Abstract
Sandy beaches are ecosystems often subjected to a variety of pollution sources, including heavy metals and polycyclic aromatic hydrocarbons, largely due to improper human activities. The sandhopper Talitrus saltator s. l. (Montagu, 1808) typically lives on supralittoral shores where it plays an important ecological role. The purpose of this study was to investigate DNA damage levels on hemocytes of T. saltator by means of the Comet assay. Firstly, we tested the sensitivity and reproducibility of the Comet assay on hemocytes of T. saltator after in vitro exposure (1 h) to the genotoxicant methyl methanesulfonate (MMS). The data demonstrated a DNA damage (defined as % DNA tail) related to MMS exposure in a concentration-dependent manner. Next, we carried out the assay on hemocytes of T. saltator that were exposed in vivo (24 h and 7 days) to (a) lead (Pb), (b) benzo(a)pyrene (B(a)P), (c) mixtures of them. Our data on exposure to Pb or B(a)P showed DNA damage on the hemocytes of T. saltator in a concentration-dependent manner, but apparently with a non-synergistic effect of exposure to their mixtures. Our results provide a background for further studies in order to verify the applicability of Comet assay on T. saltator for assessing genotoxicity levels in sandy beaches subjected to pollution.
Introduction
Heavy metals, in particular lead (Pb), are an important public health issue for their genotoxic potential, persistence and rapid bioaccumulation, affecting organisms through polluted environments. Anthropogenic emissions have resulted in an increase in lead concentrations up to several orders of magnitude higher than the estimated natural background levels in soil and water (Hoffman et al. Citation2002). Benzo(a)pyrene (B(a)P) and other polycyclic aromatic hydrocarbons (PAHs) are widespread contaminants in various environmental compartments. These compounds, well known for their carcinogenic and mutagenic properties, often have anthropogenic origin, being released into the environment via industrial processes, wood treatment facilities, household heating and road transport (Cincinelli et al. Citation2008). Moreover, organisms living in polluted environments could be exposed to complex mixtures of chemical contaminants with possible additive and synergistic combinations (Beyer et al. Citation2014).
Sandy beaches are dynamic environments subjected to a variety of perturbations and, more recently, to pollution sources mainly due to improper anthropogenic activities. The supralittoral zone is characterized by a strong input of PAHs and heavy metals, but the toxicity of these compounds has received little attention. In particular, knowledge of the possible ecogenotoxic effects in polluted sandy beaches is, despite being highly relevant to public health, still limited.
In the last years, genotoxicity assessment in both vertebrates and invertebrates has gained increasing interest in ecotoxicology (Pavlaki et al. Citation2016). The Comet assay, or single-cell gel electrophoresis assay, is widely used for the detection of DNA damage in a variety of cells. Since the Comet assay is a rapid, sensitive, relatively inexpensive method, recently we applied this assay on hemocytes of crustacean amphipods for assessing genotoxicity in contaminated freshwater (Davolos et al. Citation2015; Ronci et al. Citation2015, Citation2016, Citation2017; Di Donato et al. Citation2016).
Sandhoppers and beach fleas are amphipods of the family Talitridae with important ecological roles in the Mediterranean sandy beach ecosystems. Among the sandhoppers, Talitrus saltator sensu lato (s. l.), which digs temporary refuges a few centimeters below the surface of the damp belt of sandy beaches, represents the majority of animal biomass in the supralittoral zone and an important food source for many invertebrates. Talitrus saltator has been the subject of studies on its genetic divergence (e.g., De Matthaeis et al. Citation1998, Citation2000) and, more recently, it was also used as a bioindicator of environmental alterations including those due to heavy metal contamination (Ungherese et al. Citation2010, Citation2012, Citation2016; Ugolini et al. Citation2012a, Citation2012b; Morrison et al. Citation2017).
In the present study, the Comet assay has been applied to investigate the extent of DNA damage in hemocytes of T. saltator exposed in vivo to Pb and B(a)P. Our study provides a basis to explore the applicability of the Comet assay on T. saltator for assessing genotoxicity levels in polluted sandy beaches.
Material and methods
Talitrus saltator sampling, laboratory acclimation and hemocyte samples
Adult individuals of T. saltator s. l. were collected by hand at Coccia di Morto (41°47ʹ49” N, 12°12ʹ59” E) within the protected area “Riserva Naturale Statale del Litorale Romano”, Latium, Italy.
Sampled animals (around 100 specimens) were quickly brought to the laboratory, where they were transferred to 30 L aquarium tanks. They were kept for 15 days in sand taken from the sampling site, at a constant temperature of 23 ± 1°C, with a 16/8 h light/dark cycle. During this period, animals were fed ad libitum with a common commercial food for crustaceans (JBL Novo Prawn). Every 3 days, the vitality of T. saltator specimens was checked, and the sand was aerated and humidified.
After 15 days, hemolymph samples were collected using an insulin syringe (30-G needle) inserted between the cephalon and the first mesosomite of T. saltator. Hemocyte cells were then placed in a 1.5 mL eppendorf tube containing 100 μL of chilled phosphate-buffered saline (PBS; Sigma-Aldrich – P5493). The viability of hemocytes was assessed through the Trypan Blue exclusion method (see Ronci et al. Citation2017).
In vitro exposure to methyl methanesulfonate
The in vitro exposure (1 h) to methyl methanesulfonate (MMS) largely followed the protocol reported in Lacaze et al. (Citation2010). Briefly, 10 µL of hemocyte suspension was gently mixed with 10 µL of MMS solution in PBS at concentrations of 0.5, 2, 5 and 10 mM, while 10 µL of PBS was used for negative control purposes. Three replicates, each corresponding to a pool of hemocytes from five T. saltator specimens, were performed for in vitro exposure to both MMS and negative controls.
In vivo exposure to lead and benzo(a)pyrene
Stock solution for Pb was prepared using lead nitrate (Pb(NO3)2; Merck, 105,043) and distilled water, while for the stock solution of benzo(a)pyrene (B(a)P) dimethyl sulfoxide (DMSO) was used. Test solutions were obtained from the stock solution through serial dilutions.
For each in vivo exposure, 3 kg of sand samples taken at Coccia di Morto was used. According to the difference in weight between wet (3 kg) and dried (at 50 °C/2 h) sand sample that indicated a water volume of 150 mL, each exposure was carried out by adding 150 mL of test solutions to a dried sand sample. A dried sand sample with the addition of 150 mL of distilled water was used as a negative control.
Talitrus saltator specimens were exposed in vivo to (a) 10, 50 and 100 μg/L Pb in the forms of Pb(NO3)2 and (b) 15, 30 and 60 µg/kg B(a)P. The same concentrations were used for in vivo exposures to three different mixtures of Pb and B(a)P (i.e., 10 μg/L Pb + 15 µg/kg B(a)P; 50 μg/L Pb + 30 µg/kg B(a)P; 100 μg/L Pb + 60 µg/kg B(a)P). For each concentration of Pb and B(a)P tested, the exposures were carried out in 2-L flasks for 24 h and 7 days. Three replicates, each corresponding to five individuals, were performed. During exposure, the aforedescribed temperature and light/dark cycle conditions were maintained in the laboratory.
The Comet assay
The alkaline version of the Comet assay followed the procedure modified by Lacaze et al. (Citation2010) and Di Donato et al. (Citation2016). Briefly, microscope slides were precoated with normal melting agarose (0.8%) in PBS and dried overnight. After collection of the cells, 20 µL of 1% low melting agarose in PBS (37°C) were mixed with 20 µL cell suspension, added onto the coated slides, and finally covered with a coverslip. Slides were cooled for 5 min at 4°C for solidification of the agarose. After removing the coverslip, slides were placed in a freshly prepared lysing solution (2.5 mol/L NaCl, 100 mmol/L Na2EDTA, 10 mmol/L Tris–HCl, 1% Triton X-100, 10% DMSO, pH 10) at 4°C in the dark for 1 h; after cell lysis, slides were gently placed in a horizontal electrophoresis chamber filled with freshly prepared chilled buffer. After electrophoresis (1% low melting point agarose) conducted at 0.6 V/cm and 300 mA at 4°C for 24 min, the comet slides were transferred to a neutralizing buffer (0.4 mol/L Tris–HCl, pH 7.5) and then stained with 50 μL ethidium bromide (30 μg/mL; Sigma-Aldrich). Hemocytes with DNA strand breaks displayed DNA migration (comets) toward the anode. The comet tail length represents the distance from the center of the head (start of tail) to the end of the tail (the longer the comet tail, the greater the DNA damage). DNA damage was quantified as the percentage of DNA in the tail parameter, % DNA Tail, which is considered to be the most reliable and meaningful Comet parameter (see Ronci et al. Citation2017). Images of the Comet assay were observed under an epifluorescence microscope (Zeiss Fluorescence Microscope System) equipped with a Zeiss camera (Axio Cam ICc 1) and were analyzed using ©2006TriTekCorp™ CometScore version 1.5 software. One hundred nuclei for each replicate were captured at 40× magnification, for a total of 300 images for each experimental condition. Hemolymph cells were sorted from other somatic cells by measuring the diameter of the nucleus (around 15–20 μm). Comets showing an undistinguished head and a prominent tail were excluded from scoring.
As the Comet assay values were not normally distributed, the Kruska–Wallis non-parametric test was used for the % DNA Tail data analysis.
Results
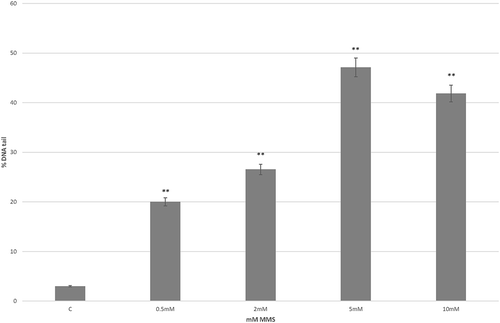
DNA damage (% of DNA tail) levels observed in hemocytes of T. saltator after in vitro exposure for 1 h to all the MMS concentrations tested were statistically higher (p < 0.01) if compared with the values found in negative controls (). As regards the exposure at 0.5, 2 and 5 mM of MMS, the % DNA tail significantly increased from 20.0 ± 0.9 to 47.1 ± 1.4, while at 10 mM of MMS, a slight reduction of % DNA tail (41.9 ± 1.0) was measured (). The reduction of DNA tail length observed at 10 mM was not significant in comparison to the value found at 5 mM.
Figure 1. DNA damage estimated by means of the Comet assay in hemocytes of Talitrus saltator. Each value is the mean with standard error considering the % DNA Tail value of the Comet assay applied to T. saltator hemocytes (1 h of exposure to different concentrations (mM) of Methyl Methanesulfonate (MMS); C stands for negative control; see text). ** indicates a significant difference (P < 0.01) when the non-parametric Kruskal–Wallis test was applied
The % of DNA tail values observed in hemocytes of T. saltator after in vivo exposure for 24 h and 7 days to Pb or B(a)P are reported in . All the Pb concentrations tested in this study after exposure for 24 h revealed DNA damage in hemocytes of T. saltator statistically higher than values found for the control (p < 0.01; ). For example, a significant increase of % DNA tail was registered at 10 and 50 µg/L Pb (2.5 ± 0.3 and 4.1 ± 0.5, respectively). After 7 days, the % DNA tail values at 50 and 100 µg/L Pb were statistically different (p < 0.01) when compared with that found in the negative control, with the value of DNA damage (7.8 ± 0.9% DNA Tail) registered at 50 µg/L higher than that found at 100 µg/L ().
Figure 2. DNA damage estimated by means of the Comet assay in hemocytes of Talitrus saltator. Each value is the mean with standard error considering the % DNA Tail value of the Comet assay applied to T. saltator hemocytes (24 h and 7 days of exposure to different concentrations of Pb (a) or benzo(a)pyrene (B(a)P) (b); C stands for negative control; see text). Grey bars refer to 24 h of exposure, while black bars refer to 7 days. ** indicates a significant difference (P < 0.01) when the non-parametric Kruskal–Wallis test was applied
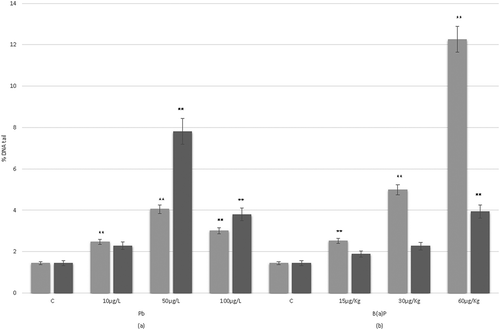
After 24 h of B(a)P exposure, the % DNA tail in hemocytes of T. saltator was statistically higher (p < 0.01) when compared with the values found for negative controls (e.g., at 30 and 60 µg/kg of B(a)P we found 5.0 ± 0.5 and 12.3 ± 1.0 in % DNA Tail, respectively; ). A statistically significant (p < 0.01) increase of DNA damage dependent on the concentration of B(a)P tested was observed (). After 7 days, the B(a)P exposure was characterized by lower DNA damage levels than those found at 24 h, with DNA damage levels generally not statistically different when compared with negative controls. However, a significant (p < 0.01) increase of DNA damage dependent at the concentration of B(a)P tested was observed ().
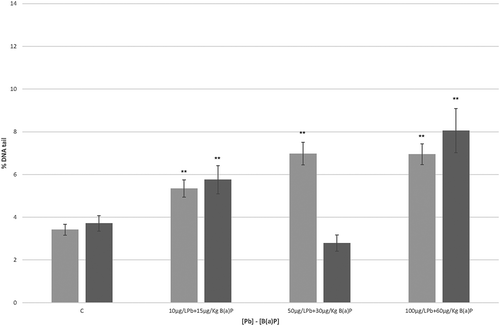
The percentages of DNA tail observed after in vivo exposure of hemocytes of T. saltator to mixtures of Pb and B(a)P are shown in . After 24 h, the DNA damage levels in hemocytes of T. saltator were statistically different (p < 0.01) when compared to values found in the control for all the mixture concentrations tested, with an increase of DNA damage dependent on the concentration (). After 7 days, the DNA damage was statistically different when compared to the control values for most of the mixture concentrations tested (). At 50 µg/L Pb + 30 µg/kg B(a)P, a reduction of % DNA tail value was evident, while the maximum % DNA tail value (8.0 ± 0.5%) was observed at 100 µg/L Pb + 60 µg/kg B(a)P ().
Figure 3. DNA damage estimated by means of the Comet assay in hemocytes of Talitrus saltator. Each value is the mean with standard error considering the % DNA Tail value of the Comet assay applied to T. saltator hemocytes (24 h and 7 days of exposure to mixtures of Pb and benzo(a)pyrene (B(a)P); C stands for negative control; see text). Grey bars refer to 24 h of exposure, while black bars refer to 7 days. ** indicates a significant difference (P < 0.01) when the non-parametric Kruskal–Wallis test was applied
Discussion
Sandhoppers such as T. saltator play an important ecological role as decomposers of the organic matter in sandy beaches. Being at the base of the food chain makes talitrid amphipods extremely well suited as bioindicators of anthropogenic contaminants in coastal regions. The assessment of their ability in bioaccumulation of various xenobiotics (e.g., heavy metals, PAHs) was the subject of recent investigations (e.g., Morrison et al. Citation2017; Ugolini et al. Citation2012a, Citation2012b; Ungherese et al. Citation2010, Citation2012, Citation2016).
In several studies, as the Comet assay resulted from a suitable tool to detect a broad spectrum of DNA lesions with high sensitivity, rapidity and low-cost, recently we used this assay on freshwater amphipods such as Gammarus elvirae that reliably allowed to assess the genotoxic potential of different sites (e.g., Davolos et al. Citation2015; Ronci et al. Citation2015, Citation2016, Citation2017; Di Donato et al. Citation2016). However, a genotoxicity study based on the Comet assay for marine talitrid amphipods living on sandy beaches was lacking.
Here, for the first time, hemocytes of the sandhopper T. saltator were exposed in vitro to MMS, a compound repeatedly served as a model substance for genotoxic study (Faßbender & Braunbeck Citation2013). The results confirmed the effectiveness of the Comet assay in measuring genotoxicity effect on hemocytes of T. saltator, in which a statistically significant DNA damage with a clear concentration-dependent manner was observed. Notably, these data were consistent with those of Lacaze et al. (Citation2010) on hemocytes of Gammarus fossarum exposed to a comparable scenario.
As regards the DNA damage observed on hemocytes of T. saltator after in vivo exposure to Pb, apparently there was a correlation between the concentration of Pb and % DNA tail measured. Other authors highlighted a strong correlation between levels of Pb-induced DNA damage and cell redox status (Jomova & Valko Citation2011). Indeed, Pb(NO3)2 can induce DNA damage, oxidative stress, necrosis and cell death. In particular, genotoxicity induced by Pb can be related to oxidative stress that occurs when there is an imbalance between the formation and removal of reactive oxygen species (ROS) (Hermes-Lima et al. Citation1991; Singh et al. Citation2017). Indeed, Duman and Kar (Citation2015) confirmed that oxidative stress can be induced by metals in the freshwater amphipod Gammarus pulex with a significant effect on the antioxidative defense enzymes. For the lower DNA damage level found after T. saltator exposure to Pb at 100 µg/L with respect to that found at 50 µg/L, one explanation may be a DNA repairing ability to Pb-induced toxic damage, in agreement with data observed in the freshwater mussel Anodonta grandis when exposed to lead (Black et al. Citation1996).
As regards the DNA damage observed on hemocytes of T. saltator after exposure in vivo to B(a)P, our data indicated a concentration-dependent manner, both at 24 h and 7 days, but a low level of DNA damage was measured after the exposure for 7 days at each of the B(a)P concentration tested. Vincent-Hubert et al. (Citation2011) measuring genotoxicity in the bivalve Dreissena polymorpha exposed to 10 μg/L of B(a)P for 10 h, 24 h, 3, 5 and 11 days, obtained similar results. Moreover, these authors explained some low DNA damage levels probably due to either an efficient detoxification or the activation of DNA repair mechanisms. Although the exposure scenarios are not comparable, our results are in agreement with DNA damage levels found after exposure to various concentrations of B(a)P reported by Vicentini et al. (Citation2017) for the chironomid Chironomus sancticaroli and Siu et al. (Citation2004) for the mussel Perna viridis. In both studies, the lower DNA damage levels after exposure to the highest B(a)P concentration indicated a possible activation of DNA repair enzymes.
Finally, under the laboratory conditions of this study, the combined in vivo exposure to Pb and B(a)P in hemocytes of T. saltator showed % DNA Tail values that indicated a concentration-dependent effect on genotoxicity when compared to controls. However, a non-synergistic effect of the exposure to the mixtures of Pb and B(a)P emerged. Apparently, our data are in agreement with those of Vincent-Hubert et al. (Citation2011), who showed DNA damage levels for D. polymorpha specimens when exposed to a combination of Cd and B(a)P. Again, the lower DNA damage levels detected by the Comet assay after T. saltator exposure to 50 µg/L Pb + 30 µg/kg B(a)P may be explained by DNA repairing ability to induced genotoxic damage in T. saltator, as previously proposed for G. elvirae by Ronci et al. (Citation2017). However, more in-depth analyses are necessary to increase our understanding of the genotoxicity on T. saltator potentially related to different concentrations of contaminant mixtures.
In conclusion, our results showed the potential applicability of the Comet assay on hemocytes of T. saltator to assess the genotoxicity impact of anthropogenic contaminants such as heavy metals and PAHs on sandy beach ecosystems.
Acknowledgements
We thank Sapienza University of Rome (Rome, Italy) for financial support.
Disclosure statement
The authors declare that they have no conflict of interest.
References
- Beyer J, Petersen K, Song Y, Ruus A, Grung M, Bakke T, Tollefsen KE. 2014. Environmental risk assessment of combined effects in aquatic ecotoxicology: A discussion paper. Marine Environmental Research 96:81–91. DOI:10.1016/j.marenvres.2013.10.008.
- Black MC, Ferrell JR, Horning RC, Martin LK. 1996. DNA strand breakage in freshwater mussels (Anodonta grandis) exposed to lead in the laboratory and field. Environmental Toxicology Chemistry 15(5):802–808. DOI:10.1002/etc.5620150528.
- Cincinelli A, Martellini T, Bittoni L, Russo A, Gambaro A, Lepri L. 2008. Natural and anthropogenic hydrocarbons in the water column of the Ross Sea (Antarctica). Journal of Marine System 73(1–2):208–220. DOI:10.1016/j.jmarsys.2007.10.010.
- Davolos D, Chimenti C, Ronci L, Setini A, Iannilli V, Pietrangeli B, De Matthaeis E. 2015. An integrated study on Gammarus elvirae (Crustacea, Amphipoda): Perspectives for toxicology of arsenic-contaminated freshwater. Environmental Science and Pollution Research International 22(20):15563–15570. DOI:10.1007/s11356-015-4727-9.
- De Matthaeis E, Davolos D, Cobolli M. 1998. Genetic divergence between populations and species of talitrids from Aegean islands. Journal of Heredity 89(1):37–43. DOI:10.1093/jhered/89.1.37.
- De Matthaeis E, Davolos D, Cobolli M, Ketmaier V. 2000. Isolation by distance in equilibrium and nonequilibrium populations of four talitrid species in the Mediterranean Sea. Evolution 54(5):1606–1613. DOI:10.1111/j.0014-3820.2000.tb00705.x.
- Di Donato G, De Matthaeis E, Ronci L, Setini A. 2016. Genotoxicity biomarkers in the amphipod Gammarus elvirae exposed in vivo to mercury and lead, and basal levels of DNA damage in two cell types. Chemistry and Ecology 32(9):843–857. DOI:10.1080/02757540.2016.1201078.
- Duman F, Kar M. 2015. Evaluation of effects of exposure conditions on the biological responses of Gammarus pulex exposed to cadmium. International Journal of Environmental Science and Technology 12(2):437–444. DOI:10.1007/s13762-013-0425-7.
- Faßbender C, Braunbeck T. 2013. Assessment of genotoxicity in gonads, liver and gills of zebrafish (Danio rerio) by use of the comet assay and micronucleus test after in vivo exposure to methyl methanesulfonate. Bulletin of Environmental Contamination and Toxicology 91(1):89–95. DOI:10.1007/s00128-013-1007-6.
- Hermes-Lima M, Pereira B, Bechara E. 1991. Are free radicals involved in lead poisoning? Xenobiotica 21(8):1085–1090. DOI:10.3109/00498259109039548.
- Hoffman DJ, Rattner BA, Burton Jr GA, Cairns Jr J, editors. 2002. Handbook of ecotoxicology. CRC Press. DOI:10.1201/9781420032505.
- Jomova K, Valko M. 2011. Advances in metal-induced oxidative stress and human disease. Toxicology 283(2–3):65–87. DOI:10.1016/j.tox.2011.03.001.
- Lacaze E, Geffard O, Bony S, Devaux A. 2010. Genotoxicity assessment in the amphipod Gammarus fossarum by use of the alkaline Comet assay. Mutation Research 700(1–2):32–38. DOI:10.1016/j.mrgentox.2010.04.025.
- Morrison L, Bennion M, McGrory E, Hurley W, Johnson MP. 2017. Talitrus saltator as a biomonitor: An assessment of trace element contamination on an urban coastline gradient. Marine Pollution Bulletin 120(1–2):232–238. DOI:10.1016/j.marpolbul.2017.05.019.
- Pavlaki MD, Araújo MJ, Cardoso DN, Silva AR, Cruz A, Mendo S, Soares AM, Calado R, Loureiro S. 2016. Ecotoxicity and genotoxicity of cadmium in different marine trophic levels. Environmental Pollution 215:203–212. DOI:10.1016/j.envpol.2016.05.010.
- Ronci L, De Matthaeis E, Chimenti C, Davolos D. 2017. Arsenic-contaminated freshwater: Assessing arsenate and arsenite toxicity and low-dose genotoxicity in Gammarus elvirae (Crustacea; Amphipoda). Ecotoxicology 26(5):581–588. DOI:10.1007/s10646-017-1791-6.
- Ronci L, Iannilli V, De Matthaeis E, Di Donato G, Setini A. 2015. Evaluation of genotoxic potential of waters from two Italian Rivers in Gammarus elvirae (Amphipoda). Water Environmental Research 87(11):2008–2017. DOI:10.2175/106143015X14212658614397.
- Ronci L, Meccoli L, Iannilli V, Menegoni P, De Matthaeis E, Setini A. 2016. Comparison between active and passive biomonitoring strategies for the assessment of genotoxicity and metal bioaccumulation in Echinogammarus veneris (Crustacea: Amphipoda). Italian Journal of Zoology 83(2):162–172. DOI:10.1080/11250003.2016.1169321.
- Singh N, Bhagat J, Ingole BS. 2017. Genotoxicity of two heavy metal compounds: Lead nitrate and cobalt chloride in Polychaete Perinereis cultrifera. Environmental Monitoring Assessment 189(7):308. DOI:10.1007/s10661-017-5993-4.
- Siu WHL, Cao J, Jack RW, Wu RSS, Richardson BJ, Xu L, Lam PKS. 2004. Application of the comet and micronucleus assays to the detection of B[a]P genotoxicity in haemocytes of the green-lipped mussel (Perna viridis). Aquatic Toxicology 66(4):381–392. DOI:10.1016/j.aquatox.2003.10.006.
- Ugolini A, Pasquali V, Baroni D, Ungherese G. 2012a. Behavioural responses of the supralittoral amphipod Talitrus saltator (Montagu) to trace metals contamination. Ecotoxicology 21(1):139–147. DOI:10.1007/s10646-011-0773-3.
- Ugolini A, Perra G, Focardi S, Somigli S, Martellini T, Cincinelli A. 2012b. Sandhopper Talitrus saltator (Montagu) as a Bioindicator of Contamination by Polycyclic Aromatic Hydrocarbons. Bulletin of Environmental Contamination and Toxicology 89(6):1272–1276. DOI:10.1007/s00128-012-0830-5.
- Ungherese G, Baroni D, Focardi S, Ugolini A. 2010. Trace metal contamination of Tuscan and eastern Corsican coastal supralittoral zones: The sandhopper Talitrus saltator (Montagu) as a biomonitor. Ecotoxicology Environmental Safety 73(8):1919–1924. DOI:10.1016/j.ecoenv.2010.06.021.
- Ungherese G, Cincinelli A, Martellini T, Ugolini A. 2012. PBDEs in the supralittoral environment: The sandhopper Talitrus saltator (Montagu) as biomonitor? Chemosphere 86(3):223–227. DOI:10.1016/j.chemosphere.2011.09.029.
- Ungherese G, Cincinelli A, Martellini T, Ugolini A. 2016. Biomonitoring of polychlorinated byphenyls contamination in the supralittoral environment using the sandhopper Talitrus saltator (Montagu). Journal of Chemical Ecology 32(4):301–311. DOI:10.1080/02757540.2015.1135908.
- Vicentini M, Morais GS, Rebechi-Baggio D, Richardi VS, Santos GS, Cestari MM, Navarro-Silva MA. 2017. Benzo(a)pyrene exposure causes genotoxic and biochemical changes in the midge larvae of Chironomus sancticaroli Strixino & Strixino (Diptera: Chironomidae). Neotropical Entomology 46(6):658–665. DOI:10.1007/s13744-017-0505-3.
- Vincent-Hubert F, Arini A, Gourlay-Francé C. 2011. Early genotoxic effects in gill cells and haemocytes of Dreissena polymorpha exposed to cadmium, B[a]P and a combination of B[a]P and Cd. Mutation Research Genetic Toxicology and Environmental Mutagenesis 723(1):26–35. DOI:10.1016/j.mrgentox.2011.03.008.
