Abstract
The environmental conditions of the habitats in which organisms live may induce a variety of plastic adaptive responses for numerous developmental, behavioural and morphological traits. One of the most relevant environmental features is the amount of available food occurring in a certain habitat. In this study, we wanted to assess whether, during development of vertebrates, diet quality may induce plastic responses at both behavioural and morphological levels. We tested whether diet may affect the rate of aggressive interactions between fire salamander larvae (Salamandra salamandra) and changes of head morphology during development. We collected 15 newborn larvae from five different localities, and we randomly assigned them to three diets (small nourishing prey, big scant prey, mixed prey). For each larva we recorded the number of snap attempts to a target larva 4 days after salamander collection, and after the 50 days of feeding treatment. We recorded and analysed head shape development using a geometric morphometrics approach. Analyses showed a significant relationship between diet and aggressiveness: larvae fed with small nourishing prey were significantly more aggressive. Diet had a significant effect in determining the quantity of head morphology changes during larval development, but did not affect the characteristics of the morphology; this means that all the larvae showed a similar shape modification, but those treated with the more nourishing prey showed a more pronounced change. These results indicate that diet features may induce both behavioural and morphological plastic responses. High-quality diets may be linked to competition for trophic resources, increasing development rates and determining higher competitive ability.
Introduction
The availability and quality of the trophic resources existing in a given environment are essential limiting factors for numerous organisms. These limiting factors lead to intense inter- and intra- specific competition to gather the amount of food necessary for survival and reproduction. In vertebrates and invertebrates, intraspecific competition is particularly strong amongst juveniles and larvae, respectively (Urban Citation2007).
One of the most common manifestations of competition is aggressiveness (Arnott & Elwood Citation2008). Multiple studies have shown that aggressive interactions between conspecifics affect not only individual survivorship, but also the composition of populations (Claessen et al. Citation2000; Ohlberger et al. Citation2012). Aggressive intraspecific interactions may occur in a wide range of contests, from behavioural displays to deadly fights, and are often linked to asymmetries occurring between conspecifics (Arnott & Elwood Citation2008). Examples of asymmetries among conspecifics include differences in internal status, and differences in assessing the value of resources (Arnott & Elwood Citation2008). Differences in size, age, experience and social status are a classical example of an asymmetry between conspecifics that is linked to the individuals own value; such differences often affect the outcome of intraspecific interactions (Arnott & Elwood Citation2008).
Theory predicts that the aggressive effort increases with the value of the resource, until costs do not exceed the resource value (Arnott & Elwood Citation2008). In environments where food resources are scarce, the aggressive individuals may be more able to increase their food intake (Manenti et al. Citation2015); moreover, smaller conspecifics may represent a major trophic supply for the larger ones as smaller individuals can be eaten by larger ones (Wissinger et al. Citation2010; Hopkins et al. Citation2011). In this case, aggression can grade into full-scale cannibalism and may have important ecological implications at both population and community levels (Delclos & Rudolf Citation2011; Kishida et al. Citation2011).
Cannibalistic populations may be found in a wide range of invertebrate and vertebrate taxa, and, usually, cannibalism is reported for predator species (Alabi et al. Citation2009; Ibanez & Keyl Citation2010; Kudo & Shirai Citation2012). A recent study showed that voracious predators cannibalise one another when enough size difference between individuals occurs (Anderson Citation2016). Cannibalism is also frequent in fish (Smith & Reay Citation1991). Fish living in an environment with poor trophic resources may have cannibalistic populations, as in the case of the Arctic char Salvelinus alpinus inhabiting Alpine lakes, which shows two distinct morphs, i.e. invertebrate feeders and cannibal morphs (Florø-Larsen et al. Citation2016). The differences between the morphs include a significant disparity in size at hatching (Florø-Larsen et al. Citation2016). Among amphibians, urodeles provide many further examples. A well-known case is represented by the salamanders of the genus Ambystoma (Wells Citation2007): individual larvae from cannibalistic populations show behavioural and morphological differences, being bigger and having proportionately larger heads with respect to the non-cannibals.
The morphological distinctiveness of cannibals is highly variable among species (Claessen et al. Citation2000). Cannibals often have larger body and head size, and these traits seem to be linked to cannibals’ success during intraspecific interactions (Wildy et al. Citation1998; Wakano Citation2004). Indeed, cannibalism may affect population size structure (van Kooten et al. Citation2007), just as a direct consequence of the trophic benefits gained by cannibals, which are likely to follow higher growth rates and reach larger size than non-cannibals.
In amphibians, full-scale cannibalism cases have been frequently observed in several salamander families and genera (Pfennig et al. Citation1994; Denoël et al. Citation2006; Buckley et al. Citation2007). The reasons for cannibalism may lie in some shared traits: all salamanders are carnivorous both at adult and at larval stages. Most species are generalist predators, usually feeding on small invertebrates but able to prey on anuran tadpoles, other salamander larvae and also juvenile fish (e.g. Dicamptodon; Parker Citation1994); during growth, larvae of most species show a dietary shift towards larger prey and favouring aggressive intraspecific interactions (Wells Citation2007). Whatever the explanation, the frequency of cannibalism among salamanders makes them particularly suitable for investigating the ecological and evolutionary meaning of this behaviour, and the fire salamander (Salamandra salamandra) may provide a particularly good study system.
This is a European widespread epigeous salamander that usually breeds in streams and small pools (Steinfartz et al. Citation2007; Manenti et al. Citation2009b), but some populations are able to breed in different kinds of subterranean environments where larvae successfully complete their development (Manenti et al. Citation2009a). In streams and pools, it is frequent to find larvae of different ages. This fact leads to the occurrence of strong asymmetries between conspecifics in terms of both size and developmental stage in the same habitat (Manenti et al. Citation2011; Romeo et al. Citation2015). Larvae of S. salamandra often display aggressive behaviours that are favoured in starvation periods and under high conspecific density (Manenti et al. Citation2015). The fact that hungry larvae display more aggressive behaviours suggests that the internal status of the starved larvae affects the apparent resource value of the conspecifics: a hungry larva would score a food resource more highly than would a satiated one (Arnott & Elwood Citation2008). Smaller fire salamander larvae may represent an important food resource and may be easily attacked by the largest ones. Indeed, the occurrence of full-grade cannibalism has been often reported, especially in pools or streams where larval density was high (Joly Citation1968). Cannibalism can thus play an important role for Salamandra salamandra in food-deprived habitats, allowing the first cohorts of larvae to feed on later-arriving cohorts and reach metamorphosis (Markman et al. Citation2009). Cannibalism may help survival in environments with limited resources, such as caves, where starvation periods may be frequent and prolonged. At the same time, at parity of starvation conditions, salamander larvae that experienced diets of higher quality are more aggressive than larvae that experienced low-quality diets (Heuring et al. Citation2017).
Diet is predicted to affect multiple larval traits during development because the trophic resources available are strongly correlated to salamander larva body condition (Heuring et al. Citation2017), behaviour (Krause et al. Citation2011) and growth rates (Limongi et al. Citation2015). In particular, the quality/composition and size of the prey are likely to affect development depending both on the nutrients levels made available for larval growth and on shape which, as recorded in the case of cannibalistic individuals of Salvelinus and Ambystoma, may enlarge mouth morphology.
In this study we evaluated the relationships between the level of aggressive interactions and morphological development of fire salamander larvae. In particular, we investigated whether the diet, in terms of composition and availability of prey, is able to promote aggressive behaviour and to induce morphological plasticity, by examining correlations between diet, aggressive behaviour and head morphology. When adaptive responses are driven by variable environmental features, they are likely to be highly plastic (Kasumovic et al. Citation2009), and we hypothesise that diet may lead to both behavioural and morphological plastic responses.
We performed laboratory experiments to answer two questions: (1) Does prey size affect the mouth and head morphology of predators? and (2) Does prey quality affect the quality of predators’ competitive response, such as aggressiveness?
Materials and methods
Larvae collection and rearing
We collected 15 larvae at developmental stage 1 (newborns: well-developed tail fin and the tip of the fin bluntly rounded; Jusczcyk & Zakrzewski Citation1981) from five epigean streams situated in the Italian Prealps in Lombardy (NW Italy; around 45°48ʹN, 9°02ʹE). Larvae were individually maintained for 50 days at a mean temperature of 18°C, exposed to the natural photoperiod, in containers of transparent plastic with a size of 10 × 11 cm; containers were perforated with perforations of 2 mm in diameter. The containers were placed in six independent water-filled blocks (40 × 50 cm, water depth: 5 cm). Larvae were randomly assigned to three diet treatments, with two blocks per treatment. The diet treatments were: “big but scant prey” diet, “small but nourishing prey” diet and “mixed prey” diet. The “big but scant prey” (hereafter “big”) diet was composed by big prey with poor nutritional value relative to size. Under this treatment, larvae were fed ad libitum with slices of fresh chicken (nutritional caloric power of 110 kcal/100 g) meat that were of the same width as their closed mouth. The “small but nourishing prey” (hereafter “small”) diet was composed by small prey with high caloric power. Under this treatment, fire salamander larvae were fed ad libitum with Chironomus sp. larvae (nutritional caloric power > 460 kcal/100 g) that were always thinner than the width of their closed mouth. The “mixed prey” (hereafter “mixed”) diet was composed of both big prey with poor nutritional power and small prey with high nutritional power, that mimicked natural conditions of larval development with smaller prey eaten in the early stages and larger prey eaten in the late stages. Under this treatment, salamander larvae were fed ad libitum with Chironomus sp. larvae thinner than their mouth for the first 25 days and with fresh chicken meat slices of the same width as their mouth for the remaining 25 days. The sequence used in this treatment was specifically developed to detect a possible effect of the prey size changing after half of the larval development.
Both at the beginning and at the end of the diet treatments, we recorded the aggressiveness of the fire salamander larvae and measured their head shape and morphology.
Aggressiveness
To test aggressiveness, we recorded for each larva (hereafter “focal larvae”) the number of snaps attempted on a target larva (Manenti et al. Citation2015). Tests started 4 days after salamander collection, and were repeated after the 50 days of diet treatment. Because when we collected the larvae we did not know their level of satiety, we fed them on the collection day and then left them without food for 3 consecutive days before starting tests, as another study found that satiety reduces aggressiveness (Manenti et al. Citation2015). We collected eight additional newborn larvae (average length: 33 mm) to be used as targets (hereafter “target larvae”); we collected them from two additional epigean streams, 3 km and > 28 km from the nearest sampling site of test larvae, respectively. To use the same length and developmental stage of the target larvae at the beginning and end of the 50-day test period, we collected eight additional newborn larvae (average length: 34 mm) from two artificial hypogean pools, at 390 m above sea level (asl) and at 1100 m. asl, 7 km and > 30 km from the nearest sampling site of test larvae, respectively. In the second test period, prey larvae were obtained from colder localities, and this allowed us to find, both at the beginning and at the end of the treatment, target larvae of similar length to the original target larvae used at the beginning of focal larvae rearing. The focal larvae and the target larvae were randomly selected, until each focal larva was tested twice both at the beginning and at the end of the rearing period. During the behavioural tests, each larva was individually placed in a 13.5 × 18.3 cm box with and illuminance intensity of 500 lux and was allowed to acclimatise for 3 min. At the end of the acclimatisation a target larva was placed in the arena and we performed the behavioural tests for 7 min, counting the number of snaps made by each focal larva toward the target larva. All observations were performed by the same observer. Due to the short duration of the trials (less than 30 min including acclimatisation), none of the larvae were physically injured by intraspecific interactions. Behaviour of target larvae was recorded, but as they never snapped in return and only a few bites were observed, we did not take into consideration for this study the target larvae’s responses.
Head shape
To record and analyse head-shape development, we used a geometric morphometrics approach (Bookstein Citation1997; Dryden & Mardia Citation1998; Rohlf Citation2000).
We photographed the dorsal view of each focal larva at the beginning and at the end of the diet treatment. Photographs were taken with a Canon PowerShot SX10 IS, after putting the larva in a transparent plastic tank filled with water to a depth of 3 cm (to hold the larvae horizontal), positioned above a sheet of graph paper (used as an absolute linear reference). On each photograph, after having measured the snout-to-vent length (SVL), we digitised nine homologous landmarks, four paired and one unpaired (LM; ), and we used an outline of the head to place 16 symmetric and equally spaced semi-landmarks (SLM; ). For all these operations, we used TpsDig 2.16 software (Rohlf Citation2010; available at http://life.bio.sunysb.edu/morph/).
Statistical analyses
We analysed the relationship between diet treatments and aggressiveness with a generalised linear mixed model expressed by the formula:
aggressiveness ~ period*treatment + SVL + dSVL + (1|id)
where aggressiveness is represented by the number of tentative snaps performed by the focal larvae, period is the time of the test (beginning/end), treatment corresponds to the diet condition (big, mixed, small), SVL is the focal larvae size, and dSVL is the size difference between focal and target larvae. We performed the comparison among levels of the treatment factor by setting a priori Helmert contrasts (big vs mixed; big + mixed vs small). We included the larval identity as a random factor acting on the model intercept. Given that the dependent variable (number of snaps) is count data including many zeros and the distribution of the data appeared over-dispersed (with the variance being more than 4 times the mean), we used zero-inflated models with negative binomial error distribution (Zuur et al. Citation2009), as implemented in the glmmADMB R package (Skaug et al. Citation2016). Significance was assessed through a likelihood ratio test (Bolker et al. Citation2008).
To explore the relationship between diet treatment and head-shape development, we conducted a two-step phenotypic trajectory analysis (Collyer & Adams Citation2013) of the head configurations (eight LM and 16 SLM) at the beginning and at the end of the diet treatment: firstly, the whole set of configurations were superimposed using a general Procrustes analysis to remove all non-shape information (Adams et al. Citation2004); secondly, a principal component analysis was performed on the new set of coordinates to extract the correct dimensionality of the shape data; finally, the vector representing the phenotypic trajectory of each larva was computed as the vectorial difference between the final and the starting step, i.e. the final and the starting head shape (Adams & Collyer Citation2009; Zuffi et al. Citation2017). Vector size expresses the amount of phenotypic variation, while vector angle expresses its direction (Adams & Collyer Citation2009). We assessed the relationship between these two components of the phenotypic change and the diet treatments using a distance-based analysis of variance (ANOVA; Adams & Collyer Citation2009) according to the model formula D ~ Growth*Treatment, where D is the pairwise distance matrices of the size and the angle component of the phenotypic trajectory, one at a time; growth is the relative increase in size of the focal larva (SVLfinal – SVLstart)/SVLstart; and treatment is the diet condition (with the same contrasts used for aggressiveness models). We assessed the significance of the effects through a permutation test (9999 permutations). We performed all analyses in R version 3.2 (R Development Core Team Citation2016).
Results
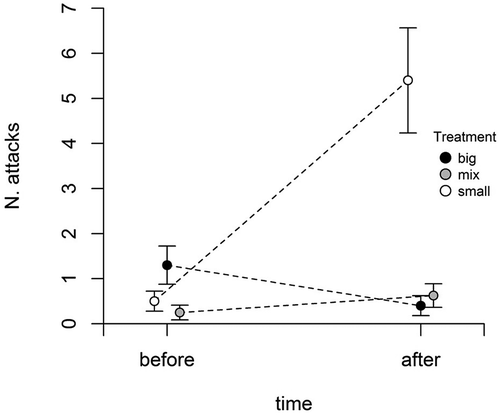
We detected a significant relationship between diet and aggressiveness (); aggressiveness changed from the pre-treatment to the post-treatment period depending on the diet treatment. The number of snaps given by the focal larvae was similar among the three groups before the diet treatment; at the end of the treatments, larvae from the big and mixed groups did not show a significant variation, while those from the “small” group significantly increased their aggressiveness ().
Table I. Relationship between feeding treatments and aggressiveness, results of the generalised linear mixed model (GLMM) analysis. For the significant factors, the statistics for the Helmert contrasts are also shown (in this case the standardised coefficients, z values, are reported instead of chi-square). SVL = Snout-to-Vent lenght; dSVL = SVL growth during the treatment period; Time = testing period (treatment beginning or ending); Treatment = size of prey ate by larvae: small, big or mix; * indicates the two-way interaction between two variables.
Figure 2. Relationship between aggressiveness and diet treatment: dots represent mean number of attacks before and after treatment; bars represent standard errors; dashed lines connect starting and ending points within each treatment.
All the diet treatments allowed larval growth. Considering just the larval SVL, the rate of growth was different between the treatments. In particular, larvae treated with small but nourishing prey grew more than larvae treated with big but scant prey F = 3.95, P = 0.04). No differences were recorded between “small” and “mixed” treatments.
Phenotypic trajectory analysis revealed a significant effect of the diet on the size component of the phenotypic vector, but not on its angular component (; ).
Table II. Relationships between geometric morphometrics variables and the feeding treatments, results of the distance-based ANOVA analyses for the components “size” and “angle” of the phenotypic change vector. Significant effects are in bold. For the significant treatment, Helmert contrasts are shown.
Figure 3. Bi-dimensional projection of the phenotypic trajectories across treatments: the chosen axes (Principal Component 1 and 4) best show the relationship between the angle and size components of the vectors. The dashed line represents the overall direction.
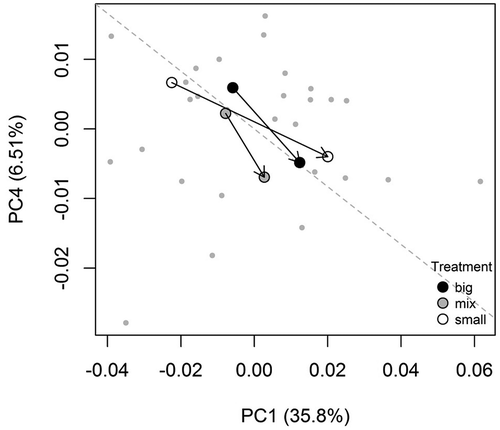
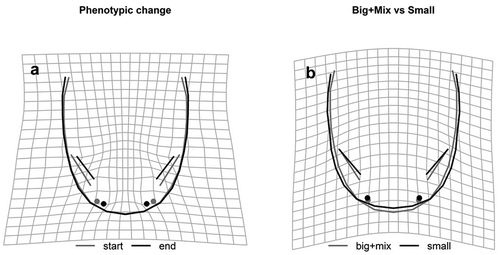
Notably, individuals from the “small” treatment showed a larger size than the other treatments (). All the larvae showed a similar shape modification, mainly loaded by the regions of the eyes and nostrils: the eyes decreased in relative size (the distance between anterior and posterior vertices) and moved away from the head edge ()); at the same time, the inter-nostril distance decreased while the eye-to-nostril distance increased ()).
Figure 4. Shape changes along the phenotypic vectors. (a) Since the angle of the trajectories does not vary among treatments, the overall shape change is shown (grey = start; black = end). (b) The final shape is compared using the significant contrast between treatments: “small” vs “big+ mixed” diet. The deformation grids highlight the areas undergoing the strongest change. Amplification factor: 1.
Discussion
Our results show that both larval aggressiveness and morphological development varied depending on the diet treatment that larvae experienced. We used a small sample size because of logistic and regional permission constraints. Further studies using more individuals may allow the identification of processes and elements that the relative power of our analyses may have not detected. However, our approach allowed us to delineate some general patterns.
First of all, considering the behavioural aspects, larvae fed with small but nourishing prey became more aggressive, while those fed with large but scanter prey were much less aggressive after the diet treatment than at the collection moment. This could be a typical case of internal state affecting aggressiveness (Arnott & Elwood Citation2008). Internal state, such as starving, may affect the apparent value of a resource for the hungry individual, which would value a food resource more than would a satiated individual (Arnott & Elwood Citation2008). In S. salamandra, larvae experiencing longer periods of starvation are much more likely to display aggressive behaviour (Manenti et al. Citation2015); when resources are scarce, more importance is generally given to the potential food occurring, and also conspecifics may be viewed as a potential food supply. At the same time, it has been shown that at the same level of starvation, the larvae of the salamander Ambystoma annulatum that experienced higher quality diets were more aggressive than larvae that experienced lower quality nutritional diets (Heuring et al. Citation2017). In the case of our experiment, focal larvae may have seen target larvae both as potential nutritional resources and as competitors for resources such as territory and prey. The higher aggressiveness levels showed by the larvae that experienced the diet with higher nutritional conditions may reflect a better internal condition and a higher competition ability than larvae that experienced lower nutritional levels. As all the larvae of each treatment were fed ad libitum, the aggressiveness was not conditioned by the amount of food eaten. However, the current information acquired by our experiments does not allow us to disentangle the relative role of size and the energy content of prey items in fire salamander larva behaviour. Larvae under the “small” treatment could have perceived only the small size of the prey and not their content with a sensitivity to an overall poor nutritional condition that is a factor generally involving aggressiveness in salamander larvae (Manenti et al. Citation2015).
In any case, the diet treatment based on insect prey had a higher nutritional power with respect to the caloric content of the diet treatment based on chicken meat items. Insects are considered a rich food for many organisms; the energy content of chironomid larvae is much higher than the energy content of the meat of chickens or other vertebrates (Armitage et al. Citation2012). Chicken nutritional power amounts only to 110 kcal/100 g, while that of chironomids exceeds 460 kcal/100 g. (Armitage et al. Citation2012; Payne et al. Citation2016). The effects of diet composition on behaviour are heavily debated in the scientific literature (Haagensen et al. Citation2014). Cases of diet composition affecting aggressiveness levels are reported in mammals such as dogs (Kocis et al. Citation2015), vervets (Bramblett et al. Citation1981) and humans (Haagensen et al. Citation2014), and even in some fish (Winberg et al. Citation2001). The effect that we detected on the fire salamander larvae confirms that higher nutrition diet levels may contribute to increase competitive interactions and aggressiveness. Based on this result, we stress that further studies should investigate the relationships between diet quality and internal factors in stimulating aggressiveness.
While behaviour is generally viewed as a highly plastic trait, especially during development, examples of morphological phenotypic plasticity are rarer (Laubichler Citation2009). However, behavioural and morphological plasticity can act jointly to produce a unique functional phenotype, and the plasticity of the morphological traits depends on which is considered the environment influencing it (Bertossa Citation2011). Behavioural plasticity can allow the expression of a wide range of responses along the life span of a single individual, but behaviour is a trait only acting in a specific moment (Bertossa Citation2011). Morphological responses can be less prompt than behavioural ones, but can have lasting consequences on the fitness of individuals. Our results indicate that in fire salamander the head morphology, at least during the larval development monitored, did not show significant variation in terms of shape. The larvae of all three diet treatments tended to reach the same pre-metamorphosis morphology, not depending on the dimensions of the prey ingested during the rearing period. However, diet treatments stimulated a plastic response in the amount of phenotypic change: the size component of the phenotypic change was significantly higher in larvae fed with small but nourishing prey than in larvae exposed to a mixed diet or fed only with larger but less nutrient prey. The development of the head shape was stronger in larvae that ate smaller but more caloric prey. Numerous biotic and abiotic elements have been reported to affect the rates of development and growth of amphibians (Rose Citation2005; Vaissi & Sharifi Citation2016). Among the biotic factors, the quantity of available food often plays a prominent role (Ogilvy et al. Citation2012). Individuals that take in more high-quality food resources are able to grow larger and more quickly, and to increase their survival possibilities (Denoël & Poncin Citation2001; Hawlena et al. Citation2011). Our results support the idea that not only the amount of food ingested, but also the diet (in terms of composition and quality) plays a major role. Our findings show that fire salamander larvae can respond adaptively to variation in the quality of the resource that is available in aquatic environments. In our study case, food typology likely affected a trait (aggressiveness) that may play an adaptive role, especially in food-deprived environments.
Our results suggest that fire salamander larvae did not show any tendency to develop specific cannibal morphs as recorded in ambystomatid salamanders (Wells Citation2007). Our analysis confirms that fire salamander larvae are capable of plastic adaptive responses depending on the environmental conditions; among these conditions the nutritional level of the available food is an important element to be considered when studying intraspecific aggressiveness. Future studies, with contrasting treatments of all combinations of small–large and high–low-energy food items, could be developed to understand whether prey size or energy content matters.
Acknowledgements
We are grateful to the PLIS Lago del Segrino and Roberto Vignarca for support in larva rearing.
The larvae collection and rearing was authorised by Lombardy Region authority, p. n.: T1.2015.0001053.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adams DC, Collyer ML. 2009. A general framework for the analysis of phenotypic trajectories in evolutionary studies. Evolution 63:1143–1154. doi: 10.1111/evo.2009.63.issue-5.
- Adams DC, Rohlf FJ, Slice DE. 2004. Geometric morphometrics: Ten years of progress following the ‘revolution’. Italian Journal of Zoology 71:5–16. doi: 10.1080/11250000409356545.
- Alabi T, Patiny S, Verheggen F, Francis F, Haubruge E. 2009. Origin and evolution of cannibalism in the animal populations: Why to eat conspecific? Biotechnologie, Agronomie. Société Et Environnement 13:409–425.
- Anderson TL. 2016. Predation risk between cannibalistic aeshnid dragonflies influences their functional response on a larval salamander prey. Journal of Zoology 300:221–227. doi: 10.1111/jzo.2016.300.issue-3.
- Armitage PD, Pinder LC, Cranston P. 2012. The Chironomidae: biology and ecology of non-biting midges. Berlin: Springer Science & Business Media.
- Arnott G, Elwood RW. 2008. Information gathering and decision making about resource value in animal contests. Animal Behaviour 76:529–542. doi: 10.1016/j.anbehav.2008.04.019.
- Bertossa RC. 2011. Morphology and behaviour: Functional links in development and evolution. Philosophical Transactions of the Royal Society B - Biological Sciences 366:2056–2068. doi: 10.1098/rstb.2011.0035.
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JS. 2008. Generalized linear mixed models: A practical guide for ecology and evolution. Trends in Ecology and Evolution 24:127–135. doi: 10.1016/j.tree.2008.10.008.
- Bookstein FL. 1997. Morphometric tools for landmark data: geometry and biology. Cambridge: Cambridge University Press.
- Bramblett C, Coelho A, Mott G. 1981. Behavior and serum cholesterol in a social group of Cercopithecus aethiops. Primates 22:96–102. doi: 10.1007/BF02382560.
- Buckley D, Alcobendas M, Garcia-Paris M, Wake MH. 2007. Heterochrony, cannibalism, and the evolution of viviparity in Salamandra salamandra. Evolution & Development 9:105–115. doi: 10.1111/j.1525-142X.2006.00141.x.
- Claessen D, de Roos AM, Persson L. 2000. Dwarfs and giants: Cannibalism and competition in size-structured populations. American Naturalist 155:219–237. doi: 10.1086/303358.
- Collyer ML, Adams DC. 2013. Phenotypic trajectory analysis: Comparison of shape change patterns in evolution and ecology. Hystrix 24:75–83.
- Delclos P, Rudolf VHW. 2011. Effects of size structure and habitat complexity on predator-prey interactions. Ecological Entomology 36:744–750. doi: 10.1111/j.1365-2311.2011.01324.x.
- Denoël M, Poncin P. 2001. The effect of food on growth and metamorphosis of paedomorphs in Triturus alpestris apuanus. Archiv Für Hydrobiologie 152:661–670. doi: 10.1127/archiv-hydrobiol/152/2001/661.
- Denoël M, Whiteman HH, Wissinger SA. 2006. Temporal shift of diet in alternative cannibalistic morphs of the tiger salamander. Biological Journal of the Linnean Society 89:373–382. doi: 10.1111/(ISSN)1095-8312.
- Dryden IL, Mardia KV. 1998. Statistical shape analysis. New York: John Wiley & Sons.
- Florø-Larsen B, Finstad AG, Berg OK, Olsen PH. 2016. Otolith size differences during early life of dwarf and cannibal Arctic char (Salvelinus alpinus). Ecology of Freshwater Fish 25:203–210. doi: 10.1111/eff.2016.25.issue-2.
- Haagensen AMJ, Sørensen DB, Sandøe P, Matthews LR, Birck MM, Fels JJ, Astrup A. 2014. High fat, low carbohydrate diet limit fear and aggression in göttingen minipigs. Plos One 9:e93821. doi: 10.1371/journal.pone.0093821.
- Hawlena D, Hughes KM, Schmitz OJ. 2011. Trophic trait plasticity in response to changes in resource availability and predation risk. Functional Ecology 25:1223–1231. doi: 10.1111/j.1365-2435.2011.01891.x.
- Heuring CA, Heuring WL, Crane AL, Mathis A. 2017. Effects of diet quality and stress on interference behaviour of larval ringed salamanders. Amphibia-Reptilia 38:89–96.
- Hopkins GR, Gall BG, Brodie ED. 2011. Ontogenetic shift in efficacy of antipredator mechanisms in a top aquatic predator, Anax junius (Odonata: Aeshnidae). Ethology 117:1093–1100. doi: 10.1111/j.1439-0310.2011.01963.x.
- Ibanez CM, Keyl F. 2010. Cannibalism in cephalopods. Reviews in Fish Biology and Fisheries 20:123–136. doi: 10.1007/s11160-009-9129-y.
- Joly J. 1968. Données écologiques sur la salamandre tachetée Salamandra salamandra. Annales Des Sciences Naturelles Zoologie - Paris 12:301–306.
- Jusczcyk W, Zakrzewski M. 1981. External morphology of larval stages of the spotted salamander Salamandra salamandra (L.). Acta Biologica Cracoviensa 23:127–135.
- Kasumovic MM, Bruce MJ, Herberstein ME, Andrade MCB. 2009. Evidence for developmental plasticity in response to demographic variation in nature. Ecology 90:2287–2296.
- Kishida O, Trussell GC, Ohno A, Kuwano S, Ikawa T, Nishimura K. 2011. Predation risk suppresses the positive feedback between size structure and cannibalism. Journal of Animal Ecology 80:1278–1287. doi: 10.1111/jane.2011.80.issue-6.
- Kocis TA, Groszler A, Zarcula S, Petruse C, Brudiu I, Cărpinișan L, Ţibru I. 2015. The impact of dog feeding on their aggressiveness. Advances in Animal and Veterinary Sciences 3:503–506. doi: 10.14737/journal.aavs/2015/3.9.503.506.
- Krause ET, Steinfartz S, Caspers BA. 2011. Poor nutritional conditions during the early larval stage reduce risk-taking activities of fire salamander larvae (Salamandra salamandra). Ethology 117:416–421. doi: 10.1111/eth.2011.117.issue-5.
- Kudo K, Shirai A. 2012. Effect of food availability on larval cannibalism by foundresses of the paper wasp Polistes chinensis antennalis. Insectes Sociaux 59:279–284. doi: 10.1007/s00040-011-0217-3.
- Laubichler MD. 2009. Form and function in evo devo: Historical and conceptual reflections. In: Laubichler MD, Maienschein J, editors. Form and function in developmental evolution. Cambridge, NY:Cambridge University Press. pp.10–46.
- Limongi L, Ficetola GF, Romeo G, Manenti R. 2015. Environmental factors determining growth of salamander larvae: A field study. Current Zoology 61:421–427. doi: 10.1093/czoolo/61.3.421.
- Manenti R, Ficetola GF, Bianchi B, De Bernardi F. 2009a. Habitat features and distribution of Salamandra salamandra in underground springs. Acta Herpetologica 4:143–151.
- Manenti R, Ficetola GF, De Bernardi F. 2009b. Water, stream morphology and landscape: Complex habitat determinants for the fire salamander Salamandra salamandra. Amphibia-Reptilia 30:7–15. doi: 10.1163/156853809787392766.
- Manenti R, Ficetola GF, Marieni A, De Bernardi F. 2011. Caves as breeding sites for Salamandra salamandra: Habitat selection, larval development and conservation issues. North-Western Journal of Zoology 7:304–309.
- Manenti R, Pennati R, Ficetola GF. 2015. Role of density and resource competition in determining aggressive behaviour in salamanders. Journal of Zoology 296:270–277. doi: 10.1111/jzo.2015.296.issue-4.
- Markman S, Hill N, Todrank J, Heth G, Blaustein L. 2009. Differential aggressiveness between fire salamander (Salamandra infraimmaculata) larvae covaries with their genetic similarity. Behavioral Ecology and Sociobiology 63:1149–1155. doi: 10.1007/s00265-009-0765-y.
- Ogilvy V, Preziosi RF, Fidgett AL. 2012. A brighter future for frogs? The influence of carotenoids on the health, development and reproductive success of the red-eye tree frog. Animal Conservation 15:480–488. doi: 10.1111/j.1469-1795.2012.00536.x.
- Ohlberger J, Langangen O, Stenseth NC, Vollestad LA. 2012. Community-level consequences of cannibalism. American Naturalist 180:791–801. doi: 10.1086/668080.
- Parker MS. 1994. Feeding ecology of stream-dwelling pacific giant salamander larvae (Dicamptodon tenebrosus). Copeia 705–718.doi: 10.2307/1447187.
- Payne CL, Scarborough P, Rayner M, Nonaka K. 2016. Are edible insects more or less ‘healthy’ than commonly consumed meats? A comparison using two nutrient profiling models developed to combat over- and undernutrition. European Journal of Clinical Nutrition 70:285–291. doi: 10.1038/ejcn.2015.149.
- Pfennig DW, Sherman PW, Collins JP. 1994. Kin recognition and cannibalism in polyphenic salamanders. Behavioral Ecology 5:225–232. doi: 10.1093/beheco/5.2.225.
- R Development Core Team. 2016. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing.
- Rohlf FJ. 2000. Statistical power comparisons among alternative morphometric methods. American Journal of Physical Anthropology 111:463–478. doi: 10.1002/(ISSN)1096-8644.
- Rohlf FJ. 2010. tpsDig, digitize landmarks and outlines, version 2.16. New York: Department of Ecology and Evolution, State University of New York at Stony Brook.
- Romeo G, Giovine G, Ficetola GF, Manenti R. 2015. Development of the fire salamander larvae at the altitudinal limit in Lombardy (north-western Italy): Effect of two cohorts occurrence on intraspecific aggression. North-Western Journal of Zoology 11:234–240.
- Rose CS. 2005. Integrating ecology and developmental biology to explain the timing of frog metamorphosis. Trends in Ecology and Evolution 20:129–135. doi: 10.1016/j.tree.2005.04.006.
- Skaug H, Fournier D, Nielsen A. 2016. glmmADMB: generalized linear mixed models using AD model builder: R package version 0.8.
- Smith C, Reay I. 1991. Cannibalism in teleost fish. Reviews in Fish Biology and Fisheries 1:41–64. doi: 10.1007/BF00042661.
- Steinfartz S, Weitere M, Tautz D. 2007. Tracing the first step to speciation: Ecological and genetic differentiation of a salamander population in a small forest. Molecular Ecology 16:4550–4561. doi: 10.1111/j.1365-294X.2007.03539.x.
- Urban MC. 2007. Risky prey behavior evolves in risky habitats. Proceedings of the National Academy of Sciences of the United States of America 104:14377–14382. doi: 10.1073/pnas.0704645104.
- Vaissi S, Sharifi M. 2016. Changes in food availability mediate the effects of temperature on growth, metamorphosis and survival in endangered yellow spotted mountain newt: Implications for captive breeding programs. Biologia 71:444–451. doi: 10.1515/biolog-2016-0054.
- van Kooten T, Persson L, Roos AM. 2007. Size-dependent mortality induces life-history changes mediated through population dynamical feedbacks. American Naturalist 170:258–270.
- Wakano JY. 2004. Drastic growth effect may explain sympatric cannibalistic polymorphism. Journal of Theoretical Biology 226:69–77. doi: 10.1016/j.jtbi.2003.08.005.
- Wells KD. 2007. The ecology and behaviour of Amphibians. Chicago: The University of Chicago Press.
- Wildy EL, Chivers DP, Kiesecker JM, Blaustein AR. 1998. Cannibalism enhances growth in larval long-toed salamanders, (Ambystoma macrodactylum). Journal of Herpetology 32:286–289. doi: 10.2307/1565312.
- Winberg S, Ø Ø, Lepage O. 2001. Suppression of aggression in rainbow trout (Oncorhynchus mykiss) by dietary L-tryptophan. The Journal of Experimental Biology 204:3867–3876.
- Wissinger SA, Whiteman HH, Denoel M, Mumford ML, Aubee CB. 2010. Consumptive and nonconsumptive effects of cannibalism in fluctuating age-structured populations. Ecology 91:549–559. doi: 10.1890/08-1366.1.
- Zuffi MAL, Mangiacotti M, Masucci GD, Sacchi R, Scali S, Sannolo M. 2017. Stable or plastic body shape? Emys orbicularis hatchlings-juveniles growth patterns under different ecological conditions. North-Western Journal of Zoology 13:262–270.
- Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. 2009. Mixed effects models and extensions in ecology with R. Berlin: Springer.

