Abstract
Pycnogonida collected monthly from September 2017 to August 2018 in the Portofino Marine Protected Area at 0–5 m depth were studied. A total of 499 specimens were collected, 457 of which were identified to species level. These were classified as belonging to 10 species: Achelia echinata*, Ascorhynchus castelli, Neotrygaeus communis*, Tanystylum conirostre*, Anoplodactylus angulatus, A. petiolatus, A. pygmaeus*, A. virescens, Callipallene phantoma and C. tiberi*. For five dominant species (those marked with an asterisk) the annual phenology was outlined. Four hundred and seventeen additional specimens, collected from the same area and depth range mainly during the 1970s and 1980s were identified to species level for completeness of information, leading to the addition of Pycnogonum pusillum and Endeis spinosa.
Keywords:
Introduction
The Pycnogonida is a poorly known class of marine arthropods represented by 1350 species described (Bamber et al. Citation2019). Lehmann et al. (Citation2014) report that 56 species are known for the Mediterranean Sea, consisting mostly of littoral species, while deep-sea ones are an exception. This is assumed to be due to geological and temperature barriers that isolate Mediterranean deep water and make its fauna relatively species-poor (see Lehmann et al. Citation2014). The Western Mediterranean fauna is better known: the Iberian and the Balearic waters host 36 species (Soler-Membrives & Munilla Citation2015), 37 species are present in the French part (Arnaud Citation1987) and 45 are those recorded in the Italian seas (Bartolino & Chimenz Citation2010). For the Eastern Mediterranean, there are less studies published and the number of species known is lower: for example, in the Turkish waters, only 11 species are recorded (Koçak Citation2013). Few cases of in-depth research on the Pantopoda of a specific area are known in the Italian literature and they are all focused on South-Central and Southern Italy (Krapp Citation1973; Chimenz Gusso et al. Citation1978; Chimenz et al. Citation1979, Citation1993; Chimenz & Cottarelli Citation1986; Piscitelli & Barone Citation2000). In the Ligurian Sea, mainly based on sporadic collections (Faraggiana Citation1940; Krapp Citation1975; Arnaud Citation1987; Chimenz Gusso Citation2000), 17 species have been recorded (Bartolino & Chimenz Citation2010).
This paper is based on data gathered in the Portofino Marine Protected Area, Ligurian Sea, during a recent continuing benthic biodiversity monitoring project. Data based on the identification of specimens from unstudied collections made in the same area during the 1970s and 1980s are incorporated in this report.
Materials and methods
Study area
The Portofino Marine Protected Area (MPA) covers a total area of 346 ha in the eastern Ligurian Sea (northern Mediterranean Sea), and is mainly characterized by conglomerate vertical underwater faces up to 60 m deep (Tortonese Citation1958, Citation1961). In recent years, some benthic taxa collected from these faces have been studied, i.e. macroalgae (Mangialajo et al. Citation2004), sponges (Pansini & Pronzato Citation1990; Pansini & Musso Citation1991; Pronzato et al. Citation1998) and cnidarians (Boero et al. Citation1986; Puce et al. Citation2009; Bavestrello et al. Citation2015). Some studies, driven by mass mortality following water warming events (e.g. Cerrano et al. Citation2000; Schiaparelli et al. Citation2007; Cerrano & Bavestrello Citation2008), focused on the long-term changes in structure and composition of the benthic communities (Bertolino et al. Citation2016; Betti et al. Citation2017; Longobardi et al. Citation2017).
Materials and methods
The sampling area extended for about 300 m along the vertical eastern side of Punta del Faro, the south-eastern cape of the Portofino Promontory (WGS 84 coordinates of the sampling site: 44°17ʹ55.70”N, 9°13ʹ7.15”E), at depths between the sea surface and 5 m. Sampling activities, conducted monthly between September 2017 and August 2018, were undertaken using SCUBA. Main macroalgae (Ellisolandia elongata, Flabellia petiolata, Dyctiota dichotoma) and hydroids (extremely variable along the year, but mainly Eudendrium spp., Aglaophenia spp., Halecium spp., Sertularella ellisii) were randomly collected on the vertical rocky cliff by visually oriented sampling. In total, around 113 g (standard error: 10.97) of organisms, covering a standard surface of about 900 cm2, were collected every month.
Samples were fixed in formaline 4% and carefully sorted using a stereomicroscope. The pycnogonids were transferred to ethanol 70% for further analysis.
Additional still unstudied material collected from the same area mainly during the 1970s and 1980s was identified to species level for completeness of information. In particular, we limited our analysis to samples collected at a maximum depth of 15 m compatible with the bathymetric range of the species more linked to surface waters among those identified in the recent collections (0–13 m in Tanystylum conirostre – see Munilla & Soler-Membrives Citation2014).
Identification of specimens was mainly based on descriptions of the Iberian fauna reported in Munilla and Soler-Membrives (Citation2014). For some details (e.g. the strigils spine formula) and diagnostic measurements an interference contrast microscope (Leica DM LB2), a Leica DFC 295 camera and Leica Application Suite (Vers. 3.8) was used.
All the studied material is conserved in the collection of the authors at the Department of Earth, Environment and Life Sciences (DISTAV) of Genoa University.
For the five dominant species collected between September 2017 and August 2018 and in the additional material, the annual phenology was outlined, based on the monthly presence of males carrying egg clusters, adults of both sexes and juveniles or subadults. Using these and additional data based on previous collections, the sex ratio (number of males/number of females) was determined. The statistical significance of the differences from the expected value (1) of the species sex ratio was assessed via the Chi-square test, using PAST software (Hammer et al. Citation2001; Vers. 3.22, last accessed December 2018).
Results
A total of 499 pycnogonids were collected, with a period of near constant abundances from summer to mid-winter and then an increase leading up to a peak abundance during spring (). Just 42 juveniles specimens were unidentified (or identified to genus level); the other 457 were classified to species level and assigned to 10 species: Achelia echinata Hodge, 1864, Ascorhynchus castelli (Dohrn, 1881), Neotrygaeus communis (Munilla & Alonso-Zarazago, 2014), Tanystylum conirostre (Dohrn, 1881), Anoplodactylus angulatus (Dohrn, 1881), A. petiolatus (Kröyer, 1884), A. pygmaeus (Hodge, 1864), A. virescens Hodge, 1864, Callipallene phantoma (Dohrn, 1881) and C. tiberi (Dohrn, 1881) (; ).
Table I. Monthly abundances of Pycnogonida species in Portofino (0–5 m depth) between September 2017 and August 2018.
Figure 1. a, Achelia echinata; b, Neotrygaeus communis; c, Tanystylum conirostre; d, Anoplodactylus virescens; e, Endeis spinosa. Scale bars: 2 mm.
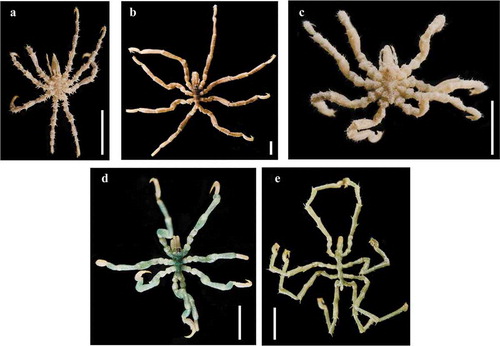
These species are known to be generally observed on algae, sponges, bryozoans and hydrozoans (see Munilla & Soler-Membrives Citation2014). The bathymetric range is narrow in N. communis (0–24 m), T. conirostre (0–13 m), A. angulatus (0–35 m), A. virescens (0–40 m), and wide in the other six species (0–537 m in A. echinata, 0–130 m in A. castelli, 0–1500 m in A. petiolatus, 0–587 m in A. pygmaeus, 0–850 m in C. phantoma and 0–523 m in C. tiberi). Moreover, A. angulatus, A. pygmaeus and A. virescens larvae are parasites of hydroids, and those of A. petiolatus can also be found on the manubrium or the bell of some jellyfish (see Chimenz Gusso Citation2000; Munilla & Soler-Membrives Citation2014).
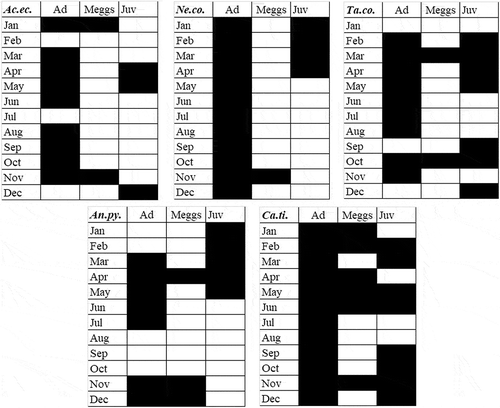
For the five dominant species, the annual phenology is outlined (). Males of A. echinata, T. conirostre and A. pygmaeus with egg clusters were recorded twice during the year; they appeared three times in C. tiberi and just once in N. communis. These observations suggest that these species are bivoltine, trivoltine and univoltine, respectively.
Figure 2. Phenology of five dominant species of sea spiders in Portofino (September 2017–August 2018): Ac.ec. = Achelia echinata; Ne.co. = Neotrygaeus communis; Ta.co. = Tanystylum conirostre; An.py. = Anoplodactylus pygmaeus; Ca.ti. = Callipallene tiberi. Ad = adults; Meggs = males with eggs clusters; Juv = Juveniles.
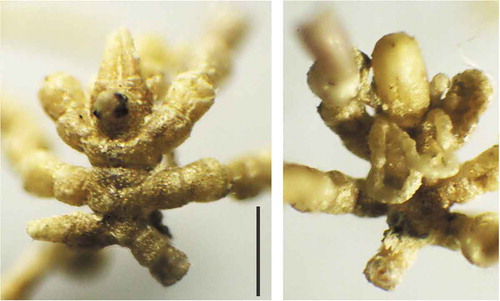
One female of N. communis collected in March 2018 was abnormal with the two central segments of the trunk modified and mutually arranged in an atypical manner and with three pairs of legs ().
Figure 3. Abnormal Neotrygaeus communis female specimen: dorsal (left) and ventral (right) view. Scale bar: 0.5 mm.
The inclusion of an additional 417 specimens confirmed the presence in the study area of nine of the species mentioned above: only A. virescens is absent in such material. However, among these pycnogonids we identified two species which we could add to the current list (): Pycnogonum pusillum, a species abundant amongst algae from 0.5 to 35 m, and Endeis spinosa (), a species found on Posidonia oceanica and algae at depths from 0 to 100 m (see Munilla & Soler-Membrives Citation2014). Larvae of this species are parasites of polyps of hydroids and jellyfish (see Chimenz Gusso Citation2000). In the phenology of the species based on the additional material is shown.
Table II. Additional material identified to species level.
Table III. Monthly phenology of Pycnogonida species in Portofino based on additional data (specimens collected mainly during the 1970s and 1980s; 0–15 m depth). A = Adults, A* = males with eggs, J = Juveniles.
Sex ratio determined for the five dominant species is shown in : in three of them (T. conirostre, A. pygmaeus and C. tiberi) a statistically significant predominance of females was recorded.
Table IV. Sex ratio of five dominant species collected in Portofino. M = number of males, F = number of females, Sex ratio = M/F, χ2 1df, P(same) and Monte Carlo P(same) = chi-square test value to assess the statistical significance of the differences from 1 of the sex ratio and relative probability of identity (also corrected based on the Monte Carlo method).
Discussion
The upper infralittoral zone of Portofino hosts a rather rich benthic fauna (e.g. Bertolino et al. Citation2016; Betti et al. Citation2017; Longobardi et al. Citation2017) and this is supported by our study of the Pycnogonida. The number of species identified in our study is close to that recorded by Chimenz et al. (Citation1979) in the Civitavecchia harbor in the Tyrrhenian Sea.
Two papers report on the collection of pycnogonids from Portofino. Faraggiana (Citation1940) recorded four species from very shallow waters: Callipallene brevirostris (probably C. spectrum, based on the taxonomical remarks of Chimenz Gusso Citation2000), Ammothea longipes, A. uniunguiculata and A. echinata. Arnaud (Citation1987) reported on three species: T. conirostre, N. communis, A. pygmaeus. Comparing our data to the list of species of the Ligurian Sea (Area 1) in Bartolino and Chimenz (Citation2010), eight species are missing in our collections: Ammothella appendiculata (Dohrn, 1881), A. longioculata (Faraggiana, Citation1940), A. longipes (Hodge, 1864), Ascorhynchus arenicola (Dohrn, 1881), Paranymphon spinosus Caullery, 1896, Pycnogonum plumipes Stock, 1968, Endeis charybdaea (Dohrn, 1881), Nymphon gracile Leach, 1814. Pycnogonum plumipes is a sciaphilous species common on coralline bottoms and present also in submarine caves and was found by Arnaud (Citation1987) near Savona (Western Liguria). All the others are species normally found in Posidonia prairies and/or on algae on sandy bottoms (see Chimenz Gusso Citation2000; Munilla & Soler-Membrives Citation2014). Two other species known from the Portofino area, Callipallene spectrum and A. uniunguiculata (Faraggiana Citation1940), were no longer detected. These species are not listed by Bartolino and Chimenz (Citation2010) amongst Ligurian Sea Pycnogonida, probably due to an oversight. The former is collected on algae between 0 and 160 m depth, while the latter is associated to Posidonia prairies and algae from 2.9 to 16 m (Chimenz Gusso Citation2000; Munilla & Soler-Membrives Citation2014). On the other hand, on the vertical rocky cliff of Portofino shallow waters, we found C. phantoma, C. tiberi and P. pusillum, which are new records for the Ligurian Sea.
The phenologies recorded for five dominant species differ at least partially from those known from literature for the same species (Munilla Leȏn Citation1980; Chimenz Gusso Citation2000), possibly indicating an influence of local and annual climatic conditions on their reproductive activity. This hypothesis seems to be confirmed also by the difference between the phenologies shown in and data about the same species in Portofino based on additional material ().
The sex ratio in favor of females evidenced in some species may be due to the particular parental care system of pycnogonids, where males carry multiple groups of eggs gathered from multiple females simultaneously (Arnaud & Bamber Citation1987). However, Achelia echinata males were slightly more abundant suggesting other influences.
Anoplodactylus angulatus postlarva is known to be a parasite of hydroids belonging to genus Tubularia (King Citation1973). No species of the genus Tubularia were found in the area during the sampling period, but they are known to be present both deeper on the same cliff face (Puce et al. Citation2009) and in the surrounding waters: for example, along a buoy chain less than 50 m distant from the survey area.
We recorded a malformation in an N. communis specimen with three segments and as many pairs of legs. Such abnormalities are not unknown in pycnogonid development and they are mainly attributable to errors in the regeneration of appendages and other body parts after being injured (except for some cases of gynandromorphism - Scholtz & Brenneis Citation2016).
Conclusions
Pycnogonids are poorly known arthropods. The preliminary results of our study of the upper infralittoral zone fauna at Portofino add to the knowledge of these animals in Italy, both as regards the geographical distribution of species identified and their phenology. It was possible to identify 12 species, three of which not previously known in the Ligurian Sea (C. phantoma, C. tiberi and P. pusillum), and for five of them (A. echinata, N. communis, T. conirostre, A. pygmaeus and C. tiberi) the annual cycle was outlined.
The continuation of this research will hopefully provide further knowledge to the species richness of the Portofino area and, thanks to future collections, could also allow us to extrapolate more detailed information on the life cycles of a larger number of species. Moreover, our purpose is to collect material also in the close Posidonia oceanica prairie to obtain data on the species related to this habitat.
Acknowledgements
The authors warmly thank Carlo Nike Bianchi, Mario Mori and Carla Morri who provided many of the specimens examined for this paper. A special thank to Dr David Staples and the other anonymous reviewer who helped us to significantly improve our paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Arnaud F. 1987. Les pycnogonides (Chelicerata) de Méditerranée: Distribution écologique, bathymetrique et biogéographique. Mésogée 47:37–58.
- Arnaud F, Bamber RN. 1987. The biology of Pycnogonida. Advances in Marine Biology 24:1–96.
- Bamber RN, El Nagar A, Arango CP Editors. 2019. Pycnobase: World Pycnogonida Database. Available: http://www.marinespecies.org/pycnobase. Accessed May 2019 9. DOI: 10.14284/360.
- Bartolino V, Chimenz C. 2010. Pycnogonida. Biologia Marina Mediterranea 17(suppl. I):394–397.
- Bavestrello G, Bo M, Bertolino M, Betti F, Cattaneo-Vietti R. 2015. Long-term comparison of structure and dynamics of the red coral metapopulation of the Portofino Promontory (Ligurian Sea): A case-study for a marine protected area in the Mediterranean Sea. Marine Ecology 36:1354–1363. DOI: 10.1111/maec.12235.
- Bertolino M, Betti F, Bo M, Cattaneo-Vietti R, Pansini M, Romero J, Bavestrello G. 2016. Changes and stability of a Mediterranean hard bottom benthic community over 25 years. Journal of the Marine Biological Association of the United Kingdom 96:341–350. DOI: 10.1017/S0025315415001186.
- Betti F, Bavestrello G, Bo M, Asnaghi V, Chiantore M, Bava S, Cattaneo-Vietti R. 2017. Over 10 years of variation in Mediterranean reef benthic communities. Marine Ecology 38:e12439. DOI: 10.1111/maec.12439.
- Boero F, Balduzzi A, Bavestrello G, Caffa B, Cattaneo-Vietti R. 1986. Population dynamics of Eudendrium glomeratum (Cnidaria: Anthomedusae) on the Portofino Promontory (Ligurian Sea). Marine Biology 92:81–85. DOI: 10.1007/BF00392749.
- Cerrano C, Bavestrello G. 2008. Medium-term effects of die-off of rocky benthos in the Ligurian Sea. What can we learn from gorgonians? Chemistry and Ecology 24:73–82. DOI: 10.1080/02757540801979648.
- Cerrano C, Bavestrello G, Bianchi CN, Cattaneo-Vietti R, Bava S, Morganti C, Morri C, Picco P, Sara G, Schiaparelli S, Siccardi A, Sponga F. 2000. A catastrophic mass-mortality episode of gorgonians and other organisms in the Ligurian Sea (North-western Mediterranean), summer 1999. Ecology Letters 3:284–293. DOI: 10.1046/j.1461-0248.2000.00152.x.
- Chimenz C, Brignoli PM, Basciano G. 1979. Pantopodi del Porto di Civitavecchia e dintorni (Italia Centrale). Cahiers de Biologie Marine XX:471‒497.
- Chimenz C, Cottarelli V. 1986. Soft bottom Pycnogonida from the Gulf of Salerno (Italy). Oebalia 13(n.s.):137‒146.
- Chimenz C, Tosti M, Cottarelli V. 1993. Taxonomical and ecological observations on Pycnogonida from Apulian coasts (southern Italy). Bolletino di Zoologia 60:339‒347. DOI: 10.1080/11250009309355834.
- Chimenz Gusso C. 2000. Picnogonidi delle coste italiane: Quadro delle conoscenze (Pycnogonida). Memorie della Società Entomologica Italiana 78:541–574.
- Chimenz Gusso C, Fresi E, Cinelli F, Mazzella L, Pansini M, Pronzato R. 1978. Evoluzione delle biocenosi bentoniche di substrato duro contro un gradiente di luce in una grotta marina superficiale. II. Pantopodi. Memorie di Biologia Marina e Oceanografia 8:91‒103.
- Faraggiana R. 1940. Pantopodi del Mar Ligure. Bollettino dei Musei di Zoologia e Anatomia comparata di Torino (ser. 3) 48:145‒158.
- Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica 4:9. Available: http://palaeo-electronica.org/2001_1/past/issue1_01.htm.
- King PE. 1973. Pycnogonids. London: Hutchinson and Co. pp. 144.
- Koçak C. 2013. Anoplodactylus digitatus (Böhm, 1879) (Arthropoda: Pycnogonida) a new addition to the Turkish fauna. Turkish Journal of Zoology 38:378‒382. DOI: 10.3906/zoo-1305-14.
- Krapp F. 1973. Pycnogonida from Pantelleria and Catania, Sicily. Beaufortia 277:55‒74.
- Krapp F. 1975. Bathyale und zirkalittorale Pantopoden (Pycnogonida) aus dem Adriatischen und dem Ligurishen Meer, mit Callipallene acribica n. sp. Bonner Zoologische Beiträge 26:280‒290.
- Lehmann T, Hess M, Melzer RR. 2014. Common littoral pycnogonids of the Mediterranean Sea. Zoosystematics and Evolution 90:163‒224. DOI: 10.3897/zse.90.7520.
- Longobardi L, Bavestrello G, Betti F, Cattaneo-Vietti R. 2017. Long-term changes in a Ligurian infralittoral community (Mediterranean Sea): A warning signal? Regional Studies in Marine Science 14:15–26. DOI: 10.1016/j.rsma.2017.03.011.
- Mangialajo L, Barberis G, Cattaneo-Vietti R. 2004. Contributo alla conoscenza della biodiversità macroalgale delle Aree Marine Protette liguri. Informatore Botanico Italiano 36:550–553.
- Munilla Leȏn T. 1980. Desarrollo anual y reproduccion de Achelia echinata Hodge, 1864 (Pycnogonida). Cahiers de Biologie Marine 21:115–121.
- Munilla T, Soler-Membrives A. 2014. Pycnogonida. Fauna Iberica. Vol. 39. Madrid: Museo National de Ciencias Naturales, Consejo Superior de Investigaciones Cientificas. pp. 292.
- Pansini M, Musso B. 1991. Sponges from trawl-exploitable bottoms of Ligurian and Tyrrhenian Seas: Distribution and ecology. Marine Ecology 12:317–329. DOI: 10.1111/j.1439-0485.1991.tb00261.x.
- Pansini M, Pronzato R. 1990. Observations on the dynamics of a Mediterranean sponge community. In: Rützler K, editor. New perspectives in sponge biology. Washington DC/London: Smithsonian Institution Press. pp. 404–415.
- Piscitelli G, Barone G. 2000. Prima nota sui Picnogonidi delle Isole Tremiti. Biologia Marina Mediterranea 7:718–722.
- Pronzato R, Bavestrello G, Cerrano C. 1998. Morpho-functional adaptations of three species of Spongia (Porifera, Demospongiae) from a Mediterranean vertical cliff. Bulletin of Marine Science 63:317–328.
- Puce S, Bavestrello G, Di Camillo CG, Boero F. 2009. Long term changes in hydroid (Cnidaria, Hydrozoa) assemblages: Effect of Mediterranean warming? Marine Ecology 30:313–326. DOI: 10.1111/j.1439-0485.2009.00283.x.
- Schiaparelli S, Castellano M, Povero P, Sartoni G, Cattaneo-Vietti R. 2007. A benthic mucilage event in North-Western Mediterranean Sea and its possible relationships with the summer 2003 European heat-wave: Short term effects on littoral rocky assemblages. Marine Ecology 28:341–353. DOI: 10.1111/j.1439-0485.2007.00155.x.
- Scholtz G, Brenneis G. 2016. The pattern of a specimen of Pycnogonum litorale (Arthropoda, Pycnogonida) with a supernumerary leg can be explained with the “boundary model” of appendage formation. The Science of Nature 103:13 (9 pp). DOI: 10.1007/s00114-016-1333-8.
- Soler-Membrives A, Munilla T. 2015. PYCNOIB: Biodiversity and biogeography of Iberian pycnogonids. PloS One 10:e0120818. DOI: 10.1371/journal.pone.0120818.
- Tortonese E. 1958. Bionomia marina della regione costiera fra Punta della Chiappa e Portofino. Archivio di Oceanografia e Limnologia 11:167–210.
- Tortonese E. 1961. Nuovo contributo alla conoscenza del benthos della scogliera ligure. Archivio di Oceanografia e Limnologia 12:163–183.
