Abstract
The great tit Parus major inhabits various types of forests, yet it prefers deciduous forests. In the urbanized areas it is one of the commonest bird species, even where only isolated trees are present, which seems to be a relatively recent phenomenon. This paper presents results of a long-term study (2002–2015) of variation in reproductive performance (number of hatchlings, hatching success, number of fledglings, fledging success, breeding success and nest failure rate) in first clutches of two great tit populations nesting in two contrasting habitats: a mature deciduous forest and an urban parkland, in central Poland. This study shows a clear and sustained spatial pattern of variation where the forest population of great tits produced more hatchlings and fledglings per successful nests than the urban park site population. Breeding success and fledging success demonstrate a similar but slightly less obvious pattern, with higher values for the forest site than the urban parkland area occurring consistently in the vast majority of years. Also hatching success was generally slightly higher in the forest than in the urban park but did not show such a clear spatial pattern. Only the nest failure rate was higher in the forest than in the urban parkland. We suggest that caterpillar richness in the forest compared to the urban parkland is one of the key drivers of the observed differences in the breeding performance between the study habitats. We also found positive correlations between fledgling numbers and caterpillar abundance and between fledgling numbers and mean May temperatures in the forest, whereas neither breeding nor fledging success showed such correlations. In the urban parkland, fledgling numbers as well as fledging and breeding successes were positively correlated with mean May temperatures. The between-population variation we found probably reflects a more general difference between urban and forest populations of tits.
Introduction
Life-history theory predicts that optimal reproductive strategies should vary across environments because organisms living in various conditions undergo functional constraints among life-history traits, resulting from limited resources that must be allocated among competing demands (Stearns Citation1992). Selection optimizes the allocation of resources to achieve the most advantageous balance of phenotypic traits under particular ecological conditions (Ricklefs Citation2000). Life-history theory explains the general features of life cycle, i.e., lifespan, growth rate, number of offspring and reproductive attempts, which are connected with constraining relationships among each other and specific environmental requirements. This classic theory has been recently supplemented by the pace-of-life syndrome hypothesis (POLS), which predicts coevolution of life-history strategy with a set of co-varying traits in physiology, metabolism and behavior shaped and favored by adaptive adjustment to specific environmental conditions (Ricklefs & Wikelski Citation2002; Réale et al. Citation2010). For example, according to this theory, species (or individuals within a population) on the slow end of the axis should be characterized by complex life-histories (e.g. high survival and lower reproduction output), physiological traits (e.g. lower metabolism) and behavioral traits (e.g. lower activity and shyness), whereas species or individuals on the fast end of the axis should demonstrate opposite characteristics (Réale et al. Citation2010). Nowadays, important modifications of life-history traits and fast eco-evolutionary processes could be noticed primarily in rapidly changing environments, such as those connected with urban development (Alberti Citation2015).
Urbanization is one of the major causes of natural habitat loss and fragmentation on the global scale, as it modifies extensively the natural landscapes, leading to rearrangement of habitats and fauna composition (McDonnell & Pickett Citation1990; Chamberlain et al. Citation2009). Habitat modification associated with urbanization creates new, often highly heterogeneous areas with novel combinations of challenges and sometimes also benefits for birds (Chace & Walsh Citation2006; Gil & Brumm Citation2013; Seress & Liker Citation2015). Advantages of urban habitats are mostly connected with additional and predictable food resources (Anderies et al. Citation2007; Kumar et al. Citation2019), high availability of nest sites (Reynolds et al. Citation2019) and, in some cases, with a reduced predation pressure (Vincze et al. Citation2017). Costs may be related to high human density and human disturbance (Fernández-Juricic & Tellería Citation2000), chemical pollution (Bichet et al. Citation2013), habitat fragmentation (Weldon & Haddad Citation2005). Numerous species suffer from these anthropogenic disturbances, which can cause changes in the diversity and abundance of an entire avian community by land transition from natural or semi-natural to urban, often driving biotic homogenization (see Chace & Walsh Citation2006; Marzluff Citation2016). Only a few species thrive in urban habitats, whilst many others sustain a mixture of advantages and costs of being urban inhabitants, whereas some species remain highly sensitive to stressors connected with environmental changes, the presence of people or have specific requirements and hence are absent or occur very rarely in urban areas (Fuller et al. Citation2009; Gil & Brumm Citation2013). In general, it has been suggested that species can be divided into three groups based on their levels of tolerance to urban disturbance – urban “avoiders”, “adapters” and “exploiters” (McKinney Citation2002). Species belonging to the “adapters” group are most interesting from the eco-evolutionary point of view because they make use of both natural and human-transformed habitats, and are thus very flexible in their lifestyles. These species evolved in natural conditions, yet rapid and drastic changes caused by urbanization force their specific adjustments, which are likely the result from phenotypic plasticity (Charmantier et al. Citation2017). The evolution of proper adaptations to novel conditions by natural selection on heritable variation may also be quite fast (Johnson & Munshi-South Citation2017). Recently, some authors have predicted that life-history traits should be formatted at the intraspecific level by particular urban ecology conditions, similarly to species communities, which are shaped by the specific ecological conditions of cities, preferring “urban-exploiter” species and eliminating sensitive species (Charmantier et al. Citation2017; Sprau et al. Citation2017; Sepp et al. Citation2018). It has been hypothesised that there exist adaptive differences in the pace of life (POLS) between urban and rural areas or even within the heterogeneous urban matrix (Sepp et al. Citation2018). It has been suggested that living in cities should lead to slower life histories (e.g. high survival, less investment in annual reproduction) in the pace-of-life continuum (from slow to fast), but in their behavioral features, urban birds should represent fast behavioral syndromes (e.g. boldness, high activity, high aggressiveness, more explorer behavior). On the other hand, the differences between urban and rural areas may also be attributed to variation in environmental conditions associated with urbanization, not necessarily as a manifestation of evolutionary adaptation, but instead, as a result of variation in food quantity and quality (Chamberlain et al. Citation2009). Alternatively, differences found between rural and urban populations, for instance reduction in the reproductive output, could be interpreted as consequences of the urban habitats being an ecological trap (Stracey & Robinson Citation2012; Sumasgutner et al. Citation2014).
Variation in reproductive performance is shaped by the interactions of extrinsic factors and intrinsic factors. Firstly, intrinsic factors connected with parental quality, age, health or experience have been proposed to explain such variation (Slagsvold & Lifjeld Citation1990; Gustafsson et al. Citation1994; Pärt Citation1995; Przybylo et al. Citation2001; Pagani-Núňez & Senar Citation2016; Caprio & Rolando Citation2017). Secondly, extrinsic factors, which are linked to differences in habitat quality (Martin Citation1987; Nager & Van Noordwijk Citation1992; Solonen Citation2001; Przybylo et al. Citation2001), food abundance (Cresswell & McCleery Citation2003; Massa et al. Citation2004; Marciniak et al. Citation2007), habitat structure and species composition of trees (Cowie & Hinsley Citation1987; Riddington & Gosler Citation1995; Mackenzie et al. Citation2014), home range size (Caprio & Rolando Citation2017), calcium abundance (Bańbura et al. Citation2010; Reynolds & Perrins Citation2010) or weather conditions (Massa et al. Citation2011; Bordjan & Tome Citation2014; Tobolka et al. Citation2015; Glądalski et al. Citation2016a) may also operate. Certainly, both types of factors influence bird reproductive success, but relations between them have often been confused.
Small urban passerines consistently exhibit earlier lay dates and smaller clutch sizes than their conspecifics in rural habitats (see reviews Chamberlain et al. Citation2009; Sepp et al. Citation2018). This is also true of great tits (Parus major) and blue tits (Cyanistes caeruleus) in our study system (Glądalski et al. Citation2015; Wawrzyniak et al. Citation2015). We have also shown that in both these tit species nestlings in the forest study area have on average better physiological condition than nestlings in the urban park area (Markowski et al. Citation2015; Kaliński et al. Citation2015a, Citation2015b, Citation2017a, Citation2017b; Glądalski et al. Citation2016b). Chamberlain et al. (Citation2009), Marzluff (Citation2016) and Sepp et al. (Citation2018) have emphasized that further comparative research on differences in reproductive parameters between urban and non-urban populations of birds is needed.
The main effort of this study is focused on effects of such environmental factors as habitat type, year, caterpillar availability, weather conditions, and also – competition on variation in hatchling and fledgling numbers and corresponding success rates of great tits during 14 consecutive breeding seasons. Our predictions are: (1) forest and urban great tits should differ in the number of hatchlings and fledglings, so that forest birds should produce more hatchlings and fledglings as a result of higher clutch sizes, caterpillar abundance and a higher habitat quality; (2) hatching success and fledging success should be similar in both areas because clutch sizes are matched to local food resources or will be higher in the optimal natural environment in the forest (3) fledgling number and especially fledging success should be sensitive to trophic conditions, weather conditions and, perhaps, to great tit densities.
Material and Methods
Study areas
The present study was carried out in 2002–2015, around the city of Łódź, central Poland. Study areas are located about 10 km apart, in two contrasting types of habitats: an urban parkland and a rich deciduous forest. The urban parkland study site (51º45ʹN; 19º24ʹE), an area of ca. 80 ha (including the zoological garden of 16 ha and the botanical garden of 64 ha), has a highly fragmented tree cover. The total length of pathways per hectare is 4.5 times greater in the parkland than in the forest, and a mesh of paths is much denser in the parkland as well (Glądalski et al. Citation2016c). Both gardens are important recreation areas of the city of Łódź and they are visited by great numbers of people, especially in spring and summer. The vegetation of the Zoological Garden has been heavily fragmented and adapted to displaying animals. The vegetation of a large part the Botanic Garden has been planted artificially to exhibit plants, with extensive tree-free spaces. Dominant native tree species are pendulate oak (Quercus robur), sessile oak (Q. petrea), birches (Betula sp.), hornbeam (Carpinus betulus), scots pine (Pinus silvestris), poplars (Populus sp.), willows (Salix sp.), limes (Tilia sp.), maples (Acer sp.), and numerous exotic tree species.
The forest study site (51º50ʹN; 19º29ʹE) is ca. 130 ha area in the interior of a mature mixed deciduous forest (1250 ha in total), neighbouring with the NE suburbia of Łódź. Oaks Q. robur and Q. petraea are predominating species. In the surrounding of nest-boxes, these oaks constituted 51.2% of all trees, while coniferous species only 9%. In general, the density of trees in the surroundings of nest-boxes is about three times lower in the urban park site than in the forest site, while in the case of the native oaks the difference is even much higher (Glądalski et al. Citation2017). The tree age structure of the forest site is mostly mature, sometimes with trees over 100 years old and some dead trees being present.
Both study sites have been supplied with wooden nest-boxes with removable front walls. All nest-boxes were placed on trees (usually on oaks) at a height of 2.5–3 m. The number of nest-boxes generally increased over the study period with current numbers being c. 200 in the parkland area and c. 300 in the forest (see Glądalski et al. Citation2016d). We currently use two types of nest-boxes that differ in height (29 cm and 32 cm, respectively) and have similar floor dimensions, 11 cm X 11 cm. In the past, we used nest-boxes from different manufacturers that might slightly differ in these dimensions. In both study areas, distances between neighboring nest-boxes were about 50 m, and the density of nest-boxes did not differ between the two study areas (see Glądalski et al. Citation2016d). The nest-boxes in both study plots were also occupied by blue tit, less frequently by other bird species: pied flycatchers (Ficedula hypoleuca) and nuthatches (Sitta europea), and, occasionally, marsh tits (Poecile palustris) and coal tits (Periparus ater).
Data on caterpillar abundance
The abundance of caterpillars during the breeding seasons was measured indirectly, using the frass fall collecting method (Marciniak et al. Citation2007). The frass fall method allows to measure the amount of frass produced by caterpillars, which indicates caterpillar numbers and biomass available for tits (Zandt Citation1994). The abundance of caterpillars was monitored over the tit breeding seasons 2003–2015. Frass fall was collected into 1 m x 1 m tissue traps, hung below tree canopies, 9 in the forest and 5 in the parkland. The traps were checked and emptied every 5 days. In the laboratory, all of the gathered samples were inspected under a microscope to separate caterpillar frass from other particles, dried and weighed to the nearest 0.1 mg. The resulting masses were established for both study plots in a given year (for details see Marciniak et al. Citation2007). The peak caterpillar amounts differed significantly between the two study areas (Glądalski et al. Citation2017). According to Glądalski et al. (Citation2017), the peak caterpillar abundance, as measured by the maximum amount of frass fall, averaged 0.21 ± 0.11 g frass/m2/day (range: 0.05–0.41) in the urban parkland and 0.59 ± 0.50 g frass/m2/day (range: 0.07–2.13) in the forest in 2003–2015. The peak amount of caterpillar frass in a season was used as a simple indicator of trophic richness in caterpillars, the fundamental food for chicks (Cholewa & Wesołowski Citation2011).
Breeding density data
Great tit (and also blue tit) nest-box breeding density data (including seasons 2002–2012) were subject of our previous analyses, and in all study years we recorded that at least 20% of nest-boxes were not occupied by any species of bird, so we concluded that availability of nest-boxes was not a limiting factor for numbers of breeding pairs (see Glądalski et al. Citation2016c). We used breeding density data (2002–2015) as one of the potentially important factors that can have an impact on the breeding performance. The evaluation of the density of great tits was based solely on breeding pairs nesting in nestboxes; the numbers may be underestimated, especially in the forest site, because of the presence of many natural holes, sometimes occupied by tits (own observations).
Data on weather conditions
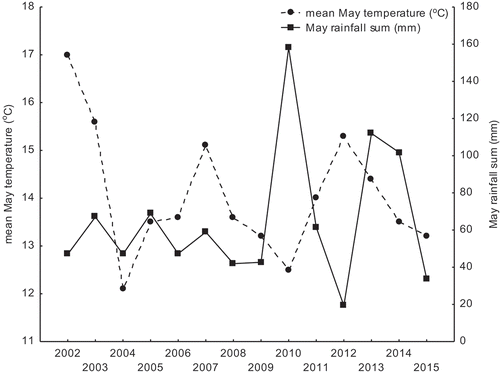
We took into account also weather conditions in years 2002–2015. Mean hatching dates in both study sites took place in May, therefore, the nestling rearing stage in all years occurred in that month in the case of most breeding pairs. Because weather conditions in May have an important role in nestling survival until leaving the nest, we decided to focus attention on this month. The local temperatures for Łódź were obtained from the TuTiempo.net climate database for Łódź (http://www.tutiempo.net/en/Climate/LODZ/124650.htm and https://en.tutiempo.net/climate/ws-121055.html).To characterize weather conditions in May we use two measures: mean temperature and total rainfall (sum of rainfall) ()
Great tit breeding data
During the breeding season, the study sites were visited daily to record nest-box occupancy, laying date, clutch size, the number of hatchlings and the number of fledglings. We calculated hatching success as the number of hatchlings in relation to clutch size, and fledging success as the number of fledglings in relation to the number of hatchlings (refers to the nests from which at least one young fledged). Breeding success was calculated as the number of fledglings in relation to clutch size.
During 2002–2015, we collected information about 1275 complete first clutches (meaning clutches that were incubated). For years when any experiments were carried out, we took into consideration only clutches which were not subjected to experimental treatments (). In the analysis, we generally focus on successful nests, whereas data on completely failed nests we only presented descriptively. Analyses of fledgling numbers in relation to years and study sites refer to the nests from which at least one young fledged, and therefore we took into account 1078 broods (628 in the parkland and 450 in the forest). We determined an exact number of hatched chicks in 976 successful broods and 103 failed broods, so analyses of hatchling numbers and hatching success were conducted on 1079 nests (616 in the parkland and 463 in the forest), while analyses of fledging success on 976 nests (569 in the parkland and 407 in the forest) for which we had accurate data on hatchling numbers.
Table I. The numbers of complete clutches and failed nests (in the brackets number of nests recognized as depredated by pine marten (1) and great spotted woodpecker (2)) of great tits in the study period 2002–2015.
Data analyses
We used general linear models to compare the mean number of hatchlings and the mean number of fledglings between study areas and years. We applied a factorial design with the number of hatchlings and the number of fledglings as dependent variables in two separate models, where study area and year were factors, with their interaction being also included.
Hatching success, i.e. the number of hatchlings in relation to clutch size, and fledging success, i.e. the number of fledglings in relation to the number of hatchlings, were considered to represent binomial distribution. Therefore, we treated them as dependent variables in two corresponding generalized linear models, where study site and year were factors whose interactions were also included in the models. These generalized linear models with binomial error distribution, logit link function and Wald Chi-squared test statistics were used to examine differences in hatching success and in fledging success between two study areas (Crawley Citation2002). Linear correlations were calculated to analyze relationships between the number of fledglings, the number of hatchlings and clutch sizes. We also tested if there were associations of the breeding performance and weather variables, the richness of the food base for chicks or population density. This was done using linear regressions. Especially, we analyzed relationships between mean fledgling numbers per nest in a given year and mean temperatures of May, total rainfall in May, peak caterpillar abundance and density of breeding pairs. Modeling was conducted using IBM SPSS Statistics 22, while linear regression analyses were done applying Statistica 12 (StatSoft Inc. 1984–2014).
Results
Breeding characteristics
Over all years of the study, 13.3% of all initiated clutches failed in the urban park site, by comparison with 18.3% in the forest site. We did not record any depredated nests in the park site, whereas in the forest, almost 14,9% of the losses were caused by the pine marten (Martes martes) predation (2,7% of all nests), and 8,9% by great spotted woodpecker (Dendrocopos major) predation (1,6% of all nests). In the urban park site, the smallest percentage of failed nests was found in 2003 (3,8%) and the largest percentage in 2010 (29.4%). During the first study year in the forest site, 2002, no failed nest was recorded, but in 2003, 4.3% nets failed. The largest percentage of losses in the forest site occurred in 2010 (42.9%) and in 2004 (40.5%). Despite these extreme records, over all study years, percentages of totally failed nests were not correlated between study sites (r = 0.31; N = 14; p = 0.27).
In total, considering clutches that gave at least one hatchling, 90.5% eggs in the urban park and 92.3% eggs in the forest hatched. Analogously, 87.7% hatchlings in the urban park and 93.7% hatchlings in the forest fledged from broods that gave at least one fledgling.
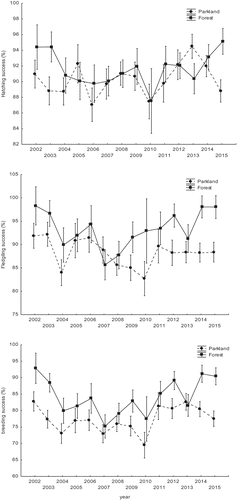
The annual mean number of hatchlings per nest during the study period differed between the study areas and was higher in the forest site. The overall mean number of hatchlings was 8.29 ± 1.72 (SD) in the park site (the lowest mean 7.59 ± 1.65 in 2013 and the highest mean 8.77 ± 1.55 in 2008) and 9.51 ± 1.93 in the forest site (the lowest mean 8.00 ± 3.56 in 2005 and the highest mean 10.86 ± 1.55 in 2003), with a significant interaction between year and study area (, ). In the forest area, year-to-year changes in the number of hatchlings were more substantial than in the parkland area. The mean number of fledglings also differed between the parkland and the forest study areas, with higher values occurring in the forest area. The overall mean number of fledglings was 7.12 ± 2.12 (the lowest mean 6.25 ± 1.85 in 2010 and the highest mean 8.31 ± 2.29 in 2002) in the parkland and 8.87 ± 2.21 (the lowest mean 7.31 ± 1.87 in 2005 and the highest mean 10.47 ± 1.84 in 2002) in the forest, with a significant interaction between year and study area (, ), resulting from year-to-year changes being greater in the forest than the park site.
Table II. Summary of two general linear models testing for the effects of year and study area on, respectively, the number of hatchlings and the number of fledglings of great tits breeding in the forest and in the urban parkland study sites.
Figure 2. Annual variation of the mean number of great tit hatchlings (upper chart) and fledglings (bottom chart) in two study areas, a parkland and a forest (breeding seasons 2002–2015). Data are presented as means ± SE.
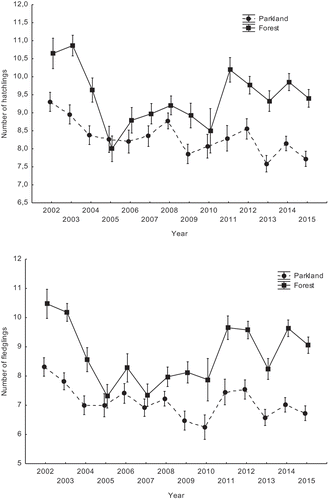
There was a positive relationship between the number of hatchlings and clutch size in both study sites: parkland (r = 0.76; n = 616; p < 0.001) and forest (r = 0.84; n = 463; p < 0.001). There was also a similar positive relationship between the number of fledglings and the number of hatchlings (parkland: r = 0.64; n = 569; p < 0.001; forest: r = 0.77; n = 407; p < 0.001).
The hatchability of eggs was affected by a significant interaction between study area and year (, ), as only in some years hatching success was significantly different between the study sites and it was always higher in the forest (except 2013 year when was opposite). The same interaction had an impact on the fledging success (, ) but in this case, differences in fledging success occurred often and almost always fledging success was higher in the forest.
Table III. Results of two generalized linear binominal models testing for the effects of year and study area on, respectively, hatching success and fledging success of great tits in the forest and the urban parkland study sites.
Breeding success
The annual mean number of fledglings per nest did not correlate with the annual peak caterpillar frass fall in the urban parkland, but there was a significant correlation in the forest (). The mean number of fledglings was not linked with the breeding pair densities (pairs/10 ha) in a given season in either study site. The mean number of fledglings was not linked with total rainfall in any study site either, but in the urban parkland, there was a non-significant negative tendency (). On the other hand, in both study areas, we found a significant positive relationship between the mean number of fledglings and mean May temperatures and, moreover, in the parkland, this correlation was high (, ).
Table IV. Correlations between mean fledgling numbers, mean fledging success, mean breeding success and four explanatory variables: maximum frass fall peak size, breeding densities, mean May temperature, May rainfall sum. Sample size for frass fall peak size N = 13; for the other variables N = 14.
Figure 4. Relationship between the mean number of great tit fledglings and mean May temperatures in the parkland and the forest (breeding seasons 2002–2015).
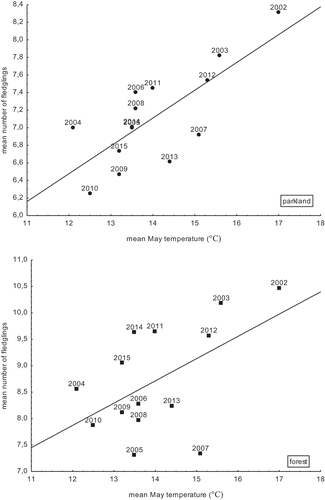
The mean fledging success and mean breeding success in the forest were not linked to any of the analysed variables, while in the case of the urban park site, fledging success and breeding success were consistently positively correlated with mean May temperatures ().
Discussion
In this study conducted in central Poland, we found that a proportion of clutches that totally failed (nests that ended at incubation stage or nests where all chicks died) was slightly lower in the urban park habitat than in the forest habitat. Symptomatically, the rate of failure changed among years independently in either study site. This suggests the impact of various and site-specific factors. Those losses had different reasons (e.g. starvation, soaking, predation), sometimes difficult to determine. One of the causes that could be relatively easily and correctly identified in the field was predation by the pine marten and great spotted woodpecker, which took place only in the forest site. The pressure exerted by these two predators clearly distinguished our two study areas, but the possibility of nest robbery by the predators was also strongly associated with the nest-box design (Skwarska et al. Citation2009; Kaliński et al. Citation2014). Similarly, nest predation by the weasel (Mustela nivalis) dependent on nest-box construction was observed in Wytham Wood, Oxfordshire, UK. In that case, the wooden nest-boxes had to be replaced with the concrete ones to avoid predation (Julliard et al. Citation1997). The literature on the pressure of predation in the context of the contrast between urban and non-urban environments is somewhat contradictory (Chamberlain et al. Citation2009; Vincze et al. Citation2017). Nonetheless, Vincze et al. (Citation2017) have concluded that cavity nests are depredated at a significantly lower rate in urban than in rural habitats and it is in line with the situation observed in our urban-non-urban habitat system.
The number of hatchlings and hatching success are important characteristics of reproductive performance in birds and it is known that the number of hatchlings is usually directly related to the number of eggs in the clutch (Koenig Citation1982). Accordingly, we found high correlations between these two variables in both study sites. Temporal and spatial patterns of variation in the mean number of hatchlings we found were very similar to variation in mean clutch size that we had previously shown (Wawrzyniak et al. Citation2015), where clutch sizes of great tits were lower in the urban park than in the forest, mostly due to differences in the site-specific food abundance and patchy environment in the parkland. Our results on hatching success are congruent with the average hatching success across passerine birds, ca. 91% shown by Koenig (Citation1982). This result is also similar to findings on other great tit populations studied in Europe, for example, 86,1–93,6% (Britt & Deeming Citation2011), 93% (Eeva & Lekihoinen Citation1995), but tends to be higher than in Frankfurt birds, 72–86% (Berresem et al. Citation1983), and Göteborg urban and rural populations, 79–80% (Isaksson et al. Citation2008).
In general, the hatchability of eggs was slightly higher in the forest than in the urban park site. Hatching success was relatively high and stable during the study period in both areas, with an exception occurring in 2010 in both places and 2006 at the park site. In the spring 2010, there was an unusually rainy period with very low temperatures (especially in May there were 24 rainy days and very high sum of rainfall, but also April was cold), which probably caused a large decrease in hatching success. In that breeding season, there was also the highest proportion of nests that completely failed in both study sites and the lowest fledging success in the urban parkland, but not in the forest where, however, only slightly more than half of the broods were successful. In general, hatching failure results from three major causes: female gametes fail to be fertilized, fertilized eggs fail to hatch, or calcium deficiency in the environment, that may cause eggshells to be thinner and prone to damage (Birkhead et al. Citation2008; Bańbura et al. Citation2010; Garcia-Navas et al. Citation2011). Embryo mortality may be caused by abiotic factors (variable temperature, humidity), female infectious disease, female condition or inbreeding (Kempenaers et al. Citation1996; Birkhead et al. Citation2008; Szulkin et al. Citation2012). Embryonic mortality can occur at any stage of development and for a variety of reasons, but generally may occur very early before egg-laying or late in the incubation period (Birkhead et al. Citation2008). Environmental factors, especially those associated with changes in temperature and humidity may impose embryo mortality in the period between egg-laying and hatching, and therefore, it seems that the abiotic factors could have a crucial role in the case of distinctly reduced hatching success like that in the 2010 year in our study.
Recent reviews of avian productivity and avian life-history evolution in the urban landscape context show equivocal findings (Chamberlain et al. Citation2009; Sepp et al. Citation2018). On the one hand, considering productivity (the number of fledglings) per successful nesting attempt Chamberlain et al. (Citation2009) found a quite clear pattern of lower productivity in urban habitats in comparison to rural ones for majority of studied species, but this general rule was less apparent when they took in to account the number of fledglings from all attempts (also failed nests). Most European studies on great tits and blue tits have shown that forest populations produce more fledglings than urban populations (Berresem et al. Citation1983; Cowie & Hinsley Citation1987; Hőrak Citation1993; Riddington & Gosler Citation1995; Solonen Citation2001; Hinsley et al. Citation2008; Bailly et al. Citation2016; Glądalski et al. Citation2017; Pollock et al. Citation2017; de Satge et al. Citation2019). Charmantier et al. (2017) have not found any difference between tit populations nesting in the city of Montpellier (France) and in a nearby rural oak forest (but does not specify if all nests were included or only successful nests). Isaksson and Andersson (Citation2007) found an opposite pattern around Göteborg, with urban tit populations being more productive than nearby forest ones.
This study shows a sustained pattern where the forest population of great tits produced more fledglings per successful nests and demonstrated higher breeding success over many breeding seasons. In our study system, breeding success was higher in the forest site in 13 out of 14 years. Fledging success also demonstrates a similar but slightly less obvious pattern, with higher values for forest than parkland area. The main reason for this interhabitat difference is probably a contrast in food availability and quality between the study habitats. Firstly, we have previously shown that mean clutch size was consistently lower in the parkland than in the forest great tit population, at least partly in response to the greater abundance of caterpillars in the forest (Wawrzyniak et al. Citation2015). Rodriguez et al. (Citation2016) in a study of a Mediterranean great tit population breeding in an orange plantation concluded that the number of hatchlings per nest was the most important predictor of fledgling production.
Our study areas significantly differed in their insect productivity, including caterpillar productivity, with caterpillars being several times more abundant at the forest site than in the parkland site (Marciniak et al. Citation2007; Glądalski et al. Citation2015). In the forest site, the mean number of fledglings was related to the amount of frass fall but fledging success was not. It seems that the caterpillar numbers in the forest influenced fledgling numbers to a greater extent via clutch sizes than by lower chick mortality. In the caterpillar-poorer urban park habitat, neither fledgling numbers nor fledging success was correlated with the peak mass of frass fall Many studies on tits indicate a relationship between the number of fledglings or fledgling/breeding success and a lower quantity and/or quality of food provided to the offspring in urban habitats in comparison with rural habitats. Poorer food supplied to nestlings in urban habitats leads to a lower offspring number or breeding/fledging success (Cowie & Hinsley Citation1987; Hőrak Citation1993; Solonen Citation2001; Bailly et al. Citation2016; Pollock et al. Citation2017). In some cases, the opposite effects were found, where an urban habitat was richer and, as a consequence, tit breeding performance was better there (Isaksson & Andersson Citation2007). Generally, it has been shown that great tits can cope with it by adjusting parental effort; however, it is hard to clearly define the mechanisms which are responsible for that. Because on the one hand it is possible that suboptimal habitat attracts poorly performing individuals and that there may be a genetic determinant beyond habitat selection and exploration abilities (for example Dingemanse et al. Citation2002; Carere et al. Citation2005). On the other hand, it is also possible that parents are able to adjust their breeding strategies to different habitat conditions (for example, Caprio & Rolando Citation2017) and it is very likely that both mechanisms matter. Connections between habitat, food richness and breeding performance in of tits seem to form a more universal phenomenon, not limited to just urban-rural gradient, but also to other food-contrasted habitat types (Blondel et al. Citation1987; Lambrechts et al. Citation1997; Massa et al. Citation2011). In caterpillar-poor habitats like coniferous forests, sclerophyllous forests, reforestations, orange plantations or, finally, urban habitats, great and blue tits not only lay fewer eggs but also are forced to use alternative prey taxa of invertebrates instead of caterpillars to feed the chicks (Blondel et al. Citation1991; Bańbura et al. Citation1994; Naef-Daenzer et al. Citation2000; Solonen Citation2001; Tremblay et al. Citation2003; Massa et al. Citation2004, Citation2011). From our previous study and from a study in progress we know that in the urban park site great tits used alternative insect prey as nestling food to a greater extent, although caterpillars were still the dominant prey (Michalski et al. Citation2011). These observations suggest that caterpillar abundance may not be as important to great tit breeding success at the park site. Because of nutrient deficiencies, switching to alternative prey may negatively affect either the survival of the chicks or their condition or both, especially when other important factors are also unfavorable (van Balen Citation1973; Naef-Daenzer & Keller Citation1999; Barba et al. Citation2004; Mackenzie et al. Citation2014; Kaliński et al. Citation2015b).
In complex ecological systems, it is unlikely that one factor, even so important for tits as the abundance of caterpillars could determine the breeding performance of birds and most probably other factors like habitat structure, weather conditions, human-induced effects or breeding densities must also directly or indirectly influence breeding results (Bańbura et al. Citation1994; Zając Citation1995; Hinsley et al. Citation2008). Bueno-Enciso et al. (Citation2016) showed that the micro-habitat surrounding nest sites and the size of the forest patch in a patchy forest area are important components of habitat quality for breeding tits, with habitat quality being a reliable index of prey availability for insectivorous birds. Mackenzie et al. (Citation2014) found a positive relationship between the number of oak trees near the nest and the fledgling weights in great tits and pointed out the importance of native vegetation for the foraging style. Hinsley et al. (Citation2008) showed that tits in a patchy environment in territories with a lower number of oaks and more gaps in canopy worked harder to raise their nestlings that finally were lighter anyway. de Satge et al. (Citation2019) found that a proportion of eggs that gave fledglings was negatively influenced by local-scale urbanisation, quantified by percentage of built up area. The vegetation in our urban parkland is heavily fragmented by lawns, paths and buildings, and it was mainly formed artificially (Marciniak et al. Citation2007). The density of trees (especially oaks) in the urban park area is much lower than in the forest (and the trees are not as mature as trees in the forest area), and, moreover, there are many non-native deciduous and coniferous trees (Glądalski et al. Citation2017). It is likely that all those factors synergistically impose worse breeding performance of the great tits in the urban park site.
It can be assumed that a high density of breeding pairs may lead to a decrease in breeding success. This may be due to, e.g. reducing the size of territories, more frequent aggressive interactions between individuals or easier transfer of parasites and pathogens. Some studies of European nest-box tit populations, where the density of breeding pairs was high, reported negative impact of densities on clutch size or other breeding indicators (Kluijver Citation1951; Perrins Citation1965; van Balen Citation1973; Dhondt Citation2010). Also, some experimental studies showed such a negative impact (Dhondt et al. Citation1992; Both Citation1998). Densities of great tits in our parkland and forest areas were rather low or medium and much lower than reported for most west-European nest-box populations of this species (Perrins Citation1965; van Balen Citation1973; Dhondt Citation2010). Dhondt (Citation2010) analyzed several Belgian tit populations for many years and concluded that density-dependent factors that affected tit reproduction are more likely to be detected in low quality sites than in high quality ones. Therefore, we expected that density-dependent effects influencing the breeding performance in our study should be small, negligible or limited rather to the urban park site. Indeed, we found no significant correlations between the breeding densities and mean numbers of fledglings or fledging success in our study sites. Despite this, it needs to be highlighted that the nest-box breeding density of great tits differed between the two study sites and was distinctly higher in the urban park every year, being nearly twice as high as in the forest area (Glądalski et al. Citation2016c).
Weather conditions during incubation and, especially, during the nestling stage may strongly affect breeding performance directly or indirectly (van Balen Citation1973; Martin Citation1987). On the one hand, low temperatures increase nestling energy demands, especially when air humidity is high. Additionally, intensive rainfall, which can lead to getting the nest material wet enhances this effect. Furthermore, temperature and heavy rainfall can influence prey abundance and availability for both adult and nestling tits (Southwood et al. Citation2004). In the present study, in the urban park site, annual mean fledgling numbers, fledging success, and breeding success were significantly positively correlated with mean daily temperatures in May. Fledgling numbers tended to be negatively correlated with rainfall sum in May. In the forest site, there was no such strong effect, and the fledgling number was significantly positively related only with mean May temperatures. Similarly, breeding success tended to be related to temperature, but fledging success did not. It is possible that in the low-quality parkland area unfavorable weather conditions may exert stronger pressures on adult birds and their nestlings. Thus, a different response of fledging success to roughly the same weather conditions at two study sites may be an indirect consequence of the difference between these habitats in their trophic richness. However, also microclimatic conditions might be slightly different, as a result of site-specific habitat features. For example, it seems that patchy structure of the habitat caused more frequent nest wetting in the urban park site in periods of heavy and prolonged rainfalls (own observations). A similar but not exactly the same pattern with a strong positive correlation between the nestling hemoglobin concentration and mean minimum daily temperatures during the nestling period within the park site, but with no relationship in the forest site, was previously shown in our study system (Kaliński et al. Citation2015b). It is in line with the results presented in this study, regarding the fledging successes. We did not find such differences in the case of fledgling numbers, perhaps because the numbers of fledglings were strongly connected with clutch sizes and hatchling numbers. Radford et al. (Citation2001) showed that female great tits significantly reduced their feeding visit rate to the nest during intensive rainfall. Lower breeding performance connected with unfavorable weather conditions that increased mortality of young great tits has been presented previously (Zając Citation1995).
Conclusions
We found a clear, consistent, long-term spatial pattern where a forest population of great tits produced more hatchlings and fledglings per successful nest and was characterized by higher breeding success than an urban park population. It may result from such habitat differences as tree species composition and spatial structure, and corresponding caterpillar abundance. Other factors may also be relevant, e.g. human disturbance indicated by the density of pathways. Annual mean number of fledglings per a successful nest was positively linked to caterpillar richness only in the forest site. On the other hand, higher temperatures during chick rearing in May were associated with a greater number of fledglings and fledging success in the parkland site, but only with a greater number of fledglings in the forest. The between-population variation we found probably reflects a more general difference between urban and forest populations of tits.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures using animals were authorized under permits from the University of Łódź, Local ethical Committe and the State Office for Environmental Protection.
Acknowledgements
We thank E. Wróblewska, A. Jaksa, D. Mańkowska, M. Janiszewska and J. Białek for collaboration and consents.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Alberti M. 2015. Eco-evolutionary dynamics in an urbanizing planet. Trends in Ecology & Evolution 30:114–126. DOI: 10.1016/j.tree.2014.11.007.
- Anderies J, Katti M, Shochat E. 2007. Living in the city: Resource availability, predation, and bird population dynamics in urban areas. Journal of Theoretical Biology 247:36–49. DOI: 10.1016/j.jtbi.2007.01.030.
- Bailly J, Scheifler R, Berthe S, Clément-Demange VA, Leblond M, Pasteur B, Faivre B. 2016. From eggs to fledging: Negative impact of urban habitat on reproduction in two tit species. Journal of Ornithology 157:377–392. DOI: 10.1007/s10336-015-1293-3.
- Bańbura J, Blondel J, de Wilde-lambrechts H, Galan M-J, Maistre M. 1994. Nestling diet variation in an insular Mediterranean population of blue tits Parus caeruleus: Effects of years, territories and individuals. Oecologia 100:413–420. DOI: 10.1007/BF00317863.
- Bańbura M, Sulikowska-Drozd A, Kaliński A, Skwarska J, Wawrzyniak J, Kruk A, Zieliński P, Bańbura J. 2010. Egg size variation in Blue Tits Cyanistes caeruleus and Great Tits Parus major in relation to habitat differences in snail abundance. Acta Ornithologica 45:121–129. DOI: 10.3161/000164510X551264.
- Barba E, Gil-Delgado JA, Monros JS. 2004. Relationship between chick diet and breeding performance of great tits in a caterpillar-poor environment. In: Rotschild M, editor. Insects and birds interactions (pp. 233–238). Hampshire, UK: Intercept Ltd.
- Berresem VKG, Berressem H, Schmidt K-H. 1983. Vergleich der Brutbiologie von Hohlenbrutern in innerstadischen und stadtrefernen Biotopen. Journal of Ornithology 124:431–445. DOI: 10.1007/BF01640362.
- Bichet C, Scheifler R, Coeurdassier M, Julliard R, Sorci G, Loiseau C. 2013. Urbanization, trace metal pollution, and malaria prevalence in the house sparrow. PLoS One 8(1):e53866. DOI: 10.1371/journal.pone.0053866.
- Birkhead TR, Hall J, Schut E, Hemmings N. 2008. Unhatched eggs: Methods for discriminating between infertility and early embryo mortality. Ibis 150:508–517. DOI: 10.1111/j.1474-919X.2008.00813.x.
- Blondel J, Clamens A, Cramm P, Gaubert H, Isenmann P. 1987. Population studies on tits in the Mediterranean region. Ardea 75:21–34.
- Blondel J, Dervieux A, Maistre M, Perret P. 1991. Feeding ecology and life history variation of the blue tit in Mediterranean deciduous and sclerophyllous habitats. Oecologia 88:9–14. DOI: 10.1007/BF00328397.
- Bordjan D, Tome D. 2014. Rain may have more influence than temperature on nest abandonment in the Great Tits Parus major. Ardea 102:79–85. DOI: 10.5253/078.102.0107.
- Both CH. 1998. Experimental evidence for density dependence of reproduction in great tits. Ecology 67:667–674. DOI: 10.1046/j.1365-2656.1998.00228.x.
- Britt J, Deeming DC. 2011. First-egg date and air temperature affect nest construction in blue tits Cyanistes caeruleus, but not in great tits Parus major. Bird Study 58:78–89. DOI: 10.1080/00063657.2010.524916.
- Bueno-Enciso J, Ferrer SE, Barrientos R, Serrano-Davies E, Sanz JJ. 2016. Habitat fragmentation influences nestling growth in Mediterranean blue and great tits. Acta Oecologica 70:129–137. DOI: 10.1016/j.actao.2015.12.008.
- Caprio E, Rolando A. 2017. Management systems may affect the feeding ecology of great tits Parus major nesting in vineyards. Agriculture, Ecosystems & Environment 243:67–73. DOI: 10.1016/j.agee.2017.03.013.
- Carere C, Drent PJ, Privitera L, Koolhaas JM, Groothuis TGG. 2005. Personalities in great tits, Parus major: Stability and consistency. Animal Behaviour 70:795–805. DOI: 10.1016/j.anbehav.2005.01.003.
- Chace JF, Walsh JJ. 2006. Urban effects on native avifauna: A review. Landscape and Urban Planning 74:46–69. DOI: 10.1016/j.landurbplan.2004.08.007.
- Chamberlain DE, Cannon AR, Toms MP, Leech DI, Hatchwell BJ, Gaston KJ. 2009. Avian productivity in urban landscapes: A review and meta-analysis. Ibis 151:1–18. DOI: 10.1111/j.1474-919X.2008.00899.x.
- Charmantier A, Demeyrier V, Lambrechts M, Perret SGrégoire A. 2017. Urbanization is associated with divergence in pace-of-life in great tits. Frontiers in Ecology and Evolution 5:53. DOI: 10.3389/fevo.2017.00053.
- Cholewa M, Wesołowski T. 2011. Nestling food of European hole-nesting passerines: Do we know enough to test the adaptive hypotheses on breeding seasons? Acta Ornithologica 46:105–116. DOI: 10.3161/000164511X625874.
- Cowie RJ, Hinsley SA. 1987. Breeding success of Blue tit and great tit in suburban garden. Ardea 75:81–90.
- Crawley MJ. 2002. Statistical computing: An introduction to data analysis using S-Plus. Chichester: Wiley.
- Cresswell W, McCleery R. 2003. How great tits maintain synchronization of their hatch date with food supply in response to long-term variability in temperature. Journal of Animal Ecology 72:356–366. DOI: 10.1046/j.1365-2656.2003.00701.x.
- de Satge J, Strubbe D, Elst J, De Laet J, Adriaensen F, Matthysen E. 2019. Urbanisation lowers great tit Parus major breeding success at multiple spatial scales. Journal of Avian Biology 50:e02108. DOI: 10.1111/jav.02108.
- Dhondt AA. 2010. Effects of competition on great and blue tit reproduction: Intensity and importance in relation to habitat quality. Journal of Animal Ecology 79:257–265. DOI: 10.1111/j.1365-2656.2009.01624.x.
- Dhont AA, Kempenaers B, Adriaensen F. 1992. Density-dependent clutch size caused by habitat heterogeneity. Journal of Animal Ecology 61:643–648.
- Dingemanse NJ, Both C, Drent PJ, van Oers K, van Noordwijk AJ. 2002. Repeatability and heritability of exploratory behaviour in great tits from the wild. Animal Behaviour 64:929–938. DOI: 10.1006/anbe.2002.2006.
- Eeva T, Lekihoinen E. 1995. Egg shell quality, clutch size and hatching success of the great tit (Parus major) and the pied flycatcher (Ficedula hypoleuca) in an air pollution gradient. Oecologia 102:312–323. DOI: 10.1007/BF00329798.
- Fernández-Juricic E, Tellería JL. 2000. Effects of human disturbance on spatial and temporal feeding patterns of Blackbird Turdus merula in urban parks in Madrid, Spain. Bird Study 47:13–21. DOI: 10.1080/00063650009461156.
- Fuller RA, Tratalos J, Gaston KJ. 2009. How many birds are there in a city of half a million people? Diversity & Distributions 15:328–337. DOI: 10.1111/j.1472-4642.2008.00537.x.
- Garcia-Navas V, Sanz JJ, Merino S, Martinez-de la Puente J, Lobato E, Del Cerro S, Rivero J, de Castaneda J, Moreno J. 2011. Experimental evidence for the role of calcium in eggshell pigmentation pattern and breeding performance in Blue Tits Cyanistes caeruleus. Journal of Ornithology 152:71–82. DOI: 10.1007/s10336-010-0551-7.
- Gil D, Brumm H, editor. 2013. Avian urban ecology: Behavioural and physiological adaptations. Oxford: Oxford University Press.
- Glądalski M, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Bańbura J. 2016a. Effects of extreme thermal conditions on plasticity in breeding phenology and double-broodness of Great Tits and Blue Tits in central Poland in 2013 and 2014. International Journal of Biometeorology 60:1795–1800. DOI: 10.1007/s00484-016-1152-9.
- Glądalski M, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Bańbura J. 2016b. Spatial variation in haemoglobin concentration of nestling Blue Tits (Cyanistes caeruleus): A long-term perspective. Journal of Ornithology 157:591–598. DOI: 10.1007/s10336-016-1325-7.
- Glądalski M, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Cyżewska I, Bańbura J. 2015. Inter-annual and inter-habitat variation in breeding performance of Blue Tits (Cyanistes caeruleus) in central Poland. Ornis Fennica 92:34–42.
- Glądalski M, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Cyżewska I, Bańbura J. 2017. Differences in the breeding success of Blue Tits Cyanistes caeruleus between a forest and an urban area: A long-term study. Acta Ornithologica 52:59–68. DOI: 10.3161/00016454AO2017.52.1.006.
- Glądalski M, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Cyżewska I, Mańkowska D, Bańbura J. 2016c. Effects of human-related disturbance on breeding success of urban and non-urban blue tits (Cyanistes caeruleus). Urban Ecosystems 19:1325–1334. DOI: 10.1007/s11252-016-0543-3.
- Glądalski M, Wawrzyniak J, Bańbura M, Kaliński A, Markowski M, Skwarska J, Zieliński P, Bańbura J. 2016d. Long-term changes in population density of nest-box breeding Great Tits and Blue Tits in two contrasting habitats in central Poland. Polish Journal of Ecology 64:385–394. DOI: 10.3161/15052249PJE2016.64.3.009.
- Gustafsson L, Nordling D, Andersson MS, Sheldon BC, Qvarnstrom A. 1994. Infectious diseases, reproductive effort and the cost of reproduction in birds. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences 346:323–331.
- Hinsley SA, Hill RA, Bellamy PE, Harrison NM, Speakman JR, Wilson AK, Ferns PN. 2008. Effects of structural and functional habitat gaps on breeding woodland birds: Working harder for less. Landscape Ecology 23:615–623. DOI: 10.1007/s10980-008-9225-8.
- Hőrak P. 1993. Low fledgling performance of urban Great Tits. Ornis Fennica 70:168–172.
- Isaksson C, Andersson S. 2007. Carotenoid diet and nestling provisioning in urban and rural great tits Parus major. Journal of Avian Biology 38:564–572. DOI: 10.1111/j.0908-8857.2007.04030.x.
- Isaksson C, Johansson A, Andersson S. 2008. Egg yolk carotenoids in relation to habitat and reproductive investment in the great tit Parus major. Physiological & Biochemical Zoology 81:112–118. DOI: 10.1086/522650.
- Johnson MTJ, Munshi-South J. 2017. Evolution of life in urban environments. Science 358(eaam):8327. DOI: 10.1126/science.aam8327.
- Julliard R, McCleery RH, Clobert J, Perrins C. 1997. Phenotypic adjustment of clutch size due to nest predation in the Great Tit. Ecology 78:394–404. DOI: 10.1890/0012-9658(1997)078[0394:PAOCSD]2.0.CO;2.
- Kaliński A, Bańbura M, Glądalski M, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Bańbura J. 2017a. Spatial and temporal variation in triglyceride concentration in the blood of nestling Blue Tits (Cyanistes caeruleus). Avian Biology Research 10:63–68. DOI: 10.3184/175815617X14836196626700.
- Kaliński A, Bańbura M, Glądalski M, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Bańbura J. 2017b. Relationships between nestling hemoglobin concentration and brood performance until fledging in Great Tits Parus major and Blue Tits Cyanistes caeruleus. Acta Ornithologica 52:141–148. DOI: 10.3161/00016454AO2017.52.2.002.
- Kaliński A, Bańbura M, Glądalski M, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Cyżewska I, Bańbura J. 2015a. Long-term variation in blood glucose concentration in nestling Great Tits (Parus major). Avian Biology Research 8:1–9. DOI: 10.3184/175815515X14294426911072.
- Kaliński A, Bańbura M, Glądalski M, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Cyżewska I, Bańbura J. 2015b. Long-term variation in hemoglobin concentration in nestling great tits Parus major. Comparative Biochemistry and Physiology A-Physiology 185:9–15. DOI: 10.1016/j.cbpa.2015.03.004.
- Kaliński A, Wawrzyniak J, Bańbura M, Skwarska J, Zieliński P, Glądalski M, Bańbura J. 2014. Does the threat of European Pine Marten (Martes martes) predation influence the height of nests built by Blue Tits (Cyanistes caeruleus) and Great Tits (Parus major)? Avian Biology Research 7:83–90. DOI: 10.3184/175815514X13983550506873.
- Kempenaers B, Adriaensen F, van Noordwijk AJ, Dhondt AA. 1996. Genetic similarity, inbreeding and hatching failure in Blue Tits: Are unhatched eggs infertile? Proceedings of the Royal Society of London Series B-Biological Sciences 263:179–185.
- Kluijver HN. 1951. The population ecology of the Great Tit, Parus major L. Ardea 39:1–135.
- Koenig WD. 1982. Ecological and social factors affecting hatchability of eggs. Auk 99:526–536.
- Kumar N, Gupta U, Malhotra H, Jhala YV, Qureshi Q, Gosler AG, Sergio F. 2019. The population density of an urban raptor is inextricably tied to human cultural practices. Proceedings of the Royal Society of London Series B-Biological Sciences 286:2018–2932.
- Lambrechts MM, Blondel J, Hurtres-Bousses S, Maistre M, Perret P. 1997. Adaptive inter-population differences in blue tit life-history traits on Corsica. Evolutionary Ecology 11:599–612. DOI: 10.1007/s10682-997-1515-0.
- Mackenzie JA, Hinsley SA, Harrison NM. 2014. Parid foraging choices in urban habitat and their consequences for fitness. Ibis 156:591–605. DOI: 10.1111/ibi.12166.
- Marciniak B, Nadolski J, Nowakowska M, Loga B, Bańbura J. 2007. Habitat and annual variation in arthropod abundance affects Blue Tit Cyanistes caeruleus reproduction. Acta Ornithologica 42:53–62. DOI: 10.3161/068.042.0113.
- Markowski M, Bańbura M, Glądalski M, Kaliński A, Skwarska J, Wawrzyniak J, Zieliński P, Bańbura J. 2015. Variation in haematocrit of nestling Blue Tits (Cyanistes caeruleus) in central Poland. Avian Biology Research 8:179–184. DOI: 10.3184/175815515X14375499328034.
- Martin TE. 1987. Food as a limit on breeding birds: A life-history perspective. Annual Review of Ecology, Evolution, and Systematics 18:453–487. DOI: 10.1146/annurev.es.18.110187.002321.
- Marzluff JM. 2016. A decadal review of urban ornithology and a prospectus for the future. Ibis 159:1–13. DOI: 10.1111/ibi.12430.
- Massa B, Cusimano CA, Margagliotta B, Galici R. 2011. Reproductive characteristics and differential response to seasonal temperatures of blue and great tits (Cyanistes caeruleus & Parus major) in three neighboring Mediterranean habitats. Review of Ecology (Terre Vie) 66:157–172.
- Massa B, Lo Valvo F, Margagliotta B, Lo Valvo M. 2004. Adaptive plasticity of blue tits (Parus caeruleus) and great tits (Parus major) breeding in natural and semi-natural insular habitats. Italian Journal of Zoology 71:209–217. DOI: 10.1080/11250000409356574.
- McDonnell MJ, Pickett STA. 1990. Ecosystem structure and function along urban–rural gradients: An unexploited opportunity for ecology. Ecology 71:1232–1237. DOI: 10.2307/1938259.
- McKinney ML. 2002. Urbanisation, biodiversity and conservation. Bioscience 52:883–890. DOI: 10.1641/0006-3568(2002)052[0883:UBAC]2.0.CO;2.
- Michalski M, Nadolski J, Marciniak B, Loga B, Bańbura J. 2011. Faecal analysis as a method of nestling diet determination in insectivorous birds: A case study in blue tits Cyanistes caeruleus and great tits Parus major. Acta Ornithologica 46:164–172. DOI: 10.3161/000164511X625937.
- Naef-Daenzer B, Keller L. 1999. The foraging performance of great and blue tits (Parus major and P. caeruleus) in relation to caterpillar development, and its consequences for nestling growth and fledging weight. Journal of Animal Ecology 68:708–718. DOI: 10.1046/j.1365-2656.1999.00318.x.
- Naef-Daenzer L, Naef-Daenzer B, Nager RG. 2000. Prey selection and foraging performance of breeding Great Tits Parus major in relation to food availability. Journal of Avian Biology 31:206–214. DOI: 10.1034/j.1600-048X.2000.310212.x.
- Nager RG, Van Noordwijk AJ. 1992. Energetic limitation in the egg-laying period of great tits. Proceedings of the Royal Society of London Series B-Biological Sciences 249:259–263.
- Pagani-Núňez E, Senar JC. 2016. More ornamented Great Tit Parus major fathers start feeding their offspring earlier. Ardea 104:167–176. DOI: 10.5253/arde.v104i2.a1.
- Pärt T. 1995. Does breeding experience explain increased reproductive success with age? An experiment. Proceedings of the Royal Society of London Series B-Biological Sciences 360:113–117.
- Perrins CM. 1965. Population fluctuations and clutch size in the Great tit, Parus major L. Journal of Animal Ecology 34:601–647. DOI: 10.2307/2453.
- Pollock C, Capilla-Lasheras P, McGill RAR, Helm B, Dominoni DM. 2017. Integrated behavioural and stable isotope data reveal altered diet linked to low breeding success in urban-dwelling blue tits (Cyaniste caeruleus). Scientific Reports 7:5014. DOI: 10.1038/s41598-017-04575-y.
- Przybylo R, Wiggins DA, Merilla J. 2001. Breeding success in Blue Tits: Good territories or good parents? Journal of Avian Biology 32:214–218. DOI: 10.1111/j.0908-8857.2001.320302.x.
- Radford AN, McCleery RH, Woodburn RJW, Morecroft MD. 2001. Activity patterns of parent great tits parus major feeding their young during rainfall. Bird Study 48:214–220. DOI: 10.1080/00063650109461220.
- Réale D, Garant D, Humphries MM, Bergeron P, Careau V, Montiglio PO. 2010. Personality and the emergence of the pace-of-life syndrome concept at the population level. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences 365:4051–4063. DOI: 10.1098/rstb.2010.0208.
- Reynolds SJ, Ibàňez-Alamo JD, Sumasgutner P, Mainwaring MC. 2019. Urbanisation and nest building in birds: A review of threats and opportunities. Journal of Ornithology 160:841–860. DOI: 10.1007/s10336-019-01657-8.
- Reynolds SJ, Perrins C. 2010. Dietary calcium availability and reproduction in birds. Current Ornithology 17:31–74.
- Ricklefs RE. 2000. Density dependence, evolutionary optimization, and the diversification of avian life histories. The Condor 102:9–22. DOI: 10.1093/condor/102.1.9.
- Ricklefs RE, Wikelski M. 2002. The physiology/life-history nexus. Trends in Ecology & Evolution 17:462–468. DOI: 10.1016/S0169-5347(02)02578-8.
- Riddington R, Gosler AG. 1995. Differences in reproductive success and parental qualities between habitats in the Great Tit Parus major. Ibis 137:371–378. DOI: 10.1111/j.1474-919X.1995.tb08035.x.
- Rodriguez S, Alvarez E, Barba E. 2016. Factors affecting fledgling output of great tits, Parus major, in the long term. Animal Biodiversity and Conservation 39:147–154. DOI: 10.32800/abc.2016.39.0147.
- Sepp T, McGraw KJ, Kaasik A, Giraudeau M. 2018. A review of impacts on avian life-history evolution: Does city living lead to slower pace of life? Global Change Biology 24:1452–1469. DOI: 10.1111/gcb.13969.
- Seress G, Liker A. 2015. Habitat urbanization and its effects on birds. Acta Zoologica Academiae Scientiarum Hungaricae 61:373–408. DOI: 10.17109/AZH.61.4.373.2015.
- Skwarska J, Kaliński A, Wawrzyniak J, Bańbura J. 2009. Opportunity makes a predator: Great Spotted Woodpacker predation on Tit broods depends on nestbox design. Ornis Fennica 86:109–112.
- Slagsvold T, Lifjeld JT. 1990. Influence of male and female quality on clutch size in tits (Parus spp.). Ecology 71:1258–1266. DOI: 10.2307/1938263.
- Solonen T. 2001. Breeding of the Great Tit and blue Tit in urban and rural habitats in southern Finland. Ornis Fennica 78:49–60.
- Southwood TRE, Wint GRW, Kennedy CEJ, Greenwood SR. 2004. Seasonality, abundance, species richness and specificity of the phytophagous guild of insects on oak (Quercus) canopies. European Journal of Entomology 101:43–50.
- Sprau P, Mouchet A, Dingemanse NJ. 2017. Multidimensional envinronmental predictors of variation in avian forest and city life histories. Behavioral Ecology 28:59–68. DOI: 10.1093/beheco/arw130.
- Stearns SC. 1992. The evolution of life histories. Oxford: Oxford University Press.
- Stracey CM, Robinson SK. 2012. Are urban habitats ecological traps for a native songbird? Season-long productivity, apparent survival, and site fidelity in urban and rural habitats. Journal of Avian Biology 43:50–60. DOI: 10.1111/j.1600-048X.2011.05520.x.
- Sumasgutner P, Nemeth E, Tebb G, Krenn HW, Gamauf A. 2014. Hard times in the city - attractive nest sites but insufficient food supply lead to low reproduction rates in a bird of prey. Frontiers in Zoology 11:48. DOI: 10.1186/1742-9994-11-48.
- Szulkin M, Patrick SC, Chapman JR, Sheldon BC. 2012. Promiscuity, inbreeding and dispersal propensity in great tits. Animal Behavior 84:1363–1370. DOI: 10.1016/j.anbehav.2012.08.030.
- Tobolka M, Zolnierowicz KM, Reeve NF. 2015. The effect of extreme weather events on breeding parameters of the White Stork Ciconia ciconia. Bird Study 62:377–385. DOI: 10.1080/00063657.2015.1058745.
- Tremblay I, Thomas DW, Lambrechts MM, Blondel J, Perret P. 2003. Variation in blue tit breeding performance across gradients in habitat richness. Ecology 84:3033–3043. DOI: 10.1890/02-0663.
- van Balen JH. 1973. A comparative study of the breeding ecology of the Great Tit Parus major in different habitats. Ardea 61:1–93.
- Vincze E, Seress G, Lagisz M, Nakagawa S, Dingemanse NJ, Sprau P. 2017. Does urbanization affect predation of bird nests? A meta-analysis. Frontiers in Ecology and Evolution 5. DOI: 10.3389/fevo.2017.00029.
- Wawrzyniak J, Kaliński A, Glądalski M, Bańbura M, Skwarska J, Zieliński P, Cyżewska I, Bańbura J. 2015. Long-term variation in laying date and clutch size of the great tit Parus major in central Poland: A comparison between urban parkland and deciduous forest. Ardeola 62:311–322. DOI: 10.13157/arla.62.2.2015.311.
- Weldon A, Haddad N. 2005. The effects of patch shape on indigo buntings: Evidence for an ecological trap. Ecology 86:1422–1431. DOI: 10.1890/04-0913.
- Zając T. 1995. Selection on laying date in the Blue Tit Parus caeruleus and Great Tit Parus major caused by weather conditions. Acta Ornithologica 30:145–151.
- Zandt HS. 1994. A comparison of three sampling techniques to estimate the population size of caterpillars in trees. Oecologia 97:399–406. DOI: 10.1007/BF00317331.