 valid species found in the literature including the present new species C. regabi. The two species C. arctica and C. inornata, although reported as valid species (http://www.marinespecies.org), are not included in this key since their original description is not documented.
1 Body length 350–1500 µm 4
2 Body length 1500–2200 µm 5
3 Body length > 2200 µm C. ceratoserolis
4.1 Dorsal tooth absent (only ventral teeth) 6
4.2 Small dorsal tooth (weak musculature) 7
4.3 Large hollow dorsal tooth 8
4.4 Large S-shaped dorsal tooth 9
5.1 Small dorsal tooth C. regabi sp. nov.
5.2 Large S-shaped dorsal tooth C. deseadensis
6.1 Cuticle homogeneous with transverse rows of punctuations… C. mucrodonta
6.2 Annulations with dots 10
7.1 Cuticle with dots that become jointed rods 11
7.2 Annulations with dots 12
8.1 Cuticle homogeneous with transverse rows of punctuations 13
8.2 Annulations with dots 14
8.3 Cuticle with dots that become jointed rods 15
8.4 Lateral dots finer than dots in the middle of the body 16
9.1 Cuticle homogeneous with transverse rows of punctuations 17
9.2 Annulations with dots C. tentabundum
10.1 Preanal papillae N = 10 C. hyalocephala
10.2* Preanal papillae absent C. heterophya*
11.1 Preanal papillae absent 18
12.1 Preanal papillae N = 8 C. leuckarti
12.2 Preanal papillae absent C. demaniana
12.2* Preanal papillae absent C. leptopharynx*
13.1 Preanal papillae N = 7–8 C. pachydema
13.2 Preanal papillae absent 19
13.3 Preanal papillae N = 2 C. dimeris
13.4 Preanal papillae N = 5 C. pentameris
14.1 Preanal papillae absent20
14.1* Preanal papillae absent C. paetzoldi*
14.2 Preanal papillae N = 9C. brachypharynx
14.3 Preanal papillae N = 7C. fennica
15.1 Preanal papillae N = 1–5 C. abnormis
15.2 Preanal papillae N = 9 C. schuurmanstekhoveni
16.1 Preanal papillae N = 8 C. guidoschneideri
16.2 Preanal papillae N = 10–13 C. tenuis
17.1 Preanal papillae absent 21
18.1 Amphid absentC. brevisetosa
18.2 Amphid large, kidney shapedC. nephramphidia
19.1 Amphid slit-like and making a spiralC. abissalys
19.2 Amphid large (60% of the head diameter) and oval C. mucrocaudata
19.3 Amphid small and oval C. nana
19.4 Amphid reduced C. macrodonta
20.1 Amphid absent C. minima
20.2 Amphid kidney shaped C. pallida
21.1 Amphid loop shaped 22
21.2 Amphid slit-like C. obliqua
22.1 Three large glands at the tail level C. minor
22.2 Presence of ventromedial papilae, arrowhead shaped C. pharetra
* Original descriptions based only on females.
Acknowledgements
We are indebted to the Chief Scientists of the cruises that allowed the sampling of the specimens used in this study – M. Sibuet and A. Khripounoff, Ifremer, Brest (BIOZAÏRE 2 & 3 Cruises) – as well as to the crew of the R/V L’Atalante and the team of the submarine ROV Victor 6000. Both cruises were arranged thanks to the multidisciplinary programme BIOZAÏRE, a partnership between Ifremer and Total Oil Company. The authors would also like to thank K. Olu for her useful suggestions on the manuscript. EB and DZ were partially supported by the project “Prokaryote–nematode Interaction in marine extreme envirONments: a uniquE source for ExploRation of innovative biomedical applications” (PIONEER) funded by the Total Foundation and Ifremer.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Andersen AC, Hourdez S, Marie B, Jollivet D, Lallier FH, Sibuet M. 2004. Escarpia southwardae sp. nov., a new species of vestimentiferan tubworm (Annelida, Siboglinidae) from West African cold seeps. Canadian Journal of Zoology 82:980–999. DOI:10.1139/z04-049.
- Appeltans W, Ahyong ST, Anderson G, Angel MV, Artois T, Bailly N, Bamber R, Barber A, Bartsch I, Berta A, Błażewicz-Paszkowycz M, Bock P, Boxshall G, Boyko C, Brandão S, Bray R, Bruce N, Cairns S, Chan T-Y, Cheng L, Collins A, Cribb T, Curini-Galletti M, Dahdouh-Guebas F, Davie PF, Dawson M, De Clerck O, Decock W, De Grave S, de Voogd N, Domning D, Emig C, Erséus C, Eschmeyer W, Fauchald K, Fautin D, Feist S, Fransen CJM, Furuya H, Garcia-Alvarez O, Gerken S, Gibson D, Gittenberger A, Gofas S, Gómez-Daglio L, Gordon D, Guiry M, Hernandez F, Hoeksema B, Hopcroft R, Jaume D, Kirk P, Koedam N, Koenemann S, Kolb J, Kristensen R, Kroh A, Lambert G, Lazarus D, Lemaitre R, Longshaw M, Lowry J, Macpherson E, Madin L, Mah C, Mapstone G, McLaughlin P, Mees J, Meland K, Messing C, Mills C, Molodtsova T, Mooi R, Neuhaus B, Ng PL, Nielsen C, Norenburg J, Opresko D, Osawa M, Paulay G, Perrin W, Pilger J, Poore GB, Pugh P, Read G, Reimer J, Rius M, Rocha R, Saiz-Salinas J, Scarabino V, Schierwater B, Schmidt-Rhaesa A, Schnabel K, Schotte M, Schuchert P, Schwabe E, Segers H, Self-Sullivan C, Shenkar N, Siegel V, Sterrer W, Stöhr S, Swalla B, Tasker M, Thuesen E, Timm T, Todaro M, Turon X, Tyler S, Uetz P, van der Land J, Vanhoorne B, van Ofwegen L, van Soest RM, Vanaverbeke J, Walker-Smith G, Walter T, Warren A, Williams G, Wilson S, Costello M. 2012. The magnitude of global marine species diversity. Current Biology 22:2189–2202. DOI:10.1016/j.cub.2012.09.036.
- Bezerra TN, Pape E, Hauquier F, Vanreusel A, Jeroen I. 2013. New genus and two new species of the family Ethmolaimidae (Nematoda: Chromadorida), found in two different cold-seep environments. Zootaxa 3692:007–027. DOI:10.11646/zootaxa.3692.1.4.
- Boetius A, Suess E. 2004. Hydrate ridge: A natural laboratory for the study of microbial life fueled by methane from near-surface gas hydrates. Chemical Geology 205:291–310. DOI:10.1016/j.chemgeo.2003.12.034.
- Copley JTP, Flint HC, Ferrero TJ, Van Dover CL. 2007. Diversity of meiofauna and free-living nematodes in hydrothermal vent mussel beds on the northern and southern East Pacific Rise. Journal of the Marine Biological Association of UK 87:1141–1152. DOI:10.1017/S0025315407055956.
- Dando PR, Austen MC, Burke Jr RA, Kendall MA, Kennicutt II MC, Judd AG, Moore DC, O’Hara SCM, Schmaljohan R, Southward AJ. 1991. Ecology of a North Sea pockmark with an active methane seep. Marine Ecology Progress Series 70:49–63. DOI:10.3354/meps070049.
- De Coninck LA. 1965. Hypodontolaiminae. In: Guilini K, Bezerra TN, Eisendle-Flöckner U, Deprez T, Fonseca G, Holovachov O, Leduc D, Miljutin D, Moens T, Sharma J, Smol N, Tchesunov A, Mokievsky V, Vanaverbeke J, Vanreusel A, Venekey V, Vincx M, editor, 2017. NeMys: world Database of Free-Living Marine Nematodes. Available: World Register of Marine Species at http://www.marinespecies.org. Accessed Dec 2017 8.
- De Man JG. 1880. Die einheimischen, frei in der reinen Erde und im siissen Wasser lebenden Nematoden. Tijdschrift Nederlandsche Dierkundige Vereeniging Tijdschr 5:1–104.
- De Vauzème R. 1834. Note sur l’Odontobius ceti de l’ordre des vers intestinaux cavitaires. Annales des Sciences Naturelles 2:326–331.
- Desbruyères D, Segonzac M, Bright M. 2006. Handbook of deep - sea hydrothermal vent fauna. 2nd ed. Linz: Denisia. p. 544.
- Dinet A, Grassle F, Tunnicliffe V. 1988. Premières observations sur la méiofaune des sites hydrothermaux de la dorsale Est-Pacifique (Guaymas, 21°N) et de l’Explorer Ridge. Proceedings of the Hydrothermalism, Biology and Ecology Symposium, Paris, 4-7 Novembre 1985. Oceanologica Acta 85:7–14.
- Dubilier N, Bergin C, Lott C. 2008. Symbiotic diversity in marine animals: The art of harnessing chemosynthesis. Nature Reviews Microbiology 6:725–740. DOI:10.1038/nrmicro1992.
- Fernandez-Leborans G, Román S, Martin D. 2017. A new deep-sea suctorian-nematode epibiosis (Loricophrya-Tricoma) from the Blanes Submarine Canyon (NW Mediterranean). Microbial Ecology 74:15–21. DOI:10.1007/s00248-016-0923-5.
- Filipjev I. 1917. Un nématode libre nouveau de la mer Caspienne, Chromadorissa gen. nov. (Chromadoridae, Chromadorini). Zoologichesky Zhurnal 2:24–30.
- Filipjev I. 1929. Classification of freeliving nematoda and relations to parasitic forms. The Journal of Parasitology 15:281–282.
- Filipjev IN. 1922. Encore sur les Nématodes libres de la mer Noire. Trudy Stavropol’skogo Naučno-Issledovatel’skogo Instituta Sel’skogo Chozjajstva 1:83–184.
- Gollner S, Riemer B, Martínez Arbizu P, Le Bris N, Bright M. 2010. Diversity of meiofauna from the 9°50′N East Pacific Rise across a gradient of hydrothermal fluid emissions. PLoS One 5:e12321. DOI:10.1371/journal.pone.0012321.
- Hauquier F, Ingels J, Gutt J, Raes M, Vanreusel A. 2011. Characterization of the nematode community of a low-activity cold seep in the recently ice-shelf free Larsen B area, Eastern Antarctic Peninsula. PLoS One 6:e22240. DOI:10.1371/journal.pone.0022240.
- Heip C, Vincx M, Vranken G. 1985. The ecology of marine nematodes. Oceanography Marine Biology Annual Review 23:399–489.
- Holovachov O, Boström S, Reid N, Warén A, Schander C. 2011. Endeolophos skeneae sp. nov. (Chromadoridae)—A free-living marine nematode epibiotically associated with deep-sea gastropod Skenea profunda (Skeneidae). Journal of the Marine Biological Association of the United Kingdom 91:387–394. DOI:10.1017/S0025315410001669.
- Humes AG, Dojiri M. 1980. A new Siphonostome family (Copepoda) associated with a vestimentiferan in deep water off California. Pacific Science 34:143–151.
- Jensen P. 1987. Differences in microhabitat, abundance, biomass and body size between oxybiotic and thiobiotic free-living marine nematodes. Oecologia 71:564–567. DOI:10.1007/BF00379298.
- Justin JL, Cassone J, Petter A. 2002. Moravecnema segonzaci gen. et sp. n. (Nematoda: Cystidicolidae) from Pachycara thermophilum (Zoarcidae), a deep-sea hydrothermal vent fish from the Mid-Atlantic Ridge. Folia Parasitologica 49:299–303. DOI:10.14411/fp.2002.055.
- Karatayev AY, Mastitsky SE, Burlakova LE, Molloy DP, Vezhnovets GG. 2003. Seasonal dynamics of endosymbiotic ciliates and nematodes in Dreissena polymorpha. Journal of Invertebrate Pathology 83:73–82. DOI:10.1016/S0022-2011(03)00043-0.
- Khripounoff A. 2003. BIOZAIRE 3 cruise, RV L’Atalante. Available: https://doi.org/10.17600/3010120. Accessed Nov 2017 10.
- Komai T, Segonzac M. 2005. A revision of the genus Alvinocaris Williams and Chace (Crustacea: Decapoda: Caridea: Alvinocarididae), with descriptions of a new genus and a new species of Alvinocaris. Journal of Natural History 39:1111–1175. DOI:10.1080/00222930400002499.
- Kuris AM, Wickham DE. 1987. Effect of nemertean egg predators on crustaceans. Bulletin of Marine Science 41:151–164.
- Lambshead PJD, Brown BJ, Ferrero T, Hawkins LE, Smith CR, Mitchell NJ. 2003. Biodiversity of nematode assemblages from the region of the Clarion-Clipperton Fracture Zone, an area of commercial mining interest. BMC Ecology 3. DOI:10.1186/1472-6785-3-1.
- Lampadariou N, Kalogeropoulou V, Sevastou K, Keklikoglou K, Sarrazin J. 2013. Influence of chemosynthetic ecosystems on nematode community structure and biomass in the deep eastern Mediterranean Sea. Biogeosciences 10:5381–5398. DOI:10.5194/bg-10-5381-2013.
- Levin LA. 2005. Ecology of cold seep sediments: Interactions of fauna with flow, chemistry and microbes. In: Gibson RN, Atkinson RJA, Gordon JDM, editors, Oceanography and marine biology—An annual review. Vol. 43. Boca Raton: Crc Press-Taylor & Francis Group. pp. 1–46.
- Longshaw M. 2011. Diseases of crayfish: A review. Journal of Invertebrate Pathology 106:54–70. DOI:10.1016/j.jip.2010.12.011.
- Lorenzen S. 1986a. Odontobius (Nematoda, Monhysteridae) from the baleen plates of whales and its relationship to Gammarinema living on crustaceans. Zoologica Scripta 15:101–106. DOI:10.1111/zsc.1986.15.issue-2.
- Lorenzen S. 1986b. Chromadorita ceratoserolis sp. n. (Chromadoridae), a free-living marine nematode epibiotically on the Isopod Ceratosolis trilobitoides from Antarctica. Polar Biology 6:247–250. DOI:10.1007/BF00443403.
- Marcon Y, Saling H, Allais AG, Bohrmann G, Olu K. 2014. Distribution and temporal variation of mega-fauna at the Regab pockmark (Northern Congo Fan), based on a comparison of videomosaics and geographic information systems analyses. Marine Ecology 35:77–95. DOI:10.1111/maec.12056.
- Martin D, Le Nourichel C, Uriz MJ, Bhaud M, Duchêne JC. 2000. Ontogenic shifts in chemical defenses of the northwest Mediterranean Sea Eupolymnia nebulosa (Polychaeta, Terebellidae). Bulletin of Marine Science 67:287–298.
- Mastitsky SE, Lucy F, Gagarin VG. 2008. First report of endosymbionts in Dreissena polymorpha from Sweden. Aquatic Invasions 3:83–86. DOI:10.3391/ai.
- Meyers TR, Elston RA, Georgi ME. 1985. A monhysterid nematode parasitizing captive American oysters (Crassostrea virginica). Journal of Invertebrate Pathology 46:205–208. DOI:10.1016/0022-2011(85)90152-1.
- Miljutin DM, Gad G, Miljutina MM, Mokievsky VO, Fonseca-Genevois V, Esteves AM. 2010. The state of knowledge on deep-sea nematode taxonomy: How many valid species are known down there? Marine Biodiversity 40:143–159. DOI:10.1007/s12526-010-0041-4.
- Mokievsky VO, Udalov AA, Azovsky AI. 2007. Quantitative distribution of meiobenthos in deep-water zones of the World Ocean. Oceanology 47:797–813. DOI:10.1134/S0001437007060057.
- Olu K, Caprais JC, Galéron J, Causse R, Von Cosel R, Budzinski H, Le Ménach K, Le Roux C, Levaché D, Khripounoff A, Sibuet M. 2009. Influence of seep emission on the non-symbiont-bearing fauna and vagrant species at an active giant pockmark in the Gulf of Guinea (Congo–Angola margin). Deep Sea Research Part II: Topical Studies in Oceanography 56:2380–2393. DOI:10.1016/j.dsr2.2009.04.017.
- Olu-Le Roy K, von Cosel R, Hourdez S, Carney SL, Jollivet D. 2007. Amphi-Atlantic cold-seep Bathymodiolus species complexes across the equatorial belt. Deep-sea Research I 54:1890–1911. DOI:10.1016/j.dsr.2007.07.004.
- Orcutt B, Samarkin V, Boetius A, Joye S. 2008. On the relationship between methane production and oxidation by anaerobic methanotrophic communities from cold seeps of the Gulf of Mexico. Environmental Microbiology 10:1108–1117. DOI:10.1111/j.1462-2920.2007.01526.x.
- Overstreet RM. 1973. Parasites of some penaeid shrimps with emphasis on reared hosts. Aquaculture 2:105–140. DOI:10.1016/0044-8486(73)90140-3.
- Pape E, Bezerra TN, Vanneste H, Heeschen K, Moodley L, Leroux F, van Breugel P, Vanreusel A. 2011. Community structure and feeding preference of nematodes associated with methane seepage at the Darwin mud volcano (Gulf of Cádiz). Marine Ecology Progress Series 438:71–83. DOI:10.3354/meps09278.
- Petter AJ. 1987. Quelques nouvelles espèces de femelles du genre Benthimermis Petter, 1980 (Benthimermithidae: Nematoda) des grans fonds de la mer de Norvège. Bulletin du Muséum National d’Histoire Naturelle 4e sér 9, section A(3):565–578.
- Ramirez-Llodra E, Segonzac M. 2006. Reproductive biology of Alvinocaris muricola (Decapoda: Caridea: Alvinocarididae) from cold seeps in the Congo Basin. Journal of Marine Biological Association of the United Kingdom 86:1347–1356. DOI:10.1017/S0025315406014378.
- Sandulli R, Miljutin D, Angeletti L, Taviani M. 2015. Meiobenthos and nematode assemblages from different deep-sea habitats of the Strait of Sicily (Central Mediterranean Sea). Mediterranean Marine Science 16:402–412. DOI:10.12681/mms.1145.
- Sarrazin J, Legendre P, De Busserolles F, Fabri MC, Guilini K, Ivanenko V, Morineaux M, Vanreusel A, Sarradin PM. 2015. Biodiversity patterns, environmental drivers and indicator species on a high-temperature hydrothermal edifice, Mid-Atlantic ridge. Deep Sea Research part II: Topical Studies in Oceanography 121:177–192. DOI:10.1016/j.dsr2.2015.04.013.
- Schratzberger M, Bolam SG, Whomersley P, Warr K, Rees HL. 2004. Development of a meiobenthic nematode community following the intertidal placement of various types of sediment. Journal of Experimental Marine Biology and Ecology 303:79–96. DOI:10.1016/j.jembe.2003.11.003.
- Segonzac M. 1992. Les peuplements associés à l’hydrothermalismeocéanique du Snake Pit (dorsale médioatlantique; 23°N, 3480 m): Composition et microdistribution de la mégafaune. Comptes Rendus de l’Académie des Sciences Paris 314:593–600.
- Setoguchi Y, Nomaki H, Kitahashi T, Watanabe H, Inoue K, Ogawa NO, Shimanaga M. 2014. Nematode community composition in hydrothermal vent and adjacent non‑vent fields around Myojin Knoll, a seamount on the Izu‑Ogasawara Arc in the western North Pacific Ocean. Marine Biology 161:1775–1785. DOI:10.1007/s00227-014-2460-4.
- Shields JD, Segonzac M. 2007. New nemertean worms (Carcinonemertidae) on Bythograeid crabs (Decapoda: Brachyura) from Pacific hydrothermal vent sites. Journal of Crustacean Biology 27:681–692. DOI:10.1651/S-2794.1.
- Shirayama Y, Ohta S. 1990. Meiofauna in a cold-seep community off Hatsushima, Central Japan. Journal of Oceanography 46:118–124.
- Sibuet M. 2001 . BIOZAIRE 2 cruise, RV L’Atalante. Available: https://doi.org/10.17600/1010130. Accessed Jul 2018 6.
- Sibuet M, Vangriesheim A. 2009. Deep-sea environment and biodiversity of the West African Equatorial margin. Deep Sea Research part II: Topical Studies in Oceanography 56:2156–2168. DOI:10.1016/j.dsr2.2009.04.015.
- Soetaert K, Muthumbi A, Heip C. 2002. Size and shape of ocean margin nematodes: Morphological diversity and depth related patterns. Marine Ecology Progress Series 242:179–193. DOI:10.3354/meps242179.
- Tchesunov AV. 2015. Free-living nematode species (Nematoda) dwelling in hydrothermal sites of the North Mid-Atlantic Ridge. Helgoland Marine Research. DOI:10.1007/s10152-015-0443-6.
- Tyler PA, German CR, Ramirez-Llodra E, Van Dover C. 2003. Understanding the biogeography of chemosynthetic ecosystems. Oceanologica Acta 25:227–241. DOI:10.1016/S0399-1784(02)01202-1.
- Van Dover CL, German CR, Speer KG, Parson LM, Vrijenhoek RC. 2002. Marine biology - Evolution and biogeography of deep-sea vent and seep invertebrates. Science 295:1253–1257. DOI:10.1126/science.1067361.
- Van Gaever S, Galeron J, Sibuet M, Vanreusel A. 2009. Deep-sea habitat heterogeneity influence on meiofaunal communities in the Gulf of Guinea. Deep-Sea Research Part II-Topical Studies in Oceanography 56:2259–2269. DOI:10.1016/j.dsr2.2009.04.008.
- Van Gaever S, Moodley L, de Beer D, Vanreusel A. 2006. Meiobenthos at the Arctic Håkon Mosby Mud Volcano, with a parental-caring nematode thriving in sulphide-rich sediments. Marine Ecology Progress Series 321:143–155. DOI:10.3354/meps321143.
- Vanreusel A, Andersen AC, Boetius A, Connelly D, Cunha MR, Decker C, Hilario A, Kormas KA, Maignien L, Olu K, Pachiadaki M, Ritt B, Rodrigues C, Sarrazin J, Tyler P, Van Gaever S, Vanneste H. 2009. Biodiversity of cold seep ecosystems along the European margins. Oceanography 22:110–127. DOI:10.5670/oceanog.2009.12.
- Vanreusel A, De Groote A, Gollner S, Bright M. 2010. Ecology and biogeography of free-living nematodes associated with chemosynthetic environments in the deep sea: A review. PLoS ONE 5:e12449. DOI:10.1371/journal.pone.0012449.
- Warwick RM, Platt HM, Somerfield PJ. 1998. Free-living marine nematodes part III Monhysterids: pictorial key to world genera and notes for the identification of British species. Synopses of the British fauna (New Series) 53. Shrewsbury, UK: Field Studies Council. 296 pp.
- Wieser W. 1968. Chromadorida astacicola (Shneider, 1932) and Chromadorida majae n. sp., Zwei mit Decapoden vergesellschaftete Nematoden. Thalassia Jugoslavia 4:39–43.
- Williams AB. 1988. New marine decapod crustaceans from waters influenced by hydrothermal, discharge, brine and hydrocarbon seepage. Fishery Bulletin 86:263–287.
- Williams JD. 2000. A new species of Polydora (Polychaeta: Spionidae) from the Indo-West Pacific and first record of host hermit crab egg predation by a commensal polydorid worm. Zoological Journal of the Linnean Society 12:537–548. DOI:10.1111/j.1096-3642.2000.tb00616.x.
- Williams JD. 2001. Polydora and related genera associated with hermit crabs from the Indo-West Pacific (Polychaeta: Spionidae), with descriptions of two new species and a second Polydorid egg predator of Hermit crabs. Pacific Science 4:429–465. DOI:10.1353/psc.2001.0037.
- Williams JD. 2002. The ecology and feeding biology of two Polydora species (Polychaeta: Spionidae) found to ingest the embryos of host hermit crabs (Anomura: Decapoda) from the Philippines. Journal of the Zoological Society of London 257:339–351. DOI:10.1017/S0952836902000948.
- Zekely J, Gollner S, Van Dover CL, Govenar B, Le Bris N, Nemeschkal H, Bright M. 2006. Nematode communities associated with tubeworm and mussel aggregations on the East Pacific Rise. Cahiers de Biologie Marine 47:477–482.
- Zeppilli D, Canals M, Danovaro R. 2012. Pockmarks enhance deep-sea benthic biodiversity: A case study in the western Mediterranean Sea. Diversity and Distribution 18:832–846. DOI:10.1111/j.1472-4642.2011.00859.x.
- Zeppilli D, Danovaro R. 2009. Meiofaunal diversity and assemblage structure in a shallow-water hydrothermal vent in the Pacific Ocean. Aquatic Biology 5:75–84. DOI:10.3354/ab00140.
- Zeppilli D, Leduc D, Fontanier C, Fontaneto D, Fuchs S, Gooday AJ, Goineau A, Ingels J, Ivanenko VN, Kristensen RM, Neves RC, Sanchez N, Sandulli R, Sarrazin J, Sørensen MV, Tasiemski A, Vanreusel A, Autret M, Bourdonnay L, Claireaux M, Coquillé V, De Wever L, Rachel D, Marchant J, Toomey L, Fernandes D. 2017. Characteristics of meiofauna in extreme marine ecosystems: A review. Marine Biodiversity. DOI:10.1007/s12526-017-0815-z
- Zeppilli D, Mea M, Corinaldesi C, Danovaro R. 2011. Mud volcanoes in the Mediterranean Sea are hot spots of exclusive meiobenthic species. Progress in Oceanography 91:260–272. DOI:10.1016/j.pocean.2011.01.001.
- Zeppilli D, Vanreusel A, Pradillon F, Fuchs S, Mandon P, James T, Sarrazin J. 2015. Rapid colonisation by nematodes on organic and inorganic substrata deployed at the deep-sea Lucky Strike hydrothermal vent field (Mid-Atlantic Ridge). Marine Biodiversity 45:489–504. DOI:10.1007/s12526-015-0348-2.
