Abstract
Accounts of the encrusting, coral-killing sponges are increasing at an alarming rate. The present paper details about a thinly encrusting red sponge Clathria (Microciona) aceratoobtusa (Carter, 1887) which is invasive or locally spreading species and create deleterious outbreaks on Gulf of Mannar coral reefs. Earlier it has also been recorded from Yemen, but not taxonomically identified. This sponge is regarded as an aggressive space-competitor locally overgrowing and killing corals, mostly belonging to the coral genera such as Porites, Acropora, Montipora, Favia and Turbinaria. We observed that Turbinaria colonies were the substrate more preferred by the sponge than other coral colonies in the entire study site. The present observation analyses detail the taxonomical and ecological data of Clathria (M.) aceratoobtusa with a comprehensive review of its ecological preference.
Introduction
The boundless competition for space, and cooperation processes, exhibited by reef communities favour physical and biological structuring and functioning of reef ecosystems (Wang et al. Citation2012). Sponges are one among the major actors of coral reef communities (Benayahu & Loya Citation1981; Acker & Risk Citation1985) and are considered as the main habitat-forming organisms (Chornesky Citation1989; Rützler Citation2002). There are various interactions happening between the sponges and the corals; the outcome of these interactions potentially may lead to large-scale destruction of coral reef communities (González-Rivero et al. Citation2011). The density and bottom cover of coral excavating sponges are proven similar to or higher than hard corals (Zea Citation1993); excavating sponges play a key role in balancing reef growth and erosion (Wilkinson Citation1983; Glynn Citation1997) and if their action intensifies and speeds up, overcoming reef growth, they may destroy the reef framework (Hutchings Citation1986; Schönberg et al. Citation2017). Sponges also intensely interact with scleractinian corals, competing for the substrate; they may overgrow and smother corals, often producing allelopathic molecules able to kill coral tissues (Coles & Bolick Citation2007; Pawlik et al. Citation2007; Benzoni et al. Citation2008; de Voogd et al. Citation2013).
Temperature increase (Rose & Risk Citation1985; Holmes Citation1997; Holmes et al. Citation2000; Rützler Citation2002), coral tissue die-off, coral disease and bleaching (Cortés et al. Citation1984; Glynn Citation1997; Williams et al. Citation1999; Rützler Citation2002; Schönberg et al. Citation2017), aggravated by climate change, may strongly affect sponge/coral interaction promoting the shift of some coral reefs towards sponge dominated reefs (Bell et al. Citation2013).
Though coral reefs have been widely studied around the words, the interaction between corals and sponges is not well understood (Peyrot-Clausade et al. Citation1995; Carreiro-Silva & McClanahan Citation2001; Zahir et al. Citation2002; Ashok et al. Citation2019). There are several reports on the sponges of the family Clionaidae (Marulanda-Gómez et al. Citation2017; Ashok et al. Citation2018), Suberitidae (Rützler Citation1971; Raj et al. Citation2018; Fromont et al. Citation2019), Chalinidae (Rossi et al. Citation2015) and Poeciloscleridae (Coles & Bolick Citation2007; Benzoni et al. Citation2008) () which can excavate or smother corals. Poecilosclerida is the largest order in the phylum Porifera with vast diversity (Bergquist & Formont Citation1988; Hajdu et al. Citation1994). They are variedly studied for the possession of various secondary metabolites (Melawaty & Pasau Citation2015) but their ecological aspects have not drawn enough attention in the Indian Ocean. To date, 26 Poecilosclerids have been described and documented from the Indian Ocean (Pattanayak Citation1999). Many of the reported species from the Indian Ocean are currently subjected to taxonomic revisions on account of a poor description, lack of stock specimens or want of confirmation for some of the identifications (Schönberg et al. Citation2017).
Table I. Sponge species able to overgrowth on hard coral species with visible negative effects; the geographic area refers to the area where interaction activity was observed; also excavating species are considered when they couple an erosion activity with a lateral growth
Corals (Pillai Citation1972, Citation1977, Citation1986, Citation1994) and sponges (Thomas Citation1968a, Citation1968b, Citation1968c, Citation1968d, Citation1968e, Citation1969, Citation1972, Citation1979, Citation1985, Citation1989) have been well documented in the Indian reefs although the interaction between them has not been given importance. The Gulf of Mannar (GoM) is one of the major reef regions of India with a significant amount of corals (Edward et al. Citation2007, Citation2012, Citation2018). The dearth of correctly identified sponges with colour images and lack of regional taxonomists makes sponges of the Gulf of Mannar as enigmatic creatures to the recent researchers. The recognition of the importance of sponges in these reef ecosystems reflects sponges are not anymore that neglected species (CARICOMP Citation1997).
Recently the encrusting sponge Clathria (Microciona) aceratoobtusa (Carter Citation1887), showy for its brilliant orange-red colour, has become abundant on the reefs of the Gulf of Mannar, and turned out to be really detrimental to various species of live corals. In the present paper, we dealt with taxonomical and ecological data of Clathria (M.) aceratoobtusa with a detailed review of its ecological preference. The data may assist in future monitoring efforts of the GoM reefs, considering the negative, long-term consequence of the proliferation of this species on dynamics and community structure of the reefs.
Materials and methods
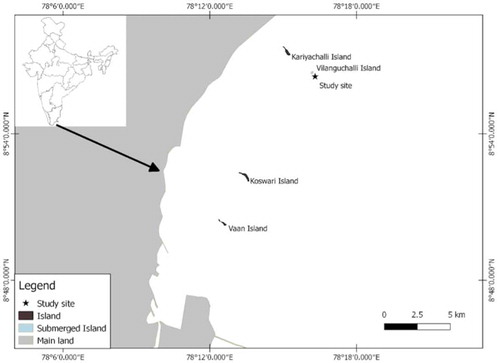
The Gulf of Mannar in southeast India is known for its coral reefs and associated biodiversity. There are 21 uninhabited coral islands in the Gulf of Mannar that come under the Gulf of Mannar Marine National Park (GoMMNP). The National Park area is a “no go” and “no take” zone. Tuticorin region of the Gulf of Mannar encompasses four islands, namely, Vaan, Koswari, Kariyachalli and Vilanguchalli (Edward et al. Citation2012). Vilanguchalli Island () is a submerged Island that occurs 6 km from the mainland. Submergence of this Island has been reported to be caused by decades of rampant coral mining coupled with sea-level rise (Raj et al. Citation2015). Parts of this Island expose during low tides. Reef type in this Island is fringing that occurs at depths between 0.5 and 5.4 m and the dominant coral genera are Acropora, Montipora, Porites and Turbinaria (Edward et al. Citation2007). Climate-driven coral mortality has caused serious damage to the reefs of the Gulf of Mannar including Vilanguchalli Island (Edward et al. Citation2018).
Figure 1. Map of the study site at the Vilanguchalli Island, Gulf of Mannar, southern coast of India
After noticing the unusual occurrence of a red patch on various coral colonies, an underwater assessment was carried out involving scuba diving. The sponge incrustations were first misinterpreted as coral disease and further analysis confirmed the sponge identity. To estimate the ecological context of the sponge colonies, ten 20 × 2 m belt transects were haphazardly laid on island reef, parallel to the shore and to each other, separated by a distance of 5 m, resulting in an area of 400 m2. The depth ranged between 2 and 4.5 m along all transects. Data were collected on the total number of coral colonies, listed by genus, and the number of corals invaded by the sponge within the belt transects. Small volumes of sponge-infested colonies were collected for the identification of the sponge species. The samples were immediately stored in 80% ethanol, and subsequent laboratory analyses, for spicule preparations, were made following Rützler (Citation1974). Spicule range was evaluated by measuring 15 spicules per type. Size is given as maximal (average sizes ± standard error) minimal, for length and width. For SEM analyses, carried out with a Philips XL 20, dissociated spicules, and coral fragments covered by sponge, were transferred onto stubs, sputtered with gold and observed.
Chi-square test was done to compare the observed proportions of infestation of C. (M.) aceratoobtusa, towards several genera of corals, to expected ones, predicted by the coral proportion observed in the transects.
Results
In Vilanguchalli patch reef we observed the occurrence of an orange-reddish, encrusting sponge overgrowing on live tissues of massive and branching corals of several genera (); it extended as a thin red sheet over the corals, leaving a red stain throughout its proliferation. A thin, white band was visible near the coral and sponge boundary ()).
Figure 2. Clathria (Microciona) aceratoobtusa at Vilanguchalli Island, Gulf of Mannar. (a) A prominent and profound dermal canal pattern was radiating all over the body upon close observation underwater; (b) Sponge infestation on live tissues of Acropora muricata; (c) Infestation on Favia sp.; (d) The sponge penetrates in a downward direction, inside the coral; (e) Intact corallites, smothered by the sponge cover, still detectable. Scale bars: a, b, c = 2 cm; d, e = 1 cm

The morphological study led to identify it as Clathria (Microciona) aceratoobtusa (Carter Citation1887). The sponge was characterized by a rough surface and tough consistency. Samples turned pale upon alcohol preservation. The oscules were contractile and poorly visible and distributed. A prominent and profound dermal canal pattern was radiating all over the body upon close observation underwater ()). Intact corallites, smothered by the sponge cover, were still detectable ()). The sponge could not be separated with moderate ease from the underlying substrate, because it penetrates in a downward direction, inside the corals ()).
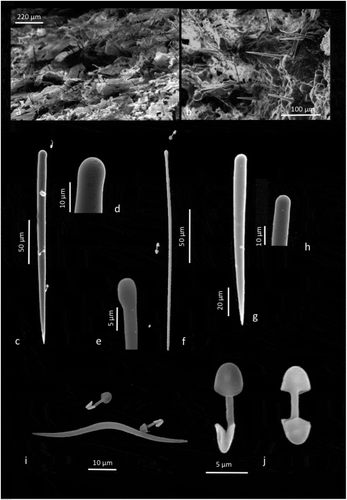
Skeleton by SEM appears typical of Clathria (Microciona), i.e. hymedesmoid-like ()). Macroscleres consist of principal long, and thick subtylostyles ()) with smooth tylo ()); very few have micro-spiny tylo; thinner, smooth ()) or microspined styles/subtylostyles ()); smooth echinating styles ()), some microspined; microscleres are palmate isochelae and toxas ()). The abundantly distributed toxas were small and delicate and also oxhorn toxas ()). Chelae often displayed contort shafts ()). Measurements of the spicules in .
Table II. Spicule dimensions of Clathria (Microciona) aceratoobtusa from Vilanguchalli Island Tuticorin group, Gulf of Mannar Marine National Park, and from Yemen (N = 15 Min. – minimum, max. – maximum, S.E. – standard error)
Figure 3. Clathria (Microciona) aceratoobtusa. (a, b) Typical hymedesmoid-like skeleton by SEM; (c) Principal long, and thick subtylostyle; (d) Smooth tylo of the thick subtylostyle; (e) Smooth tylo of the thin subtylostyle; (f) Thin auxiliary subtylostyle; (g) Smooth echinating style; (h) Smooth tylo of the echinating style; (i) Oxhorn toxa and palmate isochelae; (j) Palmate isochelae
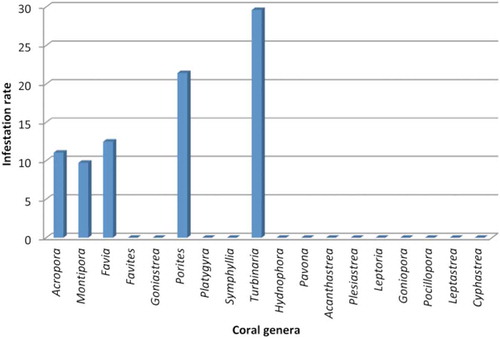
In the surveyed area, numerous coral genera are present: the most common genus, in healthy state, is Acropora with 0.64 ± 0.35 colonies/m2, followed by Turbinaria (0.22 ± 0.13 colonies/m2) and by Favia (0.07 ± 0.04 colonies/m2). Many other genera e. g Acanthastrea, Pavonia or Cyphastrea are very rare, with few, scattered colonies only (respectively, 2, 3 and 7 colonies in all the examined transects). Turbinaria colonies were the substrate more preferred by the sponge than other coral colonies in the entire study site (χ2 = 29.911, df = 4, p-value = 5.1 10−6). Sponge prevalence followed in the order Turbinaria (29.6%, n = 37) > Porites (21.43%, n = 6) > Favia (12.5%, n = 4) > Acropora (11.07%, n = 32) > Montipora (9.73%, n = 18), that was the least preferred substrate by the sponge ().
Discussion
Clathria (M.) aceratoobtusa was recorded by Carter (Citation1887) from Mergui Archipelago and later it has been variedly reported by Hentschel (Citation1911) from Shark Bay, by Thomas (Citation1985) from the Gulf of Mannar, and by Hooper (Citation1996) from Thailand and Indonesia. The sponge from Mannar fits with the species C. (M.) aceratoobtusa in its general appearance, colour, and spicule size and shape. In particular, virtually smooth spicules, as well as the oxhorn toxas and contort chelae, characterize C. (M.) aceratoobtusa (Hooper et al. Citation2000). Our specimens are very close to those described by Thomas (Citation1985) and Hentschel (Citation1911), and slightly different in respect with the neotype, illustrated by Hooper (Citation1996), characterized by macroscleres with, in general, prominent swollen heads. Anyway, Hooper (Citation1996) pointed out how the neotype slightly differed from the sponges described by Carter and Hentschel, suggesting a certain morphological variability in spicule features and size.C. (M.) aceratoobtusa was described as thinly encrusting on bivalves, rock, coral substrate, shell detritus or worm tubes (Carter Citation1887; Hentschel Citation1911; Hooper Citation1996); in 2008, Benzoni et al. reported in South Yemen a very thin encrusting Clathria (Microciona); the sponge was not determined at species level and was described as an orange-reddish band “disease” able to grow over the coral at a rate of about 1 cm/month. It was observed spreading over about 50% of the Porites lutea colonies, killing the coral. Later the same sponge was recorded also in the Maldives (Benzoni & Calcinai pers. obs.). The comparison of the old material with this new one, from Mannar, revealed that they are the same species, identified as C. (M.) aceratoobtusa. The mechanism of sponge–coral interaction appears the same described by Benzoni et al. (Citation2008) ()). The sponge covers corals by peripheral contact (Aerts & van Soest Citation1997), and the thin, dead and white line of coral along the boundary can be explained by chemical interaction as suggested for similar cases (Porter & Targett Citation1988; Rossi et al. Citation2015). Moreover, C. (M.) aceratoobtusa seems able to cover also living portions of coral (see )).
Turbinaria was the coral genus more covered by the sponge, but it was not the only; in 2008, on the contrary, Benzoni et al. found C. (M.) aceratoobtusa to be highly selective for Porites lutea in Yemen; in Mannar, the plate-like life form of this coral might have favoured the sponge overgrowth over it, even if a clear specificity is not demonstrable, considering that infected and not infected colonies may have the same pattern of growth () and C. (M.) aceratoobtusa in Yemen covered massive colonies of Porites lutea.
Table III. Clathria (Microciona) aceratoobtusa infestations on various coral growth forms from Vilanguchalli Island Tuticorin group, Gulf of Mannar Marine National Park
In the recent period, records about sponges threating coral reefs are numerous (). Since 1973 when Bryan has documented Terpios hoshinota (as Terpios sp.) in Guam, other sponge species were observed competing with corals and killing them (Coles & Bolick Citation2007; Benzoni et al. Citation2008) and to spread out across Indo-Pacific Ocean, enlarging their area of distribution (Ávila & Carballo Citation2009; Shi et al. Citation2012; de Voogd et al. Citation2013; Rossi et al. Citation2015); in particular, in the most recent period, there is an increasing number of records of T. hoshinota spreading throughout the Indo Pacific negatively affecting coral reefs (Ashok et al. Citation2018; Raj et al. Citation2018; Yang et al. Citation2018; Fromont et al. Citation2019).
As Elliot et al. (Citation1995) put in evidence, the causes of these sponge outbreaks are not clear. In some cases, it may be due to human-induced factors (Ávila & Carballo Citation2009) or due to detrimental environmental conditions; for example, high levels of nutrient pollution have been considered the main cause of rapid and catastrophic outbreaks of the sponge T. hoshinota, (Plucer-Rosario Citation1987; Rützler & Muzik Citation1993), and also sponge dispersal possibility supported by local currents may play a crucial role (Shi et al. Citation2012). Further studies are needed to elucidate the environmental factors affecting and promoting Clathria (M.) aceratoobtusa’s spread in the Gulf of Mannar. It is known that coral bleaching, driven by climate change, has become more common in the past few decades causing widespread coral mortality (Wake Citation2016; Hughes et al. Citation2018); sponges take advantage of the compromised health of corals due to environmental parameters (Rose & Risk Citation1985; Holmes Citation1997; Holmes et al. Citation2000; Rützler Citation2002; Carballo Citation2009). Corals in the Gulf of Mannar were affected significantly by coral bleaching in 2016 and are under significant stress (Edward et al. Citation2018). Hence, we may interpret the widespread occurrence of overgrowth of the coral excavating sponges as a consequence of the most recent bleaching event occurring in the Gulf of Mannar as evident from recent studies (Ashok et al. Citation2018; Edward et al. Citation2018; Raj et al. Citation2018). Sponges have been reported to be comparatively more capable of thriving in changing climatic conditions and have better regeneration capacity (Miller et al. Citation2010; Runzel Citation2016). This alarming increase of Clathria (M.) aceratoobtusa in the Gulf of Mannar deserves to be monitored, and further researches are needed to evaluate the rate and the proportion of this sponge spreading; this sponge could represent another threat to this already damaged reef, causing massive coral mortality and sponge-coral phase shift (Rützler Citation1970; Suchanek & Green Citation1981).
Conclusion
The present study provides a first account on the widespread occurrence of a coral-killing sponge in Vilanguchalli Island, Tuticorin region in Gulf of Mannar. Though climate crisis has been portrayed as a key influencer for this sponge outbreak, other important factors such as pollution and nutrient enrichment cannot be ruled out. Tuticorin is an urban area with a huge population and the domestic waste generated every day is directly routed to the sea without any treatment. This condition further upsets the ecosystem balance in favour of the sponges where they outcompete the corals and damage the reefs for long term (González-Rivero et al. Citation2011). The recently observed coral mortality due to several factors is expected to further enhance the abundances of these sponges in the near future. Coral reefs of Gulf of Mannar act as a hub for reef dependant fishery and provide livelihood to thousands of local fishermen directly.
The coral-killing sponge, Terpios hoshinota has been reported to die after the death of covered coral colonies and if no other live coral substrate is available (see Elliott et al. Citation2015). In Yemen, Benzoni et al. (Citation2008) noted that C. (M.) aceratoobtusa grows progressively over the coral, but it faded, leaving the dead coral behind. Hence, further focused studies are needed to ascertain the exact interaction between the reported sponge and its host corals. Considering the global concern for declining reef quality, the sponge assemblages on various coral communities of the ecologically sensitive coral reef system such as GoM should be regularly monitored to determine the potential of sponges to become more abundant in response to changing environmental conditions. The present study also aims to draw the attention of the reef managers to the need for focusing more research on the coral threating sponges.
Availability of data and materials
All data generated or analysed during this study are included in this published article.
Consent for publication
Being the corresponding author to this manuscript, I state that all authors agree to its submission and the Corresponding author has been authorized by the co-authors.
Ethics approval consent to participate
This article does not contain any studies with animals performed by any of the authors.
Acknowledgements
Thanks to Ministry of Environment, Forest and Climate Change, Government of India for funding support. Thanks also to Chief Wildlife Warden, Government of Tamil Nadu, India and Wildlife Warden, Gulf of Mannar Marine National Park, for research permissions (Ref.No. WL(A)/11867/2017- Permit No. 43/2017).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Acker KL, Risk MJ. 1985. Substrate destruction and sediment production by the boring sponge Cliona caribbea on Grand Cayman Island. Journal of Sedimentary Petrology 55:705–711.
- Aerts LAM, van Soest RWM. 1997. Quantification of sponge/coral interactions in a physically stressed reef community, NE Colombia. Marine Ecology Progress Series 148:125–134. DOI: 10.3354/meps148125.
- Ashok AM, Schonberg CHL, Laju RL, Edward JKP. 2019. Coral-killing sponge Terpios hoshinota in Southeast India—Bested by Acropora muricata? Marine Biodiversity 49:1069–1070. DOI: 10.1007/s12526-019-00966-8.
- Ashok AM, Schonberg CHL, Raj KD, Bhoopathi M, Bharath MS, Edward JKP. 2018. A sponge of the Cliona viridis complex invades and excavates corals of the Gulf of Mannar, south-eastern India. Marine and Freshwater Research 69:874–882. DOI: 10.1071/MF17247.
- Ávila E, Carballo JL. 2009. A preliminary assessment of the invasiveness of the Indo-Pacific sponge Chalinula nematifera on coral communities from the tropical Eastern Pacific. Biological Invasions 11:257–264. DOI: 10.1007/s10530-008-9230-5.
- Bell JJ, Davy SK, Jones T, Taylor MW, Webster NS. 2013. Could some coral reefs become sponge reefs as our climate changes? Global Change Biology 19:2613–2624. DOI: 10.1111/gcb.12212.
- Benayahu Y, Loya Y. 1981. Competition for space among coral-reef sessile organisms at Eilat, Red Sea. Bulletin of Marine Science 31:514–522.
- Benzoni F, Calcinai B, Eisinger M, Klaus R. 2008. Coral disease mimic: Sponge attacks Porites lutea in Yemen. Coral Reefs 27:695. DOI: 10.1007/s00338-008-0371-x.
- Bergquist PR, Formont PJ. 1988. The Marine Fauna of New Zealand: Porifera, Demospongiae, Part 4 (Poecilosclerida). New Zealand Oceanographic Institute Memoir 96:1–197.
- Carballo JL. 2009. Effect of natural sedimentation on the structure of tropical rocky sponge assemblages. Ecoscience 13:119–130. DOI: 10.2980/1195-6860(2006)13[119:EONSOT]2.0.CO;2.
- CARICOMP. 1997. CARICOMP monitoring of coral reefs. In: Lessios HA, Macintyre IG, editors. Proceedings of the 8th International Coral Reef Symposium. Vol. 1. Smithsonian Tropical Research Institute, Panama.
- Carreiro-Silva M, McClanahan TR. 2001. Echinoid bioerosion and herbivory on Kenyan coral reefs: The role of protection from fishing. Journal of Experimental Marine Biology and Ecology 262:133–153. DOI: 10.1016/S0022-0981(01)00288-X.
- Carter HJ. 1887. Report on the marine sponges, chiefly from King Island, in the Mergui Archipelago, collected for the Trustees of the Indian Museum, Calcutta, by Dr. John Anderson, F.R.S., superintendent of the museum. Journal of the Linnean Society, Zoology 21:61–84. DOI: 10.1111/j.1096-3642.1887.tb00381.x.
- Chornesky EA. 1989. Repeated reversals during spatial competition between corals. Ecology 70:843–855. DOI: 10.2307/1941353.
- Coles S, Bolick H. 2007. Invasive introduced sponge Mycale grandis overgrows reef corals in Kane’ohe Bay, Oahu, Hawai’i. Coral Reefs 26:911. DOI: 10.1007/s00338-007-0295-x.
- Cortés J, Murillo MM, Guzmán HM, Acuña J. 1984. Perdida de zoxantelas y muerte de corales y otros organismos arrecifales en el Caribe y Pacifico de Costa Rica. Revista de Biología Tropical 32:227–231.
- De Laubenfels MW. 1954. The sponges of the West-Central Pacific. Oregon State Monographs. Studies in Zoology 7:1–306.
- de Voogd NJ, Cleary DFR, Dekker F. 2013. The coral-killing sponge Terpios hoshinota invades Indonesia. Coral Reefs 32:755. DOI: 10.1007/s00338-013-1030-4.
- Edward JKP, Mathews G, Patterson J, Wilhelmsson D, Tamelander J, Linden O. 2007. Coral reefs of the Gulf of Mannar, southeastern India - Distribution, diversity and status. SDMRI Special Research Publication 12.
- Edward JKP, Mathews G, Raj KD, Laju RL, Selva Bharath M, Arasamuthu A, Dinesh Kumar P, Bilgi DS, Malleshappa H. 2018. Coral mortality in the Gulf of Mannar, southeastern India, due to bleaching caused by elevated sea temperature in 2016. Current Science 114:10.
- Edward JKP, Mathews G, Raj KD, Thinesh T, Patterson J, Tamelander J, Wilhelmsson D. 2012. Coral reefs of Gulf of Mannar, India - signs of resilience. Proceedings of the 12th International Coral Reef Symposium, Cairns, Australia.
- Elliot JK, Elliot JM, Mariscal RN. 1995. Host selection, location and association behaviors of anemonefishes in field settlement experiments. Marine Biology 122:377–389. DOI: 10.1007/BF00350870.
- Elliott J, Patterson M, Vitry E, Summers N, Miternique C. 2015. Morphological plasticity allows coral to actively overgrow the aggressive sponge Terpios hoshinota (Mauritius, Southwestern Indian Ocean). Marine Biodiversity 46:489–493. DOI: 10.1007/s12526-015-0370-4.
- Fromont J, Richards ZT, Wilson NG. 2019. First report of the coral-killing sponge Terpios hoshinota Rützler and Muzik, 1993 in Western Australia: A new threat to Kimberley coral reefs? Diversity 11:184. DOI: 10.3390/d11100184.
- Glynn PW. 1997. Bioerosion and coral reef growth: A dynamic balance. In: Birkeland C, editor. Life and death of coral reefs. New York: Chapman and Hall. pp. 68–94.
- González-Rivero M, Yakob L, Mumby PJ. 2011. The role of sponge competition on coral reef alternative steady states. Ecological Modelling 222:1847–1853. DOI: 10.1016/j.ecolmodel.2011.03.020.
- Hajdu E, van Soest RWM, Hooper JNA. 1994. Proposal for a phylogenetic subordinal classification of poecilosclerid sponges. In: van Soest R, van Kempen TMG, Braekman JC, editors. Sponges in time and space. Rotterdam: Balkema. pp. 123–139.
- Hentschel E. 1911. Tetraxonida. 2. Teil. In: Michaelsen W, Hartmeyer R, editors. Die Fauna Südwest-Australiens. Ergebnisse der Hamburger südwest-australischen Forschungsreise 1905. Vol. 3. Jena: Fischer. pp. 279–393.
- Holmes KE. 1997. Eutrophication and its effect on bioeroding sponge communities. Proceedings of the 8th International Coral Reef Symposium, Panama, 2.
- Holmes KE, Edinger EN, Limmon GV, Risk MJ. 2000. Bioerosion of live massive corals and branching coral rubble on Indonesian coral reefs. Marine Pollution Bulletin 40:606–617. DOI: 10.1016/S0025-326X(00)00067-9.
- Hooper JNA. 1996. Revision of the Microcionidae (Porifera: Poecilosclerida: Demospongiae) with description of Australian species. Memoirs of the Queensland Museum 40:1–626.
- Hooper JNA, Kennedy JA, van Soest RWM. 2000. Annotated checklist of sponges (Porifera) of the South China Sea region. Raffles Bulletin of Zoology 8:125–207.
- Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM. 2018. Spatial temporal patterns of mass bleaching of corals in the Anthropocene. Science 359:80–83. DOI: 10.1126/science.aan8048.
- Hutchings PA. 1986. Biological destruction of coral reefs. Coral Reefs 4:239–252. DOI: 10.1007/BF00298083.
- López-Victoria M, Zea S. 2004. Storm-mediated coral colonization by an excavating Caribbean sponge. Climate Research 26:251–256. DOI: 10.3354/cr026251.
- López-Victoria M, Zea S, Weil E. 2006. Competition for space between encrusting excavating Caribbean sponges and other coral reef organisms. Marine Ecology Progress Series 312:113–121. DOI: 10.3354/meps312113.
- Márquez JC, Zea S, Lopez-Victoria M. 2006. Is competition for space between the encrusting excavating sponge Cliona tenuis and corals influenced by higher-than- normal temperatures? Boletín De Instituto De Investigaciones Marinas Y Costera 35:259–265.
- Marulanda-Gómez Á, López-Victoria M, Zea S. 2017. Current status of coral takeover by an encrusting excavating sponge in a Caribbean reef. Marine Ecology 38:e12379. DOI: 10.1111/maec.12379.
- Melawaty L, Pasau K. 2015. The profile of secondary metabolites of sponge Clathria reinwardtii extract as a result of Fe accumulation in Spermonde Archipelago. Advances in Biological Chemistry 5:266–272. DOI: 10.4236/abc.2015.57023.
- Miller AN, Strychar KB, Shirley TC, Rützler K. 2010. Effects of heat and salinity stress on the sponge Cliona celata. International Journal of Biology 2:3–16. DOI: 10.5539/ijb.v2n2p3.
- Montano S, Chou WH, Allen CA, Galli P, Reimer JD. 2015. First record of the coral-killing sponge Terpios hoshinota in the Maldives and the Indian Ocean. Bulletin of Marine Science 91:97–98. DOI: 10.5343/bms.2014.1054.
- Pattanayak JG. 1999. Annotated checklist of marine sponges of the Indian region. Memoirs of the Queensland Museum 44:439–455.
- Pawlik JR, McMurray SE, Henkel TP. 2007. Abiotic factors control sponge ecology in Florida mangroves. Marine Ecology Progress Series 339:93−98. DOI: 10.3354/meps339093.
- Peyrot-Clausade M, Le Campion-Alsumard T, Hutchings P, Le Campion J, Payri C, Fontainee M. 1995. Initial bioerosion and bioaccretion on experimental substrates in high island and atoll lagoons (French Polynesia). Oceanologica Acta 18:531–541.
- Pillai CSG. 1972. Stony corals of the seas around India. Journal of the Marine Biological Association of India 191:216–229.
- Pillai CSG. 1977. The structure, formation and species diversity of the South Indian reefs. Proceeding of the 3rd International Symposium on Coral reef, Miami.
- Pillai CSG. 1986. Recent Corals from South-East Coast of India. In: James PSBR, editor. Recent advances in marine biology, today and tomorrow. New Delhi: Printers and Publishers. pp. 107–201.
- Pillai CSG. 1994. Coral reef ecosystems. Indian Journal of Marine Sciences 23:251–252.
- Plucer-Rosario G. 1987. The effect of substratum on the growth of Terpios, an encrusting sponge which kills corals. Coral Reefs 5:197–200. DOI: 10.1007/BF00300963.
- Porter JW, Targett NM. 1988. Allelochemical interactions between sponges and corals. Biological Bulletin 175:230–239. DOI: 10.2307/1541563.
- Raj KD, Bharath MS, Mathews G, Aeby GS, Edward JKP. 2018. Coral-killing sponge Terpios hoshinota invades the corals of Gulf of Mannar, Southeast India. Current Science 114:117–119.
- Raj KD, Mathews G, Patterson Edward JK. 2015. Vaan Island of Gulf of Mannar, Southeast coast of India - on the verge of submergence. Indian Journal of Marine Sciences 44:892–895.
- Reimer JD, Mizuyama M, Nakano M, Fujii T, Hirose E. 2011. Current status of the distribution of the coral-encrusting cyanobacteriosponge Terpios hoshinota in southern Japan. Galaxea 13:35–44. DOI: 10.3755/galaxea.13.35.
- Rose CS, Risk MJ. 1985. Increase in Cliona delitrix infestation of Montastrea cavernosa heads on an organically polluted portion of the Grand Cayman. PSZN I: MarineEcology 6:345–363.
- Rossi G, Montori S, Cerrano C, Calcinai B. 2015. The coral killing sponge Chalinula nematifera (Porifera: Haplosclerida) along the eastern coast of Sulawesi Island (Indonesia). Italian Journal of Zoology 82:143–148. DOI: 10.1080/11250003.2014.994046.
- Runzel CC. 2016. Sponge physiology: The effects of temperature on the regeneration and reaggregation of sponges (Haliclona reniera). PeerJ Preprints 4:e2654v1. DOI: 10.7287/peerj.preprints.2654v1.
- Rützler K. 1970. Spatial competition among Porifera: Solution by epizoism. Oecologia (Berlin) 5:85–95. DOI: 10.1007/BF00347624.
- Rützler K. 1971. Bredin-Archbold-Smithsonian biological survey of Dominica: Burrowing sponges, genus Siphonodictyon Bergquist, from the Caribbean. Smithsonian Contributions to Zoology 77:1–37. DOI: 10.5479/si.00810282.77.
- Rützler K. 1974. The burrowing sponges of Bermuda. Smithsonian Contributions to Zoology 165:1–32. DOI: 10.5479/si.00810282.165.
- Rützler K. 2002. Impact of crustose clionid sponges on Caribbean reef corals. Acta Geológica Hispánica 37:61–72.
- Rützler K, Muzik K. 1993. Terpios hoshinota, a new cyanobactiosponge threatening Pacific reefs. Scientia Marina 4:395–403.
- Schönberg CHL. 2003. Substrate effects on the bioeroding demosponge Cliona orientalis. Substrate colonization and tissue growth. PSZN I Marine Ecology 24:59–74. DOI: 10.1046/j.1439-0485.2003.03812.x.
- Schönberg CHL, Fang JKH, Carreiro-Silva M, Tribollet A, Wisshak M. 2017. Bioerosion: The other ocean acidification problem. ICES Journal of Marine Science 74:895–925. DOI: 10.1093/icesjms/fsw254.
- Schönberg CHL, Wilkinson CR. 2001. Induced colonization of corals by a clionid bioeroding sponge. Coral Reefs 20:69–76. DOI: 10.1007/s003380100143.
- Shi Q, Liu GH, Yan HQ, Zhang HL. 2012. Black disease (Terpios hoshinota): A probable cause for the rapid coral mortality at the northern reef of Yongxing Island in the South China Sea. Ambio 41:446–455. DOI: 10.1007/s13280-011-0245-2.
- Suchanek TH, Green DJ. 1981. Interspecific competition between Palythoa caribaeorum and other sessile invertebrates on St. Croix reef, U.S. Virgin Islands. Proceeding of the 4th International Coral Reef Symposium, Manila, 2.
- Sullivan BW, Faulkner DJ. 1985. Chemical studies of the burrowing sponge Siphonodictyon coralliphagum. In: Rützler K, editor. New perspectives in sponge biology. Washington, DC: Smithsonian Institution Press. pp. 45–50.
- Thinesh T, Jose PA, Hassan S, Selvan KM, Selvin J. 2015. Intrusion of coral-killing sponge Terpios hoshinota on the reef of Palk Bay. Current Science 109:1030–1032.
- Thomas PA. 1968a. Studies on Indian sponges - I. Two new species of silicious sponges belonging to the genera Echinodictyum Ridley and Rhadberemia Topsent (Class: Demospongiae Sollas, Order: Poecilosclerida Topsent). Journal of the Marine Biological Association of India 10:245–249.
- Thomas PA. 1968b. Studies on Indian sponges-II. Two new species of silicious sponges belonging to the genera Aka de Laubenfels and Damirina Burton. Journal of the Marine Biological Association of India 10:250–254.
- Thomas PA. 1968c. Studies on Indian sponges-III. Two species of silicious sponges of the family Ophlitaspongiidae de Laubenfels (Class: Demospongiae Sollas, Order: Poecilosclerida Topsent). Journal of the Marine Biological Association of India 10:255–259.
- Thomas PA. 1968d. Studies on Indian sponges-IV. Additions to the genus Corticum Schmidt with notes on the distribution of C. candelabrum Schmidt. Journal of the Marine Biological Association of India 10:260–263.
- Thomas PA. 1968e. Studies on Indian sponges-V. Two new records of siliceous sponges belonging to the families Myxilliadae Hentschel, and Spirastrellidae Hentschel from the Indian region. Journal of the Marine Biological Association of India 10:264–268.
- Thomas PA. 1969. Catalogue of sponges in the reference collections of the Central Marine Fisheries Research Institute, Mandapara Camp. Bulletin of the Central Marine Fisheries Research Institute 7:13–21.
- Thomas PA. 1972. Boring sponges of the reefs of Gulf of Mannar and Palk Bay. Proceedings of the1st International Symposium on Corals and Coral Reefs. Mandapam Camp (India): Marine Biological Association of India. pp. 333–362.
- Thomas PA. 1979. Boring sponges destructive to economically important molluscan beds and coral reefs in Indian seas. Indian Journal of Fisheries 26:163–200.
- Thomas PA. 1985. Demospongiae of the Gulf of Mannar and Palk Bay. In: James PSBR, editor. Recent advances in marine biology. Today and tomorrow’s. New Delhi: Printers and Publishers. pp. 205–365.
- Thomas PA. 1989. Sponge fauna of Lakshadweep. Bulletin of the Central Marine Fisheries Research Institute 43:150–161.
- Wake B. 2016. Snow white coral. Nature Climate Change 6:439–439. DOI: 10.1038/nclimate3009.
- Wang JT, Hirose E, Hsu CH, Chen YY, Meng PJ, Chen CA. 2012. A coral-killing sponge, releases larvae harboring cyanobacterial symbionts: An implication of dispersal. Zoological Studies 51:314–320.
- Wilkinson CR. 1983. Role of sponges in coral reef structural processes. In: Barnes DJ, editor. Perspectives on coral reefs. Mannka, Australia: Brian Clouston Publisher. pp. 263–274.
- Williams EH, Bartels PJ, Bunkley-Williams L. 1999. Predicted disappearance of coral-reef ramparts: A direct result of major ecological disturbances. Global Change Biology 5:839–845. DOI: 10.1046/j.1365-2486.1999.00272.x.
- Yang S, Chen H, Ho M, Chen Y, Huang Y, Chow WS, Tang S, Jeng M, Chen CA. 2018. Outbreak of coral-killing cyanobacteria sponge, Terpios hoshinota, in Taiping Island (Itu Aba), Spratlys, South China Sea. Bulletin of Marine Science 94:1543–1544. DOI: 10.5343/bms.2018.0023.
- Zahir H, Clark S, Ajla R, Saleem M. 2002. Spatial and temporal patterns of coral recruitment following a severe bleaching event in the Maldives. In: Lindén O, Souter D, Wilhelmsson D, Obura D, editors. Coral reef degradation in the Indian Ocean. Stockholm: Cordio. pp. 125–134.
- Zea S. 1993. Cover of sponges and other sessile organisms in rocky and coral reef habitats of Santa Marta, Colombian Caribbean Sea. Caribbean Journal of Science 29:75–88.