Abstract
The causes and consequences of variation in the incubation regimes of oviparous animals remain unclear, despite having important fitness consequences. Avian incubation regimes can be shortened by parents initiating incubation prior to clutch completion or prolonged when there are gaps in the laying sequence. Here, we begin by quantifying variation in the incubation regimes of three populations of blue tits Cyanistes caeruleus from the UK and Poland before examining the consequences of such variation for their hatching and fledging success. We then investigate the mechanism causing such variation by exploring the impact of local weather conditions on incubation regimes. The difference between the expected and actual hatching dates of clutches was termed the “hatching deviation” and this showed considerable variation. Hatching deviation was negatively related to local temperature and clutch size. Hatching deviation affected hatching success and hatching deviation, temperature, wind speed and clutch size affected fledging success. Deviating from the expected laying and incubation regime caused lowered reproductive success. The most successful birds were those that were able to lay one egg per day and begin incubation upon clutch completion.
Introduction
The ecological and evolutionary causes, and fitness consequences, of variation in reproductive parameters have received a huge amount of research attention over several decades (e.g. Pettifor et al. Citation2001; Massa et al. Citation2004; Vaugoyeau et al. Citation2016) and that research has yielded some crucial insights into reproductive behaviours and life-history strategies (Stearns Citation1992). However, those studies have focused disproportionately on some reproductive stages and away from others. In birds, studies have focused primarily on the period between hatching and fledging (e.g. Both & Visser Citation2001; Both et al. Citation2006; Charmantier et al. Citation2008) and away from the period between egg laying and hatching (Nilsson & Svensson Citation1993b; Kluen et al. Citation2011; García-Navas & Sanz Citation2011; Whitehouse et al. Citation2013; Glądalski et al. Citation2020). This imbalance in research attention is surprising as the conditions experienced by embryos between egg laying and hatching influence their development both before and after hatching (Meijerhof Citation1992; Birkhead et al. Citation2008; reviewed by DuRant et al. Citation2013). Further, the interval between the laying of the last egg and the hatching of the first offspring is flexible to a certain degree and under parental control (Tomás Citation2015). While the basic model for small passerines would be to lay one egg per day and start incubating on clutch completion, parents can deviate from that by initiating incubation prior to the laying of the last egg, by having laying gaps and/or by delaying the onset of incubation (Tomás Citation2015). Such parentally controlled adjustments could be seen as adaptive behaviours in response to the need to provide parental care, such as controlling hatching asynchrony (Magrath Citation1990) or fine tuning peak offspring demand in relation to local resources (García-Navas & Sanz Citation2011). Alternatively, they could be seen as limitations induced by a short-term lack of resources but either way, such adjustments could provide a mechanism facilitating adaptation to climate change (Cresswell & McCleery Citation2003; Marrot et al. Citation2017; Radchuk et al. Citation2019).
The extent of deviation away from the basic model of laying and incubation (henceforth “hatching deviation”), can have important fitness consequences. In insectivorous passerines, parentally induced variation in incubation regimes has been identified as a mechanism which allows them to fine-tune the synchrony of periods of maximal food demand by their offspring with the peak food supply in their seasonal environments (Cresswell & McCleery Citation2003; Tomás Citation2015). Further, as embryos only develop optimally within relatively narrow environmental, and more specifically thermal, limits, and prolonged periods of time spent outside of those limits result in suboptimal embryonic development and in some cases, embryonic mortality (e.g. Meijerhof Citation1992; reviewed by DuRant et al. Citation2013); then, environmental conditions should be expected to impact on incubation regimes. Additionally, hatching deviations, where there are one or more breaks in the usual egg laying interval, may be seen as a negative effect of adverse environmental conditions that constrain parental investment during the egg laying period (Nilsson & Svensson Citation1993a, Citation1993b; Vedder Citation2012).
Weather can influence various aspects of avian reproduction including the timing of egg laying (Charmantier et al. Citation2008; Hinks et al. Citation2015), the hatchability of eggs (Indykiewicz Citation2015), provoke egg ejection behaviour in open-nesting passerines (Shitikov et al. Citation2019) and influence offspring growth and survival (Goławski & Dombrowski Citation2011; Mainwaring et al., Citation2014; Mainwaring & Hartley Citation2016; Rodríguez et al., Citation2016; Nilsson & Nord Citation2017). Pertinently, local weather can have a large influence on the optimal hatching date of insectivorous passerines because it affects the timing of the peak availability of their caterpillar food supply (Perrins Citation1991; Hinks et al. Citation2015). A combination of warm weather and low rainfall provides good conditions which enable the development of plants and rich arthropod communities, while low temperatures and excessively high rainfall suppress the development of such communities (Mellanby Citation1939; Johnson et al. Citation2016). Therefore, when female birds experience a cold spell they may delay hatching (García-Navas & Sanz Citation2011; Kluen et al. Citation2011), which may be beneficial when it allows them to synchronise the peak demands for food by their nestlings and the peak availability of their caterpillar prey (Perrins Citation1991; Monrós et al. Citation1998; Cresswell & McCleery Citation2003). Females may also accelerate hatching by initiating incubation prior to laying the last egg, which increases the reproductive success of great tits Parus major (Naef-Daenzer et al. Citation2004), although delayed hatching may have costs. For example, prolonging the incubation period may decrease hatching success (García-Navas & Sanz Citation2011), may increase the risk of nest predation (Bosque & Bosque Citation1995) and negatively affect the development of nestlings (Kluen et al. Citation2011; Whitehouse et al. Citation2013). Additionally, the occurrence of hatching deviation and reduced hatching success may be interpreted to have been caused by a shortage of food or large amounts of additional energy being used to keep eggs within acceptable thermal limits during cold snap (Nilsson & Svensson Citation1993a). This idea was supported by Kluen et al. (Citation2011) who reported that females of the blue tit Cyanistes caeruleus that delayed the hatching of their clutch had lower body condition at hatching than conspecifics who did not delay hatching. Therefore, it is also important to emphasize that parental quality may also play a role in shaping incubation investment. Studies examining birds’ sensitivity to environmental conditions have increased in number in recent years as concern grows about their ability to adapt to ongoing changes in environmental conditions (e.g. more frequent weather extremes like sudden drop of temperature, long-lasting heavy rain or drought; Winkler et al. Citation2002; Charmantier et al. Citation2008; Pipoly et al. Citation2013, Citation2020; Whitehouse et al. Citation2013; Hinsley et al. Citation2016).
In this study, we begin by examining long-term variation in the hatching deviations in populations of blue tits inhabiting woodland at Lancaster, northwest UK, and urban parkland and forest at Łódź, central Poland – two contrasting climates: maritime and continental and two contrasted types of habitats: the natural and the urban, before investigating if the extent of such hatching deviation affected the hatching success and fledging success of those clutches. We then examine if pre-hatching local weather conditions during reproduction caused variation in hatching deviations. We test three predictions: first, variation will exist in the hatching deviations in blue tits because such intervals are influenced by parental behaviours; second, larger hatching deviations, whether positive or negative, will be negatively related to both hatching success and fledging success; and third, larger hatching deviations will occur in cold, rainy and windy weather conditions because females will be unable to breed optimally due to environmental stress.
Materials and methods
Study areas and routine data collection
Data were collected from populations of nestbox-breeding blue tits inhabiting deciduous and mixed woods in northwest Lancashire, UK (54°0ʹN, 02°47ʹW; for further details see Mainwaring et al. Citation2010) during the 2004–2016 breeding seasons, and deciduous woods (51º50′N, 19º29′E) and urban parkland (51º45′N, 19º24′E; for details see Glądalski et al. Citation2016a) around Łódź, Poland, during the 2002–2016 breeding seasons. In all three study sites, nestboxes were checked and repaired as necessary in January of each year. From early April onwards, nestboxes were checked at least once every 3 days to determine the day when the first egg was laid (assuming they laid one egg per day: Perrins Citation1979). Nestboxes were then checked on a daily basis after the sixth egg in clutches was laid until the parents began to incubate the clutch (although it is important to emphasize that sometimes it is hard to be sure that full incubation has started based solely on physical touch during nest visits). Whilst incubating, birds were left undisturbed for the first 11 days of incubation, and then nests were checked on a daily basis beginning on the twelfth day after incubation began so that the hatching deviations were accurately recorded, meaning that we were able to accurately determine hatching deviations to the nearest day. Briefly, after hatching, we quantified hatching success; then, after fledging, we quantified fledging success (the fledglings usually leave the nest when they are about 19–22 days old). Hatching dates were not accurately recorded at Lancaster in the 2013 and 2014 breeding seasons and so it was not possible to calculate hatching deviations in those clutches. Data were collected from 640 clutches at Lancaster, 465 clutches in forest at Łódź and 330 clutches in urban parkland at Łódź.
Quantifying hatching deviations
Hatching deviations were calculated in terms of the positive or negative deviance away from the expected model of laying and incubation, which was for blue tits to lay one egg per day with a subsequent incubation period of 13 days after clutch completion (Mainwaring et al. Citation2010; García-Navas & Sanz Citation2011; Glądalski et al. Citation2018, Citation2020). Days were used as the measurement unit of hatching deviation. Deviation from a zero value for the hatching deviation could arise from a bird starting incubation earlier than the laying of the final egg (negative values, shortened hatching deviation), or by a bird failing to lay an egg each day during the laying period or delaying the onset of incubation beyond the laying period (positive values, extended hatching deviation). The term hatching delay is also used by some authors to describe mismatch between predicted and actual hatching date (García-Navas & Sanz Citation2011) and in our definition, it is analogous but less precise than hatching deviation. Hatching dates were not accurately recorded at Lancaster in the 2013 and 2014 breeding seasons and so it was not possible to calculate hatching deviations in those clutches.
Quantifying local weather conditions
Local weather variables for Lancaster study area were supplied by Lancaster University’s Hazelrigg meteorological station (54º00′N, 02º47′W) located within 1 km of the Lancaster study area and for both study areas in Łódź from the “TuTiempo.net” climate data archive, and more specifically from the meteorological station located within 5 km of both of the study sites in Łódź (51º73′N, 19º04′E). During the analyses, the variables used were mean daily temperature, daily rainfall (mm) and mean wind speed (kilometres per hour). Mean weather variables were calculated in relation to the day before one of the key events in the breeding cycle of individual birds - the first egg date (averages were calculated per 24-h period).
Statistical analyses
Data were analysed using the SPSS v21.0 (IBM SPSS, Chicago, IL, USA) statistical package. The factors influencing variation in hatching deviations were examined using a General Linear Model which had hatching deviation as the dependent variable and year, site, and their two-way interaction term as explanatory factorial variables and the following explanatory covariates: clutch size, and mean temperature, rainfall and wind speed for 1 day before the first egg date. A Generalized Linear Model with binomial error distribution, logit link function and Wald Chi-squared test statistics was used to examine the impact of different factors on relative binomial characteristics of breeding success, hatching success (the number of hatchlings in relation to clutch size) and fledging success (the number of fledglings in relation to the number of hatchlings), study site submodels: Lancaster, the forest (Łódź) and the urban parkland (Łódź) were also added (Crawley Citation2002).
Results
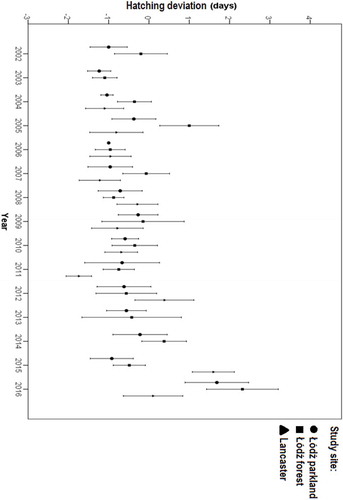
Hatching deviation varied considerably between sites and years, and a significant interaction between site and year indicated that the hatching deviation varied differently between the three sites in different years (, ). There were also effects of temperature (b = −0.113 ± 0.032, F = 12.285, P < 0.001) and clutch size (b = −0.201 ± 0.028, F = 52.814, P < 0.001) on the hatching deviation (lower temperatures and smaller clutches elongated hatching deviation (days); ).
Table I. Summary of a General Linear Model examining variation in blue tit hatching deviation in terms of year, site, clutch size and temperature, rainfall and wind speed with significant terms highlighted in bold. Temperature, rainfall and wind speed refer to the day prior to the first egg date.
Figure 1. Hatching deviation in blue tits at three study sites over 15 years, with bars representing 95% CI around the mean value.
Hatching success was negatively related to the hatching deviation (in Lancaster and in the forest (Łódź) submodels, but not in the urban parkland (Łódź), ). Hatching success was also influenced by year (in the main model and the three submodels), site (in the main model) and their two-way interaction term (in the main model). Hatching success was also negatively related to rainfall in the urban parkland (Łódź) submodel ().
Table II. Summary of a Generalized Linear Model with binomial error distribution, logit link function, and Wald Chi-squared test statistics examining variation in blue tit hatching success in terms of year, site and temperature, rainfall and wind speed with submodels: Lancaster, Łódź the forest and Łódź the urban parkland. Temperature, rainfall and wind speed refer to the day prior to the first egg date.
Fledging success varied significantly between sites and years and there was a significant interaction between these terms (the main model). Fledging success was negatively related to the hatching deviation (in Lancaster and in the forest (Łódź) submodels, and in the urban parkland (Łódź) there was a similar, but nonsignificant trend; ). Fledging success was also related to several weather variables, being positively related to the temperature in Lancaster submodel and similar, but nonsignificant trend in the forest (Łódź) and negatively related to the temperature in the parkland (Łódź). Fledging success was also positively affected by wind speed in both Łódź submodels (the forest and the urban parkland) and negatively affected by the rainfall in the urban parkland (Łódź) – there were no significant effects of rainfall or wind speed for Lancaster submodel.
Table III. Summary of a Generalized Linear Model with binomial error distribution, logit link function, and Wald Chi-squared test statistics examining variation in blue tit fledging success in terms of year, site and temperature, rainfall and wind speed with submodels: Lancaster, Łódź the forest and Łódź the urban parkland. Temperature, rainfall and wind speed refer to the day prior to the first egg date.
Discussion
The main findings of this long-term study were that considerable intraspecific variation exists in the hatching deviation of three populations of blue tits and that longer hatching deviations were negatively related to both hatching (but not in the urban parkland area) and fledging success (but also not in the urban parkland area). We also showed that longer hatching deviations were negatively related to temperature and clutch size. This suggests that adverse temperature conditions may leave some females unable to access sufficient resources which impacts on their reproductive success. The dependence on climatic variables further suggests that hatching deviations were determined by weather conditions rather than them being a deliberate strategic phenomenon induced by the parents (García-Navas & Sanz Citation2011; Glądalski et al. Citation2020). These results were similar at geographically separated study sites, in northwest England and central Poland. Minor differences (e.g. hatching success was not related to hatching deviation in the urban parkland) are probably related to differentiation of all three study areas and a mosaic of factors influencing breeding parameters in every single of those study areas. Further, by demonstrating that variation in hatching deviations in an insectivorous passerine bird is largely driven by local weather conditions during the laying period, we have highlighted that climatic conditions during very specific and short periods of the reproductive cycle may strongly influence the reproductive success of birds in temperate environments. But in this light, it is also important to emphasize that it is difficult to exclude an effect of parental quality itself.
The difference between sites may be caused by differences in phenology of blue tits in combination with a difference in phenology of the study habitats. Urban environments are usually associated with earlier clutches in tits (Seress & Liker Citation2015; Marini et al. Citation2017). Taxonomic composition of tree flora in the urban parkland results in earlier leafing and thus larvae on birches and poplars (urban parkland) appear earlier than on oaks (the forest) (Glądalski et al. Citation2018). The leafing phenology influences the availability of caterpillars for feeding nestlings. Those shifts in timing may affect the hatching deviations because when the drop of temperature happens birds in one study site may be a few days later/earlier or even after/before laying phase (Glądalski et al. Citation2018, Citation2020).
Birds such as blue tits have an opportunity to adjust the length of their breeding cycle even after egg laying has begun by adjusting the date they commence incubation (Nilsson & Svensson Citation1993b; García-Navas & Sanz Citation2011). This is important because the reproductive success of blue tits and other insectivorous passerine birds is highly dependent on their ability to synchronise their own reproductive cycle with that of their caterpillar prey (Perrins Citation1979, Citation1991; Both & Visser Citation2001). Whilst birds are able to adaptively shift their own egg laying dates quite substantially in relation to the phenology of their woodland environments, primarily through phenotypic plasticity (e.g. Charmantier et al. Citation2008) but also through microevolutionary processes (e.g. Brommer et al. Citation2005), the potential for alterations is much more limited after egg laying has begun (Cresswell & McCleery Citation2003; Glądalski et al. Citation2016b, Citation2018, Citation2020; Mainwaring et al. Citation2017). Birds that mistime their reproduction are usually later, rather than earlier, than the peak emergence of their caterpillar prey and so mechanisms allowing them to shorten the laying-hatching interval should be advantageous. Starting incubation before the final egg has been laid does bring forward the hatching of the first nestlings so that the peak demand for food better matches the peak availability, but it also results in the brood hatching asynchronously (Cresswell & McCleery Citation2003; Mainwaring et al. Citation2010). This can be suboptimal because hatching asynchrony often results in the death or suboptimal development of later-hatched and competitively inferior nestlings (Mainwaring et al. Citation2010) at least partly because parent birds often provision older and competitively superior nestlings preferentially (Mock & Parker Citation1997).
While hatching deviations with negative values show that incubation has started before the completion of the clutch, those with positive values could occur through two mechanisms: gaps in the normal egg laying process or a delay in the onset of incubation after clutch completion. An alternative possibility is that variation in hatching deviations could arise through parental incubation behaviours whilst on the nest but unfortunately, we do not have the data available to examine the influence of incubation behaviours on hatching deviations. Nevertheless, we have demonstrated that longer, positive hatching deviations were related to spells of low temperatures. Blue tits are known to have breaks in the normal laying sequence of one egg per day (Nilsson & Svensson Citation1993b). It means that blue tits and other insectivorous passerine birds in temperate environments could be particularly susceptible to adverse weather conditions for short periods if they coincide with crucial parts of the reproductive cycle, such as the middle of the laying period. This highlights the need to identify critical weather windows during reproduction rather than taking a broader approach of averaging weather variables throughout the reproductive period (van de Pol et al. Citation2016; Glądalski et al. Citation2020), particularly as it gets substantially warmer in seasonal habitats in temperate environments. This is an important consideration because once incubation has begun, embryos only develop optimally within relatively narrow thermal limits, and prolonged periods of time spent outside of those limits result in suboptimal embryonic development (reviewed by DuRant et al. Citation2013; Mainwaring et al. Citation2017). It is likely that only those birds in relatively poor condition had hatching deviations in our study because not all pairs experienced hatching deviations in periods of poor weather. It is not clear to what extent low ambient temperatures in the middle of the laying period induce positive hatching deviation, but it would be interesting to examine the effect of extreme weather events, which are those events that differ from the usual variation in climatic variables in terms of their rarity, magnitude, statistical extremity and short duration (Easterling et al. Citation2000; van de Pol et al. Citation2010; Glądalski et al. Citation2014, Citation2016a, Citation2018; Marrot et al. Citation2017; Radchuk et al. Citation2019).
Depending on the ambient temperature, the duration of hatching deviations had important consequences for fitness because pairs with longer deviations had significantly lower hatching and fledging success than pairs with shorter hatching deviations, in agreement with another study of blue tits (Kluen et al. Citation2011). Studies show also that blue tit females that had hatching deviations of zero also laid heavier and larger eggs than pairs with both negative and positive hatching deviations, thus providing further evidence that adverse weather conditions during the mid-laying period meant that females were unable to sequester sufficient resources to produce eggs (Nilsson & Svensson Citation1993a, Citation1993b; Kluen et al. Citation2011). Additionally, a previous study showed that blue tit eggs produced immediately before laying gaps were smaller than other eggs laid without the presence of gaps (Nilsson & Svensson Citation1993b). For some females during the laying period, resources (like snail shells; Bańbura et al. Citation2010, Citation2019 or food availability; Nilsson & Svensson Citation1993a, Citation1993b) are likely to be on the edge of minimum needs and a short period of cold weather at the wrong time may push them into a state where egg production is no longer possible. These females are the ones without access to sufficient resources (like snail shells or food availability) and so are likely to be producing lower quality eggs and incurring either laying gaps or the necessity to delay incubation (Nilsson & Svensson Citation1993a, Citation1993b; Bańbura et al. Citation2019).
The main findings of this study were that considerable intraspecific variation existed in the hatching deviations of blue tits from three study sites and that delays were greater following adverse thermal conditions, either due to females failing to lay an egg a day or by them delaying the onset of incubation beyond the laying period. We also found that longer hatching deviations were mainly negatively related to both hatching and fledging success, independent of local weather conditions, thereby highlighting the critical need for insectivorous passerine birds such as blue tits to time their reproduction optimally.
There are three avenues that may prove fruitful for further research. First, whilst we have shown that variation in hatching deviations exists, the exact mechanisms underlying such variation remain unclear. Our study suggests that female birds may be limited by the availability of food during the middle of the egg laying period and so studies that provide birds with supplementary food during the critical breeding phases and compare the duration of delays with conspecifics without additional food would be informative in this regard. Second, we have further demonstrated the importance of timing reproduction optimally but studies that experimentally alter the timing of reproduction and examine the fitness consequences of such altered timing would be very useful indeed. Third, anthropogenic climate change is predicted to encompass an increase in the occurrence of extreme weather events, in addition to the more gradual and incremental changes in weather conditions, and so their influence on the occurrence of hatching deviations more broadly would be informative. It is also important to emphasize that our results are descriptive and future experimental approaches, including experiments in natural settings, would be welcomed.
Ethical approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All procedures using animals were authorized under permits from the University of Lancaster and University of Łódź.
Acknowledgements
We thank E. Wróblewska, A. Jaksa, D. Mańkowska, M. Janiszewska and J. Białek for helping with data collection in Poland and T. Kurzac for logistic help in the Botanic Garden, whilst we thank M. Dickens, C. Benskin and R. Hope with data collection in England. All applicable institutional and/or national guidelines for the care and use of animals were followed. The bulk of the manuscript was written while M. Glądalski visited Lancaster University. We are very grateful to two anonymous referees and the field editor for critical comments and constructive suggestions on the previous draft of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Bańbura J, Sulikowska-Drozd A, Bańbura M, Zieliński P, Kaliński A, Wawrzyniak J, Glądalski M, Skwarska J, Markowski M. 2019. Blue Tits Cyanistes caeruleus laying smaller eggs after a decline in snail numbers: An indirect effect of slug control in a city park. Acta Ornithologica 54(2):139–148. DOI: 10.3161/00016454AO2019.54.2.001.
- Bańbura M, Sulikowska-Drozd A, Kaliński A, Skwarska J, Wawrzyniak J, Kruk A, Zieliński P, Bańbura J. 2010. Egg size variation in Blue Tits Cyanistes caeruleus and Great Tits Parus major in relation to habitat differences in snail abundance. Acta Ornithologica 45(2):121–129. DOI: 10.3161/000164510X551264.
- Birkhead TR, Hall J, Schut E, Hemmings N. 2008. Unhatched eggs: Methods for discriminating between infertility and early embryo mortality. Ibis 150(3):508–517. DOI: 10.1111/j.1474-919X.2008.00813.x.
- Bosque C, Bosque MT. 1995. Nest predation as a selective factor in the evolution of developmental rates in altricial birds. American Naturalist 145(2):234–260. DOI: 10.1086/285738.
- Both C, Bouwhuis S, Lessells CM, Visser ME. 2006. Climate change and population declines in a long-distance migratory bird. Nature 441(7089):81–83. DOI: 10.1038/nature04539.
- Both C, Visser ME. 2001. Adjustment to climate change is constrained by arrival date in a long distance migrant bird. Nature 411(6835):296–298. DOI: 10.1038/35077063.
- Brommer JE, Merilä J, Sheldon BC, Gustafsson L. 2005. Natural selection and genetic variation for reproduction reaction norms in a wild bird population. Evolution 59(6):1362–1371. DOI: 10.1111/j.0014-3820.2005.tb01785.x.
- Charmantier A, McCleery RH, Cole LR, Perrins C, Kruuk LE, Sheldon BC. 2008. Adaptive phenotypic plasticity in response to climate change in a wild bird population. Science 320(5877):800–803. DOI: 10.1126/science.1157174.
- Crawley MJ. 2002. Statistical computing: An introduction to data analysis using S-Plus. Chichester: Wiley.
- Cresswell W, McCleery RH. 2003. How great tits maintain synchronization of their hatch date with food supply in response to long-term variability in temperature. Journal of Animal Ecology 72(2):356–366. DOI: 10.1046/j.1365-2656.2003.00701.x.
- DuRant SE, Hopkins WA, Walters JR, Hepp GR. 2013. Ecological, evolutionary, and conservation implications of incubation temperature-dependent phenotypes in birds. Biological Reviews 88(2):499–509. DOI: 10.1111/brv.12015.
- Easterling DR, Meehl G, Changnon S, Parmesan C, Karl TR, Mearns LO. 2000. Climate extremes: Observations, modelling, and impacts. Science 289(5487):2068–2074. DOI: 10.1126/science.289.5487.2068.
- García-Navas V, Sanz JJ. 2011. Short-term alterations in songbird breeding schedule lead to better synchronization with food availability. Auk 128(1):146–155. DOI: 10.1525/auk.2010.10094.
- Glądalski M, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Bańbura J. 2014. Extreme weather event in spring 2013 delayed breeding time of Great Tit and Blue Tit. International Journal of Biometeorology 58(10):2169–2173. DOI: 10.1007/s00484-014-0816-6.
- Glądalski M, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Bańbura J. 2016b. Effects of extreme thermal conditions on plasticity in breeding phenology and double-bloodedness of Great Tits and Blue Tits in central Poland in 2013 and 2014. International Journal of Biometeorology 60(11):1795–1800. DOI: 10.1007/s00484-016-1152-9.
- Glądalski M, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Bańbura J. 2018. Hatching delays in great tits and blue tits in response to an extreme cold spell: A long-term study. International Journal of Biometeorology 62(8):1437–1445. DOI: 10.1007/s00484-018-1541-3.
- Glądalski M, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Bańbura J. 2020. Extreme temperature drop alters hatching delay, reproductive success and physiological condition in Great Tits. International Journal of Biometeorology 64(4):623–629. DOI: 10.1007/s00484-019-01851-6.
- Glądalski M, Bańbura M, Kaliński A, Markowski M, Skwarska J, Wawrzyniak J, Zieliński P, Cyżewska I, Mańkowska D, Bańbura J. 2016a. Effects of human-related disturbance on breeding success of urban and non-urban blue tits (Cyanistes caeruleus). Urban Ecosystems 19(3):1325–1334. DOI: 10.1007/s11252-016-0543-3.
- Goławski A, Dombrowski A. 2011. The effects of weather conditions on the numbers of wintering birds and the diversity of their assemblages in villages and crop fields in east-central Poland. Italian Journal of Zoology 78(3):364–369. DOI: 10.1080/11250003.2010.535858.
- Hinks AE, Cole EF, Daniels KJ, Wilkin TA, Nakagawa S, Sheldon BC. 2015. Scale-dependent phenological synchrony between songbirds and their caterpillar food source. American Naturalist 186(1):84–97. DOI: 10.1086/681572.
- Hinsley SA, Bellamy PE, Hill RA, Ferns PN. 2016. Recent shift in climate relationship enables prediction of the timing of bird breeding. PloS One 11(5):e0155241. DOI: 10.1371/journal.pone.0155241.
- Indykiewicz P. 2015. Egg losses caused by cold snap in the black-headed gull, Chroicocephalus ridibundus L. Polish Journal of Ecology 63(3):460–466. DOI: 10.3161/15052249PJE2015.63.3.016.
- Johnson CA, Coutinho RM, Berlin E, Dolphin KE, Heyer J, Leung A, Sabellon JL, Amarasekare P. 2016. Effects of temperature and resource variation on insect population dynamics: The bordered plant bug as a case study. Functional Ecology 30(7):1122–1131. DOI: 10.1111/1365-2435.12583.
- Kluen E, de Heij ME, Brommer JE. 2011. Adjusting the timing of hatching to changing environmental conditions has fitness costs in blue tits. Behavioral Ecology and Sociobiology 65(11):2091–2103. DOI: 10.1007/s00265-011-1218-y.
- Magrath RD. 1990. Hatching asynchrony in altricial birds. Biological Reviews 65(4):587–622. DOI: 10.1111/j.1469-185X.1990.tb01239.x.
- Mainwaring MC, Barber I, Deeming DC, Pike DA, Roznik EA, Hartley IR. 2017. Climate change and nesting behaviour in vertebrates: A review of the ecological effects and potential for adaptive responses. Biological Reviews 92(4):1991–2002. DOI: 10.1111/brv.12317.
- Mainwaring MC, Dickens M, Hartley IR. 2010. Environmental and not maternal effects determine variation in offspring phenotypes in a passerine bird. Journal of Evolutionary Biology 23(6):1302–1311. DOI: 10.1111/j.1420-9101.2010.01997.x.
- Mainwaring MC, Hartley IR. 2016. Local weather conditions have complex effects on the growth of blue tit nestlings. Journal of Thermal Biology 60:12–19. DOI: 10.1016/j.jtherbio.2016.05.005.
- Mainwaring MC, Hartley IR, Lambrechts MM, Deeming DC. 2014. The design and function of birds’ nests. Ecology and Evolution 4(20):3909–3928. DOI: 10.1002/ece3.1054.
- Marini KLD, Otter KA, LaZerte SE, Reudink MW. 2017. Urban environments are associated with earlier clutches and faster nestling feather growth compared to natural habitats. Urban Ecosystems 20(6):1291–1300. DOI: 10.1007/s11252-017-0681-2.
- Marrot P, Garant D, Charmantier A. 2017. Multiple extreme climatic events strengthen selection for earlier breeding in a wild passerine. Philosophical Transactions of the Royal Society B 372(1723):20160372. DOI: 10.1098/rstb.2016.0372.
- Massa B, Lo Valvo F, Margagliotta B, Lo Valvo M. 2004. Adaptive plasticity of blue tits (Parus caeruleus) and great tits (Parus major) breeding in natural and semi‐natural insular habitats. Italian Journal of Zoology 71(3):209–217. DOI: 10.1080/11250000409356574.
- Meijerhof R. 1992. Pre-incubation holding of hatching eggs. World’s Poultry Science Journal 48(1):57–68. DOI: 10.1079/WPS19920006.
- Mellanby K. 1939. Low temperature and insect activity. Proceedings of the Royal Society of London B 849:473–487.
- Mock DW, Parker GA. 1997. The evolution of sibling rivalry. Oxford: Oxford University Press.
- Monrós IS, Belda EJ, Barba E. 1998. Delays of the hatching dates in Great Tits Parus major: Effects on breeding performance. Ardea 86:213–220.
- Naef-Daenzer L, Nager RG, Keller LF, Naef-Daenzer B. 2004. Are hatching delays a cost or a benefit for Great Tit Parus major parents? Ardea 92:229–238.
- Nilsson J-A, Nord A. 2017. The use of the nest for parental roosting and thermal consequences of the nest for nestlings and parents. Behavioral Ecology and Sociobiology 71(12):171. DOI: 10.1007/s00265-017-2400-7.
- Nilsson J-A, Svensson E. 1993a. Energy constraints and ultimate decisions during egg-laying in the blue tit. Ecology 74(1):244–251. DOI: 10.2307/1939519.
- Nilsson J-A, Svensson E. 1993b. The frequency and timing of laying gaps. Ornis Scandinavica 24(2):122–126. DOI: 10.2307/3676361.
- Perrins CM. 1979. British tits. London: Collins.
- Perrins CM. 1991. Tits and their caterpillar food supply. Ibis 133:49–54. DOI: 10.1111/j.1474-919X.1991.tb07668.x.
- Pettifor RA, Perrins CM, McCleery R. 2001. The individual optimization of fitness: Variation in reproductive output, including clutch size, mean nestling mass and offspring recruitment, in manipulated broods of great tits Parus major. Journal of Animal Ecology 70(1):62–79. DOI: 10.1046/j.1365-2656.2001.00465.x.
- Pipoly I, Bókony V, Seress G, Szabó K, Liker A. 2013. Effects of extreme weather on reproductive success in a temperate-breeding songbird. PloS One 8(11):e80033. DOI: 10.1371/journal.pone.0080033.
- Pipoly I, Preiszner B, Sandor K, Sinkovics C, Seress G, Vincze E, Bókony V, Liker A. 2020. Effects of extreme hot weather on the reproductive output of great tits (Parus major, L.) in urban and natural habitats. bioRxiv. DOI: 10.1101/2020.01.29.924332.
- Radchuk V, Reed T, Teplitsky R, van de Pol M, Charmantier A, et al. 2019. Adaptive responses of animals to climate change are most likely insufficient. Nature Communications 10(1):3109. DOI: 10.1038/s41467-019-10924-4.
- Rodríguez S, Diez-Méndez D, Barba E. 2016. Negative effects of high temperatures during development on immediate post-fledging survival in Great Tits Parus major. Acta Ornithologica 51(2):235–244. DOI: 10.3161/00016454AO2016.51.2.009.
- Seress G, Liker A. 2015. Habitat urbanization and its effects on birds. Acta Zoologica Academiae Scientiarum Hungaricae 61(4):373–408. DOI: 10.17109/AZH.61.4.373.2015.
- Shitikov D, Samsonov S, Makarova T. 2019. Cold weather events provoke egg ejection behaviour in open-nesting passerines. Ibis 161:441–446. DOI: 10.1111/ibi.12695.
- Stearns SC. 1992. The evolution of life histories. Oxford: Oxford University Press.
- Tomás G. 2015. Hatching date vs laying date: What should we look at to study avian optimal timing of reproduction? Journal of Avian Biology 46(1):107–112. DOI: 10.1111/jav.00499.
- van de Pol M, Bailey LD, McLean N, Rijsdijk L, Lawson CR, Brouwer L. 2016. Identifying the best climatic predictors in ecology and evolution. Methods in Ecology and Evolution 7(10):1246–1257. DOI: 10.1111/2041-210X.12590.
- van de Pol M, Ens BJ, Heg D, Brouwer L, Krol J, Maier M, Exo KM, Oosterbeek K, Lok T, Eising CM, Koffijberg K. 2010. Do changes in the frequency, magnitude and timing of extreme climatic events threaten the population viability of coastal birds? Journal of Applied Ecology 47(4):720–730. DOI: 10.1111/j.1365-2664.2010.01842.x.
- Vaugoyeau M, Meylan S, Biard C. 2016. How does an increase in minimum daily temperatures during incubation influence reproduction in the great tit Parus major? Journal of Avian Biology 48(5):714–725. DOI: 10.1111/jav.01208.
- Vedder O. 2012. Individual birds advance offspring hatching in response to increased temperature after the start of laying. Oecologia 170(3):619–628. DOI: 10.1007/s00442-012-2335-7.
- Whitehouse MJ, Harrison NM, Mackenzie J, Hinsley SA. 2013. Preferred habitat of breeding birds may be compromised by climate change: Unexpected effects of an exceptionally cold, wet spring. PloS One 8(9):e75536. DOI: 10.1371/journal.pone.0075536.
- Winkler DW, Dunn PO, McCulloch CE. 2002. Predicting the effects of climate change on avian life-history traits. Proceedings of the National Academy of Sciences of the United States of America 99(21):13595–13599. DOI: 10.1073/pnas.212251999.
