Abstract
Many metabolic consequences of the excess lipid storage observed in obesity have been documented in humans and mammals, but the effects of an obese phenotype on reproductive behavior are less understood. Drosophila melanogaster (Meigan, 1830) is well suited to study the ramifications of excess energy storage as they are commonly used to explore the genes important for energy utilization. However, little is known about the reproductive behavior of mutants in these metabolic genes. Therefore, we tested, for the first time, courtship of the naturally occurring adipose (adp60) mutation which accumulates excess triglycerides and glycogen. Adipose mutants showed decreased courtship behaviors as well as copulation rate. The decrease in copulation rate could in part be explained by the decrease in courtship behaviors such as wing scissoring and wing vibration. Taken together, the data presented here suggest that the adp60 mutants may negatively affect male fly reproductive behavior and provide a system to further our understanding of the genetic and metabolic control of reproductive behaviors and reproductive potential.
Introduction
Obesity is a condition whereby excess fat is stored in the body and affects almost 40% of the United States population in 2015–2016 (CDC, Citation2019). Obesity is associated with a number of other metabolically related diseases such as Type II Diabetes, cardiovascular disease and hypertension thus increasing the rate of mortality of these individuals (reviewed in Abdelaal et al. Citation2017). Obesity and its associated metabolic syndromes are also associated with a number of reproductive problems, such as polycystic ovary syndrome and male infertility (Haslam & James Citation2005; Martins et al. Citation2019); in fact, links between nutrient storage and the physiological ability to carry out reproduction have been observed across many different species (mammals: reviewed in Gittleman & Thompson Citation1988; Bronson Citation2000; Schneider & Wade Citation2000; Schneider Citation2004; birds: reviewed in Martin Citation1987; Sibly et al. Citation2012; reptiles: Van Dyke et al. Citation2014; Lind & Beaupre Citation2015). Yet the relationship between obesity phenotypes such as excess nutrient storage, and reproductive behaviors, such as courtship, is not as well known. Such data could provide the beginnings of a framework to understand how the various mechanisms and traits associated with obesity shape reproductive drive and ability.
Recently, the fruit fly, Drosophila melanogaster, has emerged as an excellent model organism to study obesity due to its high genetic conservation to humans, its ease of obtaining large sample sizes, the ease of genetic and dietary manipulations (Musselman & Kühnlein Citation2018). In D. melanogaster, there exist mutants in metabolic genes that result in lean or obese phenotypes (Baker & Thummel Citation2007). One such gene is adipose (adp). The most well-characterized mutation of this gene is adp60, a 23-nucleotide deletion resulting in increased storage of triglycerides in the fly fat body, providing an obesity phenotype (Häder et al. Citation2003; Suh et al. Citation2007; Reis et al. Citation2010). In addition, adp60 mutants also are resistant to starvation and have decreased mobility compared to wildtype controls (Suh et al. Citation2007). While much is known about the metabolic consequences due to the loss of the adipose gene, little is known about how the adp60 mutation affects reproductive behaviors. Previous studies have characterized reproductive behaviors in flies with other metabolic perturbations such as wildtype flies with diet-induced obesity (Schultzhaus et al., 2017, 2018), and characterizing the courtship of the adp60 mutant, would complement these studies.
In this study, we compare the the courtship from adp60 male flies to wild type male flies in order to determine the effects of the adp60 mutation on reproductive behaviors. For the purposes of this study, wildtype female flies are used throughout in order to isolate any potential effects of the adp60 mutation and obesity on male courtship decisions. Previous studies have shown that wildtype male flies fed a high-fat diet show no effect of the resulting obesity on reproductive behaviors if fed the high-fat diet throughout development (Schultzhaus et al. Citation2017), but wildtype male flies do show reductions in some aspects of courtship if fed a high-fat diet only as an adult (Schultzhaus et al. Citation2018). In both cases, however, copulation rate is not affected. Altering the expression of the adp obesity gene may mimic one of these scenarios, or might have entirely different ramifications. For example, the adp60 mutation might be associated with an increase in courtship behaviors resulting in an increase in copulation rate. Alternatively, it is possible that adp60 mutants will show lower levels of reproduction compared to the wildtype, as the adp60 mutant has also been described as having a “lethargic” phenotype (i.e., a general overall lower activity level compared to wildtype; Suh et al. Citation2007) and it is unclear if this lethargy phenotype is relevant to reproductive behaviors. We believe that characterizing the reproductive behaviors of adp60 mutants will provide a better understanding of the effects of obesity on outward male sexual behavior.
Materials and methods
Fly husbandry
Flies used in this study were: OreR (BL#2376) and adp60 outcrossed into the OreR genetic background (a gift from Ronald Kuhnlein). Flies were fed cornmeal-sucrose medium (9 g Drosophila agar, 40 g sucrose, 65 g cornmeal, 25 g Red Star whole yeast, 100 mL Karo lite corn syrup in 1200 mL dH2O). For the courtship assays, 1–2-day-old adp60 male flies were separated from females and aged 4–5 days. These 5–6-day-old adp60 males were then put into the courtship apparatus (see below) with 3–5-day-old wild type OreR virgin females. Thus, each fly was only used once during the course of this study.
Courtship analysis
One male and one female were transferred to different sides of a divider in an Aktogen® courtship chamber by cooling the flies on ice until they slowed enough to capture and transfer with forceps. Cold exposure was never more than one minute. Once both male and female flies recovered from cooling and were walking normally in the chamber, the divider was removed allowing the two flies to interact. A video camera was placed over the chamber and all pairs were video recorded for 3 h. Four seperate chambers were always run together; two wildtype male trials were always paired with two trials of adp60 mutants for a total of 114 wildtype male trials, and 75 adp60 mutant trials (total numbers differ between WT and adp60 as WT flies were used for training purposes and as a result of random incidents precluding the inclusion of flies such as escapes, deaths, or injury during trials). Lastly, all flies used were from multiple different cohorts.
Videos were uploaded to a behavioral event recorder program, Observer ®. There were three videos for flies which we know copulated that were unable to be scored due to recording or uploading errors. For the remainder of the successful videos for flies that copulated, the person scoring the behaviors did so blind to which group was being scored. The male behaviors analyzed were courtship latency, orientation, wing vibration, wing scissor, tapping, thrust, and copulation (Cobb et al. Citation1985). Orientation was defined as when the male oriented at any direction close to the female’s body and is typically done for the entirety for courtship. The start of orientation signaled the start of courtship and total courtship duration was measured by the start of orientation to copulation. Therefore, the start of when the divider was removed (thus allowing a male to see a female) to when orientation began was considered the latency to courtship. Wing vibration was when the male’s wings vibrated horizontally and vertically at different angles. This behavior is known to create the “courtship song” which is important in the courtship process of Drosophila (Ewing & Bennet-Clark Citation1968). Wing scissor was when a fly’s wings were extended, crossed and re-crossed. Both wing scissoring and wing vibrations can be held for variable amounts of time. Tapping was when the male touched any female body part, usually sideways, with its tarsus. Thrusting, which occurs during copulation and copulation attempts, was when the male grasped the female’s abdomen with his foretarsi, curled his abdomen downward and forward, making contact with the female’s genitalia. Copulation occurred when a male stabilized on top of the female for a considerable amount of time, typically 10 − 15 min.
Triglyceride, glycogen and protein measurements
To confirm the adp60 metabolic phenotype, triglyceride, glycogen and protein levels were measured as previously described (Gingras et al. Citation2014). Briefly, 2 male flies were homogenized in lysis buffer (140 mM NaCl, 50 mM Tris-HCl, pH 7.4, 0.1% Triton-X 100, and 1X protease inhibitor cocktail (Roche Life Sciences)) by performing four 10-second pulses with 10-second pauses in between using a 20 kHz sonicator. Thus, one “sample” consisted of the macromolecule levels measured in two flies; 32 flies gave a N = 16 for WT, 34 flies gave a N = 17 for adp60 mutants. These flies were different individuals than those used during the behavioral experiments and came from multiple cohorts.
Triglycerides and protein were measured using the Triglycerides Liquicolor Test (Stanbio) and the BCA Protein Assay Kit (ThermoScientific), respectively, according to manufacturer’s instructions. Total glucose levels were measured using the Glucose Oxidase Reagent (Pointe Scientific) after treating samples with 8 mg/mL amyloglucosidase (Sigma) in 0.2 M citrate buffer, pH 5.0 for 2 h. Free glucose was measured in samples not treated with amyloglucosidase and glycogen levels were determined by subtracting free glucose from total glucose. Triglycerides and glycogen were normalized by dividing each by the total protein concentration of each sample.
Statistical analysis
Copulation rate between fly types was tested via Chi-Square. Only individuals whose courtship resulted in copulation were used in the courtship analysis. All courtship behaviors (except latency to court) were corrected for the total duration of an individual’s courtship (i.e., the start of orientation to copulation) and then compared statistically. That is, a rate of behavior was calculated for each courtship behavior (i.e., number of times a behavior happened / time spent in courtship) to help account for the variation in length of courtship until copulation across individuals. Variables that did not conform to parametric assumptions were ln transformed to do so and tested via t-test. These variables include latency to court, number of wing scissors performed, and number of wing vibrations performed. The t statistic and degrees of freedom for the number of wing vibrations were adjusted for equal variances not being assumed for this variable. Variables that could be not be transformed to conform to parametric assumptions were analyzed non parametrically using a Mann Whitney U test. These variables include time of courtship, rate of wing scissoring, rate of wing vibrations, number of thrusts, and rate of thrusts. Rates were calculated as the total time engaged in a behavior divided by the time of courtship. Tapping behavior did not occur frequently enough to warrant analysis and is therefore not presented. Both glycogen per protein and triglycerides per protein ratios met parametric assumptions and were analyzed across fly types with t-tests. The t statistic and degrees of freedom for triglycerides per protein ratios were adjusted for equal variances not being assumed. All analyses, transformations, and statistical adjustments were done using SPSS® version 25.
Results
Triglyceride and glycogen levels
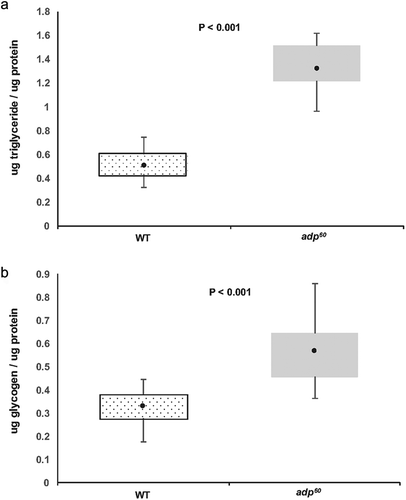
Consistent with previous reports, adp60 mutants had a higher triglyceride per protein ratio than wild type flies (t31 = 14.733, P < 0.001; . In addition, adp60 mutants had a significantly higher glycogen to protein ratio than wild type flies (t28.393 = 5.695, P < 0.001; ).
Figure 1. (a) Triglyceride and (b) glycogen content per total body protein for wild type (WT; stipple box) and adipose mutant (adp60; gray box) flies. Boxes indicate interquartile ranges (IQR), dark circles indicate means, and whiskers indicate maximum and minimum values within 1.5x IQR. Significant comparisons indicated by P values <0.05.
Copulatory and courtship behavior
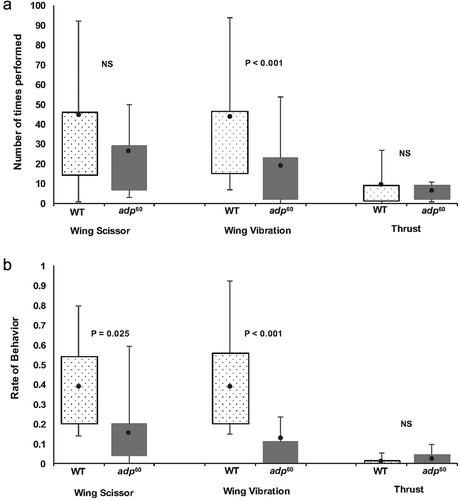
The adp60 mutants had a significantly lower rate of copulations relative to wild type flies (Χ 2 1, N = 189 = 25.95, P < 0.001) with adp60 mutants copulating at a rate of 17.3% (13/75) and wild type flies at a rate of 54.4% (62/114). Of the males who successfully copulated, there was no significant difference between fly types in total time engaged in courtship (Mann Whitney U: U = 351, Z = -0.476, N = 72, P = 0.636, nor latency to court (t70 = - 1.433, P = 0.156). However, some aspects of each courtship behavior measured did differ. There was no significant difference in the total number of wing scissors (t70 = - 1.433, P = 0.077, ), yet wild type males did spend more time wing scissoring per total time of courtship than adp60 mutants (Mann Whitney U; U = 148, Z = −3.448, N = 72, P = 0.001, ). Wild type males also did significantly more wing vibrations than adp60 mutants (t12.924 = - 2.545, P = 0.025) and spent more time doing these wing vibrations per total time of courtship than adp60 mutants (Mann Whitney U; U = 120, Z = −3.858, N = 72, P < 0.001). There was no significant difference between fly types for number of thrusts (Mann Whitney U; U = 333.5, Z = −0.737, P = 0.461) or the rate of thrusts per total time of courtship (Mann Whitney U; U = 321, Z = −0.915, P = 0.360).
Figure 2. Length of courtship and courtship latency for wild type flies and adipose mutants. See formatting and label details. “NS” indicates comparisons not significantly different at P > 0.05.
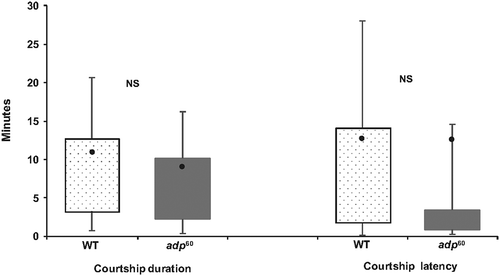
Figure 3. (a) The number of times and (b) the rate of courtship behavior (wing scissor, wing vibration, and thrusts) that occurred in wild type and adipose mutants. See for formatting and label details. Significant comparisons indicated by P values; “NS” indicates comparisons not significantly different at P > 0.05.
Discussion
In this study, the reproductive behaviors of the obese adp60 Drosophila mutant were characterized. The adp60 mutants had a lower copulation rate than the wildtype controls, consistent with the decreases in courtship behaviors observed in these animals. Therefore, it is possible that the excess nutrient storage in these mutants may be related to these behavioral differences, but also could be an independent behavioral phenotype associated with the adp60 mutation. Surprisingly, courtship latency, and total courtship time, between the wildtype and adp60 mutants were similar despite adp60 exhibiting lower levels of courtship behaviors during courtship. Previous studies have shown that wildtype flies fed a high fat diet also accumulate triglycerides (but not glycogen) and have similar courtship latencies as wildtype flies fed a normal diet (Birse et al. Citation2010; Schultzhaus et al. Citation2017, Citation2018). However, these high-fat fed flies have normal copulation frequencies (Schultzhaus et al. Citation2017) unlike what was observed from our current study between adp60 and wildtype flies (though Schultzhaus et al. (Citation2018) argues that high-fat fed flies are less attractive than wildtype flies fed a normal diet). Therefore, some traits that are unique to the adp60 phenotype affect mating success in a way not previously reported for high fat diet flies. Such traits could include the extent to which triglycerides accumulate, the accumulation of glycogen, some combination of these two characteristics, and/or some independent behavioral phenotype associated with the adp60 mutation.
Despite the similar courtship times, latency to court, and thrusting behavior, adp60 mutants showed significantly less wing scissoring and wing vibrations. These flies had a constant availability of food but do not metabolize their triglycerides and glycogen in the same way as wild type flies (thus leading to an increased amount of each in our analysis). Potentially it is the availability and ingestion of nutrients that are involved with reproductive drive (i.e. overall courtship time and courtship latency), while the appropriate storage and usage of these sources of energy, once ingested, are involved in carrying out courtship, including the courtship song caused by the wing vibration behavior. The adp60 mutants are known to have a “lethargic” phenotype where their overall mobility is less than that of wild type flies (Suh et al. Citation2007). This phenotype may be due to this altered ability to metabolize nutrients and/or the gross obesity of these animals and could be related to the decreased courtship behaviors while reproductive drive behaviors such as latency to court remain unaffected.
It is unclear why the adp60 mutant contains higher levels of glycogen and triglycerides yet courted less than wild type flies, but many possibilities exist, each of which could also contribute to the overall reduced mobility phenotype of the adp60 mutant described above. One possibility is that the physical structure of increased fat deposits decreases wing dexterity therefore affecting courtship song however, Schultzhaus et al. (2018) presented that wild type male flies fed a high-fat diet showed no significant effect on courtship song compared to wild type male flies fed a normal diet. Another possibility is that the adp60 mutation has additional unrealized effects on muscle activity. The adp60 mutation results in chronic obesity with excess fat accumulation in both the larval and adult stages of development (Häder et al. Citation2003; Suh et al. Citation2007; Reis et al. Citation2010). Potentially these mutants do not mobilize these fuels in the same way as wild type flies; lipid metabolism in these mutants is suspected to be different throughout development (see Teague et al. Citation1986 for further discussion). Any negative effect these metabolic pathways have on muscle function, in turn, could limit muscle from functioning properly and affect movement involved in courtship such as wing scissors and wing vibrations (song). Courtship song is well known to play an important role in mating within insect groups generally (reviewed in Wells & Henry Citation1998), and specifically within the Drosophila genus (Ewing & Bennet-Clark Citation1968; Spieth Citation1974; Hall Citation1994). Chakravorty et al. (Citation2014) showed that courtship song is changed in Drosophila mutants for a major wing muscle gene, and the resulting alterations to courtship song can decrease female preference for that male. The Drosophila courtship song is known to contain important components in order for it to be successful, and therefore be a “quality” song. Specifically, there are both “pulse” and “sine” aspects to song components (von Schilcher Citation1976), and alterations to these aspects of song can influence female preference for the male performing the song (von Schilcher Citation1976: Chakravorty et al. Citation2014). If the adp60 phenotype is associated with an alteration to courtship song, it is unclear what the specific alteration might be. However, if the song is altered as suggested by the decrease in wing scissors and vibrations and the subsequent lack of copulations, it is possible that adp60 mutant males might have a reduced ability for generating song or might simply be choosing to decrease performing their song (due either to a female’s lack of interest and/or the males are negatively responding to their own altered song). While we cannot address which of these mechanisms is at play (or any combination thereof), they would all lead to a reduced amount of time performing song during courtship, and/or low-quality song, that could lead to an increased rejection by the female fly. In fact, the lack of courtship song from the adp60 mutants could help explain the low copulation rates of this group as females of various Drosophila species are known to reject males on the basis of song (Hoikkala et al. Citation1998; Ritchie et al. Citation2001).
Overall, we find that courtship behaviors are altered in the obese adp60 mutant in the fruit fly, D. melanogaster as this has not been described previously. More research is needed to identify the exact physiological cue that activates reproductive behavior across species and better understand the genes and physiological processes important for regulating reproductive motivation and behavior. Species such as D. melanogaster are particularly powerful in this regard as such mutants exist both naturally, such as the adp60 mutants used here, and can be artificially created to probe the effects of specific genes and metabolic processes on organismal reproduction to better understand the reproductive ramifications the obesity phenotype and its associated traits.
copulation_picture.docx
Download MS Word (975 KB)Acknowledgements
We would like to thank Grzegorz Polak and Justin Palermo for help with fly husbandry. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abdelaal M, le Roux CW, Docherty NG. 2017. Morbidity and mortality associated with obesity. Annals of Translational Medicine 5:161. DOI: 10.21037/atm.
- Baker KD, Thummel CS. 2007. Diabetic larvae and obese flies—Emerging studies of metabolism in Drosophila. Cell Metabolism 6:257–266. DOI: 10.1016/j.cmet.2007.09.002.
- Birse RT, Choi J, Reardon K, Rodriguez J, Graham S, Diop S, Ocorr K, Bodmer R, Oldham S. 2010. High-fat-diet-induced obesity and heart dysfunction are regulated by the TOR pathway in Drosophila. Cell Metabolism 12:533–544. DOI: 10.1016/j.cmet.2010.09.014.
- Bronson F. 2000. Puberty and energy reserves: A walk on the wild side. In: Wallen K, Schneider JE, editors. Reproduction in context: Social and environmental influences on reproduction. Cambridge: MIT Press. pp. 15–33.
- Center of Disease Control (CDC). Available: https://www.cdc.gov/obesity/data/adult.html. Accessed Jan 2019 09.
- Chakravorty S, Vu H, Foelber V, Vigoreaux JO. 2014. Mutations of the Drosophila myosin regulatory light chain affect courtship song and reduce reproductive success. Plos One 9:e90077.
- Cobb M, Connolly K, Burnet B. 1985. Courtship behaviour in the melanogaster species sub-group of Drosophila. Behaviour 95:203–230. DOI: 10.1163/156853985X00136.
- Ewing AW, Bennet-Clark H. 1968. The courtship songs of Drosophila. Behaviour 31:288–301. DOI: 10.1163/156853968X00298.
- Gingras RM, Warren ME, Nagengast AA, DiAngelo JR. 2014. The control of lipid metabolism by mRNA splicing in Drosophila. Biochemical and Biophysical Research Communications 443:672–676. DOI: 10.1016/j.bbrc.2013.12.027.
- Gittleman JL, Thompson SD. 1988. Energy allocation in mammalian reproduction. American Zoologist 28:863–875. DOI: 10.1093/icb/28.3.863.
- Häder T, Müller S, Aguilera M, Eulenberg KG, Steuernagel A, Ciossek T, Kühnlein RP, Lemaire L, Fritsch R, Dohrmann C, Vetter IR. 2003. Control of triglyceride storage by a WD40/TPR‐domain protein. EMBO Reports 4:511–516. DOI: 10.1038/sj.embor.embor837.
- Hall JC. 1994. The mating of a fly. Science 264:1702-1714.
- Haslam DW, James WP. 2005. Obesity. The Lancet 366:1197–1209. DOI: 10.1016/S0140-6736(05)67483-1.
- Hoikkala A, Aspi J, Suvanto L 1998. Male courtship song frequency as an indicator of male genetic quality in an insect species, Drosophila montana. Proceedings of the Royal Society of London B: Biological Sciences 265:503–508.
- Lind CM, Beaupre SJ. 2015. Male snakes allocate time and energy according to individual energetic status: Body condition, steroid hormones, and reproductive behavior in timber rattlesnakes, Crotalus horridus. Physiological and Biochemical Zoology 88:624–633. DOI: 10.1086/683058.
- Martin TE. 1987. Food as a limit on breeding birds: A life-history perspective. Annual Review of Ecology and Systematics 18:453–487. DOI: 10.1146/annurev.es.18.110187.002321.
- Martins AD, Majzoub A, Agawal A. 2019. Metabolic syndrome and male fertility. The World Journal of Men’s Health 37:113–127. DOI: 10.5534/wjmh.180055.
- Musselman LP, Kühnlein RP. 2018. Drosophila as a model to study obesity and metabolic disease. Journal of Experimental Biology 221:jeb163881. DOI: 10.1242/jeb.163881.
- Reis T, Van Gilst MR, Hariharan IK. 2010. A buoyancy-based screen of Drosophila larvae for fat-storage mutants reveals a role for Sir2 in coupling fat storage to nutrient availability. PLoS Genetics 6:e1001206. DOI: 10.1371/journal.pgen.1001206.
- Ritchie MG, Saarikettu M, Livingstone S, Hoikkala A. 2001. Characterization of female preference functions for Drosophila montana courtship song and a test of the temperature coupling hypothesis. Evolution 55:721–727. DOI: 10.1554/0014-3820(2001)055[0721:COFPFF]2.0.CO;2.
- Schneider JE, Wade GN. 2000. Inhibition of reproduction in service of energy budget. In: Wallen K, Schneider JE, editors. Reproduction in context: Social and environmental influences on reproduction. Cambridge: MIT Press. pp. 15–33.
- Schneider JE. 2004. Energy balance and reproduction. Physiology & Behavior 81:289–317. DOI: 10.1016/j.physbeh.2004.02.007.
- Schultzhaus JN, Bennett CJ, Iftikhar H, Yew JY, Mallett J, Carney GE. 2018. High fat diet alters Drosophila melanogaster sexual behavior and traits: Decreased attractiveness and changes in pheromone profiles. Scientific Reports 8:5387. DOI: 10.1038/s41598-018-23662-2.
- Schultzhaus JN, Nixon JJ, Duran JA, Carney GE. 2017. Diet alters Drosophila melanogaster mate preference and attractiveness. Animal Behaviour 123:317–327. DOI: 10.1016/j.anbehav.2016.11.012.
- Sibly RM, Witt CC, Wright NA, Venditti C, Jetz W, Brown JH 2012. Energetics, lifestyle, and reproduction in birds. Proceedings of the National Academy of Sciences 109:10937–10941.
- Spieth HT. 1974. Courtship behavior in Drosophila. Annual review of entomology. 19: 385–405.
- Suh JM, Zeve D, McKay R, Seo J, Salo Z, Li R, Wang M, Graff JM. 2007. Adipose is a conserved dosage-sensitive antiobesity gene. Cell Metabolism 6:195–207. DOI: 10.1016/j.cmet.2007.08.001.
- Teague BD, Clark AG, Doane WW. 1986. Developmental analysis of lipids from wild‐type and adipose60 mutants of Drosophila melanogaster. Journal of Experimental Zoology 240:95–104. DOI: 10.1002/(ISSN)1097-010X.
- Van Dyke JU, Griffith OW, Thompson MB. 2014. High food abundance permits the evolution of placentotrophy: Evidence from a placental lizard, Pseudemoia entrecasteauxii. The American Naturalist 184:198–210. DOI: 10.1086/677138.
- von Schilcher F. 1976. The function of pulse song and sine song in the courtship of Drosophila melanogaster. Animal Behaviour. 24:622–625.
- Wells M, Henry CS. 1998. Songs, reproductive isolation, and speciation in cryptic species of insects. In: Howard DJ, Berlocher SH editors. In: Endless forms: species and speciation. Oxford University Press pp. 217–233
