Abstract
In the Mediterranean Sea, the two sponges of the genus Aplysina (A. aerophoba and A. cavernicola) are identified on the basis of their external morphology and the environment in which they live. During a research program on the sponge fauna in semi-submerged caves of the Italian coasts, we have sampled an abundant very small yellow sponge, often living in the tidal zone, which were attributed to the genus Aplysina. Failing to assign the samples to a species through classical taxonomic methodologies (growth form and skeleton arrangement) and for the particular environment where this sponge lives, we have decided to use the COI analysis to solve the taxonomic problem offered by these miniaturized specimens. The analysis indicated that, in spite of the morphological differences, they belong to A. aerophoba. During old detailed surveys, conducted in the ’60 years in some of the studied caves, this species was not recorded. It is possible that its abundant presence is related to the modifications occurred in the Mediterranean sponge communities occurred in the last decades in relation to global warming.
Introduction
Due to the lack of mineral skeleton (siliceous or calcareous) and the presence of chitin-spongin fibres (Ehrlich et al. Citation2007) with very similar size and shape, identification of the species of the genera belonging to the order Verongiida Bergquist, 1978 is generally critic. The two Mediterranean species of the Aplysinidae family, Aplysina aerophoba (Nardo 1833) and A. cavernicola (Vacelet Citation1959), are separated on the base of the external morphology and, in particular, surface texture, shape of digitations, presence of lateral projections and pigmentation of living specimens. Moreover, these two species were always found in different habitats: shallow-waters lighted environments for A. aerophoba while A. cavernicola lives in deep coralligenous habitats or at the entrance of shadow caves. On the other hand, the distinction of the two taxa on the base of skeletal fibres is considered very uncertain (Vacelet Citation1959).
During a research program on the sponge fauna of semi-submerged marine caves present along the Italian coasts, we have recorded abundant, very small specimens of a yellow sponge, often living in the tidal zone, that were attributed to the genus Aplysina; these miniaturised growth forms did not fit with the characteristics of the two known Mediterranean species of this genus (Costa et al. Citation2018). This taxonomic uncertainty leads us to collect specimens in different localities to solve the problem by means of morphological and genetic analysis.
Over the past decades, molecular analyses represented a powerful tool in the clarification of species boundaries in the marine realm. This was particularly useful in species whose separation on morphological base presents a high degree of uncertainty as sponges subjected to high intra-specific variation of shape and colour (Heim Citation2003): due to the incorporation of molecular techniques into the field of systematics, the discovery of morphologically cryptic but genetically divergent species has increased exponentially over the recent decades (Knowlton Citation1993; Bickford et al. Citation2006).
Heim et al. (Citation2007) tested different molecular markers, as internal transcribed spacer-1 and −2 (ITS-1, ITS-2) rDNA, mitochondrial 12S, 16S, and cytochrome oxidase subunit I (COI), in the separation of the species of the genus Aplysina of the Mediterranean Sea. Authors found that a high degree of intra-individual polymorphism within the ITS-1 and ITS-2 while, in contrast, the mitochondrial 12S and 16S are highly conserved. Authors concluded that only the COI was suitable in the separation of A. cavernicola from specimens which are regarded as A. aerophoba. Nevertheless, this last taxon probably is not a single valid species, but a cluster of species.
On this base, we have decided to use the COI analysis to solve the taxonomic problem offered by the miniaturised sponge belonging to the genus Aplysina recorded in the tidal zone of 4 semi-submerged caves along the Italian coasts.
Materials and methods
Sampling localities
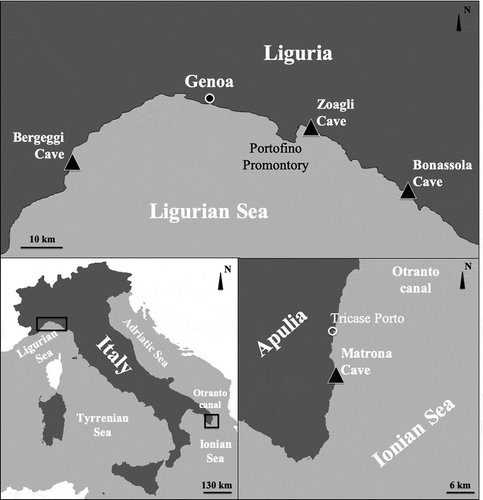
Sampling was performed by SCUBA diving in the summer 2017 and 2018 in four semi-submerged caves in different localities of the Italian coasts (); two were located in the Eastern Ligurian Riviera: “Bonassola Cave” (44°10ʹ24.60”N, 9°35ʹ25.32”E) and “Zoagli Cave” (44°20ʹ13.70ʹ’N, 9°15ʹ28.87ʹ’E); one was located in the western Ligurian Riviera: “Grotta marina di Bergeggi” (44°14ʹ33.25”N, 8°26ʹ42.90”E). The last one was located in the south Apulia coast: “Matrona Cave” (39°54ʹ22.55”N, 18°23ʹ28.01”E). The sponge-fauna of the Bonassola and Zoagli caves were already deeply studied by Sarà (Citation1964) and Costa et al. (Citation2018).
The Bonassola Cave is a semi-submerged, horizontal cavity which opens on gabbro rocks about 1 km eastward of the homonymous village. The bottom of the cave reveals irregular boulders and pebbles. The cavity is accessed through a 4–5-m long passage, 2–2.5 m wide and with a ceiling 2 m above sea level. Numerous crevices and small galleries are present on the side walls. Light conditions create a semi-obscure environment, while total darkness is reached only in parts of the cave. The Zoagli Cave is a small blind-end (cul-de sac) semi-submerged cave, which opens in a marly cliff about 1 km west of the Zoagli village. It is about 16 m long and 4 m wide at the entrance, with a height of the vault decreasing from about 3 m at the entrance to about 1 m at the end of the cave. The pebble floor steadily rises from a depth of 2 m to the end of the cave where it forms a small beach. The walls are mostly smooth. The opening is oriented to the east. The Bergeggi cave originated by karst corrosion in a dolomitic limestone of the middle Trias and its appearance was shaped by marine erosion. The main entrance, facing East, is about 10 m wide and 8 m high. The main cavity, which occupies about 350 m2, is characterized by large boulders detached from the ceiling. The sea penetrates this room forming pools of variable width and depth. The benthic communities of the Bergeggi marine cave were deeply studied through the last three decades starting from the paper of Bianchi et al. (Citation1988). The Matrona cave has a predominantly horizontal development, it is about 12 m long and about 7 m wide. It has a large collapsed semi-submerged cave. It opens with a mouth of about 7–8 m and a height varying between 1.5 and 3 m.
Morphological characterization
Sponge samples were preserved in 95% ethanol and processed by standard methods for sponge identification (Rützler Citation1978). Taxonomic decisions were taken according to the Systema Porifera (Hooper & Van Soest Citation2002) implemented by the Demosponge revision of Morrow and Cárdenas (Citation2015) and the World Porifera Database (WPD) (van Soest et al. Citation2019). The skeleton was studied measuring the length and the total diameter and the core width of at least 30 fibers. Minimum, mean (in parentheses) and maximum values (μm) of fibres and axial pith dimensions are reported.
For SEM analysis, dried tissues and skeleton were coated with gold and observed by a scanning electron microscope Vega3 TESCAN type LMU.
Molecular investigations
For genetic analysis, small portions of sponge tissues were obtained from 2 specimens of about 5-mm size sampled in Bergeggi cave (Ligurian Sea) and Matrona Cave (Ionian Sea). Total DNA was isolated using a Genomic Tissue DNA Kit (Rabbit Bio-Tek) following the manufacturer’s protocol and subsequently stored at −20°C. To perform species identification, the complete mitochondrial COI gene of 710 base pairs (bp) was amplified using two pairs of primers: PorCOI2fwd (5ʹ-aatatgngggcnccnggnatnac-3ʹ) and PorCOI2rev (5ʹ-actgcccccatngataaaacat-3ʹ) developed by Xavier et al. (Citation2010) and LCO1490 (5ʹ-ggtcaacaaatcataaagatattgg-3ʹ) and HC02198 (5ʹ-taaacttcagggtgaccaaaaaatca-3) developed by Folmer et al. (Citation1994). Polymerase chain reactions (PCR) were carried out in a 20 µl volume containing 1 µl of extracted DNA, 0.4 µl of each primer (10 µmol), 0.4 µl of dNTP mix (10 µmol each, Promega, USA), 2.4 µl of MgCl2 (25 mM), 4 µl of 5x Green GoTaq Flexi Buffer (Promega), 0.1 µl of GoTaq Taq DNA polymerase (Promega), and deionized water to volume. PCR reactions were run with the following parameters: 3 min of first denaturation at 95°C, each of 35 cycles consisted of denaturation for 30 sec at 94°C, annealing for 45 sec at 45°C, and extension at 72°C for 90 sec with a final 10 min extension at 72°C for Xavier et al. (Citation2010) primers, and initial denaturation at 94°C, each of 35 cycles consisted of denaturation for 30 sec at 94°C, annealing for 55 sec at 40°C, and extension at 72°C for 1 min with a final extension for 7 min at 72°C for Folmer et al. (Citation1994) primers. Amplification products were confirmed by using gel agarose electrophoresis and then purified using 2 µl of ExonucleaseI-Shrimp Alkaline Phosphatase (ExoSAP, USB, Cleveland, OH, USA) per 5 µl of PCR product at 37°C for 15 min, followed by deactivation at 80°C for 15 min. The run for DNA sequencing was performed with the “ABI 3730XL” Genetic Analyzer with the sanger technique. Sequences were aligned, edited, and trimmed to a common length using the DNA sequence assembly and analysis software Geneious Pro v. 9.0.4 (Biomatters, LTD, Auckland, NZ) and deposited in GenBank (accession numbers MN020524–MN020525; http://www.ncbi.nlm.nih.gov/genbank/). A similarity search (BLAST) was performed to exclude any possible contaminants such as symbionts and for species identification. For inclusion in the phylogenetic analyses, as described in (), 27 COI sequences of 8 species from Aplysina genus plus a sequence of Ailochroia crassa (Hyatt, 1875) were downloaded from the public databases (Barcode of Life Data (BOLD) Systems, www.barcodinglife.org) (Ratnasingham & Hebert Citation2007) and GenBank (https://www.ncbi.nlm.nih.gov/genbank/). All sequences were trimmed to a common length of 660 bp. A Maximum Likelihood (ML) tree was constructed using the program mega 7 (Kumar et al. Citation2016). The best fit general substitution model (as indicated by MEGA) was implemented (Tamura 3-parameter model) and all codon positions selected (Tamura Citation1992). Bootstrap support values were calculated using 1000 replicates.
Table I. Species and sequences downloaded from Genbank database with origin area and Genbank accession numbers. §Species chosen as outgroup. Accession numbers of our specimens of Aplysina aerophoba from Ligurian Sea and Ionian Sea are with asterisks (*)
Results
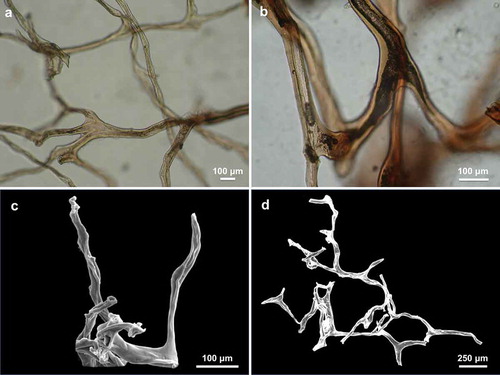
The studied specimens were small, cushion-shaped (2–10 mm in diameter; 1–3 mm thick) (). They were widely distributed in the semi-dark and dark portions of all the caves. Several specimens recorded in the tidal zone of the Zoagli and Bergeggi Caves remained completely emerged during low tides. Sometimes several small specimens fused together, forming large encrusting plates (). Most of the specimens had abundant thin branching processes even longer than 1 cm with an enlarged extremity ( and ).
Figure 2. Aplysina aerophoba (a,b) Cushion-shaped specimens joined together by thin, sometimes branching processes. (c) Samples with evident oscula indicated by arrow. (d) Magnification of the conulose surface with oscula (black arrow) and fibrous network (white arrow)

Figure 3. (a) Specimen with thin branching processes that they end up widening. (b) Magnification of the branching processes. (c) Conulose surface with evident fibrous network
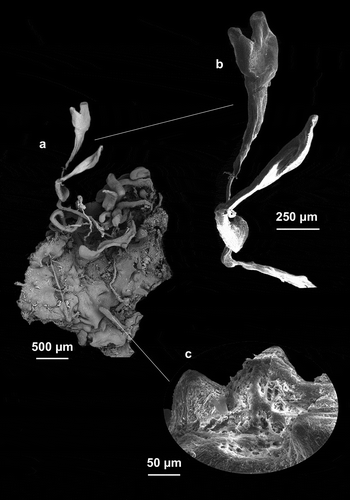
The colour in life was bright yellow () and fades to violet/black after alcohol preservation. Live specimens were soft, very fragile, with small elevated oscula (). The surface was conulose (), sometimes showing an evident fibrous network ().
The skeleton was fragile and showed the typical Aplysinidae structure. Primary and secondary fibres were indistinguishable and formed a regular three-dimensional framework (). The fibres ended inside the conules of the surface (). The fibres were laminar with a dark large axial pith (). The diameter of the fibres was 10 (47.7) 120 μm and the axial pith was 7.5 (35) 100 μm. describes the main morphological and skeletal characteristics of the specimens collected in Ligurian Sea and Apulian Sea.
Table II. Morphological and skeletal characteristics of the specimens collected in Ligurian Sea and Apulian Sea
DNA investigations
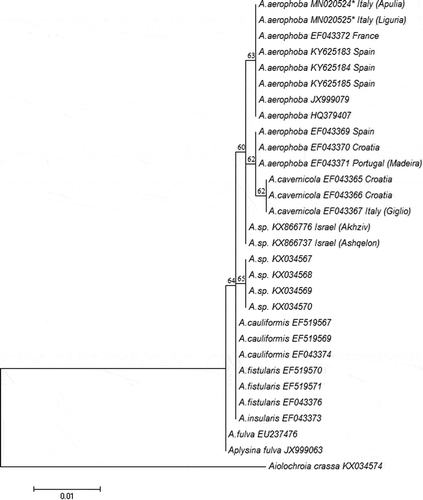
For the selected gene, aligning sequences from both sets of primers, we obtained 1110 bp from both specimens. The alignment performed with 27 sequences from genus Aplysina has generated sequences of 660 bp in length. A BLAST search performed both in GenBank and BOLD System excluded any contamination event with symbionts for our specimens, that belong to A. aerophoba (100% identity). Analysis of nucleotide sequences within the COI gene among the 8 Aplysina species showed six phylogenetically informative sites. Five of these sites represent substitutions in the third position of the respective codon. In only one, the substitution in the first position reveals an amino acid substitution of aspartic acid with asparagine for the species Aplysina sp. (). Considering these 5 sites, in the position 181st the two lineages reveal a clear difference of Adenine and Guanine nucleotide, respectively, for Mediterranean and Atlantic. The percentage identities between our A. aerophoba samples and the others Mediterranean species A. cavernicola and Aplysina sp. (this from Levantine Sea studied by Idan et al. Citation2018), have been 99.8% respectively with 3 bp of exchanges and 2 bp of exchange. Identical sites percentages and number of bp exchanges with other Atlantic species have been, respectively, 99.7% and 2 bp with A. cauliformis (Carter, 1882), A. fistularis (Pallas, 1766) and A. insulars (Duchassaing & Michelotti, 1864), 99.4% and 4 bp with A. fulva (Pallas, 1766) from Caribbean Sea, and 99.5% and 3 bp with Aplysina sp. from Brazilian coast A maximum likelihood phylogenetic trees have been calculated with Tamura 3-parameter model (). Our sequences cluster within A. aerophoba lineage. The two Mediterranean species A. aerophoba and A. cavernicola have shown the closest match and grouped together in a single clade separated from other Atlantic species (). Two sequences of Aplysina sp. from Mediterranean Sea show an uncertain position. Aiolochroia crassa has been chosen as outgroups. Aplysina fulva with the other Caribbean species (A. fistularis and A. cauliformis) represents a basal branch and cluster within the genus. The species Aplysina sp. from Brazilian archipelago has shown a separated clade between Mediterranean and Caribbean lineages.
Table III. Amino acid (upper section) a nucleotide (lower section) substitutions of the COI between different Mediterranean species of Aplysina.
Discussion
In the described case only with COI analysis, it was possible to assign our samples to Aplysina aerophoba (100% identity) confirming the usefulness of gene analysis for sponge species identification (Hebert et al. Citation2003).
A. aerophoba and A. cavernicola are confirmed to be clustered in a Mediterranean lineage clearly separated from a Caribbean and Brazilian lineages confirming the finding of Heim et al. (Citation2007).
This specific identification is not obvious in the light of the miniaturised shape of the specimens found in semi-dark caves described here for the first time. This is particularly strange when we consider that the distinction between the two Mediterranean species of the genus is mainly based on the general morphology of the specimens (Vacelet Citation1959). On the other hand, Heim et al. (Citation2007) suggested that the morphological taxon “A. aerophoba” is probably a cluster of related species. On this base and due to the peculiar morphological and ecological features of our specimens it is possible to hypothesise that, future more powerful analyses will allow to distinguish this species as a separate taxon.
However, a degree of morphological plasticity was already known both in A. aerophoba and A. cavernicola. In fact, transplantation experiments, indicated that the growth of both these species was affected by the level of solar radiation. Wilkinson and Vacelet (Citation1979) transplanted specimens of A. aerophoba in habitats characterized by different conditions of light intensity. The growth of this species, characterized by the presence of symbiotic cyanobacteria, was enhanced when solar radiation increased. On the contrary, the growth of A. cavernicola, which do not contain cyanobacteria, was inhibited by light. Similar results were obtained by Klöppel et al. (Citation2008) which reared A. aerophoba in aquaria in the aim to increase the production of bioactive compounds. When sponges are reared under lighted conditions the specimens maintained, after 150 days about the original volume. On the other hand, under dark conditions, the specimens experienced a significant reduction in size. This was not accompanied by any visible change in the structure or density of the skeleton.
The extreme reduction of the size of the specimens found in semi-submerged caves is therefore congruent with the low light intensity inside the caves. Regarding the color of the miniaturized specimens, A. aerophoba always express a dark yellow pigmentation, but our specimens show the bright yellow coloration typical of A. cavernicola that live in semi-dark zone. Very likely this is due to the loss of symbiotic cyanobacteria in dark as demonstrated by Klöppel et al. (Citation2009) during rearing experiments.
Most of the specimens showed abundant thin branching processes, sometimes branched and enlarged at the extremity. These processes are considered budding element (Vacelet Citation1959; Gerçe et al. Citation2009) and their morphology was already described by Díaz et al. (Citation2019) that suggested that their external morphology is individually genetically determined.
An open question remains the causes that induce a photophilic species to colonize a dark habitat. This aspect is particularly intriguing because, at least in the Bonassola and Zoagli caves the species was not surely present during surveys carefully conducted by Sarà (Citation1964) about 50 years ago (Costa et al. Citation2018).
It was recently suggested that during the recent decades the sponge communities of marine caves have experienced an increase in terms of specific richness and a significant change of the structural aspects. The three-dimensional growth forms decreased or remained quite similar, while numerous new two-dimensional ones were recorded (Costa et al. Citation2018; Montefalcone et al. Citation2018). We may, therefore, hypothesize that diffusion of this previously undescribed growth form of A. aerophoba is related to climatic events occurred which favoured some species and disadvantaged others.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bianchi CN, Cevasco MG, Diviacco G, Morri C. 1988. Primi risultati di una ricerca ecologica sulla grotta marina di Bergeggi (Savona). Bollettino Dei Musei E Degli Istituti Biologici dell’Università Di Genova 52:267–293.
- Bickford D, Lohman DJ, Sodhi NV, Ng PKL, Meier R, Winker K, Ingram KK, Das I. 2006. Cryptic species as a window on diversity and conservation. Trends in Ecology & Evolution 22(3):148–155. DOI: 10.1016/j.tree.2006.11.004.
- Costa G, Betti F, Nepote E, Cattaneo-Vietti R, Pansini M, Bavestrello G, Bertolino M. 2018. Sponge community variations within two semi-submerged caves of the Ligurian Sea (Mediterranean Sea) over a half-century time span. The European Zoological Journal 85(1):381–391. DOI: 10.1080/24750263.2018.1525439.
- Díaz JA, Movilla J, Ferriol P. 2019. Individualistic patterns in the budding morphology of the Mediterranean demosponge Aplysina aerophoba. Mediterranean Marine Science 20(2):282–286. DOI: 10.12681/mms.19322.
- Ehrlich H, Maldonado M, Spindler KD, Eckert C, Hanke T, Born R, Goebel C, Simon P, Heinemann S, Worch H. 2007. First evidence of chitin as a component of the skeletal fibers of marine sponges. Part I. Verongidae (Demospongia: Porifera). Journal of Experimental Zoology Part B: Molecular and Developmental Evolution 308(4):347–356. DOI: 10.1002/jez.b.21156.
- Folmer RHA, Nilges M, Folkers PJM, Konings RNH, Hilbers CW. 1994. A model of the complex between single-stranded DNA and the single-stranded DNA binding protein encoded by gene V of filamentous bacteriophage M13. Journal of Molecular Biology 240(4):341–357. DOI: 10.1006/jmbi.1994.1449.
- Gerçe B, Schwartz T, Voigt M, Rühle S, Kirchen S, Putz A, Proksch P, Obst U, Syldatk C, Hausmann R. 2009. Morphological, bacterial, and secondary metabolite changes of Aplysina aerophoba upon long-term maintenance under artificial conditions. Microbial Ecology 58(4):865. DOI: 10.1007/s00248-009-9560-6.
- Hebert PD, Ratnasingham S, de Waard JR. 2003. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proceedings of the Royal Society of London. Series B: Biological Sciences 270(suppl_1):S96–S99.
- Heim I. 2003. Synthetische Taxonomie zur Gattung Aplysina Nardo, 1834. Diploma Thesis. Stuttgart: Fakultät für Biound Geowissenschaften. Universität Stuttgart.
- Heim I, Nickel M, Brümmer F. 2007. Molecular markers for species discrimination in poriferans: A case study on species of the genus Aplysina. Porifera Research 1:361–371.
- Hooper JN, Van Soest RW. 2002. Systema Porifera. A guide to the classification of sponges. Hooper JN, Van Soest RW, editor. New York: Kluwer Academic.
- Idan T, Shefer S, Feldstein T, Yahel R, Huchon D, Ilan M. 2018. Shedding light on an East-Mediterranean mesophotic sponge ground community and the regional sponge fauna. Mediterranean Marine Science 19(1):84–106. DOI: 10.12681/mms.13853.
- Klöppel A, Pfannkuchen M, Putz A, Proksch P, Brümmer F. 2008. Ex situ cultivation of Aplysina spp. close to in situ conditions: Ecological, biochemical and histological aspects. Marine Ecology 29:159–272. DOI: 10.1111/j.1439-0485.2008.00225.x.
- Klöppel A, Putz A, Pfannkuchen M, Fritz G, Jaklin A, Proksch P, Brümmer F. 2009. Depth profile of Aplysina ssp.: Morphological, histological and biochemical aspects and their role in species distinction. Marine Biodiversity 39(2):121–129.
- Knowlton N. 1993. Sibling species in the sea. Annual Review of Ecology and Systematics 24(1):189–216. DOI: 10.1146/annurev.es.24.110193.001201.
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33(7):1870–1874. DOI: 10.1093/molbev/msw054.
- Montefalcone M, De Falco G, Nepote E, Canessa M, Bertolino M, Bavestrello G, Morri C, Bianchi CN. 2018. Thirty year ecosystem trajectories in a submerged marine cave under changing pressure regime. Marine Environmental Research 137:98–110. DOI: 10.1016/j.marenvres.2018.02.022.
- Morrow C, Cárdenas P. 2015. Proposal for a revised classification of the Demospongiae (Porifera). Frontiers in Zoology 12:1–27. DOI: 10.1186/s12983-015-0099-8.
- Ratnasingham S, Hebert PD. 2007. BOLD: The Barcode of Life Data System (http://www. barcodinglife. org). Molecular Ecology Notes 7(3):355–364. DOI: 10.1111/j.1471-8286.2007.01678.x.
- Rützler K. 1978. Sponges in coral reefs. In: Stoddart DR, Johannes RE, editors. Coral reefs: research methods. Monographs on oceanografic methodologies. Paris: UNESCO, p. 5.
- Sarà M. 1964. Distribuzione ed ecologia dei Poriferi in acque superficiali della Riviera ligure di Levante. Archivio Zoologico Italiano 49:181–248.
- Tamura K. 1992. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G + C-content biases. Molecular Biology and Evolution 9:678–687. DOI: 10.1093/oxfordjournals.molbev.a040752.
- Vacelet J. 1959. Répartition générale des éponges et systématique des éponges cornées de la région de Marseille et de quelques stations méditerranéennes. Recueil des travaux de la Station Marine d’Endoume 16:39–101.
- van Soest RWM, Boury-Esnault N, Hooper JNA, Rützler K, de Voogd NJ, Alvarez B, Hajdu E, Pisera AB, Manconi R, Schönberg C, Klautau M, Picton B, Kelly M, Vacelet J, Dohrmann M, Díaz MC, Cárdenas P, Carballo JL, Rios P, Downey R. 2019. World porifera database. Available: http://www.marinespecies.org/porifera. Accessed Apr 2018 5.
- Wilkinson CR, Vacelet J. 1979. Transplantation of marine sponges to different conditions of light and current. Journal of Experimental Marine Biology and Ecology 37:91–104. DOI: 10.1016/0022-0981(79)90028-5.
- Xavier JR, Rachello-Dolmen PG, Parra-Velandia F, Schönberg CHL, Breeuwer JAJ, Van Soest RWM. 2010. Molecular evidence of cryptic speciation in the “cosmopolitan” excavating sponge Cliona celata (Porifera, Clionaidae). Molecular Phylogenetics and Evolution 56(1):13–20. DOI: 10.1016/j.ympev.2010.03.030.