Abstract
The Southern brown howler monkey, Alouatta guariba clamitans, is one of the largest Neotropical primates. The objective of this study was to describe the origin and antimeric distribution of brachial plexus nerves in A. g. clamitans and, thereby, to provide information for comparative anatomy and for anatomy applied to loco-regional anaesthetic blocking in primates. For this purpose, a macrodissection of 10 thoracic limbs of corpses that were collected from highways was performed, and the corpses were stored in 10% formaldehyde solution. The brachial plexus was essentially formed from the ventral spinal branches of segments C5 to T1, although in one specimen the contribution of C4 and in another specimen the contribution of T2 was registered. There was a grouping of ventral branches in cranial, medium and caudal trunks, and branches of C5 and C7 were the ones that mostly contributed to the origin of nerves from the plexus. Comparatively, the brachial plexus from A. g. clamitans reflected characteristics that are typical in the primate order, but also of mammal species that require versatility and precision in movements of the thoracic limbs. On the basis of that similarity, it is proposed that anatomic landmarks for anaesthetic block techniques used in other primate species may be successfully applied to A. g. clamitans.
Introduction
The Southern brown howler monkey, Alouatta guariba clamitans Cabrera, 1940, is found in the east part of Brazil, along the Atlantic Forest, in the states of Espírito Santo, Rio de Janeiro, Minas Gerais, São Paulo, Paraná, Santa Catarina and Rio Grande do Sul, and also in the forest of Misiones Province, in Argentina (Gregorin Citation2006).
It belongs to the Atelidae family, is one of the largest Neotropical primates and feeds on a large amount of leaves (Drubbel & Gautier Citation1993). With an arboreal habitat, it takes to nearly every stratum of the forest, having a tolerance for modifications/disturbances in the environment (Bicca-Marques Citation2013; Silva & Bicca-Marques Citation2013). Adult males of A. g. clamitans weigh an average of 6.7 kg, with females weighing an average of 4.4 kg (Smith & Jungers Citation1997).
The high density of human populations in the south and south-east regions of Brazil and the consequential destruction of their habitats have decreased the broad original distribution of A. g. clamitans to a few populations, restricted in isolated forestry fragments (Chiarello & Galetti Citation1994; Crokett Citation1998). The main threats comprise the expansion of farming activities and urbanisation, yellow fever epidemics, road kills on highways, accidents with power grids and hunting (Printes et al. Citation2001; Lokschin et al. Citation2007; Almeida et al. Citation2012). For these reasons, it is considered a vulnerable species in most Brazilian states (Bicca-Marques et al. Citation2015).
The brachial plexus is a complex anatomical structure formed by a varied set of connections between the ventral ramus of the last cervical spinal nerves and the first thoracic vertebra (Martini et al. Citation2009). Although there are hundreds of Primates species, the anatomical description of the brachial plexus is limited to a few of them, as follows: Galago senegalensis senegalensis (Kanagasuntheram & Mahran Citation1960); Saimiri sciureus (Mizuno Citation1969; Araújo et al. Citation2012); Cercopithecus pygerythrus (Booth Citation1991); Papio ursinus (Booth et al. Citation1997); Cebus apella (Ribeiro et al. Citation2005); Lagothrix lagothricha (Robertson Citation1944; Cruz & Adami Citation2010); Pan paniscus (Kikuchi et al. Citation2011); Macaca mulatta (Lu at al. Citation2013; Santos-Sousa et al. Citation2016); and Callithrix jacchus and Callithrix penicillata (Santos et al. Citation2016).
The comparative study of the formation of brachial plexus among species has aroused interest since the 19th century (Paterson Citation1887), and understanding it remains one of the most challenging areas in contemporary anatomy (Johnson et al. Citation2010). Knowledge of the anatomic pattern and potential variations in the development of the brachial plexus is important in specific situations. For example, it can guide local and regional anaesthetic blocking techniques that result in the analgesia of somatic structures in thoracic limbs (Futema et al. Citation1999; Mencalha et al. Citation2016; Mistry et al. Citation2016; Shinn et al. Citation2016).
Given the importance and applicability of the plexus anatomy, combined with the lack of descriptions of the referred species, the aim of this study is to characterise the origin and distribution of the nerves in the brachial plexus of A. g. clamitans.
Materials and methods
For the present study, 10 thoracic limbs of five adult males of A. g. clamitans were dissected. They were found dead along the highway (IBAMA/SISBIO authorisation no. 33,667). Four specimens were collected from Highway BR 116 between the cities of Guapimirim and Teresópolis in Rio de Janeiro State, Brazil. The fifth was collected on Highway BR 290 in the municipality of Cachoeira do Sul, Rio Grande do Sul State, Brazil. After they were collected, the specimens were identified, fixed with intramuscular and intracavitary injections of 50% formaldehyde solution and subsequently packaged in polyethylene boxes containing the same solution.
After 2 months of fixation, macrodissections of both thoracic limbs were performed. For this, the skin and superficial fascia were removed, and the musculature of the limb was exposed. Afterwards, the latissimus dorsi muscle was reflected from its point of insertion at the medial aspect of the humerus, the pectoral muscles were sagittally sectioned 1 cm from their origin in the sternum and the thoracic portion of the ventral serratus muscle was released from its origin in the ribs. These manoeuvres increased the axillary space and allowed the dissection and individualisation of the nerves of the plexus, as well as the definition of its origins in relation with vascular structures and innervated muscles.
The muscles and nerves were named according to the International Committee on Veterinary Gross Anatomical Nomenclature (Citation2017). Schematic drawings were created and the photo-documentation was made using a Samsung® camera, model PL20, 16 megapixels.
Results
The brachial plexus was formed in three specimens by the ventral branches of C5 to T1 (60%), in another specimen by the ventral branches of C4 to T1 (20%) (), and in another specimen by those of C5 to T2 (20%). The ventral spinal branches formed the cranial, medium and caudal trunks. The cranial trunk was formed, invariably, by C5 and C6 (with the contribution of C4 in one specimen). The medium trunk was formed by C7, and the caudal one was formed, essentially, by C8 and T1 (with the contribution of T2 in a single individual). There was no antimeric variation concerning the origin of the plexus or the nerves in the same specimen.
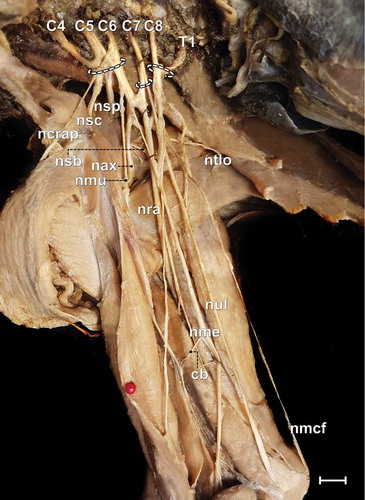
Figure 1. Photomacrograph of the axillary region and medial brachial surface of a male adult specimen of Alouatta guariba clamitans evidencing the constitution of the brachial plexus and its cranial (C4, C5 and C6), medium (C7) and caudal (C8 and T1) trunks. cb = communicating branch; nax = axillary nerve; ncrap = cranial pectoral nerve; nmu = musculocutaneous nerve; nmcf = medial cutaneous of the forearm nerve; nme = median nerve; nsb = subscapularis nerve; nsc = subclavius nerve; nsp = suprascapularis nerve; nra = radial nerve; ntlo = long thoracic nerve; nul = ulnar nerve. Scale bar: 10 mm.
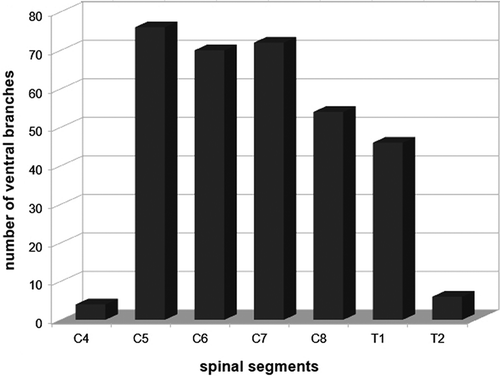
Overall, 328 ventral branches formed 120 nerves (12 nerves in 10 limbs), which resulted in an average of 2.7 branches per nerve. Of these, 64.7% of the ventral branches originated nerves to intrinsic muscles and 35.3% to extrinsic muscles. The nerves destined to the intrinsic musculature were formed, on average, from 2.7 branches, and the ones destined to the extrinsic musculature were formed from 2.3 branches. The ventral branch of C4 collaborated in the formation of 1.2% of the nerves, the ventral branch of C5 in 23.2% of the nerves, the ventral branch of C6 in 21.3%, the ventral branch of C7 in 22.0%, the ventral branch of C8 in16.5%, the ventral branch of T1 in 14.0%, and the ventral branch of T2 in 1.8% (). Thus, the branches of the cranial trunk originated 45.7% of the nerves, the medium trunk 22.0% and the caudal trunk 32.3%.
Figure 2. Total number of contributions of the ventral branches (segments C4, C5, C6, C7, C8, T1 and T2) in the formation of brachial plexus (n = 10) of Alouatta guariba clamitans.
The origin of the nerves in the brachial plexus of 10 thoracic limbs of A. g. clamitans is detailed in and , and the motor innervation is given in .
Table I. Origin and frequency of the nerves of the brachial plexus that supply the intrinsic muscles of the thoracic limb of Alouatta guariba clamitans.
Table II. Origin and frequency of the nerves of the brachial plexus that supply the extrinsic muscles of the thoracic limb of Alouatta guariba clamitans.
Table III. Muscle innervation of the brachial plexus of Alouatta guariba clamitans.
Concerning the route of the nerves, the subclavian, long thoracic, subscapular, suprascapular, cranial and caudal pectoral, thoracodorsal and axillary nerves were observed to be restricted to the most proximal portion of the limb (scapular region and gleno-humeral joint). The musculocutaneous nerve was visualised until the cubital region, while the radial, median and ulnar nerves were observed until the most distal portions of the limb, being responsible for the innervation of the muscles of the forearms and hands.
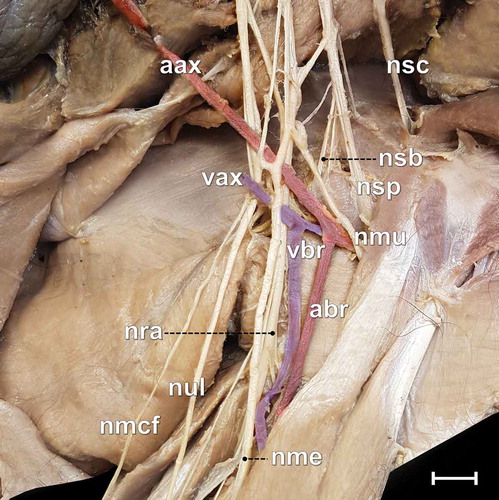
The median nerve presented two communicating branches with the musculocutaneous nerve, as follows: a proximal one on the level of cranial and medium trunks, and a distal one on the distal portion of the arm (). In both antimeres, the formation of an axillary “ansa” was observed, composed by the communication between the medium and caudal trunks for the formation of the ulnar nerve.
Figure 3. Photomacrograph of the medial surface of the right arm of an adult male specimen of Alouatta guariba clamitans, evidencing the relations between nerves and blood vessels. aax = axillary artery; abr = brachialis artery; nmcf = medial cutaneous of the forearm nerve; nme = median nerve; nmu = musculocutaneous nerve; nra = radial nerve; nsb = subscapularis nerve; nsc = subclavius nerve; nsp = suprascapularis nerve; nul = ulnaris nerve; vax = axillary vein; vbr = brachialis vein. Scale bar: 10 mm.
Discussion
Among the 10 dissected plexuses, all presented their origin in the ventral branches of C5 to T1, although in one animal there was a delicate contribution of C4, and in another one there was a delicate contribution of T2. The origin of the plexus between C5 and T1 is very nearly a rule for primates, having been reported in G. senegalensis (Kanagasuntheram & Mahran Citation1960), S. sciureus (Mizuno Citation1969), P. ursinus (Booth et al. Citation1997), L. lagothricha (Cruz & Adami Citation2010), M. mulatta (Lu et al. Citation2013; Santos-Sousa et al. Citation2016) and human beings (Parada et al. Citation1989; Zhang et al. Citation2016). However, intraspecific variations concerning the spinal segments of origin are also reported in those studies that used more specimens, as in the case of C. apella (Ribeiro et al. Citation2005) and M. mulatta (Santos-Sousa et al. Citation2016). The dissection of 10 plexus of A. g. clamitans allowed detection of intraspecific variations that may have implications for comparative anatomy inferences as well as in loco-regional anaesthesia procedures.
The participation of C4 in the formation of brachial plexus, observed in one specimen of A. g. clamitans, has been reported in human beings (Johnson et al. Citation2010; Zhang et al. Citation2016) and in non-human primates such as L. lagothricha (Robertson Citation1944), C. apella (Ribeiro et al. Citation2005), S. sciureus (Araújo et al. Citation2012) and M. mulatta (Santos-Sousa et al. Citation2016), which characterises a pre-fixed brachial plexus (Parada et al. Citation1989).
The contribution of T2, observed in one specimen of this study, was noted in a few specimens of C. apella (Ribeiro et al. Citation2005) and S. sciureus (Mizuno Citation1969), and in human beings (Johnson et al. Citation2010; Guday et al. Citation2017). Similarly, the participation of T2 configures a post-fixed type of plexus (Parada et al. Citation1989). In the existence of post-fixed plexus in humans, the inferior (caudal) trunk may be compressed by the first rib and induce neurovascular disorders in the thoracic limb (Guday et al. Citation2017). It is reasonable to assume that the morphofunctional complexity of the primates’ thoracic limbs is associated with the occurrence of pre- and post-fixed plexus (Ribeiro et al. Citation2005).
In fact, the origin of the brachial plexus in primates concentrates, mandatorily, between C5 and T1. Indeed, intraspecific variations in the origin and ramifications of each nerve of the plexus are more a rule than an exception (Johnson et al. Citation2010). Studies showed that more than half of the brachial plexus of human foetuses may present variations to the expected pattern (Uysal et al. Citation2003). In humans, the high incidence of variations may include the absence of nerves and antimeric asymmetries (Malukar & Rathva Citation2011). Such variations can be explained by changes in the signalling between the mesenchymal cells and the growing of neuronal cones, which can lead to considerable variations that persist to the postnatal period (Dent et al. Citation2003).
Antimeric variations in the origin of the plexus in the same individual were not found in the present study, although their occurrence has been reported in 1 in 10 specimens of M. mulatta (Santos-Sousa et al. Citation2016). The dissection of a higher number of specimens of A. g. clamitans would probably reveal some antimeric variation.
The brachial plexus constitutes of ventral branches of at least five segments, as in A. g. clamitans and other primates. This is typical of species whose thoracic limbs are versatile and act in, for example, climbing, excavation, running, swimming, handling food and/or prey capture. This also occurs in mammals of other orders whose demands of the thoracic limbs are not limited to terrestrial locomotion, such as the monotremes (Koizumi & Sakai Citation1997), Bradypus variegatus (Amorim Júnior et al. Citation2003), Hydrochaeris hydrochaeris (Fioretto et al. Citation2003), Agouti paca (Scavone et al. Citation2008), Tamandua tetradactyla (Cruz et al. Citation2012), Myrmecophaga tridactyla (Souza et al. Citation2014) and Myocastor coypus (Taketani Citation2017).
The plexus formed by only four ventral branches is suggested to be typical of species whose thoracic limbs are functionally limited to supporting the body mass and to specialised cursorial locomotion and who are constituently deprived of an ossified clavicle (Allam et al. Citation1952) as in, for example, the ungulates (Getty Citation1986).
The formation of three trunks observed in A. g. clamitans is frequently reported in humans (Johnson et al. Citation2010) and also in non-human primates (Cruz & Adami Citation2010; Kikuchi et al. Citation2011; Santos-Sousa et al. Citation2016). In S. sciureus, four trunks were recognised (Araújo et al. Citation2012). Non-primate mammals, such as monotremes (Koizumi & Sakai Citation1997), Hystrix cristata (Aydin Citation2003), Sciurus vulgaris (Aydin Citation2011), Hippopotamus amphibius (Yoshitomi et al. Citation2012), T. tetradactyla (Cruz et al. Citation2012) and Bradypus torquatus (Cruz et al. Citation2013), also form trunks in the plexus distribution. However, trunks are not identified in several other mammal species; their absence was noted in the domestic mammals (Getty Citation1986).
The ventral branch of C5 most individually contributed to the formation of the nerves of the brachial plexus in A. g. clamitans, the cranial trunk being the one from which most nerves originated. This type of counting is not usual between brachial plexus descriptions to allow comparisons, but it differs from the observations in M. mulatta, where the majority of the nerves received the contribution of C6 and C7 (Santos-Sousa et al. Citation2016). In the Neotropical canids Cerdocyon thous and Lycalopex gymnocercus, the ventral branches C7 and C8 predominated (Souza-Junior et al. Citation2014, Citation2017), yet C5 does not take part in the plexus of these species.
The suprascapular nerve of other primate species exhibited the participation of C6 almost as a rule, as described in Pongo sp. (Hepburn Citation1891), P. paniscus (Kikuchi et al. Citation2011), G. senegalensis (Kanagasuntheram & Mahran Citation1960), P. ursinus (Booth et al. Citation1997), L. lagotricha (Cruz & Adami Citation2010), S. sciureus (Araújo et al. Citation2012) and M. mulatta (Santos-Sousa et al. Citation2016). In L. lagotricha, the suprascapular nerve also innervated the deltoid muscle (Cruz & Adami Citation2010), which was not observed in A. g. clamitans or in the other primates.
The participation of C6 in the formation of subscapular nerve in every individual was expected, since the cranial trunk is always present in the formation of this nerve in primates (Booth et al. Citation1997; Cruz & Adami Citation2010; Santos et al. Citation2016; Santos-Sousa et al. Citation2016). The participation of C7 in forming this nerve has also been described in M. mulatta (Santos-Sousa et al. Citation2016).
The axillary nerve presented the most intraspecific variations in the samples of this study, although it always had the involvement of C6 and, therefore, of the cranial trunk. The contribution of C6 in the formation of the axillary nerve was observed in every species of primates for whose plexus a description was found (Paterson Citation1887; El-Assy Citation1966; Champneys Citation1975; Booth et al. Citation1997; Ribeiro et al. Citation2005; Cruz & Adami Citation2010; Araújo et al. Citation2012; Santos et al. Citation2016; Santos-Sousa et al. Citation2016). The innervation of the shoulder flexors muscles was similar to the descriptions of L. lagothricha (Cruz & Adami Citation2010), S. sciureus (Araújo et al. Citation2012) and M. mulatta (Santos-Sousa et al. Citation2016).
The formation of the musculocutaneous nerve of A. g. clamitans, essentially by the cranial trunk, was described in L. lagothricha (Cruz & Adami Citation2010), C. jacchus and C. penicillata (Santos et al. Citation2016), while the origin from both trunks, cranial and medium, was reported in P. ursinus (Booth et al. Citation1997), S. sciureus (Araújo et al. Citation2012), M. mulatta (Santos-Sousa et al. Citation2016) and humans (Nascimento et al. Citation2016). This nerve supplied the coracobrachialis, biceps brachii and brachialis muscles, similar to what was observed in L. lagotricha (Cruz & Adami Citation2010) and M. mulatta (Santos-Sousa et al. Citation2016), though in S. sciureus the brachialis muscle is not innervated by the musculocutaneous nerve (Araújo et al. Citation2012).
The origin of the median nerve primarily from cranial and medium trunks and eventually also from the caudal trunk was normal, since this nerve is inevitably formed by several ventral branches, including all the trunks (Natsis et al. Citation2016). This was the case for P. ursinus (Booth et al. Citation1997), L. lagotricha (Cruz & Adami Citation2010), P. paniscus (Kikuchi et al. Citation2011), C. jacchus and C. penicillata (Santos et al. Citation2016). Its motor innervation was directed to the pronators and flexors of the carpus and of the digit muscles as described for S. sciureus (Araújo et al. Citation2012). In L. lagotricha, it also innervated portions of the biceps brachii and triceps brachii muscle (Cruz & Adami Citation2010), which did not happen in A. g. clamitans.
The proximal communication between the musculocutaneous and median nerves formed the axillary ansa and the distal communication is associated with the brachial artery, according to reports concerning other primates (Booth et al. Citation1997; Cruz & Adami Citation2010; Araújo et al. Citation2012; Santos-Sousa et al. Citation2016). These communicating branches have a clinical importance in humans because they may compress the arteries and compromise blood supply to the limbs (Fazan et al. Citation2003; Deshmukh & Devershi Citation2006; El-Faloughy et al. Citation2013).
The radial nerve originated, invariably, from the three trunks, which indicates its high functional complexity. Its formation from all trunks is described in primates P. ursinus (Booth et al. Citation1997), C. apella (Ribeiro et al. Citation2005), L. lagotricha (Cruz & Adami Citation2010), P. paniscus (Kikuchi et al. Citation2011), S. sciureus (Araújo et al. Citation2012), C. jacchus and C. penicillata (Santos et al. Citation2016), but not M. mulatta in which the contribution of the cranial trunk was not observed (Santos-Sousa et al. Citation2016). The radial nerve was responsible for stimulating the extensor muscles of the elbow, carpus and fingers, as well as the supinator ones, which coincided with the description for L. lagotricha (Cruz & Adami Citation2010), S. sciureus (Araújo et al. Citation2012), and M. mulatta (Santos-Sousa et al. Citation2016). At the level of the elbow, it bifurcated in superficial and deep branches, which is usually observed in the extant placental species (Arlamowska-Palider Citation1970).
The same origin of the ulnar nerve of A. g. clamitans was also observed in every primate whose plexus was described, reinforcing that C7 or T2 participation is occasional, as in S. sciureus (Araújo et al. Citation2012). Like the median nerve, the ulnar nerve supplied the flexor muscles of the carpus and fingers, as described in S. sciureus (Araújo et al. Citation2012) and M. mulatta (Santos-Sousa et al. Citation2016).
In the species of the present study, the medial cutaneous nerve of the forearm was formed invariably in the caudal trunk, just as described in humans (Martini et al. Citation2009), P. ursinus (Booth et al. Citation1997), P. paniscus (Kikuchi et al. Citation2011), L. lagotricha (Cruz & Adami Citation2010) and S. sciureus (Araújo et al. Citation2012).
The presence of C5 in the formation of the subclavian nerve is referred to also in every specimen of M. mulatta, although the concomitant contributions of C4 or C6 were documented variations (Santos-Sousa et al. Citation2016). In S. sciureus, the subclavian nerve originated more cranially by the ventral branches of C4 (Araújo et al. Citation2012).
The findings on the origins of the thoracodorsal nerve in A. g. clamitans matched the description for P. ursinus (Booth et al. Citation1997) and L. lagotricha (Cruz & Adami Citation2010), where every trunk formed the nerve. In fact, the formation of this nerve widely varied among the different species of primates: C6 to C8 in C. apela (Ribeiro et al. Citation2005); C6 and C7 in P. paniscus (Kikuchi et al. Citation2011); and C7 and C8 in S. sciureus (Araújo et al. Citation2012) and M. mulatta (Santos-Sousa et al. Citation2016). Despite the variation in the formation of this nerve, its innervation for the latissimus dorsi muscle is invariable (Cruz & Adami Citation2010).
The nomenclature of the pectoral nerves in non-human primates differs in the literature, which is what makes it challenging to compare them. They may be named “anterior thoracic nerves” (El-Assy Citation1966), “major and minor pectoral nerves” (Ribeiro et al. Citation2005), “medial and lateral pectoral nerves” (Kikuchi et al. Citation2011), or there may be no distinction made between them (Cruz & Adami Citation2010; Santos et al. Citation2016). In the present study, the Nomina Anatomica Veterinaria (ICVGAN Citation2017) was followed that determines the “cranial pectoral nerve” as being the one directed to the superficial pectoral muscle and the “caudal pectoral nerve” as the one directed to the deep pectoral muscle.
The formation of the cranial pectoral nerve included the cranial and median trunk (from C5 to C7), varying in just one specimen in which the contribution of C5 did not happen. The participation of the cranial and median trunks is in agreement with the description for Pan sp., Pongo sp., Hylobates sp. and Gorilla sp. (Hepburn Citation1891; Champneys Citation1975), and P. paniscus (Kikuchi et al. Citation2011). In M. mulatta the most frequent formation was by the cranial and median trunks, although in some cases caudal trunk participation occurred, with or without the contribution of the cranial one (Santos-Sousa et al. Citation2016). The caudal pectoral nerve originated, invariably, from the median and caudal trunks in 80% of the plexus of this study, as in M. mulata (Santos-Sousa et al. Citation2016). In P. paniscus there was no contribution from the median trunk (Kikuchi et al. Citation2011).
The constitution of the long thoracic nerve of A. g. clamitans, primarily by the cranial and medium trunks, was the same as reported in chimpanzees (Champneys Citation1975) and C. apella (Ribeiro et al. Citation2005). In L. lagotrichia it is described as being formed only by C7 (Cruz & Adami Citation2010), which was observed in one specimen of A. g. clamitans. In M. mulatta the long thoracic nerve originated from C6 and C7 in the majority of the individuals, although C8 contributed in some individuals (Santos-Sousa et al. Citation2016). It innerved the thoracic portion of the serratus ventralis muscle, as described in other primates (Hepburn Citation1891; Hill Citation1972; Booth et al. Citation1997; Ribeiro et al. Citation2005; Cruz & Adami Citation2010; Santos-Sousa et al. Citation2016).
The anatomical similarity in the origin and distribution of the brachial plexus between A. g. clamitans and primates (humans and non-humans) suggests that the anaesthetic blocking techniques developed for humans may succeed when applied to howler monkeys. This was confirmed by Santos et al. (Citation2017) who used the anatomical landmarks recommended for humans (Muñoz et al. Citation2010) in one male specimen of A. g. clamitans, and obtained analgesia and muscular relaxation in the distal region to the gleno-humeral joint. Another report describes the use of the subscapular technique with palpation of the axillary pulse to create an anaesthetic block of the brachial plexus in an A. g. clamitans specimen (Ido Citation2016). In this case, the same landmarks proposed in the anaesthetic technique developed for domestic carnivores were adopted (Klaumann et al. Citation2013). Certainly, there is a gap in the anatomical basis of anaesthetic techniques not only for A. g. clamitans but also for wildlife species in general (Ido Citation2016; Santos et al. Citation2017).
Lastly, it may be concluded there was an antimeric symmetry associated with the origin and the resulting nerves in every animal of the present study. The brachial plexus of A. g. clamitans was largely formed by the contributions of the ventral spinal branches of C5 to T1, with eventual participation of C4 and T2. The branches of C5 and C7 were the ones that most contributed to the formation of the nerves of the brachial plexus.
Acknowledgements
We thank Parque Nacional da Serra dos Órgãos (PARNASO) for the donation of four specimens, and Pro-rectory of Research, Post-graduation and Innovation (PROPPI) from UNIPAMPA for the financial support for this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Allam MW, Lee DG, Nulsen FE, Fortune EA. 1952. The anatomy of the braquial plexus of the dog. The Anatomical Record 114:173–179. DOI: 10.1002/ar.1091140205.
- Almeida MAB, Santos E, Cardozo JC, Fonseca DF, Noll CA, Silveira VR, Maeda AY, Souza RP, Kanamura C, Brasil RA. 2012. Yellow fever outbreak affecting Alouatta populations in Southern Brazil (Rio Grande do Sul State), 2008-2009. American Journal of Primatology 74:68–76. DOI: 10.1002/ajp.21010.
- Amorim Júnior AA, Amorim MJAAL, Silva DR, Pimentel DS, Araújo FP, Alvim MMS. 2003. Origem do plexo braquial no bicho-preguiça (Bradypus variegatus Shinz, 1825). International Journal of Morphology 21:357–362.
- Araújo EB, Lima AR, Pinheiro LL, Muniz JAPC, Imbeloni A, Branco E. 2012. Origem e distribuição do plexo braquial de Saimiri sciureus. Pesquisa Veterinária Brasileira 32:1351–1354. DOI: 10.1590/S0100-736X2012001200022.
- Arlamowska-Palider A. 1970. Morphological studies on the main branches of the radial nerve in mammals. Acta Theriologica 15:185–197. DOI: 10.4098/AT.arch.70-12.
- Aydin A. 2003. Brachial plexus of the porcupine (Hystrix cristata). Veterinarni Medicina 48:301–304. DOI: 10.17221/5783-VETMED.
- Aydin A. 2011. The spinal nerves that constitute the brachial plexus in the red squirrel (Scurus vulgaris). Veterinarni Medicina56:405–408. DOI: 10.17221/1556-VETMED.
- Bicca-Marques JC. 2013. How do howler monkeys cope with habitat fragmentation? In: Marsh LK, Chapman CA. editors. Primates in fragments: complexity and resilience. New York: Springer Press. pp. 283–303.
- Bicca-Marques JC, Alves SL, Ingberman B, Buss G, Fries BG, Alonso A, Cunha RGT, Miranda JMD 2015. Avaliação do Risco de Extinção de Alouatta guariba clamitans Cabrera 1940 no Brasil. Processo de avaliação do risco de extinção da fauna brasileira. ICMBio. Available: http://www.icmbio.gov.br/portal/faunabrasileira/lista-de-especies/7179-mamiferos-alouatta-guariba-clamitans-guariba-ruivo. Accessed May 2018 07.
- Booth KK. 1991. The brachial plexus in the vervet monkey (Cercopithecus pygerythrus). Journal of Medical Primatology 20:23–28.
- Booth KK, Amorim MJAAL, Silva DR, Pimentel DS, Araújo FP, Alvim MMS. 1997. The brachial plexus in the Chacma baboon (Papio ursinus). Journal of Medical Primatology 26:196–203. DOI: 10.1111/j.1600-0684.1997.tb00052.x.
- Champneys F. 1975. On the muscles and of a Chimpanzee (Troglodytes niger) and a Cynocephalus anubis. Journal of Anatomy and Physiology 6:176–211.
- Chiarello AG, Galetti M. 1994. Conservation of the brown Howler monkey in Southeast Brazil. Oryx 28:37–42. DOI: 10.1017/S0030605300028271.
- Crockett CM. 1998. Conservation biology of the genus Alouatta. International Journal of Primatology 19:549–578. DOI: 10.1023/A:1020316607284.
- Cruz GAM, Adami M. 2010. Anatomia do plexo braquial de macaco-barrigudo (Lagothrix lagothricha). Pesquisa Veterinária Brasileira 30:881–886. DOI: 10.1590/S0100-736X2010001000012.
- Cruz GAM, Adami M, Almeida AEFS, Silva EAAC, Faria MMMD, Pinto MGF, Silva RDG. 2012. Características anatômicas do plexo braquial de tamanduá-mirim (Tamandua tetradactyla Linnaeus, 1758). Revista Brasileira De Saúde E Produção Animal13:712–719. DOI: 10.1590/S1519-99402012000300011.
- Cruz GAM, Adami M, Oliveira VL. 2013. Características anatômicas do plexo braquial de bicho-preguiça-de-coleira (Bradypus torquatus Illiger, 1811). Biotemas 26:195–201.
- Dent EW, Tang F, Kalil K. 2003. Axon guidance by growth cones and branches: Common cytoskeletal and signaling mechanisms. Neuroscientist 9:343–353. DOI: 10.1177/1073858403252683.
- Deshmukh AG, Devershi DB. 2006. Bilateral variation in the formation of median nerve. Journal of Mahatma Gandhi Institute of Medical Sciences 11:36–39.
- Drubbel RV, Gautier JP. 1993. On the occurrence of nocturnal and diurnal loud calls, differing in structure and duration, in red howlers (Alouatta seniculus) of French Guiana. Folia Primatologica 60:195–209. DOI: 10.1159/000156693.
- El-Assy YS. 1966. Beiträge zur morphologie des periphren nerven systems der Primaten. Gegenbaurs Morphologisches Jahrbuch 108:476–567.
- El-Falougy H, Selmeiova P, Kubikova E, Stenova J, Haviarova Z. 2013. The variable communicating branches between musculocutaneous and median nerves: A morphological study with clinical implications. Bratislavské Lekárske Listy 114:290–294.
- Fazan VPS, Amadeu ADS, Caleffi AL, Rodrigues Filho OA. 2003. Brachial plexus variations in its formation and main branches. Acta Cirúrgica Brasileira 18:14–18. DOI: 10.1590/S0102-86502003001200006.
- Fioretto ET, Castro MFS, Guidi WL, Mainardi R, Souza RR, Ribeiro AACM. 2003. Gross anatomic organization of the capybara’s (Hydrochaeris hydrochaeris) brachial plexus. Anatomia, Histologia, Embryologia 32:169–174. DOI: 10.1046/j.1439-0264.2003.00453.x.
- Futema F, Fantoni DT, Auler Jr JOC, Cotorpassi SRG, Acaui A, Stopiglia AJ. 1999. Nova técnica de bloqueio do plexo braquial em cães. Ciência Rural 29:63–69. DOI: 10.1590/S0103-84781999000100012.
- Getty R. 1986. Anatomia dos Animais Domésticos. 5th. Rio de Janeiro: Guanabara Koogan. 2000 pp.
- Gregorin R. 2006. Taxonomia e variação geográfica das espécies do gênero Alouatta Lacépède (Primates, Atelidae) no Brasil. Revista Brasileira De Zoologia 23:64–144. DOI: 10.1590/S0101-81752006000100005.
- Guday E, Bekele A, Muche A. 2017. Anatomical study of prefixed versus postfixed brachial plexuses in adult human cadaver. ANZ Journal of Surgery 87:399–403. DOI: 10.1111/ans.13534.
- Hepburn D. 1891. The comparative anatomy of the muscles and nerves of the superior and inferior extremities of the anthropoid apes Part I. Journal of Anatomy and Physiology 26:149–185.
- Hill WCO. 1972. Primates: comparative anatomy and taxonomy V Cebidae - Part B. Edinburgh: University Press.
- Ido CK. 2016. Bloqueio do plexo braquial em bugio (Alouatta caraya): relato de caso. Trabalho de Especialização (Programa de Aprimoramento Profissional - SES-SP). Jaboticabal: Universidade Estadual Paulista, Faculdade de Ciências Agrárias e Veterinárias.
- International Committee on Veterinary Gross Anatomical Nomenclature. 2017. Nomina Anatomica veterinaria. 6th. Knoxville, TN: World Association of Veterinary Anatomists, Editorial Committee.
- Johnson EO, Vekris M, Demesticha T, Soucacos PN. 2010. Neuroanatomy of the brachial plexus: Normal and variant anatomy of its formation. Surgical and Radiologic Anatomy 32:291–297. DOI: 10.1007/s00276-010-0646-0.
- Kanagasuntheram R, Mahran ZY. 1960. Observations on the nervous system of the lesser bush baby (Galago senegalensis senegalensis). Journal of Anatomy 94:512–527.
- Kikuchi Y, Oishi M, Shimizu D. 2011. Morphology of brachial plexus and axillary artery in bonobo (Pan paniscus). Anatomia, Histologia, Embryologia 40:68–72. DOI: 10.1111/ahe.2011.40.issue-1.
- Klaumann PR, Portela DA, Vilani RGDC, Otero PE. 2013. Anestesia locorregional do membro torácico. In: Klaumann PB, Otero PE. editors. Anestesia locorregional em pequenos animais. São Paulo: Roca. pp. 177–212.
- Koizumi M, Sakai T. 1997. On the morphology of the brachial plexus of the platypus (Ornithorhynchus anatinus) and the echidna (Tachyglossus aculeatus). Journal of Anatomy 190:447–455. DOI: 10.1046/j.1469-7580.1997.19030447.x.
- Lokschin LX, Printes RC, Cabral JNH, Buss G. 2007. Power lines and howler conservation in Porto Alegre, RS, Brazil. Neotropical Primates 14:76–80. DOI: 10.1896/044.014.0206.
- Lu Q, Gu L, Jiang L, Qin B, Fu G, Li X, Yang J, Huang X, Yang Y, Zhu Q, Liu X, Zhu J. 2013. The upper brachial plexus defect model in rhesus monkeys: A cadaveric feasibility study. Neuroreport 24:884–888. DOI: 10.1097/WNR.0000000000.000011.
- Malukar O, Rathva A. 2011. A study of 100 cases of brachial plexus. National Journal of Community Medicine 2:166–170.
- Martini FH, Timmons MJ, Tallitsch RB. 2009. Anatomia Humana. 6th. Porto Alegre: Artmed.
- Mencalha R, Santos-Sousa CA, Costa OM, Abidu-Figueiredo M. 2016. Ultrasound and gross anatomy of the brachial plexus and major nerves of the forelimb: An anesthetic approach using the domestic rabbit (Oryctolagus cuniculus) as an experimental model. Acta Cirúrgica Brasileira 31:218–226. DOI: 10.1590/S0102-865020160040000001.
- Mistry T, Mangal V, Sharma G, Agrawal A. 2016. Assessment of variation in depth of brachial plexus using ultrasound for supraclavicular brachial plexus block in patients undergoing elective upper limb surgery. Indian Journal of Anaesthesia 60:393–397. DOI: 10.4103/0019-5049.183385.
- Mizuno N. 1969. The brachial plexus in the squirrel monkey (Saimiri sciureus). Primates 10:37–40. DOI: 10.1007/BF01730807.
- Muñoz CM, Rot G, Navas AM, Moreno ME. 2010. Estudio comparativo de la eficácia del bloqueo supraclavicular em la artroscopia de hombro. Revista De La Sociedad Española Del Dolor 17:366–371. DOI: 10.1016/j.resed.2010.09.005.
- Nascimento SR, Ruiz CR, Pereira E, Andrades L, De Souza CC. 2016. Rare anatomical variation of the musculocutaneous nerve - Case report. Revista Brasileira De Ortopedia 51:366–369. DOI: 10.1016/j.rbo.2015.08.005.
- Natsis K, Paraskevas G, Tzika M. 2016. Five roots pattern of median nerve formation. Acta Medica (Hradec Kralove) 59:26–28. DOI: 10.14712/18059694.2016.52.
- Parada H, Pineda UH, Lagunas EM, Vidal HA. 1989. Variaciones anatômicas de las ramas raquídeas que constituyen los troncos de origen del plexo braquial. Anales De Anatomía Normal 7:22–26.
- Paterson AM. 1887. The limb plexuses of mammals. Journal of Anatomy and Physiology 21:611–634.
- Printes RC, Liesenfeld MVA, Jerusalinsky L. 2001. Alouatta guariba clamitans Cabrera, 1940: A new southern limit for the species and for Neotropical primates. Neotropical Primates 9:118–121.
- Ribeiro AR, Prada ILS, Barros RAC, Silva DCO. 2005. Origem do plexo braquial do macaco Cebus apella. Brazilian Journal of Veterinary Research and Animal Science 42:143–149. DOI: 10.11606/issn.1678-4456.bjvras.2005.26445.
- Robertson DF. 1944. Anatomy of the South American woolly monkey (Lagothrix). Part 1: The forelimb. Zoologica 29:169–192.
- Santos ER, Barni BS, Colombi1 LAF, Braga1 CS, Mombach VS, Muccillo MS, Alievi MM, Contesini EA. 2017. Bloqueio de plexo braquial em um bugio-ruivo (Alouatta guariba): Relato de caso. Arquivo Brasileiro De Medicina Veterinária E Zootecnia 69:1186–1190. DOI: 10.1590/1678-4162-9303.
- Santos PRS, Silva MHR, Rodrigues AR, Assis Neto AC. 2016. Descrição anatômica do plexo braquial de Callithrix jacchus e Callithrix penicillata. Pesquisa Veterinária Brasileira 36:901–904. DOI: 10.1590/s0100-736x2016000900017.
- Santos-Sousa CA, Gomes MS, Carvalho NC, Souza-Júnior P, Dos Santos CM, Abidu-Figueiredo M. 2016. Origin and antimeric distribution of brachial plexus nerves in Macaca mulatta (Zimmermann, 1780) (Primates: Cercopithecidae). Italian Journal of Zoology 83:469–481. DOI: 10.1080/11250003.2016.1258438.
- Scavone ARF, Machado MRF, Guimarães GC, Oliveira FS, Gerbasi SHB. 2008. Análise da origem e distribuição dos nervos periféricos do plexo braquial da paca (Agouti paca Linnaeus, 1766). Ciência Animal Brasileira 9:1046–1055.
- Shinn HK, Kim BG, Jung JK, Kwon HU, Yang C, Won J. 2016. Prolonged hemidiaphragmatic paresis following continuous interscalene brachial plexus block: A case report. Medicine (Baltimore) 95:1–5. DOI: 10.1097/MD.0000000000003891.
- Silva FE, Bicca-Marques JC. 2013. Do patch size and interpatch distance influence the distribution of Brown Howler Monkeys (Alouatta guariba clamitans) in a fragmented landscape in South Brazil? In: Marsh LK, Chapman CA. editors. Primates in fragments: complexity and resilience. New York: Springer Science Business Media. pp. 137–145.
- Smith RJ, Jungers WL. 1997. Body mass in comparative primatology. Journal of Human Evolution 32:523–559. DOI: 10.1006/jhev.1996.0122.
- Souza Junior P, Carvalho NC, Mattos K, Abidu-Figueiredo M, Santos ALQ. 2017. Brachial plexus in the Pampas Fox (Lycalopex gymnocercus): A descriptive and comparative analysis. The Anatomical Record 300:537–548. DOI: 10.1002/ar.23509.
- Souza Junior P, Carvalho NC, Mattos K, Santos ALQ. 2014. Origens e ramificações do plexo braquial no cachorro-do-mato Cerdocyon thous (Linnaeus, 1766). Pesquisa Veterinária Brasileira 34:1011–1023. DOI: 10.1590/S0100736X2014001000015.
- Souza PR, Cardoso JR, Araujo LBM, Moreira PC, Cruz VS, Araujo EG. 2014. Gross anatomy of the brachial plexus in the Giant Anteater (Myrmecophaga tridactyla). Anatomia, Histologia, Embryologia 43:341–345. DOI: 10.1111/ahe.2014.43.issue-5.
- Taketani M. 2017. Comparative anatomy of the brachial plexus in Coypu (Myocastor coypus; Rodentia). Naturalistae 21:17–22.
- Uysal II, Seker M, Karabulut AK, Buyukmumcu M, Ziylan T. 2003. Brachial plexus variations in human fetuses. Neurosurgery 53:676–684. DOI: 10.1227/01.NEU.0000079485.24016.70.
- Yoshitomi S, Kawashima T, Murakami K, Takayanagi M, Inoue Y, Aoyagi R, Sato F. 2012. Anatomical architecture of the brachial plexus in the common hippopotamus (Hippopotamus amphibius) with special reference to the derivation and course of its unique branches. Anatomia, Histologia, Embryologia 41:280–285. DOI: 10.1111/ahe.2012.41.issue-4.
- Zhang L, Dong Z, Zhang CL, Gu YD. 2016. Surgical anatomy of the radial nerve at the elbow and in the forearm: Anatomical basis for intraplexus nerve transfer to reconstruct thumb and finger extension in C7 - T1 brachial plexus palsy. Journal Reconstructive Microsurgery 32:670–674. DOI: 10.1055/s0036-1584687.
