Abstract
We used a combination of culture-dependent and independent approaches to study in depth the microbial community associated with the digestive tract of the terrestrial isopod Porcellionides pruinosus (Brandt, Citation1833). Specimens from different sampling sites in Tunisia harbored distinct microbiota profiles indicating the impact of both host origin and environmental factors on shaping the microbial flora within P. pruinosus. Our results revealed unexpected bacterial diversity especially via metagenomic analysis; a total of 819 operational taxonomic units (OTUs) assigned to two major bacterial phyla; Proteobacteria and Bacteroidetes. We used Nutrient Agar to isolate the cultivable fraction of bacteria associated with the gut of three geographically distant populations of P. pruinosus. The isolated bacteria belong to Actinobacteria, Firmicutes and Proteobacteria. Enrichment cultures on carboxymethylcellulose (CMC) medium gave evidence that the gut of this Oniscidea harbors cellulolytic Firmicutes and Proteobacteria probably involved in the lignocellulose degradation and then in mediating the functional role of terrestrial isopods as litter decomposers and regulators of nutrient cycling in soil ecosystems.
Introduction
Originating from an aquatic environment, terrestrial isopods (Isopoda: Oniscidea) have successfully colonized terrestrial habitats differently to other crustacean taxa. This colonization took place in the light of morphological, behavioral and ecophysiological adaptations (Schmalfuss Citation1984; Carefoot Citation1993; Hornung Citation2011) to the terrestrial environment.
Oniscidea represent a keystone actor for litter degradation in terrestrial ecosystems beyond their contribution to the nutrient cycling and the regulation of the soil microbial activity (Zimmer & Topp Citation1999; Zimmer Citation2002a; Crowther et al. Citation2015). They feed on decaying plant litter that mainly consists of recalcitrant polymers as lignin and cellulose characterized by a low nutrient content. So, given the lack of the necessary enzymatic machinery for the degradation of these compounds, Oniscidea rely on symbiont-derived enzymes (Kukor & Martin Citation1986; Zimmer & Topp Citation1998; Zimmer Citation2002a). Recent studies showed that terrestrial isopods depend on numerous Carbohydrate Active EnZymes (CAZymes) affiliated to several families to degrade lignocellulose mainly composed by cellulose, lignin and hemicellulose. These enzymes were provided by the host and especially by its associated microbiota (Bredon et al. Citation2018, Citation2020).
In fact, the acquisition of bacterial symbionts seems to be a crucial step to facilitate isopods progression on land areas by acquiring new digestive capabilities especially the oxidative breakdown of phenolic compounds like lignin and tannin (Zimmer & Topp Citation1998) and the hydrolysis of cellulose (Zimmer Citation2002a). In addition, bacterial symbionts provide a high-quality nutritional source that allows isopods to overcome the dietary deficiencies of terrestrial leaf litter (Horváthová et al. Citation2016).
The digestive system of terrestrial isopods consists of a hindgut and a midgut or hepatopancreas where the secretion of digestive enzymes and the absorption of nutrients take place (Kostanjšek et al. Citation2002). It has been shown that the hepatopancreas can host two kinds of symbiont “Candidatus Hepatoplasma crinochetorum” (Mollicutes, hereafter “Hepatoplasma”) and “Candidatus Hepatincola porcellionum” (Rickettsiales, hereafter “Hepatincola”) (Wang et al. Citation2004a, Citation2004b). Despite the mechanism of mutual exclusion, these bacteria are introduced as mutualistic partners that enhance the nutritive utilization of the food sources of isopods (Zimmer Citation2002a). Fraune and Zimmer (Citation2008) showed that Hepatoplasma improves the survival rate of Oniscidea under conditions of poor diet. Thus, the acquisition of the hepatopancreatic bacteria Hepatoplasma and Hepatincola is considered a potential evolutionary prerequisite for the terrestrial colonization by isopods (Bouchon et al. Citation2016).
Among Crustacea, Oniscidea are the most successful colonizers of terrestrial habitats. In contrast to several species of marine isopods which don’t show microbiota in their digestive tract (Ray & Julian Citation1952; Besser et al. Citation2018), all the studied terrestrial species showed diverse and rich microbial communities in their digestive tract (Zimmer & Topp Citation1998; Kostanjšek et al. Citation2002; Zimmer Citation2002a, Citation2004; Dittmer et al. Citation2016; Horváthová et al. Citation2016; Bredon et al. Citation2020). A comparative study of the bacterial community associated with the hepatopancreas showed a clear difference between freshwater, semi-terrestrial and terrestrial isopods suggesting that the symbionts acquisition may occur in response to nutritional habits (Wang et al. Citation2007).
The terrestrial isopod P. pruinosus is a synanthropic cosmopolitan species that tolerates a wide range of environmental conditions (Loureiro et al. Citation2006). This species was used in bioremediation tests like pesticides degradation (Loureiro et al. Citation2002) and as a bioindicator species of soil contamination (Loureiro et al. Citation2005, Citation2009). In addition, many investigations were realized using genetic (Grandjean et al. Citation2005; Delhoumi et al. Citation2019a) and biochemical approaches (Achouri et al. Citation2012) to study the interpopulation polymorphism of P. pruinosus. Despite this, little is known about the gut microbiota structure, dynamic and role within this species.
This study aims to (1) investigate the bacterial community associated with the digestive tissues of P. pruinosus using a combination of culture-dependent and independent approaches, (2) isolate cellulolytic bacteria from the gut and (3) discuss the functional role of the host-associated microbiota and its contribution to the adaptation of isopods to their environment.
Materials and methods
Sampling
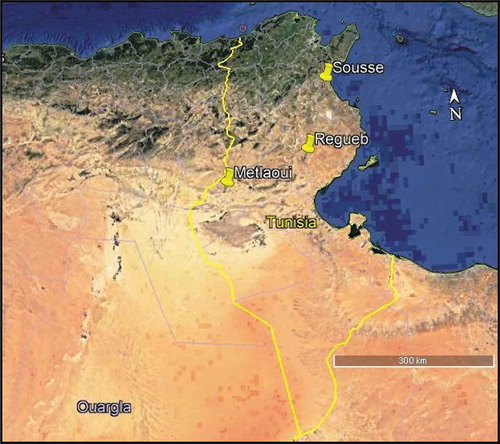
Adult specimens of P. pruinosus from Tunisia were manually collected from three geographically distant regions, Metlaoui, Regueb and Sousse in autumn 2016 (). Samples from Sousse were collected from a grassland parcel. Animals were sampled from an agricultural area and a phosphate mine in Regueb and Metlaoui respectively (). Before dissection, isopods were kept alive in plastic boxes under laboratory conditions (a temperature between 20 and 25°C and a daily photoperiod of 12 h) and fed on leaf litter from the sampling localities.
Table I. Characteristics of the sampling sites in Tunisia.
Bacterial counts and isolation from the digestive tissues
From each region, five guts of healthy adult isopods were extracted with fine-tipped sterile forceps (Butera et al. Citation2016), pooled and homogenized to be serially diluted in a sterile physiological solution and plated on Nutrient Agar (NA; Difco, Franklin Lakes, NJ) culture medium. After 72 h of incubation at 37°C, the colonies were counted and the mean number of bacteria per gut was determined. Single colonies of the most diluted NA plates were purified and characterized by colony morphology and microscopy and then subjected to gram staining. The isolates were stored in 20% glycerol at −20°C for further characterization.
Isolation of cellulolytic bacteria from the gut
In order to assess the presence of cellulolytic bacteria in the gut of P. pruinosus, enrichment cultures on carboxymethylcellulose substrate were prepared as described in (Butera et al. Citation2012). 200 µl of each pooled guts homogenate were suspended in enrichment culture flasks containing 20 ml of Medium 1 (Wenzel et al. Citation2002) enriched with 0.1 g of carboxymethylcellulose (CMC; Sigma).
After 4 weeks of aerobic incubation at 37°C on a rotary shaker, cultures were serially diluted and inoculated onto Petri dishes containing Medium 2 (Wenzel et al. Citation2002) supplemented with CMC. Plates were incubated at 37°C for 3–5 days, all single colonies of the most diluted plates were streaked to purity on fresh plates of the same medium and tested for cellulose degradation. The isolates were stored as described above for further characterization.
Congo red dye test
To test the ability of gut isolates and of isolates from the enrichment cultures to degrade cellulose, plates with Medium 2 supplemented with CMC were covered with a Congo red dye solution (Cacciari & Quatrini Citation2002; Delalibera et al. Citation2005). After dye removal, a clear zone around the colonies indicates the carboxymethylcellulose (CMC) degradation. The hydrolysis activity was determined by the calculation of the ratio of the clearance zone diameter (mm) to the colony diameter (mm) (Huang et al. Citation2012).
Identification of the isolated strains
All isolates were grouped into Operational Taxonomic Units based on ITS length polymorphisms. The primers ITSF (5ʹ-TCGTAACAAGGTAGCCGTA-3ʹ) and ITSReub (5ʹ-GCCAAGGCATCCACC-3ʹ) (Cardinale et al. Citation2004) were used to amplify the ribosomal 16 S-23 S rRNA gene intergenic spacer region (ITS) as described in (Catania et al. Citation2016). The resulting ITS patterns were used to assign the isolates to operational taxonomic units and the ribosomal 16 S rRNA gene of one representative isolate of each OTU was amplified with bacterial universal primers 27 f (5ʹAGAGTTTGATCCTGGCTCAG-3ʹ) and 1492 r (5ʹ-TACGYTACCTTGTTACGACTT-3ʹ) (Frank et al. Citation2008) and then sequenced. The 16 S rDNA sequences were analyzed as described in Djahnit et al., (Citation2019). Sequences were deposited in GenBank under accession numbers MH594510 to MH594526.
DNA extraction from digestive tissues
Adult isopods (both males and females) were surface-sterilized with 70% ethanol, washed twice with distilled water and surface dried; their guts were dissected and used freshly for DNA extraction (Butera et al. Citation2016). From each population, five pooled guts were used for total DNA extraction and purification using the QIAamp® DNA Stool Kit (QIAGEN, Venlo, The Netherlands) according to the manufacturer’s instructions. DNA concentration and quality was determined using the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies). The extracted DNA was utilized for the metagenomic analysis of the microbiota.
Metagenomic sequencing of the 16 S rRNA
Procaryotic primers Pro 341 f and Pro 805 r were used to amplify the 16 S rRNA hypervariable region V3-V4 of procaryotes. A final volume of 25 µl of reaction mixture contained 10 ng of genomic DNA and 0.25 µM of each primer. The PCR program was: 98°C for 2 min followed by 35 cycles beginning at 65°C and ending at 55°C for 15 s and a final extension at 68°C for 30 s (Takahashi et al. Citation2014). Sequencing was performed on Miseq Illumina with 300 bp paired-end reads.
The sequencing reads were processed and analyzed using QIIME version 1.9 (Caporaso et al. Citation2010a). Reads of quality higher than 20, with a minimum number of consecutive high quality base calls to include a read of 0.95 as a fraction of the input read length were analyzed further. USEARCH61 algorithm (Edgar Citation2010) was used to detect chimeras using a taxonomy assignment based approach. The remaining sequences were clustered into OTUs using a 97% sequence identity threshold. A representative sequence set was formed by picking the most abundant from each OTU and aligned against the Greengenes core set database (DeSantis et al. Citation2006) (August 2013 version) by PyNAST (Caporaso et al. Citation2010b) with a minimum sequence length of 75% of the median input sequence length and a minimum identity of 75%. The Ribosomal Database Project (RDP) classifier program was used to assign taxonomy to the aligned sequences.
Results
Bacterial isolation from the gut of P. pruinosus
The bacterial communities colonizing isopods guts sampled from three geographically distant regions in Tunisia (Metlaoui, Regueb and Sousse) () was analyzed by cultural methods. A total of 29 isolates were obtained on Nutrient agar medium from Metlaoui and Regueb regions, while no isolates were obtained from Sousse region. Counting of bacteria colonizing isopod gut showed an abundance ranging between 1 and 5x105 CFU/gut of P. pruinosus. Four isolates were obtained from CMC enrichments. Both NA isolates and CMC isolates were grouped in twenty-one operational taxonomic units (OTUs) by ribosomal 16 S-23 S intergenic spacer region analysis. The representative isolates of each OTU were affiliated to the 3 phyla: Actinobacteria, Firmicutes and Proteobacteria. The bacterial community isolated from the gut of specimens from Metlaoui showed the highest bacterial diversity, while that of isopods sampled from Regueb was represented only by Actinobacteria (). The NA isolates were affiliated to Klebsiella, Bordetella, Microbacterium, Staphylococcus, Promicromonospora, Cellulosimicrobium, Streptomyces and Brachybacterium genera.
Table II. Phylogenetic identification of intestinal bacteria isolated from P. pruinosus by direct plating on Nutrient Agar (NA) and enrichment on carboxymethylcellulose.
Cellulolytic isolates in the gut of P. pruinosus
The degrading cellulose activity was defined as the ratio between the clearance zone diameter and the colony diameter on CMC plates. 51% of the NA isolates were positive to Congo red test with a ratio fluctuating between 1 and 5.6. This ratio ranged between 1 and 3.3 for the carboxymethylcellulose enrichment culture isolates that were 100% positive to Congo red test. The 16 S rDNA sequencing analysis exhibited 99–100% of nucleotide homology with bacterial strains available in the NCBI database which belong to Firmicutes and Proteobacteria ().
Metagenomic analysis of the microbiota associated with P. pruinosus
A total of 69,233 raw reads (30,170; 15,888 and 23,175 for Metlaoui, Regueb and Sousse samples respectively) were processed using QIIME. A final number of 57,847 reads were grouped into 819 OTUs at 97% of similarity ().
Table III. Sequencing information obtained from metagenomic sequencing of the digestive tract of isopod sampled at three out of the four sites of study.
Unsurprisingly, Proteobacteria phylum was unique in guts of P. pruinosus from Sousse and Regueb and predominant from Metlaoui samples ()). The Proteobacteria phylum was dominated by representatives of Alphaproteobacteria and Gammaproteobacteria in the microbiota profile of three samples. However Bacteroidetes phylum was represented by members of Flavobacteria ()).
Figure 2. Relative abundance of bacterial groups at the phylum level (a) and most represented genera (b) within the digestive tract of P. pruinosus.
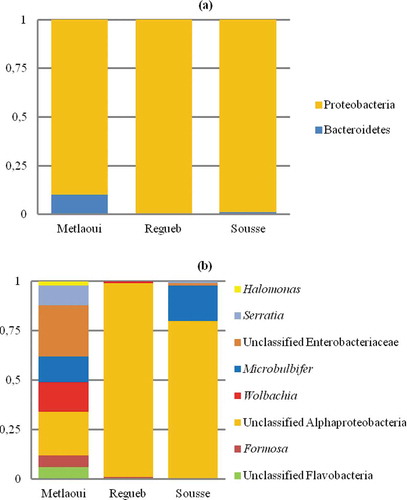
Apart from classical bacteria widely associated with arthropods like Wolbachia (15% and 1% in Metlaoui and Regueb samples respectively) and Serratia (10.4% and 1% in Metlaoui and Sousse samples profiles), the metagenomic analysis revealed the presence of a large spectrum of environmental bacteria ()). About genera, Halomonas, Microbulbifer and Wolbachia were the most shared between the three samples. Halomonas represented 3%, 1.1% and 1.1% of the bacterial community associated with digestive tracts of samples from Metlaoui, Regueb and Sousse respectively. However, Microbulbifer represents 13% and 18% of the microbiota of samples from Metlaoui and Sousse respectively.
In order to identify the unassigned Alphaproteobacteria sequences, a representative sequence was analyzed using BLAST 2.7.1 (Zhang et al. Citation2000; Morgulis et al. Citation2008). With a query cover of 99% and identity of 98% a hepatopancreatic symbiont of Porcellio scaber clone 1 was identified (Candidatus Hepatincola porcellionum” (Rickettsiales, Alphaproteobacteria) Accession number AY189806.1) in the sample of Regueb region.
Discussion
In order to deepen our insight into the gut microbiota structure within the terrestrial isopod P. pruinosus and its cellulolytic activity, we used a combination of cultivation-dependent and independent approaches. The cultivable fraction of bacteria was targeted by direct isolation on NA and after enrichment culture on CMC.
Herein, the isolated bacteria belong to Proteobacteria, Actinobacteria and Firmicutes phyla which are usually present in the arthropod microbiota such as insects (Huang et al. Citation2012; Vargas-Asensio et al. Citation2014; Dantur et al. Citation2015) ().
The most represented genera among the NA media isolates from P. pruinosus are Microbacterium, Streptomyces and Staphylococcus. It has been suggested that, beyond carbohydrate degradation, Microbacterium could play other roles in the host intestine (Vargas-Asensio et al. Citation2014). Members of Streptomyces genus, isolated from the gut of Regueb samples, were already isolated from a wide spectrum of arthropods where they contribute efficiently to the degradation of phenolic components like lignin and cellulose in addition to the production of secondary metabolites (Bibb Citation2005; Vargas-Asensio et al. Citation2014). Promicromonospora sp., isolated also from the same sample, showed the highest cellulolytic activity. It has been mentioned that this cellulolytic bacterium was isolated from the gut of the scarab beetle Pachnoda marginata where it was involved in the degradation of plant polymers (Cazemier et al. Citation2003).
All NA media isolates from the Regueb sample belong to Actinobacteria phylum. In fact, representatives of Actinobacteria are known to adjust physiological and metabolic equilibrium by production of extracellular enzymes and secondary metabolites (Schrempf Citation2001). However, representatives of Actinobacteria, Proteobacteria (Klebsiella and Bordetella) and Firmicutes (Staphylococcus) characterize the isolates from the Metlaoui sample. Members of Staphylococcus were previously detected in the gut wall of P. scaber (Kostanjšek et al. Citation2002; Lapanje et al. Citation2010) and other arthropod taxa (Zhang et al. Citation2014).
The lack of isolated bacteria from Sousse samples on NA media and the low number of isolated cellulolytic bacteria in this study can be ascribed to the difficulty of isolating and cultivating a wide spectrum of bacteria (Park et al. Citation2007).
Three of the five strains isolated from the enrichment cultures on CMC shared 99 to 100% of similarity with species from the genus Bacillus widely associated with the digestive tract of invertebrates. Bacillus cereus group constitute true residents symbionts of the digestive tissues of terrestrial arthropods among which the Oniscidean species P. scaber (Swiecicka & Mahillon Citation2006). Indeed, it has been ensured that Bacillus genus produces large quantities of extracellular enzymes involved in the cellulose degradation and then litter breakdown (Gupta & Verma Citation2015).
Bacteria of the genus Pseudomonas, isolated from the Sousse samples, had long been associated with the hepatopancreas of Oniscidea where they are introduced as a primary specific endosymbiont with a probable role in the degradation of cellulose and phenol (Bouchon et al. Citation2016). In accordance with our findings, members of Pseudomonas and Bacillus genera were previously detected in the hindgut of other species of terrestrial isopods like P. scaber (Kostanjšek et al. Citation2002; Lapanje et al. Citation2010; Horváthová et al. Citation2016), and Armadillidium vulgare (Dittmer et al. Citation2016). In order to quantify the capacity of isolates to degrade cellulose we utilized the test Congo red. This test established that isolated bacteria belonging to the Bacillus (Bacillus cereus, Bacillus wiedmannii and Bacillus sp) and Pseudomonas genera exhibited a cellulolytic degrading activity suggesting a close link between these gut colonizers and the digestion process within P. pruinosus.
Microbacterium, Staphylococcus and Bacillus, resistant bacteria to contamination soil, were the most isolated from the gut of Metlaoui sample collected from a phosphate mine. Thus, they are potentially involved in the resistance of P. pruinosus to the contamination induced by the production of phosphogypsum. Consistently with our findings, Bacillus, Pseudomonas, Klebsiella Microbacterium, Staphylococcus, Cellulosimicrobium and other members of Actinobacteria were the most isolated bacteria from the gut of Oniscidean species P. scaber sampled from a polluted area. These bacteria are introduced as resistant microorganisms to mercury pollution and may be involved in the physiological resilience of their host against contaminants (Lapanje et al. Citation2010). In the same manner, it appears that the isolated bacteria from the gut of P. pruinosus from Regueb were closely related to the inhabiting environment marked by agricultural practices. Indeed, Cellulosimicrobium and Streptomyces genera have been detected in agricultural soil and plants where they are used as growth promoters and biofertilizers in several crops (Kenzaka et al. Citation2017; Vurukonda et al. Citation2018). We can ensure through the culture dependant approach that the intestinal bacteria aid isopods to adapt to their specific environment (contaminant area and agricultural environment for the samples of Metlaoui and Regueb respectively). Furthermore, we found that the digestive tissues of P. pruinosus harbor cellulolytic bacteria involved in the breakdown of cellulose and potentially in the degradation of terrestrial leaf litter mainly composed of lignocelluloses. The fact that the microbiota associated with the digestive tissues of P. pruinosus includes cellulolytic bacteria producing enzymes involved in the degradation of cellulose makes this species useful in industrial processing of lignocellulose and then biofuel production from biomasses as suggested within plant feeding animals like terrestrial isopods (Bredon et al. Citation2020) and lepidoptera (Dantur et al. Citation2015).
The sequencing analysis revealed that intestinal isolated bacteria from P. pruinosus belong mainly to Proteobacteria, Actinobacteria and Firmicutes phyla. These phyla dominate the gut microbiota of several invertebrates where they play symbiotic functions like the nitrogen fixation, denitrification, carbohydrate degradation, detoxification, apart from defensive trend against pathogens (Schloss et al. Citation2006; Shao et al. Citation2014). Along the same line, Bredon et al. (Citation2018) showed that Proteobacteria, Actinobacteria phyla characterize the composition of the bacterial community associated with the degradation of lignocelluloses within the terrestrial isopod A. vulgare.
The NGS analysis showed the presence of only two bacterial phyla (Proteobacteria and Bacteroidetes) in comparison to 19 and 20 phyla identified within the Oniscidea species A. vulgare (Dittmer et al. Citation2016) and P. scaber (Horváthová et al. Citation2016) respectively.
On the other hand, the screening of the bacterial community involved in the breakdown of lignocellulose revealed the presence of four phyla within P. pruinosus, five phyla in A. vulgare, four phyla in Porcellio dilatatus dilatatus and Porcellio dilatatus petiti respectively and three phyla within the freshwater isopod Asellus aquaticus (Bredon et al. Citation2020). The same study showed that Proteobacteria and Bacteroidetes phyla dominate the microbiota of the five studied species especially P. pruinosus where they encode the most part of lignocellulose degrading CAZymes. In the present study, the three screened samples of P. pruinosus exhibited a high bacterial diversity comparable to that found in other litter decomposers like termites (Hongoh Citation2010; Brune Citation2014), as demonstrated with the species A. vulgare (Dittmer et al. Citation2016), and in freshwater crustaceans like Daphinia (Qi et al. Citation2009).
As the most of the isolated bacteria were from the sample of Metlaoui, the metagenomic analysis demonstrated that the highest number of raw sequences was identified within samples from the same site. Herein, the high salinity induced particularly by the phosphogypsum dissolution (Yermani et al. Citation2003) may explain the presence of halotolerant bacteria like Formosa, Microbulbifer and Halomonas. Formosa, polysaccharides degrading bacteria, can be found in various marine habitats rich in organic matter in association with invertebrates (Riedel et al. Citation2013). Likewise, Microbulbifer representatives are salt-dependent bacteria involved in the breakdown of cellulose and xylan (Yoon et al. Citation2007).
The difference of microbiota structure between Metlaoui, Regueb and Sousse samples can be ascribed to both host diet and environmental factors. In accordance, it has been argued that the host origin is the most important factor shaping the microbiota composition in Oniscidea (Dittmer et al. Citation2016; Delhoumi et al. Citation2018). Indeed, the microbiome reacts more rapidly than its host to environmental variability which aids it to evolve and conquer new niches (Bredon et al. Citation2019, Citation2020). Moreover, several studies showed that environmental bacteria are involved in Oniscidea performances like reproductive success, growth rate, survival and fertility (Zimmer Citation2002b; Horváthová et al. Citation2015; Delhoumi et al. Citation2019b) given their high nutritive value (Zimmer & Topp Citation1998). With the same manner, Horváthová et al. (Citation2016) demonstrated the key role the external microbes forming a biofilm on the growth of the isopod P. scaber; individuals fed on the a diet source supplemented with a large amount of biofilm gained more mass in comparison with those feeding on a diet source with a marginal biofilm. In line with this interaction (isopod-symbiont), Des Marteaux et al. (Citation2020) found that P. scaber and O. Asellus modify the microbial abundance and bacterial community structure in the leaf litter which alter the decomposition process.
Interestingly, we evidenced for the first time the presence of Hepatincola in the microbiota of P. pruinosus. Effectively, the hepatopancreatic symbiont in P. scaber Candidatus Hepatincola porcellionum (Rickettsiales) was identified in the gut of Regueb sample. Hepatincola has been detected only in species of the Crinocheta group (Oniscus asellus, Philoscia muscorum, P. scaber and A. vulgare) (Fraune & Zimmer Citation2008; Dittmer et al. Citation2016). Despite its negative effect on host longevity, it has been suggested that Hepatincola as a hepatopancreatic symbiont is mainly involved in the provisioning of cellulolytic enzymes necessary for the breakdown of lignin and tannins (Zimmer & Topp Citation1998) and then the adaptation to plant material the typical nutritional source in the terrestrial environment. Hence, despite its importance in the evolutionary progression of terrestrial isopods on land, this bacterium showed a low prevalence in some populations of Oniscidea and this may refer to its environmental transmission (Wang et al. Citation2007). In the context of terrestrialization process and the importance of intestinal flora, it has been showed that the major part of lignocellulose degrading CAZymes in terrestrial isopods are provided by the microbiota which enhances the food utilization and then the adaptation to their environment (Bredon et al. Citation2020). So, the complementarity and the diversity of the host’s enzymes repertoire and that of their microbiota allow Oniscidea to utilize several kind of plant litter and subsequently to occupy new ecological niches on land. However in marine species, data ensured the endogenous origin of enzymes involved in the breakdown of lignocellulose (King et al. Citation2010; Besser et al. Citation2018).
The metagenomic analysis of the microbiota associated with P. pruinosus revealed an important fraction of environmental bacteria acquired usually via ingested nutrients and feces. In fact, it’s well known that terrestrial isopods ingest bacteria colonizing the plant material and their own feces in order to provide microbial enzymes necessary for digestion (Zimmer & Topp Citation1998; Zimmer Citation2002a) and to meet their feeding requirements on the other side (Delhoumi et al. Citation2019b) given the low nutritive quality of terrestrial leaf litter. The combined use of cultivation-dependent and independent approaches established that environmental bacteria may constitute a source of variability between populations of P. pruinosus since the structure of the associated microbiota changes depending on the environment and the origin of the isopod.
Conclusion
The culture-dependent approach allowed the identification of cellulolytic bacteria involved in the breakdown of cellulose within P. pruinosus and potentially in the degradation of terrestrial leaf litter. Metagenomic analysis revealed a high bacterial diversity with a variable structure depending on the host origin. Hence, apart from reproductive manipulators (Wolbachia, Serratia …) and cellulolytic bacteria involved in the digestive process, the microbial flora associated with the digestive tissues of P. pruinosus includes a wide spectrum of environmentally transmitted bacteria with unknown function. A major finding of this study is the detection of the hepatopancreatic symbiont Hepatincola that may mediate the role of Oniscidea in the ecosystem functioning. Further researches on other isopod species are required in order to get a complete image on the microbiota and to advance our knowledge on its role on the land colonization by Oniscidea.
Acknowledgements
The authors express their sincere thanks to the Editor and anonymous referees for their valuable comments, corrections and language assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Achouri MS, Bouslema MF, Hmaied S, Charfi-Cheikhrouha F. 2012. Genetic differentiation and gene flow of Porcellionides pruinosus. Journal of Natural History 46:33–43. doi:10.1080/00222933.2012.707235.
- Besser K, Malyon GP, Eborall WS, da Paro Cunha G, Filgueiras JG, Dowle A, Garcia LC, Page SG, Dupree R, Kern M, Gomez LD, Li Y, Elias L, Sabbadin F, Mohamad SE, Pesante G, Steele-King C, de Azevedo ER, Polikarpov I, Dupree P, Gragg SM, Bruce NC, McQueen-Mason SJ. 2018. Hemocyanin facilitates lignocellulose digestion by wood-boring marine crustaceans. Nature Communications 9:5125. doi:10.1038/s41467-018-07575-2.
- Bibb M. 2005. Regulation of secondary metabolism in Streptomycetes. Current Opinion in Microbiology 8:208–215. doi:10.1016/j.mib.2005.02.016.
- Bouchon D, Zimmer M, Dittmer J. 2016. The terrestrial isopod microbiome: An all-in-one toolbox for animal–microbe interactions of ecological relevance. Frontiers in Microbiology 7:1472. doi:10.3389/fmicb.2016.01472.
- Brandt IF. 1833. Conspectus monographiae crustaceorum oniscodorum Latreillii. Bulletin del la Societé Imperiale des Naturalistes de Moscou 6:171–193.
- Bredon M, Dittmer J, Noël C, Moumen B, Bouchon D. 2018. Lignocellulose degradation at the holobiont level: Teamwork in a keystone soil invertebrate. Microbiome 6:162. doi:10.1186/s40168-018-0536-y.
- Bredon M, Herran B, Bertaux J, Grève P, Moumen B, Bouchon D. 2020. Isopod holobionts as promising models for lignocellulose degradation. Biotechnology for Biofuels 13:49. doi:10.1186/s13068-020-01683-2.
- Bredon M, Herran B, Lheraud B, Bertaux J, Grève P, Moumen B, Bouchon D. 2019. Lignocellulose degradation in isopods: New insights into the adaptation to terrestrial life. BMC Genomics 20:462. doi:10.1186/s12864-019-5825-8.
- Brune A. 2014. Symbiotic digestion of lignocellulose in termite guts. Nature Reviews Microbiology 12:168–180. doi:10.1038/nrmicro3182.
- Butera G, Ferraro C, Alonzo G, Colazza S. 2016. The gut microbiota of the wood-feeding termite Reticulitermes lucifugus (Isoptera; Rhinotermitidae). Annals of Microbiology 66:253–260. doi:10.1007/s13213-015-1101-6.
- Butera G, Ferraro C, Colazza S, Alonzo G. 2012. The culturable bacterial community of frass produced by larvae of Rhynchophorus ferrugineus Olivier (Coleoptera: Curculionidae) in the Canary island date palm. Letters in Applied Microbiology 54:530–536. doi:10.1111/j.1472-765X.2012.03238.x.
- Cacciari I, Quatrini P. 2002. I gruppi fisiologici del ciclo del carbonio. In: Angeli F, editor. “Metodi di analisi microbiologica del suolo” G Picci e P Nannipieri (coord.). Vol. 9. Roma, Italy: Ministero delle politiche agricole e forestali, Soc Ital Scienza del Suolo Parte III. pp. 74–75.
- Caporaso JG, Bittinger K, Bushman FD, Desantis TZ. 2010b. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 26:266–267. doi:10.1093/bioinformatics/btp636.
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K. 2010a. QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7:335–336. doi:10.1038/nmeth.f.303.
- Cardinale M, Brusetti L, Quatrini P, Borin S. 2004. Comparison of different primer sets for use in automated ribosomal intergenic spacer analysis of complex bacterial communities. Applied and Environmental Microbiology 70:6147–6156. doi:10.1128/AEM.70.10.6147-6156.2004.
- Carefoot TH. 1993. Physiology of terrestrial isopods. Journal of Comparative Biochemistry and Physiology A 106:413–429. doi:10.1016/0300-9629(93)90235-V.
- Catania V, Sarà G, Settanni L, Quatrini P. 2016. Bacterial communities in sediment of a Mediterranean marine protected area. Canadian Journal of Microbiology 63:303–311. doi:10.1139/cjm-2016-0406.
- Cazemier AE, Verdoes JC, Reubsaet FAG, Hackstein JHP. 2003. Promicromonospora pachnodae sp. nov., a member of the (hemi)cellulolytic hindgut flora of larvae of the scarab beetle Pachnoda marginata. Antonie Van Leeuwenhoek 83:135–148. doi:10.1023/A:1023325817663.
- Crowther TW, Thomas SM, Maynard DS, Baldrian P, Covey K, Frey SD, van Diepen LT, Bradford MA. 2015. Biotic interactions mediates oil microbial feed backs to climate change. Proceedings of the National Academy of Sciences USA 112:7033–7038. doi:10.1073/pnas.1502956112.
- Dantur KI, Enrique R, Welin B, Castagnaro AP. 2015. Isolation of cellulolytic bacteria from the intestine of Diatraea saccharalis larvae and evaluation of their capacity to degrade sugarcane biomass. AMB Express 5:15. doi:10.1186/s13568-015-0101-z.
- Delalibera I, Handelsman JJ, Raffa KF. 2005. Contrasts in Cellulolytic Activity of gut microorganisms between the wood borer Saperda vestita (Coleoptera: Cerambycidae), and the bark beetle Ips pini, and Dendroctonusfrontalis (Coleoptera: Curculionidae). Environmental Entomology 34:541–547. doi:10.1603/0046-225X-34.3.541.
- Delhoumi M, Zaabar W, Bouslama MF, Achouri MS. 2018. Characterization of the dominant bacterial communities associated with terrestrial isopod species based on 16S rDNA analysis by PCR-DGGE. Open Journal of Ecology 8:495–509. doi:10.4236/oje.2018.89030.
- Delhoumi M, Zaabar W, Bouslama MF, Achouri MS. 2019b. Effect of symbiont acquisition on growth, survival and fertility of the terrestrial isopod Porcellionides pruinosus (Crustacea, Oniscidea). Invertebrate Reproduction & Development 63:60–66. doi:10.1080/07924259.2018.1544174.
- Delhoumi M, Zaabar W, Bouslama MF, Zayani D, Achouri MS. 2019a. High level of genetic variation in mitochondrial 16S rDNA among populations of Porcellionides pruinosus (Brandt, 1833) (Crustacea: Isopoda: Oniscidea) in Tunisia. Italian Journal of Zoology 86:1–8.
- Des Marteaux LE, Kullik SA, Habash M, Schmidt JM. 2020. Terrestrial isopods Porcellio scaber and Oniscus asellus (Crustacea: Isopoda) increase bacterial abundance and modify microbial community structure in leaf litter microcosms: A short-term decomposition study. Microbial Ecology. DOI: 10.1007/s00248-020-01527-4.
- DeSantis TZ, Hugenholtz P, Larsen N, Rojas M. 2006. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Applied and Environmental Microbiology 72:5069–5072. doi:10.1128/AEM.03006-05.
- Dittmer J, Lesobre J, Moumen B, Bouchon D. 2016. Host origin and tissue microhabitat shaping the microbiota of the terrestrial isopod Armadillidium vulgare. FEMS Microbiology Ecology 92:fiw063. doi:10.1093/femsec/fiw063.
- Djahnit N, Chernai S, Catania V, Hamdi B, China B, Cappello S, Quatrini P. 2019. Isolation, characterization and determination of biotechnological potential of oil-degrading bacteria from Algerian centre coast. Journal of Applied Microbiology 126(3):780–795.
- Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26(19):2460–2461.
- Frank JA, Reich CI, Sharma S, Weisbaum JS. 2008. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Applied and Environmental Microbiology 74:2461–2470. doi:10.1128/AEM.02272-07.
- Fraune S, Zimmer M. 2008. Host-specificity of environmentally transmitted Mycoplasma-like isopod symbionts. Environmental Microbiology 10:2497–2504. doi:10.1111/j.1462-2920.2008.01672.x.
- Grandjean F, Gouini N, Verne S, Delaunay C. 2005. Characterization of polymorphic microsatellite loci in the terrestrial isopod Porcellionides pruinosus. Molecular Ecology Notes 5:507–509. doi:10.1111/j.1471-8286.2005.00972.x.
- Gupta A, Verma JP. 2015. Sustainable bioethanol production from agro-residues: A review. Renewable and Sustainable Energy Reviews 41:550–567. doi:10.1016/j.rser.2014.08.032.
- Hongoh Y. 2010. Diversity and genomes of uncultured microbial symbionts in the termite gut. Biosciences, Biotechnology and Biochemistry 74:1145–1151. doi:10.1271/bbb.100094.
- Hornung E. 2011. Evolutionary adaptation of oniscidean isopods to terrestrial life: Structure, physiology and behaviour. Terrestrial Arthropod Reviews 4:95–130. doi:10.1163/187498311X576262.
- Horváthová T, Babik W, Bauchinger U. 2016. Biofilm feeding: Microbial colonization of food promotes the growth of a detritivorous arthropod. ZooKeys 577:25–41. doi:10.3897/zookeys.577.6149.
- Horváthová T, Kozłowski J, Bauchinger U. 2015. Growth rate and survival of terrestrial isopods is related to possibility to acquire symbionts. European Journal of Soil Biology 69:52–56. doi:10.1016/j.ejsobi.2015.05.003.
- Huang S, Sheng P, Zhang H. 2012. Isolation and identification of cellulolytic bacteria from the gut of Holotrichia parallela larvae (Coleoptera: Scarabaeidae). International Journal of Molecular Sciences 13:2563–2577. doi:10.3390/ijms13032563.
- Kenzaka T, Ishimoto Y, Tani K. 2017. Draft genome sequence of multidrug-resistant Cellulosimicrobium sp. Strain KWT-B, isolated from feces of Hirundo rustica. Genome Announcements 5(28):e00641–17. DOI: 10.1128/genomeA.00641-17.
- King AJ, Cragg SM, Li Y, Dymond J, Guille MJ, Bowles DJ, etal. 2010. Molecular insight into lignocellulose digestion by a marine isopod in the absence of gut microbes. Proceeding of the Natlional Academy of Sciences U.S.A. 107:5345–5350. DOI: 10.1073/pnas.0914228107.
- Kostanjsek R, Lapanje A, Rupnik M, Strus J, Drobne D, Avgustin G. 2004. Anaerobic bacteria in the gut of terrestrial isopod Crustacean Porcellio scaber. Folia Microbiologica. (Praha) 49:179–182. doi:10.1007/BF02931397.
- Kostanjšek R, Trus J, Avgustin G. 2002. Genetic diversity of bacteria associated with the hindgut of the terrestrial crustacean Porcellio scaber (Crustacea: Isopoda). FEMS Microbiology Ecology 40:171–179. doi:10.1111/j.1574-6941.2002.tb00950.x.
- Kukor JJ, Martin MM. 1986. The effect of acquired microbial enzymes on assimilation efficiency in the common woodlouse, Tracheoniscus rathkei. Oecologia 69:360–366. doi:10.1007/BF00377057.
- Lapanje A, Zrimec A, Drobne D, Rupnik M. 2010. Long-term Hg pollution-induced structural shifts of bacterial community in the terrestrial isopod (Porcellio scaber) gut. Environmental Pollution 158:3186–3193. doi:10.1016/j.envpol.2010.07.001.
- Loureiro S, Amorim MJB, Campos B, Rodrigues SMG. 2009. Assessing joint toxicity of chemicals in Enchytraeus albidus (Enchytraeidae) and Porcellionides pruinosus (Isopoda) using avoidance behaviour as an endpoint. Environmental Pollution 157:625–636. doi:10.1016/j.envpol.2008.08.010.
- Loureiro S, Sampaio A, Brandão A, Nogueira AJA. 2006. Feeding behaviour of the terrestrial isopod Porcellionides pruinosus Brandt, 1833 (Crustacea, Isopoda) in response to changes in food quality and contamination. Science of the Total Environment 369:119–128. doi:10.1016/j.scitotenv.2006.05.023.
- Loureiro S, Soares A, Nogueira A. 2005. Terrestrial avoidance behaviour tests as screening tool to assess soil contamination. Environmental Pollution 138:121–131. doi:10.1016/j.envpol.2005.02.013.
- Loureiro S, Sousa JP, Nogueira AJA, Soares AMVM. 2002. Assimilation efficiency and toxicokinetics of 14C-lindane in the terrestrial isopod Porcellionides pruinosus: The role of isopods in degradation of persistent soil pollutants. Ecotoxicology 11:481–490. doi:10.1023/A:1021013519330.
- Morgulis A, Coulouris G, Raytselis Y, Madden TL. 2008. Database indexing for production MegaBLAST searches. Bioinformatics 24:1757–1764. doi:10.1093/bioinformatics/btn322.
- Park DS, Oh HW, Jeong WJ, Kim H. 2007. Culture-based study of the bacterial communities within the guts of nine longicorn beetle species and their exo-enzyme producing properties for degrading xylan and pectin. Journal of Microbiology (Seoul,korea) 45:394–401.
- Qi W, Nong G, Preston JF, Ben AF, Ebert D. 2009. Comparative metagenomics of Daphnia symbionts. BMC Genomics 10:172. doi:10.1186/1471-2164-10-172.
- Ray DL, Julian JR. 1952. Occurrence of cellulase in Limnoria. Nature 169:32–33. doi:10.1038/169032a0.
- Riedel T, Gómez-Consarnau L, Tomasch J, Martin M. 2013. Genomics and physiology of a marine flavobacterium encoding a proteorhodopsin and a xanthorhodopsin-like protein. PLoS One 8(3):e57487. DOI: 10.1371/journal.pone.0057487.
- Schloss PD, Delalibera I, Handelsman J, Raffa KF. 2006. Bacteria associated with the guts of two wood-boring beetles: Anoplophora glabripennis and Saperda vestita (Cerambycidae). Environmental Entomology 35:625–629. doi:10.1603/0046-225X-35.3.625.
- Schmalfuss H. 1984. Eco-morphological strategies in terrestrial isopods. Symposia of the Zoological Society of London 53:49–63.
- Schrempf H. 2001. Recognition and degradation of chitin by Streptomycetes. Antonie Van Leeuwenhoek 79:285–289. doi:10.1023/A:1012058205158.
- Shao Y, Arias-Cordero E, Guo H, Bartram S. 2014. In vivo Pyro-SIP assessing active gut microbiota of the cotton leafworm, Spodoptera littoralis. PLoS One 9:e85948. doi:10.1371/journal.pone.0085948.
- Swiecicka I, Mahillon J. 2006. Diversity of commensal Bacillus cereus sensu lato isolated from the common sow bug (Porcellio scaber, Isopoda). FEMS Microbiology Ecology 56:132–140. doi:10.1111/j.1574-6941.2006.00063.x.
- Takahashi S, Tomita J, Nishioka K, Hisada T. 2014. Development of a prokaryotic universal primer for simultaneous analysis of bacteria and archaea using next-generation sequencing. PLoS One 9:e105592. doi:10.1371/journal.pone.0105592.
- Vargas-Asensio G, Pinto-Tomas A, Rivera B, Hernandez M. 2014. Uncovering the cultivable microbial diversity of Costa Rican beetles and its ability to break down plant cell wall components. PLoS One 9:e113303.
- Vurukonda SSKP, Giovanardi D, Stefani E. 2018. Plant growth promoting and biocontrol activity of Streptomyces spp. as endophytes. International Journal of Molecular Sciences 19(4):952. DOI: 10.3390/ijms19040952.
- Wang Y, Brune A, Zimmer M. 2007. Bacterial symbionts in the hepatopancreas of isopods: Diversity and environmental transmission. FEMS Microbiology Ecology 61:141–152. doi:10.1111/j.1574-6941.2007.00329.x.
- Wang Y, Stingl U, Anton-Erxleben F, Geisler S. 2004b. Candidatus Hepatoplasma crinochetorum., a new, stalk-forming lineage of Mollicutes colonizing the midgut glands of a terrestrial isopod. Applied and Environmental Microbiology 70:6166–6172. doi:10.1128/AEM.70.10.6166-6172.2004.
- Wang Y, Stingl U, Anton-Erxleben F, Zimmer M. 2004a. Candidatus Hepatincola porcellionum. gen. nov., sp. nov., a new, stalk-forming lineage of Rickettsiales colonizing the midgut glands of a terrestrial isopod. Archives of Microbiology 181:299–304. doi:10.1007/s00203-004-0655-7.
- Wenzel M, Radek R, Brugerolle G, König H. 2002. Identification of the ectosymbiotic bacteria of Mixotricha paradoxa involved in movement symbiosis. European Journal of Protistology 39:11–23. doi:10.1078/0932-4739-00893.
- Yermani M, Zouari K, Michelot JL, Mamou A, Moumni L. 2003. Geochemical approach to the functioning of the Gafsa North deep aquifer (central Tunisia). Hydrological Sciences Journal 48:95–108. doi:10.1623/hysj.48.1.95.43482.
- Yoon JH, Jung SY, Kang SJ, Oh TK. 2007. Microbulbifer celer sp. nov., isolated from a marine solar saltern of the Yellow Sea in Korea. International Journal of Systematic and Evolutionary Microbiology 57:2365–2369. doi:10.1099/ijs.0.65184-0.
- Zhang Z, Schwartz S, Wagner L, Miller W. 2000. A greedy algorithm for aligning DNA sequences. Journal of Computational Biology 7:203–214. doi:10.1089/10665270050081478.
- Zhang ZQ, He C, Li ML. 2014. Analysis of intestinal bacterial community diversity of adult Dastarcus helophoroides. Journal of Insect Science 14:114. doi:10.1093/jis/14.1.114.
- Zimmer M. 2002a. Nutrition in terrestrial isopods (Isopoda: Oniscidea): An evolutionary-ecological approach. Biological Reviews 77:455–493.
- Zimmer M. 2002b. Postembryonic ontogenetic development in Porcellio scaber (Isopoda: Oniscidea): The significance of food. Invertebrate Reproduction and Development 42:75–82. doi:10.1080/07924259.2002.9652512.
- Zimmer M, Topp W. 1998. Microorganisms and cellulose digestion in the gut of the woodlouse Porcellio scaber. Journal of Chemical Ecology 24:1397–1408. doi:10.1023/A:1021235001949.
- Zimmer M, Topp W. 1999. Relationships between woodlice (Isopoda: Oniscidea) and microbial density and activity in the field. Biology and Fertility of Soils 30:117–123. doi:10.1007/s003740050597.