Abstract
Because of the geological history of North Africa, populations of Great Tits Parus major existing in this region experienced less geographical isolation from populations of continental Europe than populations of Afrocanarian Blue Tits Cyanistes teneriffae. As a result, North African Great Tits are more ephemeral in their distribution and habitat choice as well as less ecologically generalist than their European continental conspecifics. By contrast, the Afrocanarian Blue Tit forms a separate species which is closely related to the Blue Tit Cyanistes caeruleus of Europe, including Mediterranean islands. Afrocanarian Blue Tits are a versatile generalist species. Here we compare some aspects of the breeding ecology of Great Tits and Afrocanarian Blue Tits in a highland area of the Algerian Aures Mountains facing the Sahara in the south. For both the tit species this area constitutes peripheral part of their distributions. We found that in two breeding seasons Great Tits started laying eggs on similar average dates as Afrocanarian Blue Tits, whereas in one year they preceded the latter species. Great Tits laid larger clutches than Afrocanarian Blue Tits. Fledging success was similar between the two species, with Great Tits prospering slightly better in the year when broods of Great Tits were also less infested by blow flies than broods of Afrocanarian Blue Tits. The subtle differences we found between the breeding ecology of Great Tits and Afrocanarian Blue Tits seem to be consistent with Snow’s idea that these species in North Africa differ in a degree of adaptation to local conditions.
Introduction
The potential range of distribution of any avian species depends on a set of abiotic environmental conditions, which physically limit a possibility of the species existence, and on biotic factors, which result from evolutionary history and ecology of biotic communities (Andrewartha & Birch Citation1954; Gaston Citation2003; Newton Citation2003). In some cases there is a sharp physical limit for the distribution of species, while in other cases a gradual change of multiple ecological factors may determine gradual disappearance of habitats suitable to inhabit (Rapoport Citation1982). For example, the Sahara has been a strong barrier for the breeding distribution of most of the Western Palearctic birds, as well as for exchange between the Western Palearctic and sub-Saharan breeding avifaunas in general (Rapoport Citation1982; Newton Citation2003). This barrier has been operating at least for forest-nesting birds through the Pleistocene and later, in spite of some wetter periods affecting Sahara (Blondel et al. Citation2010; Larrasoana et al. Citation2013; Husemann et al. Citation2014). Because in some periods of the Pleistocen the north part of the Sahara received more rain, forests extended on the lowlands south of the Atlas Mountains before getting to the desert barrier (Larrasoana et al. Citation2013; Husemann et al. Citation2014), which might enable forest birds to inhabit those forests.
During the Pleistocene Ice Age the climatic and ecological conditions of North Africa were temperate, hence similar to those prevailing in peninsular regions of southern Europe (Blondel et al. Citation2010; Husemann et al. Citation2014). Therefore, the regions together provided several somewhat mutually isolated refugia for Western Palearctic plants and animals (Husemann et al. Citation2014). The subdivision of different species into populations occupying the temporarily isolated refugia lead to the evolution of different adaptations of species or subspecies of birds, which shaped their present geographic distribution developed after the glacial retreat (Newton Citation2003). The present distribution and variation of Mediterranean parids is a legacy of the biogeographic history of this region. North African and Canarian populations of the Afrocanarian Blue Tit Cyanistes teneriffae are a monophyletic clade of the species level, with European Blue Tits Cyanistes caeruleus as the sister species (Illera et al. Citation2011, Gohli et al. Citation2015; Stervander et al. Citation2015). On the other hand, the North African Great Tit Parus major excelens is part of the wide within-species geographic variation of the Great Tit Parus major (Cramp & Perrins Citation1993). Accordingly, the North African region of the Western Palearctic has a unique species of the genus Cyanistes, the Afrocanarian Blue Tit, while the Great Tit occurs as one broadly distributed species over most of the Western Palearctic, including its North African part. This confirms Snow’s (Citation1954) conclusion that Blue Tits were isolated in North-West Africa for a very long time, resulting in the evolution of the separate species of the Afrocanarian Blue Tit.
Snow (Citation1952, Citation1954) have emphasised that habitat-specialisation of the Blue Tit species complex and the Great Tit is geographically variable. Across most of its European range Great Tits are generalist with respect to a wide range of woody habitats, including coniferous stands, whereas Blue Tits are more confined to deciduous tree-dominated habitats. By contrast, in North Africa, Afrocanarian Blue Tits are a widely distributed generalist species, while Great Tits are more ephemerally distributed, avoiding drier areas (Snow Citation1952, Citation1954). It seems that the Iberian Peninsula is the area of switch in habitat-specialisation between Great Tits and Blue Tits, as suggested by Blue Tits becoming more generalist and Great Tits more specialist in this area (Snow Citation1954; Barrientos et al. Citation2016). North African Great Tits are also morphologically very similar to the Iberian conspecifics (Snow Citation1954). All this suggests that Afrocanarian Blue Tits are better adapted to North African habitats than Great Tits (Snow Citation1954).
Because habitat substantially determines ecological strategies of species/populations through abiotic and biotic effects (Southwood Citation1977, Citation1988), the differences in the distribution of Great Tits as compared to Afrocanarian Blue Tits in North Africa may have consequences for reproductive strategies of both these parids. Different aspects of the breeding biology of Afrocanarian Blue Tits have been studied in all areas of their geographical distribution, including both West-North Africa and Canary Islands (Isenmann Citation1987; Moali & Isenmann Citation1990; Chabi et al. Citation1995, Citation2000; Chabi & Isenmann Citation1997; Lo Valvo & Massa Citation1995; Ziane et al. Citation2006; Garcia-del-Rey et al. Citation2006, Citation2007; Brahmia et al. Citation2013; Adamou et al. Citation2015). On the other hand, only very limited data on the breeding of Great Tits in North Africa are available (Snow Citation1952; Sanz Citation1998).
A major idea of this study was to compare the timing of the onset of laying, clutch size and fledging success between Great Tits and Afrocanarian Blue Tits (Cyanistes teneriffae ultramarinus) coexisting in a highland forest in NE Algeria. Because mountainous areas are characterised by harsh climatic conditions, the timing of the tit breeding would be expected to depend on the weather during the pre-laying time. In general, Blue Tits in the Mediterranean area have been known to be affected by ectoparasitic blow flies (Calliphoridae) (Hurtrez-Bousses et al. Citation1997a, Citation1997b, Citation1999, Citation2000), which seems also to be true of Afrocanarian Blue Tits (Bouslama et al. Citation2001, Citation2002). Because nothing is known on infestations of Great Tit broods by blow flies in North Africa, we used our quantitative data on ectoparasitism to examine potential effects of blow flies on Great Tits in comparison with Afrocanarian Blue Tits. Our general prediction was that Great Tits should be more ecologically sensitive to different factors than Afrocanarian Blue Tits.
Materials and methods
Study area
The study site was located in a forested area of the Belezma National Park near Aures, Batna, Algeria (35°35ʹN, 06°03ʹE, mean altitude 1437 m). This mountainous part of Algeria is called the Belezma Range, which is a sub-range of the Aures Mountains that constitute the eastern range of the Saharan Atlas. The area faces the Sahara Desert with only some forestless transitory belt of separation. The Atlas cedars Cedrus atlantica are a prevailing tree species in the study forest. Holm oaks Quercus ilex, ash trees Fraxinus xanthoxyloides, junipers Juniperus oxycedrus occur in the understory. The forests of the area have been greatly degraded by human influence over the last 5 thousand years (Djema & Messaoudene Citation2009; Blondel et al. Citation2010), resulting in rather patchy distribution of habitats for tits to nest.
Climatic conditions of the study area are determined by the mountainous character and the closeness of the Sahara. They are characterised by cool winters and warm summers, with mean yearly temperatures 11–15°C, precipitation 300–350 mm, and de Martonne index of aridity 11.9–14.6, indicating a semi-arid status of the area (Doerr Citation1962).
Data collection and analysis
The study was conducted on the nest-box-nesting populations of Great Tits and Afrocanarian Blue Tits in 2007–2009. Wooden nest-boxes (n = 90) of internal dimensions 25 × 15 X 15 cm were set up on the tree trunks at the height of 3–4 m. The nest-boxes were visited at least twice a week through April-June to record the nesting species and basic breeding characteristics, including the onset of laying, clutch size, brood size and the number of fledglings. For this study we collected data on 89 broods of Afrocanarian Blue Tits (23–38 per year) and 30 broods of Great Tits (8–14 per year).
We used general and generalised linear models to analyse the data. General linear models were applied to analyse clutch size and laying date data. A generalised linear model with the Poisson error structure and log-link function was applied for the blow fly (Protocalliphora) count data, while the binomial error structure and logit-link function were applied for fledging success (the number of fledglings in relation to the number of hatchlings) (Crawley Citation2002). In both general and generalised linear modeling, we started with initial models that included all main explanatory variables and their two-way interactions, which were then simplified by removing non-significant interactions (Crawley Citation2002). All the statistical computations were performed using IBM SPSS Statistics 22.
Results
The average monthly temperature in the study area in the pre-breeding month, April, was highest in 2008 (14.4°C), lowest in 2009 (11°C) and intermediate in 2007 (13.5°C). The total rainfall in April was 32.5 mm in 2007, 3 mm in 2008 and 75.1 mm in 2009.
Laying eggs started between 5 May and 16 May in Great Tits and between 4 May and 20 May in Blue Tits. In 2007 and 2008, laying dates were very similar between the two study species, while in 2009 Great Tits started laying 11 days earlier on average than Afrocanarian
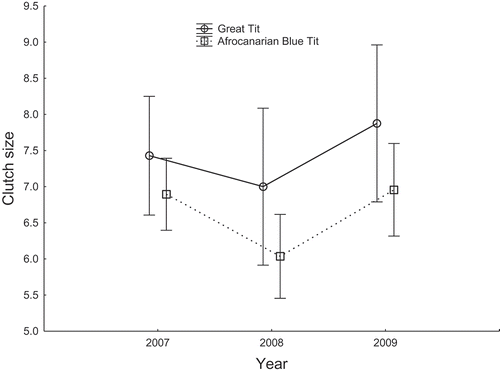
Blue Tits (9 May v. 20 May, respectively) (), resulting in a significant interaction between the species and year factors (). Mean clutch size differed among years in parallel between both species (non-significant interaction), with greater clutches being always laid by Great Tits (total mean 7.43 ± 0.29 (SE)) than Afrocanarian Blue Tits (total mean 6.96 ± 0.17 (SE)) (, ). Maximum individual clutch sizes we found were 11 eggs in the Great Tit and 9 eggs in the Afrocanarian Blue Tit.
Table I. Summary of a general linear model testing for differences in laying dates between Great Tits and Afrocanarian Blue Tits in cedar stands of Belezma National Park during 2007–2009.
Table II. Summary of a general linear model testing for differences in clutch size between Great Tits and Afrocanarian Blue Tits in cedar stands of Belezma National Park during 2007–2009.
Figure 1. Comparison of the dates of the onset of egg laying (means ± 95% CI) between Great Tits and Afrocanarian Blue Tits in cedar stands of Belezma National Park during 2007–2009.
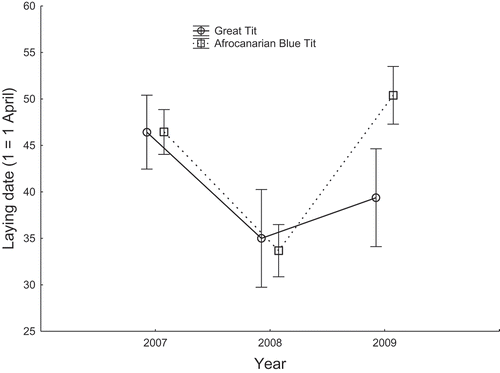
Figure 2. Comparison of clutch size (means ± 95% CI) between Great Tits and Afrocanarian Blue Tits in cedar stands of Belezma National Park during 2007–2009.
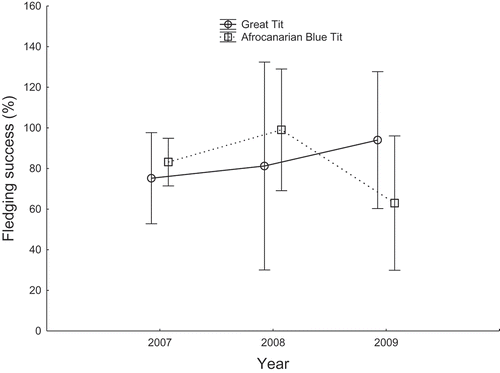
Numbers of blow flies in the nests were affected by a significant interaction between the species and year factors and an interaction between laying date and year (, ). The species-year interaction resulted from significant yet reversed differences between Afrocanarian Blue Tits and Great Tits in the number of blow flies per nest in 2007 (5.23 ± 0.46(SE) v. 8.02 ± 1.09 (SE), respectively; Wald X21 = 6.73, p = 0.009) and 2009 (27.85 ± 6.55(SE) v. 0.47 ± 0.29(SE), respectively; Wald X21 = 34.38, p < 0.0001), but any difference lacking in 2008 (0.92 ± 0.25(SE) v. 0.41 ± 0.41(SE), respectively; Wald X21 = 0.61, p = 0.44). An influence of the tit laying dates on the number of blow flies in nests differed between years, as indicated by the year-laying date interaction (). In 2007 and 2008, the number of blow flies per nest was not affected by the laying date (Wald X21 = 0.77, p = 0.38 and Wald X21 = 2.13, p = 0.14, respectively). In 2009, the number of blow flies was negatively related with the progress of the breeding season, as indicated by the laying date (2009: b = −0.241 ± 0.060, Wald X21 = 16.1, df = 1, p < 0.0001), meaning that the later laying date, the less numerous blow flies. However, this in fact resulted from a significant relation between the number of blow flies and laying date in Blue Tits (b = −0.234 ± 0.060, Wald X21 = 14.40, p = 0.001), with no relation in Great Tits (Wald X21 = 2.62, p = 0.11). The level of brood infestation with blow flies did not affect fledging success, whereas it was affected by an interaction between the year and species factors (, ). This interaction resulted from a slight switch in the order of the values of fledging success between Great Tits and Blue Tits in 2009 in comparison with 2007–2008 (), while in general over the 3-year period, the successes were very similar between the species (83.48% and 81.72%, respectively).
Table III. Summary of a generalized linear model testing for differences in the number of blow flies (Protocalliphora) found in the nests between Great Tits and Afrocanarian Blue Tits in relation to year and laying date in cedar stands of Belezma National Park during 2007–2009. The Poisson error distribution was assumed for the number of blow flies per brood, with the log link function being applied.
Table IV. Summary of a generalized linear model testing for differences in fledging success between Great Tits and Afrocanarian Blue Tits s in relation to year and the number of blow flies (Protocalliphora) found in the nests in cedar stands of Belezma National Park during 2007–2009. The binomial error distribution was assumed for the fledging success rate (the number of fledglings in relation to the number of hatchlings), with the logit link function being applied.
Figure 3. Comparison of the number of blow fly larvae (Protocalliphora) per brood (means ± 95% CI) between Great Tits and Afrocanarian Blue Tits in cedar stands of Belezma National Park during 2007–2009.
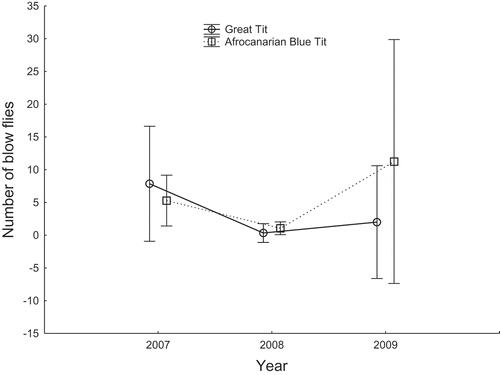
Figure 4. Comparison of fledging success expressed as the percentage of hatchlings that fledged (means ± 95% CI) between Great Tits and Afrocanarian Blue Tits in cedar stands of Belezma National Park during 2007–2009.
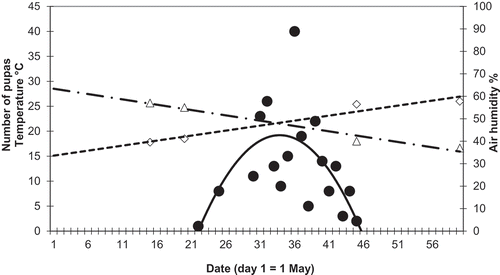
The occurrence of blowfly pupas is non-random with respect to ambient temperature and air humidity, with the maximum intensity of infestation taking place for a combination of these variables at c. 25°C and 50% humidity, which took place in early June in 2007 ().
Discussion
We found that in two breeding seasons Great Tits started laying eggs on very similar average dates as Afrocanarian Blue Tits, whereas in one year they substantially preceded the latter species. Great Tits laid on average larger clutches than Afrocanarian Blue Tits. Fledging success was similar between the two species, with Great Tits prospering slightly better in the year of the phenological discrepancy between the study species (2009), when broods of Great Tits were also less infested by blow flies then broods of Afrocanarian Blue Tits.
In our study area located in highlands of NE Algeria, both Great Tits and Afrocanarian Blue Tits occur at the south border of their distribution in this part of North Africa. Because of its mountainous character and closeness to the Sahara in the south, the Bolezma Natural Park is characterised by greatly seasonal climate, with cool winters, variable springs and warm summers. Especially springs are greatly variable and unpredictable with respect to temperature and rainfall (Adamou, unpublished). The resulting harshness and unpredictability of the physical conditions of this area is analogous to the harshness of conditions at the northern limit of tits in Scandinavia (Eeva et al. Citation1989; Veistola et al. Citation1994). Populations existing at the borders of species distribution are often unstable, with reproductive parameters differing from those in the core part of the range (Slagsvold Citation1981; Rapoport Citation1982; Jarvinen Citation1986; Gaston Citation1996, Citation2003).
We are not aware of any studies specifically addressing population density and stability in the case of North African Great Tits or Blue Tits, but it seems clear that Great Tits are less frequent and less numerous than Afrocanarian Blue Tits (Snow Citation1952, Citation1954; Adamou et al. Citation2016). The distribution of Afrocanarian Blue Tits is relatively narrow, restricted to North Africa and the Canary Islands, which suggests that they should be well adapted to environmental conditions and habitats of the area (Snow Citation1954), although the kind of forests in which the species probably originally evolved have completely disappeared under climatic and human-related pressures (Zaimeche Citation1994; Blondel et al. Citation2010). On the other hand, Great Tits do not seem to be so well fit to the North African habitats (Snow Citation1954). It is also characteristic that Great Tits in North Africa lay larger clutches than Afrocanarian Blue Tits, as we found in this study and as found in a study by Chabi reported by Sanz (Citation1998). In most of the area of sympatric distribution, continental Blue Tits produce larger clutches than Great Tits (Cramp & Perrins Citation1993; Sanz Citation1998, Citation2002; Fargallo Citation2004; Massa et al. Citation2011).
Our data suggest that both species start laying relatively late as for the latitude of the area (cf. Snow Citation1952; Moali & Isenmann Citation1990; Lo Valvo & Massa Citation1995; Sanz Citation1998, Citation2002; Fargallo Citation2004; Massa et al. Citation2011), even in late May, which is likely to be associated with the harsh climatic conditions of the highland study area and delayed availability of food resources. Indication of much earlier laying dates is known from lowland areas of Algeria (Snow Citation1952; Ziane et al. Citation2006). Similar altitude-related patterns have been reported for Great Tits and Blue Tits in Spain (Gil-Delgado et al. Citation1992; Belda et al. Citation1998).
During two years of this study mean laying dates of Great Tits were the same as mean laying dates of Afrocanarian Blue Tits, while in one year there was a substantial delay of 11 days in the latter species. It seems justified to suppose that the reason for this delay in Afrocanarian Blue Tits was an adaptive adjustment of the timing of breeding to a relatively late spring, as indicated by low temperature and heavy rainfall in April 2009. In our study area, April constitutes the pre-laying period, when weather is highly variable between years, but it may provide a good indication of conditions during the breeding season, and when the final location of nest site and territory are established. Relatively low temperature accompanied by heavy rainfall in April may have a different meaning as a clue for well-adapted Afrocanarian Blue Tits and less-adapted or differently-adapted Great Tits. Such conditions may be a clue for Afrocanarian Blue Tits to delay laying, whereas they may not be a reliable clue for Great Tits, leading to just a random response. It cannot be excluded that the response of Great Tits is not random but adaptive, yet based on the rainfall rather than temperature. The rainfall could have triggered early laying in Great Tits, as they are known to be more sensitive to draught and dry habitats in North Africa (Snow Citation1954).
We found that the interannual pattern of variation in blow fly numbers infesting broods of Great Tits and Afrocanarian Blue Tits was similar to the pattern of variation in laying dates. The mean numbers of blow flies per nest were very similar between the tit species in 2007 and 2008, whereas they were substantially different in 2009. More blow fly larvae infested broods of Afrocanarian Blue Tits than Great Tits in 2009, which seemed to be linked to the late and early breeding of the tits, respectively. This makes sense because we found that the timing of blow fly infestation is connected with a combination of temperature and humidity, which is attained later in a cooler spring. Thus, the level of brood infestation may vary with the laying and hatching date, as shown also in this study. Therefore Great Tits that started breeding early accidentally avoided the infestation by blow flies. Actually, the higher infestation of Afrocanarian Blue Tit broods only slightly deteriorated their fledging success in 2009 in comparison with broods of Great Tits. This is consistent with a weak effect of blow fly larvae on fledging success that has been reported for Blue Tits in some Mediterranean areas, where, however, a clear effect on nestling condition has occurred (Hurtrez-Bousses et al. Citation1997a, b, Citation1999, Citation2000).
The subtle differences we found between some aspects of the breeding ecology of Great Tits and Afrocanarian Blue Tits seem to be consistent with Snow’s (Citation1954) idea that these species in North Africa differ in a degree of adaptation to local conditions as a result of distinct evolutionary history. Because comparative data on Great Tits in North Africa are hardly available, further studies are needed.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Acknowledgements
The study was financially and logistically supported by the National Park of Belezma. We thank the Headquarters of the Park for permissions. We thank D. Summers for linguistic consultation.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Adamou AE, Kouidri M, Bensaci M, Ouakid ML. 2016. Analyse biogéoraphique de l’avifaune nicheuse dans une cédraie dégradée des Aurés (Nord-Est de l’Algerie). Alauda 84:221–230.
- Adamou AE, Kouidri M, Bańbura A, Ouakid ML, Chabi Y, Bańbura J. 2015. Variation in the timing of reproduction and clutch size of Afrocanarian Blue Tits Cyanistes teneriffae ultramarinus in the Saharan Atlas of Algeria. Pol J Ecol. 63:599–607. DOI:10.3161/15052249PJE2015.63.4.012.
- Andrewartha HG, Birch LC. 1954. The distribution and abundance of animals. Chicago: University of Chicago Press.
- Barrientos R, Bueno-Enciso J, Sanz JJ. 2016. Hatching asynchrony vs foraging efficiency: The response to food availability in specialist vs. generalist tit species. Scientific Reports 6:37750. DOI: 10.1038/srep37750.
- Belda EJ, Barba E, Gil-Delgado JA, Domingo JI, López GM, Monrós JS. 1998. Laying date and clutch size of Great Tits (Parus major) in the Mediterranean region: A comparison of four habitat types. Journal of Ornithology 139:269–276. DOI:10.1007/BF01653337.
- Blondel J, Aronson J, Bodiou JY, Boeuf G. 2010. The Mediterranean region: Biological diversity in space and time. 2nd ed. Oxford: Oxford University Press.
- Bouslama Z, Chabi Y, Lambrechts MM. 2001. Chicks resist high parasite intensities in an Algerian population of blue tits. Ecoscience 8:320–324. DOI:10.1080/11956860.2001.11682659.
- Bouslama Z, Lambrechts MM, Ziane N, Djenidi R, Chabi Y. 2002. The effect of nest ectoparasites on parental provisioning in a north‐African population of the Blue Tit Parus caeruleus. Ibis 144:E73–E78. DOI:10.1046/j.1474-919X.2002.00070_5.x.
- Brahmia Z, Scheifler R, Crini N, Maas S, Giraudoux P, Benyacoub S. 2013. Breeding performance of blue tits (Cyanistes caeuleus ultramarinus) in relation to lead pollution and nest failure rates in rural, intermediate, and urban sites in Algeria. Environmental Pollution 174:171–178. DOI:10.1016/j.envpol.2012.11.028.
- Chabi Y, Benyacoub S, Bańbura J. 2000. Egg-size variation in Algerian populations of the Blue Tit (Parus caeruleus ultramarinus): Effects of altitude and habitat. Revue d’Ecologie 55:183–192.
- Chabi Y, Isenmann P. 1997. La reproduction de la Mesange Bleue Parus caeruleus ultramarinus dans des suberaies Quercus suber à trois differentes altitudes en Algerie. Alauda 65:13–18.
- Chabi Y, Isenmann P, Benyacoub S, Samraoui B. 1995. Breeding ecology of the North-African Blue Tit (Parus caeruleus ultramarinus) in two semi-evergreen oak forests in Algeria. Revue d’Ecologie 50:133–140.
- Cramp S, Perrins CM, eds. 1993. The birds of the Western Palearctic. Vol. 7. Oxford: Oxford University Press.
- Crawley MJ. 2002. Statistical Computing. Chichester: Wiley.
- Djema A, Messaoudene M. 2009. The Algerian forest: Current situation and prospects. EFI Proceedings 57:17–27.
- Doerr AH. 1962. De Martonne’s index of aridity and Oklahoma’ climate. Proceedings of the Oklahoma Academy of Science 1962:211–213.
- Eeva T, Lehikoinen E, Veistola S. 1989. Habitat preference and breeding performance in four hole-nesting passerines at the northern limit of their range. Ornis Fennica 66:142–150.
- Fargallo JA. 2004. Latitudinal trends of reproductive traits in the Blue Tit Parus caeruleus. Ardeola 51:177–190.
- Garcia-del-Rey E, Cresswell W, Perrins CM, Gosler AG. 2006. Variable effects of laying date on clutch size in the Canary Island Blue Tits (Cyanistes teneriffae). Ibis 148:64–567. DOI:10.1111/j.1474-919X.2006.00569.x.
- Garcia-del-Rey E, Cresswell W, Perrins CM, Gosler AG. 2007. Evolutionary trends and extreme cases of life history traits in the Canary Island Blue Tit Cyanistes teneriffae on oceanic islands (Canary Islands). Ardeola 54:27–39.
- Gaston KJ. 1996. Species-range-size distributions: Patterns, mechanisms and implications. TREE 11:197–201. DOI:10.1016/0169-5347(96)10027-6.
- Gaston KJ. 2003. The structure and dynamics of geographic ranges. Oxford: Oxford University Press.
- Gil-Delgado JA, López G, Barba E. 1992. Breeding ecology of the Blue Tit Parus caeruleus in Eastern Spain: A comparison with other localities with special reference to Corsica. Ornis Scandinavica 23:444–450. DOI:10.2307/3676675.
- Gohli J, Leder EH, Garcia-Del-Rey E, Johannessen LE, Johnsen A, Laskemoen T, Popp M, Lifjeld JT. 2015. The evolutionary history of Afrocanarian blue tits inferred from genomewide SNPs. Molecular Ecology 24:180–191. DOI:10.1111/mec.13008.
- Hurtrez-Bousses S, Blondel J, Perret P, Renaud F. 1997a. Relationship between intensity of blowfly infestation and reproductive success in a Corsican population of Blue Tits. Journal of Avian Biology 28:267–270. DOI:10.2307/3676980.
- Hurtrez-Bousses S, de Garine-wichatitsky M, Perret P, Blondel J, Renaud F. 1999. Variations in prevalence and intensity of blow fly infestations in an insular Mediterranean population of blue tits. Canadian Journal of Zoology 77:337–341. DOI:10.1139/z98-213.
- Hurtrez-Bousses S, Perret P, Renaud F, Blondel J. 1997b. High blowfly parasitic loads affect breeding success in a Mediterranean population of blue tits. Oecologia 112:514–517. DOI:10.1007/s004420050339.
- Hurtrez-Bousses S, Renaud F, Blondel J, Perret P, Galan MJ. 2000. Effects of ectoparasites of young on parents’ behaviour in a Mediterranean population of Blue Tits. Journal of Avian Biology 31:266–269. DOI:10.1034/j.1600-048X.2000.310219.x.
- Husemann M, Schmitt T, Zachos FE, Ulrich W, Habel J. 2014. Palaearctic biogeography revisited: Evidence for the existence of a North African refugium for Western Palaearctic biota. Journal of Biogeography 41:81–94. DOI:10.1111/jbi.12180.
- Illera JC, Koivula K, Broggi J, Packert M. 2011. A multi-gene approach reveals a complex evolutionary history in the Cyanistes species group. Molecular Ecology 20:4123–4139. DOI:10.1111/j.1365-294X.2011.05259.x.
- Isenmann P. 1987. Geographical variation in clutch size: The example of the Blue Tit (Parus caeruleus) in the Mediterranean area. Vogelwarte 34:93–99.
- Jarvinen A. 1986. Clutch size of passerines in harsh environments. Oikos 46:365–371. DOI:10.2307/3565836.
- Larrasoana JC, Roberts AP, Rohling EJ. 2013. Dynamics of Green Sahara periods and their role in hominin evolution. PloS One 8:e76514. DOI: 10.1371/journal.pone.0076514.
- Lo Valvo F, Massa B. 1995. Breeding performance of Parus caeruleus ultramarinus on Pantelleria Island (Sicilian Channel). Rivista Italiana di Ornitologia 65:129–135.
- Massa B, Cusimano CA, Margagliotta B, Galici R. 2011. Reproductive characteristics and differential response to seasonal temperatures of Blue and Great Tits (Cyanistes caeruleus & Parus major) in three neighbouring mediterranean habitats. Revue d’Ecologie 66:157–172.
- Moali A, Isenmann P. 1990. The timing of breeding and clutch size of Blue tits (Parus caeruleus) in two montane habitats in Algeria. In: Blondel J, Gosler A, Lebreton J-D, McCleery R, editors. Population biology of Passerine birds. An integrated approach. Berlin: Springer. pp. 117–120.
- Newton I. 2003. Speciation and biogeography of birds. London: Academic Press.
- Rapoport EH. 1982. Areography. Oxford: Pergamon Press.
- Sanz JJ. 1998. Effects of geographic location and habitat on breeding parameters of Great Tits. Auk 115:1034–1051.
- Sanz JJ. 2002. Climate change and breeding parameters of great and blue tits throughout the western Palearctic. Global Change Biology 8:406–422. DOI:10.1046/j.1365-2486.2002.00496.x.
- Slagsvold T. 1981. Clutch size and population stability in birds: A test of hypotheses. Oecologia 49:213–217. DOI:10.1007/BF00349190.
- Snow DW. 1952. A contribution to the ornithology of North-West Africa. Ibis 94:473–498. DOI:10.1111/j.1474-919X.1952.tb01846.x.
- Snow DW. 1954. The habitats of Eurasian tits (Parus spp.). Ibis 96:565–585. DOI:10.1111/j.1474-919X.1954.tb05478.x.
- Southwood TRE. 1977. Habitat, the templet for ecological strategies? The Journal of Animal Ecology 46:337–365. DOI:10.2307/3817.
- Southwood TRE. 1988. Tactics, strategies and templets. Oikos 52:3–18. DOI:10.2307/3565974.
- Stervander M, Illera JC, Kvist L, Barbosa P, Keehnen NP, Pruisscher P, Bensch S, Hansson B. 2015. Disentangling the complex evolutionary history of the Western Palearctic blue tits (Cyanistes spp.) - phylogenomic analyses suggest radiation by multiple colonization events and subsequent isolation. Molecular Ecology 24:2477–2494. DOI:10.1111/mec.13145.
- Veistola S, Lehikoinen E, Iso-Iivari L. 1994. Breeding biology of The Great Tit Parus major in a marginal population in northernmost Finland. Ardea 83:419–420.
- Zaimeche SE. 1994. The consequences of rapid deforestation: A North African example. AMBIO 23:136–140.
- Ziane N, Chabi Y, Lambrechts MM. 2006. Breeding performance of Blue Tits Cyanistes caeruleus ultramarinus in relation to habitat richness of oak forest patches in nort-eastern Algeria. Acta Ornithologica 41:163–169. DOI:10.3161/068.041.0201.