Abstract
Species diversity assessments are an important step to evaluate the conservation status of a community, both in marine and terrestrial ecosystems. These assessments are pivotal if related to both, the constant increase of human pressure on ecosystems and the anthropogenic climate change occurring nowadays. Sharks and rays are globally threatened, and the situation is particularly alarming in the Mediterranean Sea where more than 50% of species are listed at risk of extinction by the International Union for Conservation of Nature (IUCN). In this paper, we revise and discuss the chondrichthyan species richness of the Mediterranean and the Black Sea. Through an accurate review of published taxonomic studies, historical data on species occurrence, analyses of scientific survey data and biodiversity databases and other scientific papers, we produced a revised list of species whose presence in the Mediterranean Sea is confirmed or highly probable and discussed on current taxonomic and occurrence disputes on the species that are instead rarer or claimed to be locally extinct. We listed a total of 88 species, representing 30 families and 48 genera that are currently present in the Mediterranean and the Black Sea. This number includes 48 shark species, 38 batoids, and 2 chimaeras. The review represents a reference for future conservation assessments of cartilaginous fish in the region and a guide for decision-makers when promoting the sustainable exploitation of fisheries resource within an ecosystem-based framework. This paper can help to set a baseline of the Mediterranean species and thus resolve some uncertainties regarding their conservation status, explaining the reasons for their prolonged absence in the reports. Indeed, failure to record over time may not be due to grubbing up, but because after careful review this species was not really part of the Mediterranean fauna.
Introduction
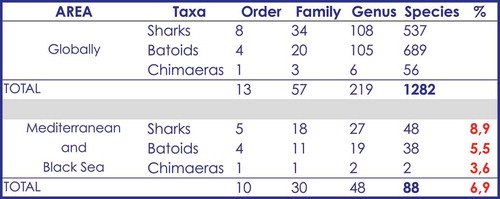
Sharks, batoids (rays and skates), and chimaeras are grouped in the class Chondrichthyes. 1,282 species of chondrichthyans are described globally. These include 1,226 of Elasmobranchii species (537 shark species belonging to 34 Families, and 689 batoid species belonging to 20 families), and 56 species of Holocephali belonging to 3 families (Ebert et al. Citation2013; Last et al. Citation2016a; Nelson et al. Citation2016; Scharpf & Lazara Citation2019; Roskov et al. Citation2020). Chondrichthyans are widely distributed in marine ecosystems, and sometimes they are also found in freshwater environments (Ebert et al. Citation2013). However, chondrichthyans’ classification is still unclear and debated for several species and genera (Compagno et al. Citation2005; Ebert & Stehmann Citation2013; UNEP/MAP SPA/RAC Citation2018). Taxonomic issues are even more severe in the Mediterranean Sea (Serena Citation2005; Ebert et al. Citation2013), where lack of nomenclature stability and taxonomic resolution frequently occurs (Iglésias Citation2014). In this region, Chondrichthyes diversity is still debated due to the ongoing changes in species occurrence and abundance in the different basins and sub-basins. Although the strong fishing pressure has led to local species depletion or even extinctions (Ferretti et al. Citation2008, Citation2013), the arrivals of new species through the Suez Canal, especially of Indo-Pacific origin, is increasing species richness in the eastern basin (Golani et al. Citation2002; Zenetos et al. Citation2010). While, the Mediterranean Sea is considered a Condrichthyes-rich basin, here there is the highest proportion of threatened species in the world (Dulvy et al. Citation2016); at least 53% of the species are classified by the IUCN as Vulnerable, Endangered and Critically Endangered (Dulvy et al. Citation2014; Otero et al. Citation2019). Quite a large proportion of species (20%) are still classified as Data Deficient.
Recognizing that elasmobranchs are by far the most endangered group of fishes in the Mediterranean Sea, the Contracting Parties to the Barcelona Convention, within the framework of the Action Plan for the Protection of the Marine Environment and the Sustainable Development of the Coastal Area of the Mediterranean (MAP Phase II), prioritized their protection entrusting SPA/RAC with the task of elaborating an action plan for the conservation of the chondrichthyan populations of the Mediterranean. This Action Plan was adopted in 2003 and updated in 2019 (UNEP/MAP SPA/RAC Citation2003, Citation2020) with the main objective being the facilitation and implementation of the UN FAO IPOA-Sharks (http://www.fao.org/ipoa-sharks/en/) by identifying the main priorities and actions to be undertaken at the national and international level to ensure that targeted and non-targeted fisheries are sustainable. Currently, 24 species listed in the Annex II (list of endangered or threatened species) of the SPA/BD Protocol are already protected in the Mediterranean Sea, meaning they cannot be trans-shipped, landed, transferred, stored, retained on board, sold or displayed or offered for sale, and must be released unharmed and alive to the extent if possible (GFCM Citation2018a).
Protecting cartilaginous fish diversity is part of the more comprehensive policy of the Convention on Biological Diversity (CBD 1992) which stems from the growing recognition that biological diversity is a world heritage for present and future generations across the world. Reducing the extinction risk for sharks, batoids and chimaeras assumes particular importance in an ecosystem-based approach of biodiversity conservation considering the key role these species play in marine communities. Many large pelagic sharks are apex predators whose overexploitation can affect the abundance of lower-level consumers (Myers et al. Citation2007). Overexploitation of shark populations can have negative consequences both on the ecosystem functioning and even the human activities relying on it (Ferretti et al. Citation2010; Britten et al. Citation2014).
Even though the Mediterranean is a semi-enclosed sea covering less than 1% of the surface of the global oceans, it constitutes a general richness hotspot of total species on a global scale (Tortonese Citation1989; Bianchi & Morri Citation2000; Coll et al. Citation2010; Valavanidis & Vlachogianni Citation2011; Derrick et al. Citation2020) as a result of the climatic events of the Quaternary, which acted as a sort of “biodiversity pump” (Bianchi & Morri Citation2000). During the Pleistocene, the Mediterranean Sea developed its current oceanographic features, showing a close affinity with the nearby Atlantic Ocean. This affinity is to be related to the fact that the Strait of Gibraltar never constituted a rigid boundary. For this reason, any biodiversity study should not strictly be limited to the Mediterranean area. Ekman (Citation1953) proposed two main biogeographical regions in the Atlantic (Lusitanic and the Mauritanian regions) close to the Mediterranean region; thus, according to this classical conception of biogeography, biodiversity studies should also take into account the area between the English Channel and Cape Verde. Furthermore, proximity to tropical regions favors the thermophilic Mediterranean fauna, which is further promoted by the current climate change through increasing water temperature (Coll et al. Citation2010; Azzurro et al. Citation2019). The present richness of cartilaginous and bony fishes is likely the result of the recolonization of the Mediterranean basin after the Messinian Salinity Crisis. As demonstrated for the great white shark (Leone et al. Citation2020), pulses of species immigrations occurred during the glacial and interglacial periods of the Quaternary, contributing to extinctions and speciations (Bianchi et al. Citation2012). Although the expansion of non-indigenous species with Indo-Pacific origin is growing in the Eastern Mediterranean, species distribution in the basin is mainly linked to the differences in environmental conditions across the Mediterranean Sea with species richness decreasing along a longitudinal gradient from west to east (Melendez et al. Citation2017).
A relevant decrease of sharks and batoids populations was observed during the last 50 years in different Mediterranean sectors (Aldebert Citation1997; Maynou et al. Citation2011; Guijarro et al. Citation2012; Ferretti et al. Citation2013; Barausse et al. Citation2014; Colloca et al. Citation2017a; Moro et al. Citation2019). This was likely as an effect of the increasing trend in fishing effort (Garcia Citation2011) and low population resilience to harvesting, observed also for the most common species (Quetglas et al. Citation2016).
According to official statistics on capture production, countries contributing more to the elasmobranch landings in the Mediterranean Sea are Turkey, Tunisia, Greece, Italy, Spain, Croatia, and Egypt. The most commonly caught species are skates (Rajidae) and catsharks (Scyliorhinus spp. and Galeus spp.) (Walker et al. Citation2005; Cashion et al. Citation2019). Different species of pelagic sharks, as well as eagle rays (Myliobatidae) and stingrays (Dasyatidae), are bycatch of pelagic and demersal fisheries (Megalofonou et al. Citation2000; Damalas & Megalofonou Citation2012; Echwikhi et al. Citation2013).
In the last 50 years, monitoring programs aimed at evaluating the status and distribution of Mediterranean demersal resources promoted the collection of information on chondrichthyan presence and abundance that are useful to detect anthropogenic impact and changes in marine communities (Quignard & Capapé Citation1971; Fredj & Maurin Citation1987; Aldebert Citation1997; Moranta et al. Citation1998; Bertrand et al. Citation2000; Megalofonou et al. Citation2000; Relini et al. Citation2000; Jukic-Peladic et al. Citation2001; Garofalo et al. Citation2003; Schembri et al. Citation2003; Fromentin & Farrugio Citation2005; Relini et al. Citation2010a; Serena et al. Citation2011; Maravelias et al. Citation2012; Tserpes et al. Citation2013; Follesa et al. Citation2019). However, there are still knowledge gaps for both species richness and abundance of sharks, batoids and chimaeras in the different sectors of the Mediterranean Sea. These gaps often make hard for international organizations such as FAO, UNEP, and IUCN to assess the conservation status of populations. Knowledge of chondrichthyan abundance and richness is also relevant to any future strategic plan for the conservation of marine biodiversity in the region and for this group, which has played a key ecological role in Mediterranean trophic webs for thousands of years (Menesini Citation1968; Bellocchio et al. Citation1991; Marsili Citation2007; Ramírez-Amaro et al. Citation2017).
The present study aims at increasing our understanding of the current status of the chondrichthyan species diversity in the Mediterranean Sea and at exploring possible drivers of change across different subregions, as well as at setting an important baseline for Mediterranean chondrichthyan diversity.
The Mediterranean area
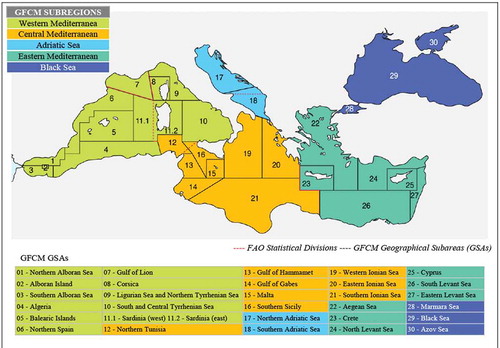
On a global scale, the Mediterranean Sea is placed at the center of a three-continent convergence point: Europe, Africa, and Asia. It connects to the Atlantic Ocean through the Strait of Gibraltar (maximum depth 1,092 meters and minimum depth 300 meters), while it is linked eastward to the Black Sea through the Turkish Straits System, composed by the Marmara Sea, the Strait of the Dardanelles (91 meters deep), and the Bosporus Strait (72.8 meters deep). The Black Sea communicates with the Sea of Azov via the Kerč Strait. The Azov Sea constitutes a small basin, with a maximum length from east to west of 1,150 km, and a maximum width of 600 km. The Black Sea encloses a total surface of 463,000 km2 and reaches a maximum depth of 2,300 meters (Murray Citation1991; Oguz et al. Citation1993). By contrast, the Mediterranean Sea spans over an overall area of nearly 2.5 million km2, reaching a maximum depth of 5,267 in front of the southern coast of Greece, in the Calypso Pit. Since the opening of the Suez Channel, in 1869, the Mediterranean Sea is also connected with the Red Sea (Hopkins Citation1985; Pinardi et al. Citation2006). The Strait of Sicily divides the Mediterranean Sea in a western and an eastern sub-basin. Details on these two sub-regional areas are provided by the General Fisheries Commission for the Mediterranean (GFCM), which also considers aspects linked to fisheries ().
Figure 1. GFCM subregions and geographical subareas from SOMFI 2018 (FAO Citation2018c). Reproduced with permission of FAO
The average salinity in the Mediterranean Sea is 36.2–39‰, with higher average values recorded in the eastern area (37.5–39.5‰). Annually, surface temperatures range between 11°C and 32°C, with some local differences. Large portions of the Mediterranean basin are classified as deep sea, even if they show specific features that differ from the nearby Atlantic waters, such as the high homeothermy detected from 300–500 meters depth to the bottom. The bottom temperature varies from 12.8°C–13.5°C in the western basin, and 13.5°C–15.5°C in the eastern side (Emig & Geistdoerfer Citation2005).
Due to hydrological and climatic characteristics, the Mediterranean average primary production is much lower than what is commonly observed in most oceanic areas (130–140 gC m−2 g−1) (Bosc et al. Citation2004). Exceptions are the Adriatic Sea, the Gulf of Lion, the Northern Aegean and the Alboran Sea, where this value ranges from 160 to 300 gC m−2 g−1 due to an extraordinary concentration of medium nutrients coming from the rivers’ run-off that enriches the coastal ecosystems (Agenzia Europea Dell’Ambiente (AEA) Citation2000; Bosc et al. Citation2004). Primary productivity in the Black Sea is also higher (140–150 g C m−2 g−1) (Demidov Citation2008) compared to other Mediterranean sectors since it is influenced by high productivity along the coasts of Ukraine, Romania, and Bulgaria (Shlyakhov & Daskalov Citation2008). Even if primary productivity in the Black Sea is higher than in the Mediterranean Sea, eutrophication affected marine ecosystems in both basins, with cross-border repercussions on biological diversity. Some fish stocks showed declines over the last 30 years as a consequence of high eutrophication (European Commission Citation2009).
Materials and methods
This study was developed following two lines of investigations. One was based on a careful bibliographic and museum search for occurrence records and preserved individuals indicating the presence of chondrichthyan species in the region (Mancusi et al. Citation2000; Nicolosi et al. Citation2019). The second line examined the taxonomic records of data collected by monitoring commercial fisheries and in scientific surveys carried out throughout the Mediterranean and Black Sea (Bertrand et al. Citation1997, Citation2000; GFCM Citation2018b). This allowed us to establish and investigate the diversity patterns in the different sub-regions of the Mediterranean and Black seas.
Data collection
Data collected primarily came from national and international scientific programs, targeted to evaluate fish resources. The most important of which was the European Union-Data Collection Framework (EU DCF), that includes both echo and trawl surveys (Ninni Citation1912; Mancini Citation1922; D’Ancona Citation1949; Aloncle Citation1972; Fredj & Maurin Citation1987; Gil De Sola Citation1994; Aldebert Citation1997; Bertrand et al. Citation2000; Megalofonou et al. Citation2000; Jukic-Peladic et al. Citation2001; Schembri et al. Citation2003; Fromentin & Farrugio Citation2005; Serena et al. Citation2005a, Citation2011, Citation2014; Relini et al. Citation2010a, Citation2010b). The MEDIterranean Trawl Survey (MEDITS) programme of the EU DCF (since 1994) covers on average about 543,000 km2, with just over 1,280 tows per year conducted according to the standardized protocol covering the European countries bordering the Mediterranean Sea (Albania, Croatia, Cyprus, France, Greece, Italy, Malta, Montenegro, Slovenia, and Spain) (Spedicato et al. Citation2019). Tows allocation in trawl surveys were done according to a random-stratified sampling scheme including five bathymetric strata: 10–50 m, 51–100 m, 101–200 m, 201–500 m, and 501–800 m (Relini et al. Citation2000; Bertrand et al. Citation2002; Massutí & Moranta Citation2003; Tserpes et al. Citation2013; Serena et al. Citation2014). Additional data came from samplings from landings of industrial and small-scale fisheries, in particular for what concern data from North African non-European countries (Tunisia, Egypt, Lebanon, Libya, Syria, Turkey, and Morocco), as well as from the Black Sea (GFCM Citation2018b). As opposed to the MEDITS surveys, most of the latter information came from research programs focused on resource evaluation of the relative studied areas. Nevertheless, such data have been very useful to provide important baselines of biological diversity in the investigated area (El Sayed Citation1994; Golani Citation1996; Öztürk Citation1999; Mejuto et al. Citation2002; Schembri et al. Citation2003; Shlyakhov & Daskalov Citation2008; Bariche & Fricke Citation2020). Final reports, scientific publications, and in many cases even raw data were used for the general analysis.
To explore spatial patterns of species distribution, all data were geographically organized to refer to the Subregions identified by GFCM (Western Mediterranean, Central Mediterranean, Eastern Mediterranean, Adriatic Sea, and the Black Sea) ( and ).
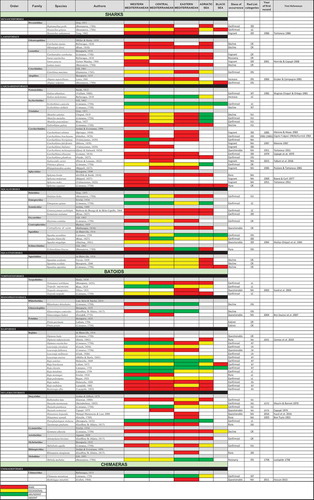
Figure 2. Faunistic list of the chondrichthyans of the Mediterranean and Black seas. When available the year of the first finding is shown. Colours correspond to the kind of occurrence in the subregions. The Red List categories is also considered for which species assessed. The status of occurrence suggests only the probability to meet the species and does not want represent the conservation status. Therefore, this evaluation is only the result of our observations made during the development of this synthesis study
Data analysis
To get an overall picture of a biodiversity status for the Mediterranean chondrichthyans, while dealing with the heterogeneity of available data and lack of information for some areas, we used a traffic light classification system (Caddy Citation1999, Citation2015). This approach was considered as the most appropriate for this preliminary and semi-quantitative assessment of the species present in the Mediterranean and Black seas. According to the collected information (e.g., number or biomass or presence/absence of specimens in the area), discrete levels of abundance (represented as traffic light colors) were assigned to the presence frequency of each species in each Mediterranean sector: red to indicate rare, yellow for occasional and green for abundant species. White was assigned to indicate the absence of the species in the considered area. For each species, an indication of their occurrence status such as confirmed or rare, or common, or questionable, or vagrant was also included (), where vagrant means occasional adult visitor, in agreement with Golani et al. (Citation2002) and in contrast with immigrant species that come to inhabit permanently in the Mediterranean area. lists the species that can be found in the Mediterranean and the Black Sea. Almost all of these species have been confirmed as valid for the Mediterranean basin. However, some of them remain questionable and need confirmations (FAO Citation2018a, Citation2018b; Otero et al. Citation2019). A few species have seen only once. Others seem to be present in the Mediterranean only occasionally. In this review, special attention was given to discuss the status of the dubious, rare, vagrant, declining, recovering, or extinct species in the Mediterranean. Species without taxonomic issues and showing a fairly assessed and stable occurrence status were not described in detail.
To better describe the chondrichthyan biodiversity of the Mediterranean and Black seas, a semi-quantitative biodiversity score was given to each sub-region. First, we assigned a score traffic to the light categories as follows: 0 for absent species, 0.33 for rare species, 0.66 for occasional species and, 1 for abundant species. Secondly, all values obtained by species in each Mediterranean sector were added up. Finally, to standardize this overall biodiversity score, we divided the summed values by the total surface (expressed as thousands square kilometers) covered by each region.
A chi-square test (α = 0.05) was performed to evaluate the independence between the region and the traffic light abundance index. Independence was tested for the entirety of chondrichthyan species as well as separate shark species and batoids. Given that only two species of chimaeras are represented in the Mediterranean Seas they were not tested as separate groups. Since the values of the chi-square tests for sharks and batoids were expected to be particularly low, p-values were computed using a Monte-Carlo simulation with 2000 replicates.
The whole set of FAO publications relevant for the region, such as Synopses, Identification Field Guides, and Identification Cards (Compagno Citation1984a, Citation1984b; Fisher et al. Citation1987; Bonfil & Abdallah Citation2003; Serena Citation2005; FAO Citation2007, Citation2009; Ebert et al. Citation2013), were used as main references for taxonomic issues. Moreover, other taxonomic references (Tortonese Citation1956; Bini Citation1967; Hureau & Monod Citation1979; Whitehead et al. Citation1984; Bouchot Citation1987; Lloris & Rucabado Citation1998; Compagno et al. Citation2005; Last et al. Citation2016a; Bariche & Fricke Citation2020, etc.) and scientific contributions (Ben–Tuvia Citation1971; Quignard & Capapé Citation1971; Aloncle Citation1972; Capapé Citation1989; El Sayed Citation1994; Ferguson Citation1994; Papaconstantinou et al. Citation1994; Moreno Citation1995; Aldebert Citation1997; Kabasakal Citation1998; Mate et al. Citation1999; Bertrand et al. Citation2000; Relini et al. Citation2000; Jukic-Peladic et al. Citation2001; Bradaï et al. Citation2002; Başusta et al. Citation2005; Compagno et al. Citation2005; Golani Citation2005; Hemida Citation2005; Saad et al. Citation2005; Serena Citation2005; Soldo & Peirce Citation2005; Capapé et al. Citation2006; Bradaï et al. Citation2012; etc.) ensured the technical support needed to confirm the validity of species collected from different sources.
The DNA barcoding library of the regional network for Mediterranean chondrichthyans was consulted to support the current identification keys and the reliable identification of specimens. The main reference for comparisons and confirmations of species identification was the “Barcoding Gap Analysis” tool on BOLD (Ratnasingham & Hebert Citation2007).
Regarding the scientific nomenclature, we followed the Online Database of Eschmeyer’s Catalog of Fishes, which is the authoritative reference for taxonomic fish names, together with World Register of Marine Species (WoRMS) and FishBase (Froese & Pauly Citation2019; Fricke 2020a; WoRMS Editorial Board Citation2020). However, the family-group names of chondrichthyans are ultimately regulated by the International Code of Zoological Nomenclature (Code) and published by the International Commission on Zoological Nomenclature (ICZN Citation1999) as the 4th edition of the Code (on 1 January 2000).
Results
A total of 88 chondrichthyan species, representing 30 families and 48 genera, were listed using the information from historical data, scientific campaigns, scientific papers, and observations at landing sites all around the Mediterranean Sea (). This list includes 48 species of sharks, belonging to 18 families and 27 genera, 38 species of batoids, belonging to 11 families and 19 genera and two chimaeras belonging to two different genera ().
Figure 3. Synoptic table showing the number of species belonging to each systematic group of chondrichthyans. The relationship between Mediterranean chondrichthyans and global one is compared in terms of percentage
Taxonomic account
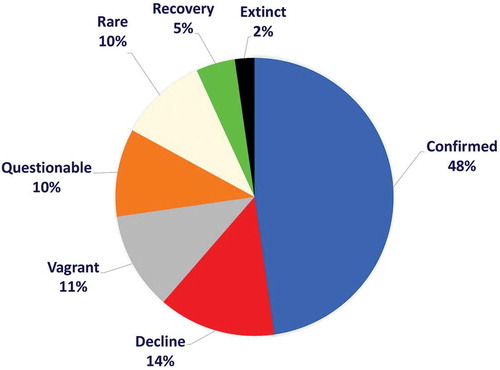
Approximately, 48% of these species are constantly recorded, while the remaining are characterized by 14% of species increasingly rare. About 20% of these species are rare or questionable, and 11% can be considered vagrant (10 species of which 4 are views once) (). Most of this 52% is composed of species that were not anymore recorded in recent years, in particular, the two species belonging to the genus Pristis Linck 1790 can be considered extinct in the Mediterranean basin. However, Mobula mobular (Bonnaterre, 1788) and Isurus oxyrinchus Rafinesque, 1810 show an apparent increase in the relative abundance that needs further investigations to be confirmed. More than half of the species listed in the Mediterranean basin require special consideration to clarify their current occurrence status in this area.
Figure 4. Percentage representation of the species occurrence according to a consideration linked to the analysis made in the text and which takes into account the date of first finding. The period between the first finding and subsequent records of these species highlights the peculiarities of the Mediterranean basin. To these presences specific codes have been assigned
The current inferred abundance, frequency of occurrence and geographical distribution of sharks, batoids and chimaeras in the Mediterranean and Black seas is described by family according to the ordination suggested by the Eschmeyer’s Catalog of Fishes and WoRMS (WoRMS Editorial Board Citation2020; Fricke et al. Citation2020b). Additional details are provided for the species whose presence in the area is still considered uncertain or rare. Whenever possible, the year of the first Mediterranean record for uncertain or rare species is reported, as well as the time elapsed without additional sightings ().
CLASS ELASMOBRANCHII
Cohort Selachii (Sharks)
Lateral gill slits, pectoral fin not attached to the side of the head. Tapered body.
Order Hexanchiformes – Cow sharks
Only one family in the Mediterranean and the Black Sea.
Family Hexanchidae Gray, 1851
Sharks with six or seven pairs of gill slits; single dorsal fin without spines; anal fin present. Eyes without a nictitating eyelid.
The Hexanchidae family is represented in the Mediterranean by two genera and three species. Heptranchias perlo (Bonnaterre, 1788) and Hexanchus griseus (Bonnaterre, 1788) are distributed in the entire Mediterranean Sea. H. griseus is also found in the Black Sea. Conversely, Hexanchus nakamurai Teng, 1962 is very rare and apparently differs from the congeneric only in the position of the dorsal fin with respect to the ventral and anal fins. Only three records are available to date. Indeed, Tortonese (Citation1986), also referring to Whitehead et al. (Citation1984), mentioned a specimen of H. nakamurai preserved at the Museum of Natural History of the University of Florence (Vanni Citation1992). More recently, in May 2001, another specimen was caught off the western coast of Crete Island (Damalas & Megalofonou Citation2012). Lastly, in 2017, an individual of H. nakamurai was accidentally caught by a set gillnet near Himara (South Albania), at a depth of 550 m. No genetic analyses were carried out to confirm the exact identity of the individual (Bakiu et al. Citation2018). H. nakamurai can surely be considered vagrant.
Order Lamniformes – Mackerel sharks
Four families in the Mediterranean and the Black Sea. Eyes without a nictitating eyelid.
Family Odontaspididae Müller & Henle, 1839
Sharks with trunk compressed-cylindrical and stout. Snout pointed and conical; gill openings moderately large, not extending onto the dorsal surface. Anal and ventral fins about as large as the dorsal fins or somewhat smaller.
The Odontaspididae family is represented in the Mediterranean by two genera and two species: Carcharias taurus Rafinesque, 1810 and Odontaspis ferox (Risso 1810). Both species seem to have declined in the Mediterranean and their sightings are very rare (Fergusson et al. Citation2002; Bargnesi et al. Citation2020).
Family Lamnidae Bonaparte, 1835
Sharks with gill openings large, just extending onto the dorsal surface, gill rakers absent. Two dorsal fins, caudal fin nearly symmetrical; strong lateral keel on the caudal peduncle.
The Lamnidae family is represented by three genera and four species: Carcharodon carcharias (Linnaeus, 1758), I. oxyrinchus, Isurus paucus Guitart Manday, 1966 and Lamna nasus (Bonnaterre, 1788). Despite being part of protection agreements of the main Conventions such as Barcelona, C. carcharias and L. nasus have shown clear signs of decline (Ferretti et al. Citation2008; Moro et al. Citation2019). Conversely I. oxyrinchus seems to be in a recovery phase (Serena & Silvestri Citation2018; Mancusi et al. Citation2020). For this reason, they require careful monitoring. In particular, Biscoito and Wirtz (Citation1994) and Moreno (Citation1995) reported the presence of I. paucus in the Mediterranean waters, later confirmed by Hemida and Capapé (Citation2008). Indeed, in 2001 two specimens of I. paucus were sold at the Algiers’ fish market. Whether I. paucus (commonly known as longfin mako shark) is a permanent resident of the Mediterranean Sea remains unclear. These recent records, and the historical accounts, are too sporadic and isolated to constitute sufficient proof that the species regularly occur off the Algerian coasts or in other parts of the Mediterranean Sea. Therefore, we consider this species vagrant.
Family Cetorhinidae Gill, 1861
Sharks with extremely long gill slits nearly encircling the head, extending onto the dorsal surface, internal gill openings with prominent gill rakers. Caudal fin nearly symmetrical; strong lateral keel on the caudal peduncle.
The Cetorhinidae family is represented globally by only one species: Cetorhinus maximus (Gunnerus, 1765). In the Mediterranean Sea, it occurs mainly in the western part, being a frequent component of the bycatch of artisanal fishery especially in the spring season (Serena et al. Citation2000; Mancusi et al. Citation2005, Citation2020). C. maximus is absent in the Black Sea; the species is declared Endangered by the IUCN Red List (Sims et al. Citation2016).
Family Alopiidae Bonaparte, 1835
Sharks with extremely elongated upper lobe of the caudal fin. No keels on the caudal peduncle.
The Alopidae family is represented in the Mediterranean by only one genus and two species. Alopias superciliosus Lowe, 1841 is a highly cosmopolitan, migratory, epi-mesopelagic species, occurring circumglobally in tropical and temperate seas. Hence, it is likely to hypothesize the entry of this species in the Mediterranean Sea from the Atlantic Ocean via the Strait of Gibraltar. The presence of this species in the Mediterranean Sea was unknown until the early 1980s (Tortonese 1937–1938; Hureau & Monod Citation1979; Gruber & Compagno Citation1981; Quèro Citation1984). Gruber and Compagno (Citation1981) mentioned a specimen captured in 1966 by a longline in the Ionian Sea, but they did not provide any further detail. Cigala Fulgosi (Citation1983a) cited four specimens captured in the Strait of Sicily (Italy), while Corsini-Foka and Sioulas (Citation2009) published two findings of bigeye thresher shark caught in 1952 and 1954 in the waters of the Dodecanese. These records, therefore, represent the first official reports of the presence of the species for the entire Mediterranean Sea. Furthermore, Golani (Citation1996) detected the species in Israeli waters. Megalofonou et al. (Citation2005) mentioned it for the Aegean Sea. More recently, this species was also reported in the Marmara Sea (Kabasakal & Karhan Citation2007). In recent years, records have considerably increased and various authors do not exclude that the species may have a stable population in the Mediterranean Sea, though this species is much rarer than the congener Alopias vulpinus (Bonnaterre, 1788), which occurs more frequently in the area (Zenetos et al. Citation2005, Citation2008; Clò et al. Citation2008; Sperone et al. Citation2018).
Order Carcharhiniformes - Ground sharks
Five families in the Mediterranean, one (Scyliorhinidae) also present in the Black Sea. Eyes with a nictitating eyelid.
Family Pentanchidae Smith, 1912
Sharks with dorsal fins similar and positioned in the posterior part of the body. Nictitating eyelids rudimentary. Supraorbital crests on the chondrocranium absent.
The Pentanchidae family, previously included as a subfamily in the Schyliorhinidae, is represented in the Mediterranean by two species of small deep-water sharks belonging to the genus Galeus Rafinesque, 1810. In 2005, Iglésias et al. proposed a new classification for the group, with the redefinition of the family Scyliorhinidae sensu stricto and the resurrection of the family Pentanchidae. The validity of this family is still questioned by some taxonomic authorities, and it is not recognized by certain organizations like the Integrated Taxonomic Information System (Roskov et al. Citation2020), which prefer to classify its genera in the Scyliorhinidae family. Finally, the Catalog of Fishes and WoRMS consider the Pentanchidae as a valid Family (Fricke 2020a; WoRMS Editorial Board Citation2020).
Regarding the genus Galeus, Muñoz-Chápuli and Ortega (Citation1985) described for the first time some important morphological differences to distinguish some specimens of Galeus melastomus Rafinesque, 1810 and reassigning them to the congener species Galeus atlanticus (Vaillant, 1888). Indeed, G. atlanticus, has been synonymous with G. melastomus for a long time until Muñoz-Chápuli and Ortega (Citation1985) disputed this status. Distinguishing the two species by using morphological features is somewhat problematic, if not impossible. The identification key used so far probably does not guarantee the exact specific determination since the morphological differences are very subjective. However, the subsequent genetic analysis confirmed the presence of G. atlanticus in the Mediterranean Sea, even though in a very restricted area corresponding to the Alboran Sea (Rey et al. Citation2010).
Family Scyliorhinidae Gill, 1862
Sharks with dorsal fins similar and positioned in the posterior part of the body. Nictitating eyelids rudimentary. Supraorbital crests on the chondrocranium present.
The Scyliorhinidae family is represented in the Mediterranean by only one genus and two species.
Scyliorhinus canicula (Linnaeus, 1758) is a common species in the Mediterranean and relatively abundant in various areas, while Scyliorhinus stellaris (Linnaeus, 1758), retaining more coastal habits on hard bottoms, have shown declines in abundance in different Mediterranean sectors (Abella et al. Citation2017d). In their recent study on the genus Scyliorhinus Blainville, 1816 Soares and De Carvalho (Citation2019) analyzed samples from the Adriatic and Algerian coasts and opted for the resurrection of the species Scyliorhinus duhamelii (Garman 1913), following the Garman’s assumptions based on differences in color pattern, the shape of anterior nasal flaps, and clasper morphology in comparison to S. canicula. Garman (Citation1913) distinguished S. duhamelii from S. canicula and S. stellaris simply by the position of the dorsal fins, which would be more posterior than the other two species.
S. duhamelii has never been detected during the several campaigns of the MEDITS trawl survey program (Bertrand et al. Citation2002) and in the ELASMOMED program (Cariani et al. Citation2017). Therefore, currently, this species is considered invalid for the Mediterranean Sea, in disagreement with the Catalog of Fish (Fricke et al. Citation2020b) and in agreement with both WoRMS and FishBase, not accepting S. duhamelli (Froese & Pauly Citation2019; WoRMS Editorial Board Citation2020). They consider Catulus duhamelii (Garman 1913) as junior synonym of S. canicula (Compagno Citation1984b). To clarify this issue, we suggest that more detailed studies, that include molecular taxonomy analysis are undertaken.
Family Triakidae Gray, 1851
Sharks with dorsal fins different in shape and positioned centrally on the body. The second dorsal fin is always slightly smaller.
The Triakidae family is represented in the Mediterranean by only two genera and four species. In particular, the genus Mustelus Linck, 1790 is poorly represented in the Western Mediterranean subregion, especially in the northern area. However, in the northern Adriatic Sea, there are frequent large catches of Mustelus mustelus (Linnaeus, 1758) and Mustelus punctulatus Risso, 1827, while Mustelus asterias (Cloquet, 1819) is to be considered rare (Marino et al. Citation2017). On the other hand, the species Galeorhinus galeus (Linnaeus, 1758) has now become extremely rare in all subregions. However, it is worth noting the re-capture of two adult females of G. galeus off the southern coast of Sicily in 2014 and 2017 respectively, which were tagged in Scotland and Ireland, showing unprecedented migratory routes for this species (Colloca et al. Citation2019).
Family Carcharhinidae Jordan & Evermann, 1896
Sharks with the dorsal fins positioned centrally on the body. The second dorsal fin always much smaller. Eyes round with nictitating eyelids well developed.
The Carcharhinidae family is represented in the Mediterranean by four genera and eleven species (). Requiem sharks are distributed in temperate and tropical seas and oceans, with several species widely distributed. Their habitats range from the open sea to coastal areas, but they are also found in inland waters, rivers, and freshwater lakes (e.g., Carcharhinus leucas [Valenciennes, 1839]). The genera belonging to the Carcharhinidae family are among the most difficult to identify, due to the very similar morphological features that they share, such as shape and livery. They also show overlapping distributions, which further complicate their identification easily solvable using the shark identification keys and field guides (Bigelow & Schroeder Citation1948; Springer Citation1950; Casey Citation1964; Schwartz & Burgess Citation1975; Compagno Citation1984b; Gàrrick Citation1985; Grace Citation2001; etc.). Today, we count 11 species belonging to the Carcharhinidae family likely to be present in the Mediterranean Sea. Some of these species are resident (e.g., Carcharhinus obscurus [Lesueur, 1818]; Carcharhinus plumbeus [Nardo, 1827]; Prionace glauca [Linnaeus, 1758]). While others occasionally enter in the Mediterranean Sea and can be considered vagrant, such as Galeocerdo cuvier (Péron & Lesueur, 1822) or Sphyrna mokarran (Rüppell, 1837). Finally, the presence of some species has to be confirmed (e.g., Carcharhinus melanopterus [Quoy & Gaimard, 1824]). Some details related to vagrant and dubious species are reported below.
Moreno and Hoyos (Citation1983) described the first capture of Carcharhinus altimus (Springer 1950) off the coasts of Morocco in the Alboran Sea and he considered this specimen as came in from the Strait of Gibraltar. The species was also mentioned in the Algerian waters (Hemida & Labidi Citation2001). Azab et al. (Citation2019) collected 14 specimens of Carcharhinus altimus (Springer 1950) at the Alexandria fish market. The fishes were caught in Egyptian waters but the authors did not include any detail on the utilized fishing gear. A single record is also reported in the Levantine waters (Golani Citation1996) and recently Ayas et al. (Citation2020) report a juvenile specimen of C. altimus for the Mersin Bay (Turkey). To conclude, C. altimus can be considered as a vagrant species for the Mediterranean Sea.
Carcharhinus brachyurus (Günther, 1870) is mainly distributed in temperate waters of the Pacific and Atlantic oceans and could be considered an Atlantic immigrant that probably reached the Levantine Sea. Gàrrick (Citation1982), in his revision of the genus Carcharhinus (Blainville, 1816) cites a female embryo of C. brachyurus preserved in the Museum of Natural History collection in Vienna. This specimen, caught in 1889 in front of the coasts of Nice (France), represents the first mention of the species in the Mediterranean Sea. In the same period (1862–1892), Döderlein deposited two dry jaws of C. brachyurus in the collections of the Zoological Museum of the University of Palermo (Italy). These specimens were caught in the Sicilian waters and probably represent the first historical records of the species in the Mediterranean Sea (Psomadakis et al. Citation2009). Cigala Fulgosi (Citation1983b), described a female landed at the Mazara del Vallo fish market, that was caught in the area of the Strait of Sicily in 1981. Later on, other specimens were reported, especially in the north-western area of the Mediterranean Sea (e.g., Vacchi et al. Citation1996; Orsi Relini Citation1998; Zava et al. Citation2006). Recently, Azab et al. (Citation2019) collected one specimen of C. brachyurus at the Alexandria (Egypt) fish market and its presence was also confirmed in the Turkish waters (Kabasakal & Bilecenoğlu Citation2020). The presence of C. brachyurus in the Mediterranean Sea has been confirmed even before historical times: referring to fossil teeth. Marsili (Citation2007) cites the observations of Menesini (Citation1968) and Bellocchio et al. (Citation1991) of C. brachyurus teeth collected in Miocene and Pliocene deposits.
Carcharhinus falciformis (Bibron, 1839) is an abundant species in the Eastern Atlantic, but it was reported only sporadically in the Alboran Sea, as well as along the Algerian and southern Spanish coasts (Moreno Citation1987; Barrul & Mate Citation2002; Hemida et al. Citation2002). Recently, other records of this species were reported: one from the Ligurian Sea (Garibaldi & Orsi-Relini Citation2012), two specimens from the Egyptian waters (Azab et al. Citation2019), and one from Turkey (Kabasakal & Bilecenoğlu Citation2020). C. falciformis can be considered a vagrant species in the Mediterranean Sea. Fossil teeth of this species were found in some Italian Miocene and Pleistocene deposits (Menesini Citation1968; Bellocchio et al. Citation1991).
Carcharhinus limbatus (Valenciennes, 1839) has a few records in the Mediterranean Sea and some authors consider its occurrence as occasional (Cadenat & Blache Citation1981). It is reported to occur in the whole Mediterranean basin except in the Adriatic (Serena Citation2005) as well south-eastern sectors all along the North African coasts, Levantine basin until the Turkish coasts (Branstetter Citation1984; Bi̇lecenoğlu et al. Citation2002). Fergusson (Citation1994) reported the species as “fairly common”. In the western basin, the blacktip shark has been sporadically recorded. Capapé et al. (Citation2004) cited only one individual from the Algerian coasts and Morey et al. (Citation2008) another specimen from the Balearic Islands (Spain). In the central Mediterranean Sea C. limbatus seems to be more frequent (Capapé Citation1975; Quignard & Ben Othman Citation1978; Capapé et al. Citation2004). Ben-Tuvia (Citation1953) and Fredj and Maurin (Citation1987) considered, instead, the blacktip shark mainly associated with the eastern Mediterranean basin, although Golani (Citation2006) listed the species as rare off Israel. We believe that further investigations are warranted for this species.
Carcharhinus melanopterus (Quoy & Gaimard, 1824) is a species with an Indo-Pacific origin, likely entering the Mediterranean Sea via the Suez Canal. If this species is confirmed in the Mediterranean Sea, its presence is likely limited to the Levantine basin. Compagno (Citation1984b, Citation2005) considered the species as valid for the Levantine basin and incurring from the Red Sea. This observation was possibly based on Tortonese (Citation1951), who reported the species as present along the coasts of Egypt. However, no specimens are preserved for any comparisons (Golani et al. Citation2002). Ben-Tuvia (Citation1966) expressed a doubt by stating that the main ecological features of this species are also found in endemic Mediterranean species, such as Carcharhinus brevipinna (Valenciennes, 1839). Serena (Citation2005) considered the presence of this species in the Mediterranean basin doubtful and we think that further investigations are needed to clarify the situation.
Carcharhinus obscurus (Lesueur, 1818) was not part of the Mediterranean species list compiled by Gàrrick (Citation1982) and Tortonese (Citation1956). Tortonese eventually confirmed its presence in Sicilian waters (Tortonese Citation1987). The first finding of C. obscurus in the Mediterranean Sea was published by Capapé et al. (Citation1979). The specimens observed by these authors was a female caught along the Tunisian coasts. It exhibited 92 precaudal vertebrae, which constitutes the main diagnostic feature useful to distinguish C. obscurus from Carcharhinus galapagensis (Snodgrass & Heller, 1905).
Tobuni et al. (Citation2016) described the first well-documented occurrence of G. cuvier in the southern Mediterranean Sea. Although this record cannot suggest the stable occurrence of the species in the area, it can confirm its sporadic occurrence, supporting also some previous records considered doubtful: one from Malaga (Spain) (Pinto de la Rosa Citation1994), and the second from Sicily (Italy) (Celona Citation2000). The identification of these specimens was based only on the jaws description with no documented evidence on their origin. Moreover, both publications did not provide any photographic documentation of the entire individual.
In 2001, a specimen of Rhizoprionodon acutus (Rüppell, 1837) was caught off the western coasts of Crete Island (Greece) (Damalas & Megalofonou Citation2012). This represented the second record for the species in the Mediterranean Sea. The first was cited by Pastore & Tortonese (Citation1985) off the Calabrian coasts of the Ionian Sea. Two other specimens of this species have recently been caught incidentally by artisanal fisheries in Tunisian and Albanian waters (Ben Amor et al. Citation2016; Kousteni et al. Citation2019). A fifth specimen was probably captured in the waters of Cyprus in 2019 (Giovos pers. comm.). This species is to be considered vagrant.
Other species such as C. leucas and Carcharhinus longimanus (Poey, 1861) cannot be included in the Mediterranean fauna, though they were found as fossils in the Miocene and Pleistocene deposits of the Italian, Maltese and Spanish continental territories (Menesini Citation1968, Citation1974; Cigala Fulgosi Citation1986; Bellocchio et al. Citation1991; Cappetta & Nolf Citation1991). There are no confirmed reports of living individuals in the Mediterranean Sea.
Carcharhinus amboinensis (Müller & Henle, 1839) has only one record in the Mediterranean Sea and at present cannot be considered valid species of the Mediterranean Sea. This species was reported by De Maddalena and Della Rovere (Citation2005) based on the description of jaws belonging to a specimen caught as bycatch in 2003 from commercial fishery off Crotone (Italy). Unfortunately, the authors did not provide detailed information on some key morphological features of the jaws that are useful for species identification and there is not track of the collected specimen. For these reasons, we consider the presence of this species, at the moment, anecdotic in agreement with Ebert et al. (Citation2013). However, we cannot reject the possibility that individuals may incur in the Mediterranean Sea from the Red Sea. Indeed, Spaet et al. (Citation2011), described a single specimen of C. amboinensis caught in 2010 in the Al-Qunfudah waters (Saudi Arabia).
Finally, Carcharhinus acarenatus Moreno & Hoyos 1983 is now considered as a valid species, as reported by Almeida and Biscoito (Citation2019) and in the Eschmeyer’s Catalog of Fishes (Fricke et al. Citation2020b). However, this species is considered as a junior synonym of C. brachyurus by Compagno (Citation1984b) and Froese and Pauly (Citation2020a) and invalid by Ebert et al. (Citation2013). Therefore, it is currently considered doubtful and invalid for the Mediterranean.
Family Sphyrnidae Bonaparte, 1840
Sharks with the dorsal fins positioned centrally on the body. The second dorsal fin always much smaller than the first. Eyes round with nictitating eyelids well developed. Peculiar head laterally expanded.
The Sphyrnidae family is represented in the Mediterranean by one genus and, probably, four species.
S. mokarran and Sphyrna tudes (Valenciennes 1822) were reported only once in the Mediterranean Sea. S. mokarran was recorded by Boero and Carli (Citation1977), who described a specimen caught in the “tonnarella” (tuna trap) of Camogli (Italy) in 1969.
S. tudes was described by Valenciennes (Citation1822) in the Mediterranean waters and later was reclassified as Sphyrna couardi (Cadenac 1951) by Cadenat and Blache (Citation1981) and Compagno (Citation1984b). Eventually, Compagno et al. (Citation2005) reconsider S. couardi as a non-valid species. The specimen described by Valenciennes (Citation1822) was captured in Nice and preserved at the Natural History Museum in Paris. This description was accepted McEachran and Sèret (Citation1987) who recognized the possible occasional presence of S. tudes in the Mediterranean Sea. Yet, Compagno (Citation1984b) and Compagno et al. (Citation2005) did not confirm the presence of this species in the eastern Atlantic and the Mediterranean Sea. Ebert et al. (Citation2013) suggested that S. tudes occurred only along the South American Atlantic coasts. However, Tortonese (Citation1951) described an additional S. tudes specimen, which was confirmed by Serena (Citation2005) who observed the specimen preserved at the Calci Museum of Natural History (University of Pisa, Italy), with catalog number 2347. This second specimen was bought by Tortonese at the Livorno (Italy) fish market; hence, we cannot establish with complete certainty if the individual was caught within Mediterranean waters. In conclusion, S. tudes remains a doubtful inhabitant of the Mediterranean and further investigations need to be undertaken.
Vasil’eva (Citation2007) cites the hammerhead species Sphyrna zygaena (Linnaeus, 1758) as present in the Romanian coasts of the Black Sea. However, this report was not documented and, certainly, its casual presence in the Black Sea must be confirmed. Regarding the Mediterranean Sea, there is no debate on the occurrence of this species in the region (Tortonese Citation1987, Bello Citation1999; De la Serna et al. Citation2002; Di Natale et al. Citation2005) even though its population has experienced steep declines under the impact of fishing (Ferretti et al. Citation2008).
Order Squaliformes - Dogfish sharks
Seven families in the Mediterranean Sea, one of which (Squalidae) also in the Black Sea.
Family Dalatiidae Gray, 1851
Sharks with two dorsal fins without spines, pectoral fin with characteristic rounded edge.
The Dalatiidae family is represented in the Mediterranean by only one species Dalatias licha (Bonnaterre 1788). It is a demersal species living up to 1,000 m of depth. More frequent in western than eastern Mediterranean but never abundant, absent in the north Adriatic and Black seas (Bertrand et al. Citation2000; Baino et al. Citation2001).
Family Etmopteridae Fowler, 1934
Sharks with two small dorsal fins with spines, pectoral fin with rounded edge.
The Etmopteridae family is represented in the Mediterranean by only one species, Etmopterus spinax (Linnaeus, 1758). This is a demersal species living up to 1,000 m of depth. More frequent in the western than eastern Mediterranean but relatively abundant, absent in the north Adriatic and Black seas (Bertrand et al. Citation2000; Baino et al. Citation2001; Serena et al. Citation2005b).
Family Somniosidae Jordan, 1888
Sharks with two dorsal fins with or without very small spines, inner edge broadly rounded.The Somniosidae family is represented in the Mediterranean by two genera and two species:Centroscymnus coelolepis Barbosa du Bocage & de Brito Capello, 1864 and Somniosus rostratus (Risso, 1827). Both species are benthic living up to 1,000 and 2,700 m of depth respectively. They are present mainly in the western Mediterranean however not abundant. Rare in the eastern sector and absent in the north Adriatic and Black seas (Cigala Fulgosi & Gandolfi Citation1983; Bertrand et al. Citation2000; Baino et al. Citation2001; Barrull & Mate Citation2001; Clò et al. Citation2002; Serena et al. Citation2005a).
Family Oxynotidae Gill, 1863
Sharks with two high sail-shaped dorsal fins with spines.
The Oxynotidae family is represented in the Mediterranean by only one species. Oxynotus centrina (Linnaeus, 1758) is common throughout the entire Mediterranean, although not abundant, absent in the Black Sea (Bertrand et al. Citation2000; Baino et al. Citation2001).
Family Centrophoridae Bleeker, 1859
Sharks with two dorsal fins different in shape with spines with lateral grooves.
The Centrophoridae family is represented in the Mediterranean by one genus and probably only one species.The taxonomy of the genus Centrophorus Müller & Henle, 1837 has not been settled, and some species are still under investigation (White et al. Citation2013, Citation2017a). Also, the validity of Centrophorus granulosus (Bloch & Schneider, 1801) vs. Centrophorus uyato (Rafinesque, 1810) is still debated among taxonomists. Some researchers stated that the Mediterranean species of Centrophorus should be named correctly (and definitively) as C. uyato (Bonaparte 1834), while others proposed a new description of the species and to establish a neotype for C. uyato (Guallart pers. comm.). Recently, molecular approaches, in agreement with the studies of Verissimo et al. (Citation2014), demonstrated the presence of a unique mitochondrial clade within the Mediterranean Sea (Benvenuto Citation2019). Such results, confirmed by morphometric analyses, suggest that a unique morphological and distinct group characterize all the Mediterranean Centrophorus sp. and indicate the occurrence of a single Centrophorus species (probably C. uyato) in the Mediterranean Sea. In this sense, a revision of the genus taxonomy in the area is needed (Cariani et al. Citation2017). Based on White et al. (Citation2013), we consider Centrophorus cf. uyato as the valid species in the Mediterranean Sea.
Family Squalidae de Blainville, 1816
Sharks with two dorsal fins different in shape with long spines without grooves.
The Squalidae family is represented in the Mediterranean and Black seas by the only genus Squalus Linnaeus, 1758 and, probably, three species: Squalus acanthias Linnaeus, 1758; Squalus blainville (Risso, 1827) and Squalus megalops (Macleay, 1881). Several scientific studies such as MEDITS trawl survey contributed to clarifying the real presence of these species in the Mediterranean Sea. S. acanthias and S. blainville are mostly found in the northern part of the Mediterranean Sea, and the Adriatic including the Black Sea (Bat et al. Citation2005; Serena et al. Citation2009; Bonello et al. Citation2015; Giarruso Citation2019). S. megalops seems to be present mainly along the African coasts of Tunisia (Marouani et al. Citation2012). Some taxonomic and nomenclatural problems affect the group of the species in question. Excluding S. acanthias, easily recognizable thanks to its specific pattern, characterized by small white spots on the body, the correct identification of the other two species requires the observations of the dermal denticles, meristic features or even genetic analysis. Certain authors use incorrectly the denomination Squalus blainvillei (Risso, 1827) (Marouani et al. Citation2012; Viana et al. Citation2016), but the name must be considered senior synonym of S. blainville, as indicated by Froese and Pauly (Citation2019), Fricke (Citation2020a) and WoRMS Editorial Board (Citation2020).
Regarding S. megalops, described for the first time by Muñoz-Chápuli et al. (Citation1984), its occurrence in the Mediterranean is still debated and needs to be confirmed (Muñoz-Chápuli & Ramos Citation1989; Marouani et al. Citation2012; Bonello et al. Citation2015; Kousteni et al. Citation2016; Verissimo et al. Citation2016; Vella et al. Citation2017; Bellodi et al. Citation2018; Giarruso Citation2019). In any case, specimens of S. megalops for which the identification is considered feasible were rarely reported in the catches (Bonello et al. Citation2015; Bellodi et al. Citation2018). Most of the catches of this species are recorded from the northern coasts of Canary Islands, Morocco and southern Spain (Malaga). Furthermore, the first records of this species in the northern Atlantic and the Mediterranean Sea refer to another scientific name, that successively entered into synonymy: Squalus acutipinnis Regan, 1908. The firsts records of the species in the African equatorial zone are reported by Poll (Citation1951), and in the south African area by Krefft (Citation1968). However, the real presence of S. megalops is still unclear not only for the Mediterranean Sea but also for the neighboring Atlantic area (Last et al. Citation2007; Verissimo et al. Citation2016) and some evidences confirm the inconsistency of the species identification keys to distinguish between the Atlantic and Mediterranean Squalus, concerning S. blainville and S. megalops (Verissimo et al. Citation2016). Muñoz-Chápuli et al. (Citation1984) stated that, once solved these taxonomic issues, the species would probably be listed as present also in Tunisian, Libyan, and Italian waters. Marouani et al. (Citation2012) confirmed the presence of this species in the Gulf of Gabés (Tunisia). In conclusion, a taxonomic uncertainty still exists for the genus Squalus. This means that more studies are required to confirm the validity of the different morphological features of these sharks.
Family Echinorhinidae Gill, 1862
Sharks with the body covered with irregularly dermal denticles on the skin that form flat shields, varying in diameter, with large finely ridged spines. Two dorsal fins with apex rounded are located in the rear part of the body over pelvic fins.
The Echinorhinidae family is represented in the Mediterranean Sea (absent in the Black Sea) by only one species: Echinorhinus brucus (Bonnaterre, 1788). This species is very rare in the area.
Order Squatiniformes - Angel sharks
One family in the Mediterranean Sea included the Black Sea
Family Squatinidae de Blainville, 1816
Sharks with a dorso-ventrally depressed body, pectoral fins greatly expanded along sides of the head as a free triangular lobe; no anal fin.
The Squatinidae family is represented in the Mediterranean by the genus Squatina Duméril, 1805 with three different species: Squatina aculeata Cuvier, 1829; Squatina oculata Bonaparte, 1840; Squatina squatina (Linnaeus, 1758). They are all Critically Endangered in the Mediterranean Sea according to IUCN Red List criteria (Ferretti et al. Citation2016a, Citation2016b; Soldo & Bariche Citation2016). No specimens of Squatina were recorded in some Mediterranean areas for many years, such as in the North-Western Mediterranean Sea (Lawson et al. Citation2019) except the seven specimens found in Corsica Island (Lapinsky & Giovos Citation2019). In the Black Sea, only one species of angel shark (S. squatina) is present, with few sporadic reports. The last record from the area is a specimen found at the Istanbul fish market in 2005 (Pers. obs.). Previously, Kabasakal (Citation2002) reported an incidental capture of S. squatina in the waters in front of the city of Şile (Turkey), in the southwestern Black Sea. Since the 1960s, this species was officially quoted in the waters of the Black Sea (Geldiay Citation1969; Whitehead et al. Citation1984; Mater & Meriç Citation1996; Golani Citation1996; Öztürk Citation1999; Bi̇lecenoğlu et al. Citation2002; Fricke et al. Citation2007; Keskin Citation2010; Bi̇lecenoğlu et al. Citation2014; Yankova et al. Citation2014).
In order to preserve the angel shark populations several international and national initiatives have been activated (Lauria et al. Citation2015; Lawson et al. Citation2019). Currently, a study coordinated by Shark Trust is in progress to promote an Action Plan for the conservation of the angel sharks in the Mediterranean, including the Black Sea area (Gordon et al. Citation2019).
Cohort Batoidea (Rays)
Ventral gill slits, pectoral fin attached to the side of the head. Flattened body
Order Torpediniformes - Electric rays
One family in the Mediterranean Sea absent in the Black Sea
Family Torpedinidae Henle, 1834
Batoids with pectoral fins greatly enlarged, forming a large oval disc. Large electric organ on each side of the head.
The Torpedinidae family is represented in the Mediterranean by two genera and probably four species. Saad et al. (Citation2005) surveyed along the Syrian coasts, reported the first finding of Torpedo sinuspersici Olfers, 1831, a Lessepsian migrant. However, the authors neither collected any morpho-biometric information nor added any precise indications about the sighting location. Moreover, to date, no genetic analyses have been carried out to confirm the species identification, which is very difficult to distinguish on a morphometric basis with the congener Torpedo marmorata Risso 1810.
With exception of Torpedo torpedo (Linnaeus, 1758), which have a very characteristic dorsal side pattern, the genus Torpedo Duméril, 1805 has identification issues in general. The other genus, Tetronarce Gill, 1862, represented by a single species, Tetronarce nobiliana (Bonaparte, 1835), always shows a dark blue pattern that is almost black, easy to identify.
As mentioned above, genetic analysis can provide valuable help in solving many identification problems, although it may be insufficient. Some species in certain cases, such as Torpedo alexandrinsis Mazhar, 1987 and Torpedo fuscomaculata Peters, 1855 are still erroneously reported in the Mediterranean Sea (Quignard & Tomasini Citation2000; Psomadakis et al. Citation2009; Muammer Oral Citation2010; Zenetos et al. Citation2010; Almeida & Biscoito Citation2019; Froese & Pauly Citation2020b). In particular, the description of T. alexandrinsis is based on five specimens found in Alexandria (Egypt) but the types are not available. Therefore, Serena (Citation2005) and Weigmann (Citation2016) later considered T. alexandrinsis an invalid species for the Mediterranean Sea since its presence was based on anecdotal information. Subsequently, de Carvalho et al. (Citation2016) excluded it from the world’s fish fauna. More recently, Fricke (2020a) considered this species valid for the Red Sea, hypothesizing also an endemism in the northern part of this sea supported by DiBattista et al. (Citation2015), even though Bonfil and Abdallah (Citation2003) did not list it among the chondrichthyans of the Red Sea. T. fuscomaculata was listed today in the Southeast Atlantic and Western Indian Ocean from South Africa to Tanzania, and off Madagascar, Mauritius, and Seychelles (Last et al. Citation2016a) and later confirmed by Fricke (2020a). Though, Muammer Oral (Citation2010) cited it for the Turkish waters of the Eastern Mediterranean. Despite T. fuscomaculata being mentioned in the Lessepsian immigrant group by Quignard and Tomasini (Citation2000), based on one record collected in Alexandria (Egypt) (Mazhar Citation1982), its validity for the Mediterranean remains questionable. Serena (Citation2005) suggested that its presence in the Mediterranean was probably linked to a misidentification of T. marmorata due to a very similar pattern of the dorsal side. No further evidence of these species in the Mediterranean has been published nor have genetic analyses been carried out on the specimen previously collected. Therefore, we do not consider T. fuscomaculata a valid species for the Mediterranean Sea.
Order Rhinopristiformes - Guitarfhishes/Sawfishes
Three families in the Mediterranean Sea, absent in the Black Sea
Family Rhinobatidae Last, Séret & Naylor, 2016
Batoids with pectoral fins enlarged, and a strongly depressed trunk. Snout elongate and pointed.
The Rhinobatidae family is represented in the Mediterranean by only one species: Rhinobatos rhinobatos (Linnaeus, 1758) Historically common throughout the northern Mediterranean Sea, this species has gone through substantial declines in population abundance of range contractions. Its conservation status in the Mediterranean is considered Endangered by the IUCN Red List and today the species is likely still present in the least exploited parts of the southern and eastern Mediterranean Sea (Bradai & Soldo Citation2016).
Family Glaucostegidae Bonaparte, 1835
Batoids with pectoral fins enlarged and strongly depressed trunk; the position of the dorsal fins is more anterior compared to those of R. rhinobatos. Snout elongate and pointed.
The Glaucostegidae family is represented in the Mediterranean by one genus and probably two species.
Glaucostegus halavi (Forsskål, 1775), was identified for the first time by Vinciguerra (Citation1884) in the Gulf of Tunis. However, Quignard and Capapé (Citation1971), on the base of morphological features, modified the determination in Rhinobatos cemiculus (Geoffroy St. Hilaire, 1817). Tortonese (Citation1951) recorded the presence of G. halavi along the Egyptian coasts, but no specimens were described or preserved and, consequently, their presence was not confirmed (Ben-Tuvia Citation1966). In 2004, a female of G. halavi was caught by a longline 16 nm off Zarzis, in the Gulf of Gabes, and the capture was reported by Ben Souissi et al. (Citation2007). Nevertheless, this record seems to be doubtful and might require a reconsideration of the specimen classification (Bradaï et al. Citation2012). Hence, the presence of this species in the Mediterranean still needs confirmation. By contrast, Glaucostegus cemiculus (Geoffroy St. Hilaire, 1817) is certainly a Mediterranean species, which has now disappeared from the northern areas probably due to the strong fishing effort (Colloca et al. Citation2020).
Family Pristidae Bonaparte, 1835
Batoids with a strongly depressed trunk and snout elongate and pointed with a long rostrum having 15–32 teeth.
The Pristidae family is represented in the Mediterranean by one genus and two species: Pristis pectinata Latham, 1794 and Pristis pristis (Linnaeus, 1758) The presence of stable populations for these two sawfishes in the Mediterranean Sea has been debated for decades (Tortonese Citation1956; Whitehead et al. Citation1984; Bi̇lecenoğlu et al. Citation2002; Leone et al., Citation2014). Both species have alternatively been included in regional faunal lists of the Mediterranean Sea (Tortonese Citation1956; Whitehead et al. Citation1984) or cited as dubious/questionable for the area (Tortonese Citation1987; Serena Citation2005). Last et al. (Citation2016a) considered the species P. pectinata part of the Mediterranean fauna even if they stated that the population is now fragmented, and the species have to be considered locally extinct over almost its entire original range. Regarding P. pristis, the authors did not include the species in the Mediterranean faunal list. Recently, Faria et al. (Citation2013) suggested that sawfishes have never been resident populations in the Mediterranean basin. Similarly, Harrison and Dulvy (Citation2014) stated that the Mediterranean Sea has never been included in the sawfishes’ historical distribution boundaries proposing that the few occurrences reported in the literature have to be considered vagrant specimens coming from outside the Mediterranean Sea.
These disputes were eventually reviewed by Ferretti et al. (Citation2015) who also carried out further bibliographic and archival/museum investigations. This study reported that between 1576 and 1959, there have been numerous accounts documenting the occurrence of both sawfish species (P. pristis and P. pectinata) in the Mediterranean Sea, including 24 catches also pertaining to juvenile specimens. Extinction models on these historical records revealed that the species became likely extinct in the Mediterranean Sea between the 1960s-70s (Ferretti et al. Citation2015).
Order Rajiformes - Skates and rays
Only one family in the Mediterranean and the Black Sea
Family Rajidae de Blainville, 1816
Batoids with a depressed body almost circular to rhombic disc. Tail well demarcated from the disc.
The Rajidae family is represented in the Mediterranean by four genera and sixteen species, some of which are questionable (). Dipturus batis (Linnaeus, 1758) shows evident phenotypic differences among the individuals, indicating that a careful re-examination of its taxonomy is required. In the past, two distinct species were gathered under the single scientific name D. batis. Today more careful studies tend to separate the common skate D. batis species-complex into two nominal species, the blue skate (temporarily called Dipturus cf. flossada [Risso, 1826]) and the flapper skate (Dipturus cf. intermedia [Parnell, 1837]) (Iglésias et al. Citation2010). More recently, Last et al. (Citation2016a) recognized D. batis, also referred to as Dipturus sp. cf. flossada, as a valid species. This species was previously confused with the larger sympatric flapper skate, D. intermedius, now considered as a species apart (Last et al. Citation2016b). Although these authors included the Mediterranean Sea in the distribution range of both species, they did not give any indication about their spatio-temporal distribution. Looking at previous classification attempts, Tortonese (Citation1956) provided a very good description of the species based on juvenile specimens from museum collections, but, at the same time, he stated there were not enough elements to distinguish the Mediterranean individuals from the Atlantic ones. In this sense, he indicated a single taxonomic entity: Raja batis (Linnaeus, 1758), calling the Mediterranean individuals Raja macrorhynchus Rafinesque, 1810, which was later abandoned. Döderlein (Citation1879) considered the presence of R. batis as uncertain in the Mediterranean Sea. Similarly, Clark (Citation1926) and Norman (Citation1935) expressed doubts on the existence of R. batis in the Mediterranean Sea, in contrast with Risso (Citation1810), who firstly described R. batis from specimens caught in the area. Last et al. (Citation2016a) did not consider D. batis as a complex species and they did not include it among the Mediterranean skates. Given this long history of debates, more effort in clarifying the current status and taxonomy of this species is necessary. It is important, not only to investigate whether the species is currently present in the area but also to verify the documentation about the specimens recorded in the past. To this end, a review of the museum collections in the Mediterranean area could provide useful and definitive information on the real occurrence of the species in these waters (Nicolosi et al. Citation2019).
Regarding Dipturus oxyrinchus (Linnaeus 1758) Iglésias (mentioned in Ebert and Stehmann [Citation2013]) indicated that this species could appear to be a composite species as well, just like D. batis, namely one smaller form occurring in the North-Eastern Atlantic and the Mediterranean Sea, and a larger form only found in the North-Eastern Atlantic. A recent publication by Griffiths et al. (Citation2011) suggests that the Mediterranean stock may be genetically isolated from stocks outside the Mediterranean Sea. The reasons for such stock differentiation should be based on differences in maximum size and egg capsule size.
Dipturus nidarosiensis (Storm, 1881) was known as an endemic species of the North-Eastern Atlantic Ocean, even though it was rarely caught (Stehmann & Bürkel Citation1984; Williams et al. Citation2008). However, its distribution range seems to be enlarged. Indeed, in recent times, some individuals linked to this species have also been caught in the Mediterranean Sea during research expeditions carried out between 2005 and 2008 (Cannas et al. Citation2010; Follesa et al. Citation2012; Ramírez-Amaro et al. Citation2017; Carbonara et al. Citation2019; Geraci et al. Citation2019). Although Cannas et al. (Citation2010) and Carbonara et al. (Citation2019) confirmed the species identification by genetics, Ebert and Stehmann (Citation2013) suggested that individuals caught in the Mediterranean might belong to a smaller morphotype of Dipturus sp. not described yet. Despite the presence of this species in the Mediterranean has been confirmed, it is necessary to deepen the rather complex structure of the genus Dipturus (Rafinesque, 1810) and its several composite species distributed in the North-Eastern Atlantic Ocean and the Mediterranean Sea.
Both Whitehead et al. (Citation1984) and Last et al. (Citation2016a) mentioned Leucoraja fullonica (Linnaeus, 1758) as present in the North-Eastern Atlantic coasts, from Madeira and Morocco, having a wide northward distribution, reaching the Southern Iceland, Norway, and the northern North Sea and Skagerrak. They also included the species in the Mediterranean Sea, especially in its western areas. Previously, Tortonese (Citation1956) had reported the presence of L. fullonica in the Mediterranean Sea, asserting its rarity in the Adriatic Sea and indicating, oddly for Last et al. (Citation2016a), the Suez Canal as the way of entrance. Indeed, the genus Leucoraja (Malm, 1877) has never been recorded in the Red Sea (Bonfil & Abdallah Citation2003). However, this species is difficult to find in professional fisheries catches, especially for trawlers. Since the start of the Barcoding program in 2005, this species has never been recorded (Cariani et al. Citation2017). Therefore, we need confirmation of the current presence of this species in the Mediterranean Sea.
Leucoraja melitensis (Clark 1926) is an endemic species of the Mediterranean Sea, described for the first time in the waters around Malta (Clark Citation1926). Its distribution is limited to a restricted area of the Central Mediterranean Sea (Serena Citation2005). Recently, it is cited on the coasts of Tunisia, it is considered rare off Algeria, and just one record from Italian waters is available (Dieuzeide et al. 1953-55; Tortonese Citation1956; Torchio Citation1960; Capapé Citation1977a). L. melitensis is considered relatively simple to identify, but not abundant in the area except for the Strait of Sicily (Ragonese et al. Citation2003; Serena et al. Citation2011). There are still several knowledge gaps for this species.
Raja africana Capapé, 1977 is sometimes reported in the taxonomic lists of the Mediterranean Sea and cited as an immigrant species from the Atlantic Ocean. This species, previously defined as dubious by Compagno (Citation1999) and Serena (Citation2005), has not been confirmed by the recent genetic study carried out by Cariani et al. (Citation2017). Therefore, this species is invalid for the Mediterranean Sea. The indication reported in Last et al. (Citation2016a) should also be considered a mistake.
Raja asterias Delaroche, 1809 is a very common species and it is mentioned as endemic inside the Mediterranean Sea, both in the past and in a recently annotated global checklist of chondrichthyans (Tortonese Citation1956; Serena Citation2005; Weigmann Citation2016). Several scientific contributions citing R. asterias as present in the Atlantic waters can be considered anecdotal since they did not report any morphological or genetic features useful for confirmation (Coelho et al. Citation2005; Serghini et al. Citation2008; Tai et al. Citation2010). However, Ordines et al. (Citation2017) has recently confirmed the presence of R. asterias in the Atlantic waters after studying a specimen captured in the Gulf of Cadiz.
Raja clavata (Linnaeus, 1758) is a species with a wide geographical distribution. It inhabits coastal and upper slope waters of the North-Eastern Atlantic Ocean, from the north-western part to the south of the African continent where Stehmann (Citation1995) defined its taxonomic status as certain for African populations. Its geographical range includes the European coasts of the Atlantic Ocean, the Baltic Sea, the Mediterranean Sea, and the Black Sea. It was also found in the South-Western Indian Ocean (Stehmann & Bürkel Citation1984).
Regarding its taxonomic status, it must be said that recently some authors (e.g., Dulvy et al. Citation2016; Bradaï et al. Citation2018), have used an incorrect nomenclature referring to Malacoraja clavata (Linnaeus, 1758). In agreement with the Catalog of Fish, WoRMS, and FishBase (Froese & Pauly Citation2019; Fricke Citation2020a; WoRMS Editorial Board Citation2020) as well as the indications of the Zoological Record, we believe that the use of this scientific name is improper and, at the moment, to be rejected. In fact, M. clavata is to be considered a senior synonym of R. clavata (McEachran & Dunn Citation1998).
The two species Raja montagui Fowler, 1910 and Raja polystigma Regan, 1923 have been confused for each other, according to a misspecification of diagnostic features required for the correct identification. The dichotomous key based on the presence or absence of small black spots on the pectoral fins edge does not seem to allow a good distinction between the two species. Even in recent years, they have often been considered a single species (Raja montagui). As already known, R. montagui is widely distributed mainly in the North-Eastern Atlantic, from Norway to Morocco, including the Canarian Islands (Serena Citation2005; Ebert & Stehmann Citation2013; Last et al. Citation2016a). Recent genetic studies clarified the real distinction between the two species and their distribution in the Mediterranean Sea: R. montagui is limited to the north African coasts of Algeria and Tunisia, while R. polystigma is endemic and distributed along the whole Mediterranean coasts (Frodella et al. Citation2016). A deeper investigation of genetic and morphological aspects concerning these two species is required to obtain simplified identification keys.
Order Myliobatiformes – Stingrays, butterfly rays, eagle rays, and devilrays
Six families occur in the Mediterranean Sea, two of which are also in the Black Sea (Dasyatidae and Gymnuridae). Tail well demarcated from the disc, slender or whip-like, with one or several spines; usually with a single dorsal fin, but no caudal and anal fins.
Family Dasyatidae Jordan & Gilbert, 1879
Batoids with depressed body almost circular to rhombic disc.
The Dasyatidae family is represented in the Mediterranean by five genera and eight species ().
Tortonese (Citation1956) reports how Garman (Citation1913) uselessly ascribed the European species to the subgenus Dasybatus (Garman, 1885) defining Pastinachus marinus (= Dasyatis centroura [Mitchill, 1815]). CLOFNAM (Check-list of the Fishes of the NE Atlantic and the Mediterranean) and FNAM (Fishes of the NE Atlantic and the Mediterranean) refer explicitly to D. centroura whose distribution includes the Atlantic coasts of Spain, Portugal, Africa, and the entire Mediterranean Sea, as well as the Western Atlantic Ocean (Hureau & Monod Citation1979; Whitehead et al. Citation1984). Ebert and Stehmann (Citation2013) confirm this geographical distribution. Furthermore, Iglésias (Citation2014) in his “Handbook of the marine fishes of Europe” considers D. centroura as a valid species. Afterward, Weigmann (Citation2016), on the basis of few specimens, introduces Dasyatis lata (Garman, 1880) for the waters of Taiwan, distinguishing it from D. centroura of the coasts off Quilon, India. Séret (Citation2016) still considers D. centroura to be in the Western-North Atlantic from Cape Cod to the Gulf of Mexico, and in the South-Western Atlantic from Brazil to Argentina as well as in the eastern Atlantic Ocean from Bay of Biscay to Angola, including the Mediterranean Sea. Finally, Last et al. (Citation2016b) declared D. centroura as a synonymy, preferring the genus of Bathytoshia Whitley, 1933. Above all, they make a clear distinction regarding the geographical distribution of Bathytoshia centroura (Mitchill, 1815), which is not included in the Mediterranean Sea but only to the western Atlantic. At the same time, Bathytoshia lata (Garman, 1880) is indicated for the Mediterranean Sea.
Eventually, the authors in agreement with Last et al. (Citation2016b) consider B. lata for the Mediterranean as a rare species. However, the collection of a significant number of specimens is absolutely necessary to deepen morphological and genetic studies in order to confirm the species occurrence within this sea.
Last et al. (Citation2016a) reports Dasyatis marmorata (Steindachner, 1892) as present in the Eastern Central Atlantic Ocean, from Mauritania to Congo, also considering a possible presence in the eastern part of the Mediterranean Sea. In fact, after a long and complex comparison between D. marmorata and Dasyatis chrysonota (Smith, 1828), where the first one was previously considered a junior synonym of the blue stingray (D. chrysonota), the actual geographic distribution of these species has been clarified. D. chrysonota is distributed in the South Eastern Atlantic Ocean and the South Western Indian Ocean, while D. marmorata can be considered a tropical Atlantic species that has rapidly spread throughout the Mediterranean Sea (Quignard & Tomasini Citation2000). The first finding of D. marmorata was recorded in the southern part of Tunisia by Maurin and Bonnet (Citation1970), later confirmed by Capapé and Zaouali (Citation1992, Citation1995)). Other records were from the Mediterranean coasts of Israel (Golani et al. Citation2002; Golani & Capapé Citation2004), Turkey (Erguden et al. Citation2014) and Lebanon (Lteif pers. comm.).
Capapé (Citation1977b) and Beveridge et al. (Citation2004) believed that a new Mediterranean species of stingray, called Tortonese’s stingray, could also be present along the eastern coasts of the Atlantic Ocean, but it was mentioned to be outside the Mediterranean Sea only once during the last century until its first real recording by Diatta et al. (Citation2001) off the coasts of Senegal. The first description of Dasyatis tortonesei Capapé, 1975 in the Mediterranean Sea was given by Capapé (Citation1974, Citation1975, Citation1977b) on the basis of a specimen caught in Tunisian waters. This species was considered a junior synonym of the common stingray Dasyatis pastinaca (Linnaeus, 1758) and its taxonomic status questioned by various authors (Séret & McEachran Citation1986; Tortonese Citation1987; Compagno Citation1999; Schrynmakers Citation2001; Serena Citation2005; Ebert & Stehmann Citation2013). However, it was reassigned as a valid species by some other authors referring to specimens collected in the eastern Mediterranean Sea (Golani Citation1996; Neifar et al. Citation2000; Golani & Capapé Citation2004; Golani Citation2005; El Kamel et al. Citation2009; Saadaoui Citation2010). Recently, Last et al. (Citation2016a) stated that this species differs from D. pastinaca on some important features, such as the shape of the dorsal skinfold, aspect, and number of oral papillae. This species needs to be more clearly confirmed as valid for the Mediterranean Sea.
Himantura uarnak (Gmelin, 1789), commonly known as honeycomb stingray, is a widely distributed species. Its presence is known from the Indo-Pacific to the Red Sea, from eastern to northern Africa, and in the Philippines (Last et al. Citation2016a). Recently, its geographical boundaries were expanded from the Red Sea to the Eastern Mediterranean Sea through the Suez Canal (Golani et al. Citation2002). The first record in the Mediterranean Sea is reported by Ben-Tuvia (Citation1955). Later on, other records were reported, all from the Levantine Basin (Ben-Tuvia Citation1966; Mouneimne Citation1977; El Sayed Citation1994; Başusta et al. Citation1998; Golani Citation2005; Ali et al. Citation2010, Citation2013). More recently, another species of honeycomb stingray, Himantura leoparda Manjaji-Matsumoto & Last, 2008, was reported in the Mediterranean Sea by Yucel et al. (Citation2017). They used both morphological and molecular techniques, providing the first DNA barcode of the species in the Mediterranean Sea (Manjaji-Matsumoto & Last Citation2008). Analyzing more specimens is recommended to confirm the presence and distribution of this species in the Mediterranean Sea. In fact, both Last et al. (Citation2016a) and Golani and Fricke (Citation2018) do not consider this species in the Red Sea.
Taeniurops grabatus (Geoffroy St. Hilaire, 1817) is a Dasyatidae present on the Atlantic coasts of West Africa (Whitehead et al. Citation1984), and it is especially found along the African coasts of Morocco, Mauritania, Senegal, Angola, and also in the Cape Verde Islands, Canary, and Madeira (Biscoito & Wirtz Citation1994; Monteiro et al. Citation2008). The presence of this species in these areas was confirmed since the Miocene (Antunes et al. Citation1999). In the Mediterranean Sea, it is present mainly along the North African coasts. Contrary to statements by some authors, it is not present in the Red Sea (Bonfil & Abdallah Citation2003; Serena Citation2005), where it is probably confused with the congeneric species Taeniurops meyeni (Müller & Henle, 1841). Some individuals of T. meyeni likely entered the Mediterranean Sea from the Suez Canal, but their presence is still not documented (Adi Barash pers. com). T. grabatus has settled down along the coasts of North Africa, where it is relatively abundant, especially in Tunisian waters and in the Gulf of Gabès (Tortonese Citation1956; Capapé Citation1983). There are also reports from the Levantine basin, where, in 1998, a young male was caught in Iskenderun Bay, Turkey (Başusta et al. Citation1998). In the previous year, a female was captured along the coasts of Tuscany, in the northernmost part of the Western Mediterranean Sea. A second specimen, caught in the same area with an artisanal fishing net was released, still alive, after an accurate identification (Serena et al. Citation1999).
Gymnuridae Fowler, 1934
Batoid with a depressed body and rhombic disc. Snout obtuse, disc about twice as broad as long, no dorsal fin.
The Gymnuridae family is represented in the Mediterranean by only one species. Gymnura altavela (Linnaeus, 1758) is benthic on sandy and muddy bottoms, inhabiting the inshore waters of Atlantic Ocean, Mediterranean and Black seas (Robins & Ray Citation1986; Serena Citation2005; Menni & Lucifora, Citation2007; Ebert & Stehmann Citation2013; Last et al. Citation2016a).
Aetobatidae Agassiz, 1858
Large batoid with moderately long fleshy rostral lobe, connecting to the head at eye level. Caudal sting behind the dorsal fin.
The Aetobatidae family is represented in the Mediterranean by only one species. Aetomylaeus bovinus (Geoffroy St. Hilaire, 1817), has dorsal surface greenish brown with transverse bars, more evident in juveniles, becoming faded in large specimens. Since White (Citation2014), who considered this genus as synonym of Aetomylaeus Garman, 1908, this species belonged to the genus Pteromylaeus Garman, 1913. Coastal demersal species, sometimes on the posidonia beds. Inhabiting in Eastern Atlantic and South West Indian oceans, including Mediterranean, absent in the Black Sea (Serena Citation2005; Ebert & Stehmann Citation2013; Last et al. Citation2016a)
Myliobatidae Bonaparte, 1835
Batoid with a short fleshy rostral lobe, connecting to pectoral fin by a ridge at eye level. Large caudal sting behind the dorsal fin.
The Myliobatidae family is represented in the Mediterranean by only one species. Myliobatis aquila (Linnaeus, 1758) has dorsal surface uniformly brown. It is benthic on sandy and muddy bottoms, inhabiting the shallow waters of Eastern Atlantic, South West Indian oceans and Mediterranean, absent in the Black Sea (Serena Citation2005; Ebert & Stehmann Citation2013; Last et al. Citation2016a).
Family Rhinopteridae Jordan & Evermann, 1896
Batoids with a depressed body almost circular to rhombic disc. Head marked off from the trunk, two subrostral fins incised.
The Rhinopteridae family is represented in the Mediterranean by only one species.
Rhinoptera marginata (Geoffroy St. Hilaire, 1817) is a bathypelagic batoid species inhabiting the inshore waters of the Eastern Atlantic Ocean from southern Spain to Senegal, including the entire Mediterranean basin but not the Black Sea (McEachran & Capapé Citation1984; McEachran & Séret Citation1990; Serena Citation2005; Ebert & Stehmann Citation2013). Commonly known as Lusitanian cownose ray, it is considered a rare and, hence, a “Data Deficient” species in the Mediterranean Sea (Başusta & Erdem Citation2000; Ferretti et al. Citation2016c), according to the IUCN regional Red List (Dulvy et al. Citation2016). It has been absent from the catches for a long time in the North-Western Mediterranean Sea, probably due to overfishing. Very little is known about the species in the area, as only a few sightings from the Levantine basin are available (Barrul & Mate Citation2002; Başusta et al. Citation2012). Recently, in the Eastern Mediterranean Sea (Mersin Bay in Turkey), 129 specimens have been accidentally caught in a single haul at a depth of 30 m by a commercial purse seiner (Mumtaz & Başusta Citation2018). Thirty-six specimens were pregnant, each bearing one embryo at a terminal phase of gestation. For this reason, Mumtaz and Başusta (Citation2018) suggested that the area can be considered as breeding and/or nursery area. This reinforces the importance of the Levantine basin in terms of biodiversity conservation.
Family Mobulidae Gill, 1893
Batoids with a depressed body and rhomboidal disc. Two characteristic prominent cephalic lobes extending forward on the head
The Mobulidae family is represented in the Mediterranean by only one species.
Tortonese (Citation1956), mentioning Duhamel Du Monceau (Citation1777) and Lacépéde (Citation1798), which first described M. mobular based on the observation of Mediterranean specimens, stated that this species is present in the eastern Mediterranean Sea and the Atlantic Ocean, from England to Senegal, and occasionally in the Western Atlantic Ocean. Currently, the species is considered rare. More recently, many authors wrote about real population distribution and provided elements for its distinction from the congener species Mobula japanica (Müller & Henle, 1841) (Serena Citation2005; Couturier et al. Citation2012; Holcer et al. Citation2012; Ebert & Stehmann Citation2013; Fortuna et al. Citation2014; Notarbartolo Di Sciara et al. Citation2015; Poortvliet et al. Citation2015; Last et al. Citation2016a; White et al. Citation2017b; Hosegood et al. Citation2018; etc.). Ebert and Stehmann (Citation2013) stated that the presence of M. mobular outside the Mediterranean Sea should still be considered uncertain, while Poortvliet et al. (Citation2015) affirmed that M. mobular has a geographical range restricted to the Mediterranean Sea. Moreover, they suggested a morphological affinity between M. mobular and M. japanica. Although differences in teeth morphology suggest that M. japanica and M. mobular are separate species (Adnet et al. Citation2012), more detailed studies on the morphology and genetic of the species are necessary to confirm whether M. japanica is a valid species. Hosegood et al. (Citation2018), in agreement with the conclusion of White et al. (Citation2017b), found no proofs to support the thesis of two different species. Their cluster analyses indicated modest differences between the Indo-Pacific and the Atlantic (including the Mediterranean) groups. Consequently, they upheld M. mobular as a single species, with M. japanica considered as a junior synonym of it. Therefore, M. mobular can be assessed as a circumglobally distributed species. Perhaps it would be necessary to concentrate more studies on the possibility to recognize subpopulations between the Indo-Pacific and the Atlantic and/or between the latter and the Mediterranean regions (Notarbartolo di Sciara pers. comm.). However, all these studies require a statistically reliable number of specimens to be analyzed, both for morphological features and molecular techniques.
CLASS HOLOCEPHALI
Order Chimaeriformes - Chimaeras
One family in the Mediterranean Sea, absent in the Black Sea.
Family Chimaeridae Rafinesque, 1815
A single external gill opening on each side of head; naked skin; first dorsal fin with a long and hard spine; elongate tapering tail.
The Chimaeridae family is represented in the Mediterranean by two genera and two probably species.
Chimaera monstrosa Linnaeus, 1758 has always represented the group of chimaeras in the Mediterranean. However, nine years ago another species of chimaera was fished for the first time in Syrian and subsequently in Egyptian waters. In fact, Hassan (Citation2013) cited a large female specimen of the chimaera Hydrolagus mirabilis (Collett, 1904) caught for the first time in the Mediterranean Sea along the Syrian coast in 2011. The specimen was caught by a bottom trawler operating at 500-meter depth. Later, in 2015, ten additional specimens of H. mirabilis were caught during a deep-sea research fishing cruise carried out along the Egyptian coasts with a commercial bottom trawl at depths between 300–800 m (Farrag Citation2016). These last captures could confirm the occurrence of H. mirabilis in the Mediterranean Sea. Anyway, this immigrant species from the Atlantic Ocean must be considered a rare or doubtful species as of now. Further confirmations are needed.
Overall Elasmobranchs biodiversity in the Mediterranean and Black seas
In general, the highest number of elasmobranch species (84%) were recorded in the western Mediterranean Sea, and followed, along a west-east gradient, by the Central Mediterranean Sea (81%), the Eastern Mediterranean Sea (78%), Adriatic Sea (60%) and by the Black Sea, with only 13% of the species represented. This west-east gradient applies even if we just refer to the 48 Mediterranean shark species. When only batoids were considered, a greater richness is detected in the Eastern and Central Mediterranean Sea (79% each) than the western region (74%). By applying the traffic light classification scheme, “rare” species represented the largest portion in all considered regions, from 50% in the Western Mediterranean to 5% in the Black Sea. “Occasional” species showed the highest values in the Central Mediterranean Sea (27%), while “abundant” species were relatively more frequent in the Western and Central Mediterranean (16% each). Considering sharks and rays separately, sharks are more frequently classified as “rare” (40.8%) than rays (26.8%) ().
Figure 5. Relative species abundance in the five Mediterranean sectors considered in the present study (a). Relative abundance if considering separately sharks, rays and chimaeras (b). Relative number of species occurring in each sector according to the traffic light classification (c-d)
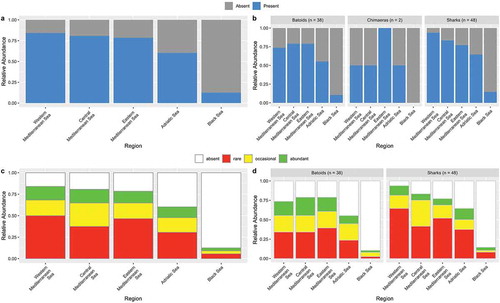
The Black Sea was the area with the lowest diversity, with eleven species of cartilaginous fishes () (Tortonese 1969; Whitehead et al. Citation1984; Bouchot Citation1987; Fredj & Maurin Citation1987; Bi̇lecenoğlu et al. Citation2002; Yankova et al. Citation2014). Some are considered very rare such as S. squatina or doubt such as Raja brachyura Lafont, 1873 while others (such as S. zygaena and A. vulpinus) were recorded just once or a few times.
Figure 6. Number of species involved in each subregion. The status of occurrence is utilized to express their trend from west to east
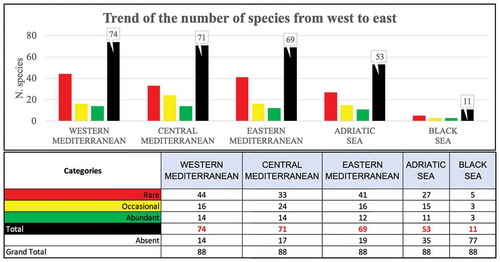
Even if western, central, and eastern Mediterranean regions showed a high number of species recorded, the Adriatic Sea was the main Mediterranean biodiversity hotspot for elasmobranchs in terms of diversity score (; Dulčić et al., Citation2005). Given that the diversity score is weighted by the surface of each sector, this result is mainly due to the relatively small size of the Adriatic basin. Fifty-three species of cartilaginous fish have been recorded in the Adriatic area and, it represents the lowest number of species after the Black Sea. In general, the occurrence of cartilaginous fish is lower in the northern part of the Adriatic Sea, where its shallower waters reduce the presence of deep-sea species. Few species are widely distributed in the Adriatic Sea such as S. canicula, Mustelus spp, P. glauca, S. acanthias, R. clavata, etc., while some bathyal species inhabit exclusively the central and southern area around the Pomo Pit (Jardas Citation1984).
Figure 7. Relative abundance of species in all sub-regions (a). Biodiversity score in all sub-regions (b). Biodiversity score in all considered sub-regions taking only into account batoid (c) and shark species (d) respectively
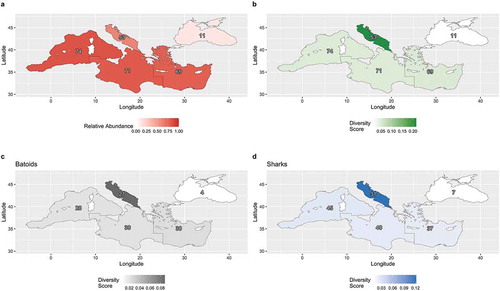
P-values of all chi-square tests highlighted a significant dependency between sub-region and abundance index for the entire taxonomic group (p-value: 6.26*10-24) and, separately considering sharks (p-value: 0.00045) and rays (p-value: 0.00045).
Notes on demersal species
Taking into account the demersal species collected during the scientific trawl surveys (e.g., MEDITS surveys), which has systematically sampled European Mediterranean Waters since 1994, some species were commonly recorded in both the Mediterranean and the Black Sea (S. canicula; R. clavata; S. acanthias, etc.). Other species are common in the west sub-region (G. melastomus; E. spinax; S. blainville) or the east portion, including the Black Sea (S. acanthias and R. brachyura). By contrast, other species were confined in restricted areas: Raja miraletus Linnaeus, 1758 mostly in the Tyrrhenian Sea, R. brachyura and Raja undulata Lacepède, 1802 in the Aegean Sea and G. atlanticus in the Alboran Sea. The deep-water species such as E. spinax, C. monstrosa, S. rostratus, C. coelolepis, G. melastomus and C. cf. uyato were absent in some areas such as the northern Adriatic Sea, due to relatively low depths. S. canicula was widely distributed on sandy, muddy or gravelly bottoms, from coastal waters down to 550 m depth, and it was more frequently recorded on the continental shelf between 50 and 250 m. S, canicula also showed very low abundances in terms of overall biomass in some restricted areas such as the North Adriatic Sea, Eastern Sicily, and Northern Ionian Sea. R. clavata occurred preferentially at the edge of the shelf and in the upper slope (200–500 m) showing an irregular pattern of distribution and likely absent in some East-Central Mediterranean areas (Gil De Sola Citation1994; Relini et al. Citation2000; Massutí & Moranta Citation2003; Sion et al. Citation2004; Massutí & Reñones Citation2005; Megalofonou et al. Citation2005; Serena Citation2005, Citation2007; Serena et al. Citation2009; Damalas & Vassilopoulou Citation2011; Ragonese et al. Citation2013; Tserpes et al. Citation2013; Ramírez-Amaro et al. Citation2015; Abella et al. Citation2017a, Citation2017b, Citation2017c, Citation2017d; Melendez et al. Citation2017; Bradaï et al. Citation2018; Serena et al. Citation2018; Follesa et al. Citation2019).
In general, within the framework of the (MEDITS) trawl surveys, 45 species of chondrichthyans (18 sharks, 2 angel sharks, 4 stingrays, 3 skates, 14 rays, 3 electric rays, and 1 chimaera) were recorded and included in a taxonomic list (Anonymous Citation2017). In addition to the species mentioned above, single or sporadic captures were also recorded (e.g., H. griseus, Mustelus spp., Squatina spp., R. marginata) (Bertrand et al. Citation2000; Relini et al. Citation2000, Citation2010a).
Discussion
The Mediterranean Sea is a heterogeneous biogeographic area that shows a high level of biological diversity. This sea constitutes a complex marine ecosystem within which elasmobranchs play a basic role in controlling trophic relationships (Katsanevakis et al. Citation2014). This is related to multiple factors from its geological history to its peculiar oceanographic and ecological features. Human impact, however, has become a driving force in shaping the spatio-temporal patterns of species abundance and distribution through direct and indirect effects of fishing exploitation, climate change - which also has promoted the immigration and expansion of thermophilic taxa - habitat destruction and pollution. These changes also affected the populations of cartilaginous fish.
The Mediterranean and the Black Sea contribute to the 6.9% of the global chondrichthyan species diversity with at least 48 species of sharks, 38 species of batoids (some of them need to be confirmed), and two chimaeras. Only 10–11 elasmobranch species are listed in the Black Sea (Bi̇lecenoğlu et al. Citation2002; Serena Citation2005; Hassan Citation2013; Yankova et al. Citation2014; Mahmoud Citation2016; FAO Citation2018a, Citation2018b) ( and ). Endemism is low and recorded only in skates. Four species (L. melitensis, R. asterias, R. polystigma and Raja radula Delaroche 1809) could be considered indigenous, even if R. asterias has also been found outside the Gibraltar Strait and along the Portuguese coasts of the Gulf of Cádiz (Ordines et al. Citation2017).
Comparing the results of the present study with the taxonomic list provided by the FAO field guide (Serena Citation2005), this investigation suggests that at least 14 new species (8 sharks, 5 batoids, and one chimaera) have been added to the Mediterranean chondrichthyans fauna since 2005. Nine of these species remain questionable, ten are occasionally recorded (with just one or a few records), and some species still need to be confirmed ().
To confirm the current presence of many species in the Mediterranean and the Black Sea for which very few records are available, here we carried out a thorough revision of the literature and museum collections and examined over 30 years of data proceeding from fishery resource monitoring carried out in the Mediterranean Sea. This work allowed us to take an important snapshot of the local chondrichthyan biodiversity and update previous references, and try to settle discussions, on the occurrence of chondrichthyans species in the Mediterranean basin. This work was necessary as the presence of important taxonomic issues, combined with critical conservation status, for many species make developing standardized protocols for chondrichthyan monitoring difficult in the region. Yet we were challenged by the variable level of the scientific accuracy of the scientific literature and the multitude of languages that sometimes make key references not readily available to research efforts. Many published studies indicating the first occurrence of species in the region lacked detailed descriptions of the specimens. In other cases, specimens deposited in Museum collections lacked important information such as location, date, type of acquisition, as well as fishing gear involved in the capture.
There are several species of , that still require an important review aimed at confirming their actual occurrence in the Mediterranean Sea. It was necessary to distinguish between species that have been constantly included in the taxonomic lists from others that have only occasionally been mentioned. For the latter species, it was necessary to provide some clarification on the causes of this scarcity of records. For example, D. batis has been frequently cited in old texts, but it is completely absent in the catches of any Mediterranean scientific survey programs and of any commercial fisheries for at least 50 years. This species was the subject of taxonomic discussions as it was considered a complex species (Iglésias et al. Citation2010; Last et al. Citation2016b). For this nomenclatural issue, it is problematic to consider D. batis as a valid species for the Mediterranean Sea. In this sense, museum collections can provide useful support to solve some of these doubts. Conversely, D. nidarosiensis officially appeared in the Mediterranean faunistic lists only recently (Cannas et al. Citation2010) and subsequently was confirmed by different authors (Follesa et al. Citation2012; Massi et al. Citation2017; Geraci et al. Citation2019; Carbonara et al. Citation2019) particularly for the western and central Mediterranean and the Adriatic Sea. Although, it should be said that further information on its status in the Mediterranean is recommended.
More than 20% of the Mediterranean species are considered questionable and vagrant (). Many of the vagrant species reported in the literature have not been recorded for a long time such as the two species of hammerhead shark, S. mokarran and S. tudes. Other notable examples are Mediterranean sawfishes (P. pristis and P. pectinata), which have not been recorded in the region since the end of the 1960s. Data modeling performed on historical records of the species suggested that both species went extinct in the Mediterranean Sea between the 1960s and 1970s (Ferretti et al. Citation2015). In these cases, the challenge is recognizing when the lack of record of these occasionally recorded species are of ecological or biogeographic nature or due to the historical and undocumented depletion of the species in the area.
Another aspect we focused on in this study is the spatio-temporal changes of Mediterranean chondrichthyan biodiversity. We detected a general decrease in biodiversity from north-western to south-eastern regions. This is a well-established general pattern in Mediterranean species diversity (Coll et al. Citation2010) probably due to a decreasing gradient of productivity from west to east and the presence of a threshold-strait or canal effect (e.g., Gibraltar, Sicily-Tunisia, Bosphorus and Suez) influencing species movements (Coll et al. Citation2010). Species richness was lower in the Adriatic and the Black Sea with 53 and 10–11 species respectively () (Fredj & Maurin Citation1987; Garibaldi & Caddy Citation1998; Bi̇lecenoğlu et al. Citation2002; Yankova et al. Citation2014; and present study). Yet when these values are standardized by the area of the Mediterranean subregions, the Adriatic Sea has the highest number of species per unit surface ().
There is a multitude of factors affecting the differences observed in the chondrichthyan biodiversity across subregions (Guisande et al. Citation2013). These range from morpho-edaphic or oceanographic characteristics to levels of anthropogenic pressures such as fishing, habitat destruction, and pollution, to changes in ocean temperature and species invasions from surrounding areas such as the Red Sea through the Suez Canal and the Atlantic Ocean. These factors are often difficult to disentangle but some elements appear evident.
Fishing is one of the prevalent drivers for conditioning biodiversity of the marine ecosystem. The Mediterranean Sea is one of the most exploited ocean sectors on the planet (Colloca et al. Citation2017b; Fortibuoni et al. Citation2017; Kroodsma et al. Citation2018; Corrales et al. Citation2019; Giménez et al. Citation2020). Multiple species that are now extremely rare or no longer present in different subsectors were subject to prolonged and intense fishing exploitation in the region (Ferretti et al. Citation2013). For most of these species, scientific records may have detected just the tail end of their declining population trajectories. Hence, it is paramount now trying to reconstruct their population abundance and distribution baselines by collecting and analyzing more historical information on their occurrence in the region and data on the intensity and distribution of current and historical Mediterranean fisheries (McClenachan et al. Citation2012; Ferretti et al. Citation2014).
Incidental catches of chondrichthyan species are still recorded in coastal and offshore fisheries. Large pelagic elasmobranchs are mostly caught as bycatch of longline fisheries. Drifting longlines targeting swordfish produces relatively high bycatch of P. glauca and I. oxyrinchus (FAO Citation2018c). Demersal species are mainly caught by semi-industrial bottom trawlers (FAO Citation2018c). The most abundant catch is now S. canicula, G. melastomus, D. oxyrhinchus, E. spinax, M. mustelus, C. monstrosa, R. miraletus, and R. clavata (Follesa et al. Citation2019). Small-scale fisheries mainly catch large pelagic species such as M. mobular, C. maximus, etc. (Mejuto et al. Citation2002; Peristeraki et al. Citation2008; Barone & Serena Citation2018; Mancusi et al. Citation2020). In general, as Mediterranean biodiversity is higher in coastal and shelf waters than in deep-sea environments (Coll et al. Citation2010), most of the chondrichthyan catches result from coastal fishing activities. Spatially, in north-western Mediterranean areas where industrialized fisheries are more developed, chondrichthyan species occurrence shows critical concerning levels. While, in other areas such as the Levantine basin, where industrial fishing is still limited or almost absent, the cartilaginous fish are not exploited thus showing more stable situations in terms of occurrence and abundance.
Deepening our knowledge of elasmobranchs ecology, distribution, and abundance in the Mediterranean Sea is paramount for assessing their conservation status and eventually, for planning more efficient conservation actions. Particular attention needs to be paid on species currently listed as “Data Deficient” by the IUCN (Serena et al. Citation2019; Walls & Dulvy Citation2020). The lack of complete information on commercial catches, including historical records, precludes the possibility to perform quantitative analyses (Cashion et al. Citation2019). Finally, the lack of enough biological information (e.g., food preferences, competition among species, trophic levels, etc.) is another problem for understanding some of the observed changes in the presence, abundance, and distribution of these species in the area.
In the last twelve years, assessments carried out on the conservation status of chondrichthyans in the Mediterranean (Dulvy et al. Citation2016) showed an increase in the number of assessed species. Among the 88 species of Mediterranean chondrichthyans considered, of which 73 assessed, 53.4% are classified as threatened (CR, EN, and VU) and therefore at high risk of extinction (). More than one-third of the species were classified either as Data Deficient (17.8%), or not evaluated at all (17% or 15 species). These results underline the need to increase efforts in acquiring more information on the conservation status of Mediterranean chondrichthyans which is crucial to support necessary actions to preserve this important marine biodiversity component.
Figure 8. Conservation status of the Mediterranean chondrichthyans following the IUCN Red List categories
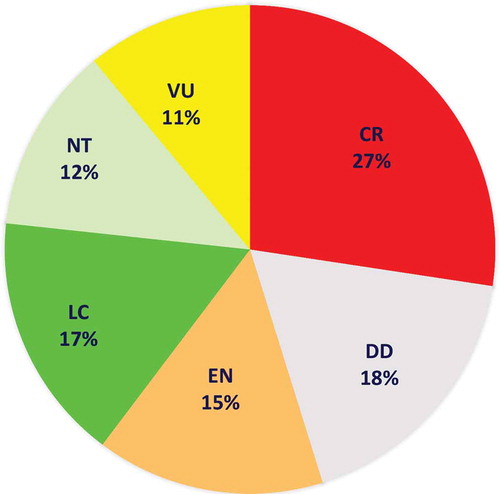
Mediterranean and Black Sea water masses are warming, and this is affecting the presence, abundance, and distribution of its marine species, as well as promoting the immigration of other species from the warmer Red Sea through the Suez Canal. This recent gateway is just an example of how human activities had an important role in the observed changes in the species diversity. It is worth noting that the exchange of species between the Red Sea and the Mediterranean is expected to increase in the near future due to the effects of both climate change and the enlargement of the Suez Canal which doubled its size in 2015.
Conclusion
Fishing activities, together with habitat destruction and climate change are profoundly altering the Mediterranean marine biodiversity, and, in particular, chondrichthyan species which show very low resilience to perturbations (Ferretti et al. Citation2008; Dulvy et al. Citation2016).
Among the multiple threats chondrichthyans face in the Mediterranean Sea, fishing is currently the prevalent one. Elasmobranchs are often an important bycatch of multiple Mediterranean fisheries, and this, combined with their low biological productivity (Ricklefs Citation1979), puts these animals at a high risk of extinction (Kindsvater et al. Citation2016). Technical measures as simply widening of the mesh size of the fishing nets are absolutely inappropriate measures for sound management of elasmobranch resources. In fact, in order to keep the populations of these fishes in balance, incisive management programs are required to guarantee stability for the populations. Pushing for a ban of the activities in nursery and mating fishing grounds is certainly the best if not a winning solution for sustainable exploitation of renewable resources. Consequently, these solutions also guarantee the conservation process of the most vulnerable species as the elasmobranchs. Among these management measures, the elimination or at least the reduction of the bycatch cannot be missing.
On the other hand, to guarantee high biodiversity in the Mediterranean Sea, we also need cautious management throughout all the basin, especially in the areas where fisheries are not fully developed yet, such as the Eastern Mediterranean Sea. These actions must be addressed to reduce fishing effort on chondrichthyans, mitigate bycatch, and reduce the impact on marine habitats. The GFCM must play an important role in coordinating all Mediterranean countries towards the development of an innovative and concerted approach for a sustainable fishery. The GFCM can enforce policy decisions and exert the right pressure on countries to adopt best practices for sustainable fishing. This aspect is strictly linked with the adoption of laws and regulations aimed to protect the renewable resources that are affected by all fishing activities, including recreational fishing. To gather as much information as possible, all of these considerations need to be confirmed with the use of more accurate monitoring programs, for both the Mediterranean and the Black Sea. Fortunately, it has been observed that some management measures are promoting recovery of shark biodiversity, such as the ban of drifting nets, which occurred eighteen years ago (Council Regulation EC 894/97).
To conclude, it is important to stress the great importance of a continuous collection of reliable data and the enforcement of data collection programs in areas where such activities are not well developed. The use of the information collected within the European Data Collection Framework (DCF) and the Data Collection Reference Framework (DCRF) of the GFCM, provided, for the first time, an overall description of the Mediterranean species diversity of chondrichthyans. We focused on the main issues affecting our knowledge so far. Such descriptions will be useful for future assessments of any diversity change in the area and to better address management measures to keep biological diversity stable in space and time. In this sense, it is necessary to affirm the importance of a policy that operates seriously and that puts the precautionary approach into practice. In fact, Mediterranean elasmobranchs, perhaps more than others, are threatened and suffer overfishing and habitat degradation. Moreover, the Mediterranean basin is a very heterogeneous and critical area from many points of view. Economic interests prevail over everything and often it is not possible to adopt a common policy for the management of resources, especially fish stocks. The European Union has the possibility of intervening only in a part of the Mediterranean, often causing contradictory situations regarding the countries of North Africa and the Levant. Taking all this into account, the role of the GFCM assumes a basic importance not only in the policies of management of fish resources but also as regards the conservation of vulnerable species such as elasmobranchs and consequently of the biodiversity of the marine ecosystem (FAO Citation2019). In particular, the GFCM is the unique regional body able to monitor and to enforce, current protective instruments that may reduce further harm to highly threatened populations such as the elasmobranchs.
Acknowledgements
We would like to thank the EU Data Collection Framework research program for allowing the collection of the most important data set in the Mediterranean and FAO for allowing us to use some of its maps. For the valuable suggestions and collaboration demonstrated over time, we give thanks to Adi Barash, University of Haifa (Israel); Agostino Leone, University of Bologna (Italy); Alessia Cariani University of Bologna (Italy); Alice Ferrari University of Bologna (Italy); Cecilia Mancusi, Environmental Protection Agency of Tuscany Region (Italy); Bernard Séret Ichtyo-Consult, (France); Bruno Zava, Wilderness Environmental Studies (Italy); Carlotta Mazzoldi, University of Padova (Italy); Christian Capapé, Université de Montpellier (France); Daniel Golani, University of Jerusalem (Israel); David A. Ebert, Moss; Landing Marine Laboratories, (USA); Emilio Sperone, University of Calabria (Italy); Farid Hemida, ENSSMAL (Algeria); Francesco Tiralongo, University of Catania (Italy); Fulvio; Garibaldi and Luca Lanteri, University of Genoa (Italy); Gabriel Morey, Balearic Islands Government (Spain); Giulio Relini, University of Genoa (Italy); Guallart Javier, University of Valencia (Spain); Hakan Kabasakal, Independent Researcher (Turkey); Halit Filiz, Muğla Üniversitesi (Turkey); Ilija Cetkovic, University of Montenegro (Montenegro); Ioannis Giovos, iSea & Marine and Environmental Research (Greece); John Randall, Private Consultant (USA); Karla D.A. Soares, Universidade de São Paulo (Brazil); Mahmoud M.S. Farrag, Al-Azhar University (Egypt); Mark Dimech, Private Consultant (Malta); Matthias F W Stehmann, Ichthys (Germany); Michel Bariche, American University of Beirut (Lebanon); Mohamad; Hassan, University of Tishreen (Syria); Mohamed Nejmeddine Bradai, INSTM (Tunisia); Mumtaz E. Tirasin, Dokuz Eylu ̈l University (Turkey); Myriam Lteif, CNRS, (Lebanon); Nuri Başusta, Firat University (Turkey); Persefoni Megalofonou, University of Athens (Greece); Peter Nick Psomadakis, FAO (Italy); Rigers Bakiu, University of Tirana (Albania); Samuel P. Iglésias, Muséum National d’Histoire Naturelle (France); Shakman Esmail, University of Tripoli (Lybia); Simon Weigmann, University of Hamburg (Germany); Simone Niedermueller and Giulia Prato, WWF (Italy); Stefano Lelli and Polo Carpentieri, FAO (Italy); William Toby White, CSIRO (Australia).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abella JA, Mancusi C, Mannini A, Serena F. 2017a. Galeus melastomus. Biology Marine Mediterranean 24(Suppl. 1):136–143. In: Sartor P., Mannini A., Carlucci R., Massaro E., Queirolo S., Sabatini A., Scarcella G., Simoni R. (eds), Synthesis of the knowledge on biology, ecology and fishery of the halieutic resources of the Italian seas.
- Abella JA, Mancusi C, Serena F. 2017b. Raja asterias. Biology Marine Mediterranean 24(Suppl. 1):144–149. In: Sartor P., Mannini A., Carlucci R., Massaro E., Queirolo S., Sabatini A., Scarcella G., Simoni R. (eds), Synthesis of the knowledge on biology, ecology and fishery of the halieutic resources of the Italian seas.
- Abella JA, Mancusi C, Serena F. 2017c. Raja clavata. Biology Marine Mediterranean 24(Suppl. 1):150–156. In: Sartor P., Mannini A., Carlucci R., Massaro E., Queirolo S., Sabatini A., Scarcella G., Simoni R. (eds), Synthesis of the knowledge on biology, ecology and fishery of the halieutic resources of the Italian seas.
- Abella JA, Mancusi C, Serena F. 2017d. Scyliorhinus canicula. Biology Marine Mediterranean 24(Suppl. 1):157–164. In: Sartor P, Mannini A, Carlucci R, Massaro E, Queirolo S, Sabatini A, Scarcella G, Simoni R. (eds), Synthesis of the knowledge on biology, ecology and fishery of the halieutic resources of the Italian seas.
- Adnet S, Cappetta H, Guinot G, Notarbartolo Di Sciara G. 2012. Evolutionary history of the devilrays (Chondrichthyes: Myliobatiformes) from fossil and morphological inference. Zoological Journal of the Linnean Society 166(1):132–159.
- Agenzia Europea Dell’Ambiente (AEA). 2000. Situaciones y presiones del medio ambiente marino y del litoral mediterráneo.
- Aldebert Y. 1997. Demersal resources of the Gulf of Lions (NW Mediterranean). Impact of Exploitation on Fish Diversity. Vie Et Milieu 47:275–284.
- Ali M, Saad A, Ben Amor MM, Capapé C. 2010. First records of the Honeycomb Stingray, Himantura uarnak (Forskål, 1775), off the Syrian coast (eastern Mediterranean) (Chondrichthyes: Dasyatidae). Zoology in the Middle East 49(1):104–106. DOI:10.1080/09397140.2010.10638397.
- Ali M, Saad A, Reynaud C, Capapé C. 2013. Additional records of honeycomb stingray Himantura uarnak (Chondrichthyes: Dasyatidae) off the Syrian coast (Eastern Mediterranean). Tishreen University Journal for Research and Scientific Studies - Biological Sciences Series 35(4):215–221.
- Almeida AJ, Biscoito M. 2019. Chaves para a identificação dos peixes de oceano Atlântico oriental, Mar Mediterrâneo e Mar Negro. Boletim, Museu De Historía Natural Do Funchal Suplemento No 15:1–195.
- Aloncle H. 1972. Descriptive catalogue of fishes in Moroccan seas. Part 1: Cyclostomata, selachii, Holocephali. Holocephali or Chimaeridae. Bulletin Institute Pêches Maritime Maroc 19:161–163.
- Anonymous. 2017. MEDITS Handbook. Version n. 8, MEDITS Working Group. pp. 177.
- Antunes MT, Balbino AC, Cappetta H. 1999. Sélaciens du Miocène terminal du bassin d’Alvalade (Portugal) Essai de synthèse. Ciencia Da Terra (UNL) N 13:115–129.
- Ayas D, Ciftci N, Yalcin E, Akbora HD, Bakan M, Erguden D. 2020. First record of the big nose shark, Carcharhinus altimus (Springer, 1950) from Mersin Bay. International Journal of Fisheries and Aquatic Studies 8(2):132–136.
- Azab AM, HMM K-A, Sarhan MMH, El-Tabakh MAM. 2019. Carcharhinid shark species (Family Carcharhinidae), with special reference to the first records in the Egyptian Mediterranean Waters, Alexandria, Egypt. Egyptian Journal of Aquatic Biology & Fisheries 23(3):545–559. DOI:10.21608/ejabf.2019.48531.
- Azzurro E, Sbragaglia V, Cerri J, Bariche M, Bolognini L, Ben Souissi J, Busoni G, Coco S, Chryssanthi A, Fanelli E, Ghanem R, Garrabou J, Gianni F, Grati F, Kolitari J, Letterio G, Lipej L, Mazzoldi C, Milone N, Pannacciulli F, Pešić A, Samuel‐Rhoads Y, Saponari L, Tomanic J, Topçu NE, Vargiu G, Moschella P. 2019. Climate change, biological invasions, and the shifting distribution of Mediterranean fishes: A large‐scale survey based on local ecological knowledge. Glob Change Biol 00:1–14. DOI: ORG/10.1111/GCB.14670.
- Baino R, Serena F, Ragonese S, Rey J, Rinelli P. 2001. Catch composition and abundance of elasmobranchs based on the MEDITS program. Rapp. Communication Intitute Mer Méditerranean. 36:234.
- Bakiu R, Cakalli M, Giovos I. 2018. The first record of bigeyed sixgill shark, Hexanchus nakamurai Teng, 1962 in Albanian waters. Journal Black Sea/Mediterranean Environment 24(1):74–79.
- Barausse A, Correale V, Curkovic A, Finotto L, Riginella E, Visentin E, Mazzoldi C. 2014. The role of fisheries and the environment in driving the decline of elasmobranchs in the northern Adriatic Sea. ICES Journal of Marine Science 71(7):1593–1603. DOI:10.1093/icesjms/fst222.
- Bargnesi F, Gridelli S, Cerrano C, Ferretti F. 2020. Reconstructing the history of the sand tiger shark (Carcharias taurus) in the Mediterranean Sea. Aquatic Conservation Marine and Freshwater Ecosystems 30(5):915–927. DOI:ORG/10.1002/AQC.3301.
- Bariche M, Fricke R. 2020. The marine ichthyofauna of Lebanon: An annotated checklist, history, biogeography, and conservation status. Zootaxa 4775(1):001–157. DOI:10.11646/zootaxa.4775.1.1.
- Barone M, Serena F. 2018. MEDLEM archive to evaluate the by-catch of elasmobranchs in the Mediterranean basin. Poster REF.FF2018_TS1-1_ SERENA. In: FAO. 2018. Fish Forum 2018: Book of abstract. Rome: FAO, Licence: CC BY-NC-SA 3.0 IGO. pp. 338
- Barrul J, Mate I. 2002. Tiburones del Mediterràneo. Arenys de Mar: EI Set-ciències. pp 290
- Barrull J, Mate I. 2001. First record of a pregnant female little sleeper shark Somniosus rostratus (Risso, 1826) on the Spanish Mediterranean coast. Boletin Institute Espanola Oceanography 17(3 y 4):323–325.
- Başusta A, Ozer EI, Sulikowski JA, Başusta N. 2012. First record of a gravid female and neonate of the Lusitanian cownose ray, Rhinoptera marginata, from the eastern Mediterranean Sea. Journal of Applied Ichthyology 28(4):643–644. DOI:10.1111/j.1439-0426.2012.01941.x.
- Başusta N, Erdem U. 2000. A study on the pelagic and demersal fishes in Iskenderun Bay. Turkish Journal of Zoology 24:1–19.
- Başusta N, Erdëm U, Kumlu M. 1998. Two new records for the Turkish seas: Round stingray Taeniura grabata and skate stingray Himantura uarnak (Dasayatidae). Journal of Zoology 44:65–66.
- Başusta N, Keskin Ç, Serena F, Séret B, Eds. 2005. The Proceedings of the International Workshop on Mediterranean Cartilaginous Fish with Emphasis on Southern and Eastern Mediterranean. Istanbul: Turkish Marine Research Foundation. pp. 209–213.
- Bat L, Erdem Y, Ustaoğlu S, Yardım Ö, Hüseyin Satılmış H. 2005. A Study on the Fishes of the Central Black Sea Coast of Turkey. Journal Black Sea/Mediterranean Environment 11:281–296.
- Bello G. 1999. The chondrichthyans of the Adriatic Sea. Acta Adriatica. 40:65–76.
- Bellocchio G, Carboni MG, Nami M, Pallini G. 1991. Fauna di ittiodontoliti del Pliocene di Allegrona (Terni, Umbria). Bollettino Society Natural Napoli 100:41–73.
- Bellodi A, Porcu C, Cau A, Marongiu M, Melis R, Mulas A, Pesci P, Follesa M, Cannas R. 2018. Investigation on the genus Squalus in the Sardinian waters (Central-Western Mediterranean) with implications on its management. Mediterranean Marine Science:256–272. DOI:10.12681/mms.15426.
- Ben Amor MM, Diatta Y, Diop M, Ben Salem M, Capapé C. 2016. Confirmed occurrence in the Mediterranean Sea of milk shark Rhizoprionodon acutus (Chondrichthyes: Carcharhinidae) and first record off the Tunisian coast. Cahiers de Biologie Marine 57:145–149.
- Ben Souissi J, Golani D, Mejri H, Ben Salem M, Capape C. 2007. First confirmed record of the Halave’s Guitarfish, Rhinobatos halavi (Forsskål 1775) (Chondrichthyes: Rhinobatidae) in the Mediterranean Sea with a description of a case of albinism in elasmobranchs. Cahiers de Biologie Marine. 48(1):67–75.
- Ben-Tuvia A. 1953. Mediterranean fishes of Israel. Bulletin Sea Fisheries Research Static Haifa 8:1–40.
- Ben-Tuvia A. 1955. Two Indo-Pacific fishes, Dasyatis uarnak and Upeneus moluccensis, in the eastern Mediterranean. Journal Nature 176(4494):1177–1178. DOI:10.1038/1761177b0.
- Ben-Tuvia A. 1966. Red Sea fishes recently found in the Mediterranean. Copeia 2:254–276.
- Ben–Tuvia A. 1971. Revised list of the Mediterranean fishes of Israel. Israel Journal of Zoology 20:1–39.
- Benvenuto A. 2019. Taxonomic uncertainty in genus Centrophorus in the Mediterranean Sea: Results from the integration of molecular and morphological taxonomy. Thesy, Alma Mater Studiorum Università di Bologna. pp. 74.
- Bertrand J, Gil De Sola L, Papakonstantinou C, Relini G, Souplet A. 2000. Contribution on the distribution of the Elasmobranchs in the Mediterranean Sea (from the MEDITS Surveys). Biologia Marina Mediterranea 7:385–399.
- Bertrand J, Gil de Sola L, Papakostantinou C, Relini G, Souplet A. 1997. An international bottom trawl survey in the Mediterranean: The MEDITS Program. ICES CM 1997/Y: 03 16
- Bertrand JA, Gil De Sola L, Papaconstantinou C, Relini G, Souplet A. 2002. The general specifications of the Medits surveys. Scientia Marina 66(S2):9–17 66 DOI:10.3989/scimar.2002.66s29
- Beveridge I, Neifar L, Euzet L. 2004. Eutetrarhynchid cestodes from Atlantic and Mediterranean elasmobranch fishes, with the description of two new species of Dollfusiella Campbell & Beveridge, 1994 and redescriptions of Prochristianella papillifer (Poyarkoff, 1909) Dollfus, 1957 and Parachristianella trygonis Dollfus, 1946. Systematic Parasitology 59(2):81–102. DOI:10.1023/B:SYPA.0000044426.65921.44
- Bianchi CN, Morri C. 2000. Marine biodiversity of the Mediterranean Sea: Situation, problems and prospects for future research. Marine Pollution Bulletin 40(5):367–376. DOI:10.1016/S0025-326X(00)00027-8.
- Bianchi CN, Morri C, Chiantore M, Montefalcone M, Parravicini V, Rovere A. 2012. Mediterranean Sea biodiversity between the legacy from the past and a future of change. Life in the Mediterranean Sea: A Look at Habitat Changes 1:55.
- Bigelow HB, Schroeder WC. 1948. Fishes of the western North Atlantic. Memoir Sears Found. Marine Research Yale Universität Part 1. pp. 576.
- Bi̇lecenoğlu M, Kaya M, Ci̇hangi̇r B, Çi̇çek E. 2014. An updated checklist of the marine fishes of Turkey .Turk Journal Zoology 38:901–929 © TÜBİTAK 10.3906/ZOO-1405-60
- Bi̇lecenoğlu M, Taskavak E, Mater S, Kaya M. 2002. Checklist of the marine fishes of Turkey. Zootaxa 113. New Zealand: Magnolia Publishers, Auckland. pp. 194.
- Bini G. 1967. Atlante dei Pesci delle Coste italiane. Milano: Mondo Sommerso 1:206. Leptocardi, Ciclostomi, Selaci.
- Biscoito MJ, Wirtz P. 1994. Two new records of stingray (Pisces: Dasyatidae) from the Archipelago of Madeira (NE Atlantic). Bocagiana 169:1–4.
- Boero F, Carli C. 1977. Prima segnalazione mediterranea di Sphyrna mokarran (Rüeppel, 1837) (Selachii, Sphyrnidae). Bollettino dei Musei e degli Istituti dell’Università di Genova 45:91–93.
- Bonello JJ, Bonnici L, Ferrari A, Cariani A, Schembri PJ. 2015. Not all that clear cut: Intraspecific morphological variability in Squalus blainville (Risso, 1827) and implications for identification of the species. Journal of the Marine Biological Association of the United Kingdom 12. DOI: 10.1017/S0025315415001915.
- Bonfil R, Abdallah M. 2003. Field identification guide to the sharks and rays of the Red Sea and Gulf of Aden. Rome, FAO: FAO Species Identification Guide for Fishery Purposes. pp. 71. 12 colour plates.
- Bosc E, Bricaud A, Antoine D. 2004. Seasonal and interannual variability in algal biomass and primary production in the Mediterranean Sea, as derived from 4 years of SeaWiFS observations. Journal of Geophysical Research. DOI: 10.129/2003GB002034.
- Bouchot M-L. 1987. Requins. In: Fischer W, Schneider M, Bauchot M-L,editors. Méditerranée et Mer Noir. Fiches FAO d’identification des especes puor les besoins de la pêche. Rome: FAO. pp. 1529 Vol II.
- Bradaï MN, Saïdi B, Enajjar S. 2012. Elasmobranchs of the Mediterranean and Black Sea: Status, ecology and biology bibliographic analysis. In: Studies and Reviews. Rome, FAO: General Fisheries Commission for the Mediterranean. Rome: FAO. No. 91. pp. 103
- Bradaï MN, Saidi B, Ghorbel M, Bouain A, Guelorget O, Capapé C. 2002. Observations sur les requins du golfe de Gabes (Tunisie meridionale, Mediterranee centrale). Mesogée 60:61–77.
- Bradaï NM, Saidi B, Enajjar S. 2018. Overview on Mediterranean Shark’s Fisheries: Impact on the Biodiversity. In: Türkoğlu M, Önal U, Ismen A, editors. Marine Ecology - Biotic and Abiotic Interactions. London: Intechopen. pp. 211–230. DOI: ORG/10.5772/INTECHOPEN.74923.
- Bradai MN, Soldo A. 2016. Rhinobatos rhinobatos. In: The IUCN Red List of Threatened Species. E.T63131A16527789.
- Branstetter S. 1984. Carcharinidae, Hemigaleidae et Triakidae. Paris: UNESCO 1:102–121. In: Fishes of the North-Eastern atlantic and the Mediterranean (Whitehead P.J.P., Bauchot M.-L., Hureau J.-C., Nielsen J. & E. Tortonese, eds).
- Britten GL, Dowd M, Minto C, Ferretti F, Boero F, Lotze HK. 2014. Predator decline leads to decreased stability in a coastal fish community. Ecology Letters. 17:1518–1525. https://doi.org/10.1111/ele.12354
- Caddy JF. 1999. Deciding on precautionary management measures for a stock based on a suite of limit reference points (LRPs) as a basis for a multi-LRP harvest law. NAFO Science Council Studies 32:55–68.
- Caddy JF. 2015. The traffic light procedure for decision making: Its rapid extension from fisheries to other sectors of the economy. Global Journal of Science Frontier Research: I Marine Science, 15(1):30. DOI: 10.13140/RG.2.2.22857.75361
- Cadenat J, Blache J. 1981. Requins de Méditerranée et d’atlantique (plus particulièrement de la côte occidentale d’afrique). Paris: ORSTOM, Faune Trop 21:1–330.
- Cannas R, Follesa MC, Cabiddu S, Porcu C, Salvadori S, Iglésias SP, Deiana AM, Cau A. 2010. Molecular and morphological evidence of the occurrence of the Norwegian skate Dipturus nidarosiensis (Storm, 1881) in the Mediterranean Sea. Marine Biology Research 6(4):341–350. DOI:10.1080/17451000903428496.
- Capapé C. 1974. Systématique, écologie et biologie de la reproduction des sélaciens des côtes tunisiennes. VI. Thèse de 3 cycle. Paris: Université de Paris. pp. 140. (Fascicule 1: Etude systématique – texte), 46 planches (fascicule 2: Etude systématique – atlas commenté) & 96 pp. (fascicule 3: Ecologie).
- Capapé C. 1975. Sélaciens nouveaux et rares le long des côtes tunisiennes. Premières observations biologiques. Archives de l’Institut Pasteur de Tunis 51(1–2):107–138.
- Capapé C. 1977a. Contribution à la biologie des Rajidae des côtes tunisiennes. Vll. Raja melitensis Clark, 1926: Sexualité reproduction, fécondité. Cah. Biol. Mar 18(2):177–190. 5 fig., 1 pl
- Capapé C. 1977b. Les espèces de genre Dasyatis Rafinesque, 1810 (Pisces, Rajiformes) des côtes tunisiennes. Cybium 3(2):75–105.
- Capapé C. 1983. Données nouvelles sur la morphologie des Dasyatidae (Pisces, Rajiformes) des côtes tunisiennes. Bull. Inst. natn. scient. tech. océanogr. Pêche Salammbò 10:69–98.
- Capapé C. 1989. Les Sélaciens des côtes méditerranéennes: aspects généraux de leur écologie et exemples de peuplements. Océanis (Paris) 15:309–331.
- Capapé C, Guélorget O, Vergne Y, Marquès A, Quignard JP. 2006. Skates and rays (Chondrichthyes) from waters off the Languedocian coast (southern France, northern Mediterranean). Annales, Series Historia Naturalis 16:165–178.
- Capapé C, Seck AA, Diatta Y, Reynaud C, Hemida F, Zaouali J. 2004. Reproductive biology of the black-tip shark, Carcharhinus limbatus (Chondrichthyes: Carcharhinidae) off West and North African coasts. Cybium 28(4):275–284.
- Capapé C, Zaouali J. 1992. Le régime alimentaire de la pastenague marbrée, Dasyatis marmorata (Steindachner, 1892) (Pisces, Rajiformes, Dasyatidæ) des eaux tunisiennes. Vie et Milieu 42(3–4):269–276.
- Capapé C, Zaouali J. 1995. Reproductive biology of the marbled stingray, Dasyatis marmorata (Steindachner, 1982) (Pisces: Dasyatidae) in Tunisian waters (Central Mediterranean). Journal of Aquariculture and Aquatic Sciences 7:108–119.
- Capapé C, Zaouali J, Desoutter M. 1979. Note sur la présence en Tunisie de Carcharhinus obscurus (Lasueur, 1818) (Pisces, Pleurotremata) avec clé de determination des Carcharinidae des côtes tunisiennes. Bulletin Offical Nation 2:171–182. Pêches Tunisie, Tunis 3, n.
- Cappetta H, Nolf D. 1991. Les Sélaciens du Pliocén Inferieur de Le Puget-sur-Argent (Sud-Est de la France). Paleont. Abt. A 218:49–67.
- Carbonara P, Cannas R, Donnaloia M, Melis R, Porcu C, Spedicato MT, Zupa W, Follesa MC. 2019. On the presence of Dipturus nidarosiensis (Storm, 1881) in the Central Mediterranean area. PeerJ 7:e7009. DOI: 10.7717/peerj.7009.
- Cariani A, Messinetti S, Ferrari A, Arculeo M, Bonello JJ, Bonnici L, Cannas R, Carbonara P, Cau A, Charilaou C, El Ouamari N, Fiorentino F, Follesa MC, Garofalo G, Golani D, Ilaria Guarniero I, Hanner R, Hemida F, Omar Kada O, Lo Brutto S, Mancusi C, Morey G, Schembri PJ, Serena F, Sion L, Stagioni M, Angelo T, Vrgoc N, Steinke D, TintI F. 2017. Improving the Conservation of Mediterranean Chondrichthyans: The ELASMOMED DNA Barcode Reference Library. PLoS ONE 12(1):e0170244. DOI:10.1371/JOURNAL.PONE.0170244.
- Casey JG. 1964. Anglers’ guide to sharks of the northeastern United States, Maine to Chesapeake Bay. Bureau of Sport Fisheries and Wildlife Circ 179:32.
- Cashion MS, Bailly N, Pauly D. 2019. Official catch data underrepresent shark and ray taxa caught in Mediterranean and Black Sea fisheries. Marine Policy 105:1–9. DOI: 10.1016/J.MARPOL.2019.02.041.
- Celona A. 2000. First record of a tiger shark Galeocerdo cuvier (Peron and LeSueur, 1822) in the Italian waters Annales for Istrian and Mediterranean studies, Series. Hist Natural 2(21):207–210.
- Cigala Fulgosi F. 1983a. First Record of Alopias superciliosus (Lowe, 1840) in the Mediterranean, with Notes on Some Fossil Species of the Genus Alopias (Pisces, Selachii, Alopiidae). Estr Annali Musium Civil. Storage Natural Genova 84:211–229.
- Cigala Fulgosi F. 1983b. Confirmation of the presence of Carcharinus brachyurus (Guenther, 1870) (Pisces, Selachii, Carcharhinidae) in the Mediterranean Doriana. Genova 249:1–5.
- Cigala Fulgosi F. 1986. A deep water elasmobranch fauna from a lower Pliocene outcropping (Nrthern Italy). In: Uyeno T, Arai R, Taniuchi T, Matsuura K, editors. Indo-Pacific Fish Biology: Proceedings of Second International Conference on Indo-Pacific Fishes. Japan: Ichthyol. Soc. pp. 133–139.
- Cigala Fulgosi F, Gandolfi G. 1983. Re-description of the external morphology of Somniosus rostratus (Risso, 1826), with special reference to its squamation and cutaneous sensory organs, and aspects of their functional morphology (Pisces Selachii Squalidae). Monitor Zoology. Ltal. (Nuov.ser.) 17:27–70.
- Clò S, Bonfil R, De Sabata E 2008. Additional records of the bigeye thresher shark, Alopias superciliosus, from the central and eastern Mediterranean Sea. JMBA2, Biodiversity Records 6168.
- Clò S, Dalù M, Danovaro R, Vacchi M 2002. Segregation of the Mediterranean population of Centroscymnus coelolepis (Chondrichthyes: Squalidae): A description and survey Elasmobranch Fisheries: Managing for Sustainable Use and Biodiversity Conservation (Hosted by NAFO), 11-13/9/2002, Santiago de Compostela, Spain poster (136 k).
- Clark RS 1926. Rays and Skates, a revision of the European species. Fisheries, Scotland, Scient. Invest. (1): 1-66, fig. 1-42, pl. 1–36.
- Coelho R, Erzini K, Bentes L, Correia C, Lino PG, Monteiro P, Ribeiro J, Gonçalves JMS. 2005. Semi-pelagic longline and trammel net elasmobranch catches in southern Portugal: Catch composition, catch rates and discards. Journal of Northwest Atlantic Fishery Science 35:531–537. DOI:10.2960/J.v35.m482.
- Coll M, Piroddi C, Steenbeek J, Kaschner K, Ben Rais Lasram F, Aguzzi J, Ballesteros E, Bianchi CN, Corbera J, Dailianis T, Danovaro R, Estrada M, Froglia C, Galil BS, Gaso JM, Gertwagen R, Gil J, Guilhaumon F, Kesner-Reyes K, Kitsos M-S, Koukouras A, Lampadariou N, Laxamana E, López-fé de la Cuadra CM, Lotze HK, Martin D, Mouillot D, Oro D, Raicevich S, Rius-Barile J, Saiz-Salinas JI, San Vicente C, Somot S, Templado J, Turon X, Vafidis D, Villanueva R, Voultsiadou E. 2010. The Biodiversity of the Mediterranean Sea: Estimates, Patterns, and Threats. PLoS ONE 5(8):e11842. DOI:10.1371/JOURNAL.PONE.0011842.
- Colloca F, Carrozzi V, Simonetti A, Di Lorenzo M. 2020. Using Local Ecological Knowledge of Fishers to Reconstruct Abundance Trends of Elasmobranch Populations in the Strait of Sicily. Frontiers in Marine Science 7:508. DOI: 10.3389/FMARS.2020.00508.
- Colloca F, Enea M, Ragonese S, Di Lorenzo M. 2017a. A century of fishery data documenting the collapse of smooth-hounds (Musteluspp.) in the Mediterranean Sea. Aquatic Conservation: Marine and Freshwater Ecosystems 27(6):1145–1155. DOI:10.1002/aqc.2789.
- Colloca F, Scarcella G, Libralato S. 2017b. Recent Trends and Impacts of Fisheries Exploitation on Mediterranean Stocks and Ecosystems. Frontiers in Marine Science 4:244. DOI: 10.3389/FMARS.2017.00244.
- Colloca F, Scannella D, Geraci M.L, Falsone F, Giusto B, Vitale S, Di Lorenzo M, Bono G. 2019. British sharks in Sicily: records of long-distance migration of tope shark (Galeorhinus galeus) from the north-eastern Atlantic to the Mediterranean Sea. Medit. Mar. Sci., 20/2:309–313. DOI: http://dx.DOI.org/10.12681/mms.18121
- Compagno LJV. 1984a. FAO species catalogue. Vol. 4. Sharks of the world. An annotated and illustrated catalogue of sharks species known to date. Part 1. Hexanchiformes to Lamniformes. FAO Fisheries Synopsis 125(4):249. Pt.1.
- Compagno LJV. 1984b. FAO species catalogue. Vol. 4. Sharks of the world. An annotated and illustrated catalogue of sharks species known to date. Part 2. Carcharhiniformes. FAO Fisheries Synopsis 125(4):251–655. Pt. 2.
- Compagno LJV. 1999. Checklist of living elasmobranchs. In: Hamlett WC, editor. Sharks, skates, and rays: The biology of elasmobranch fishes. Baltimore and London: The Johns Hopkins University Press. pp. 471–498 515 pp.
- Compagno LJV, Dando M, Fowler S. 2005. A Field Guide to the Sharks of the World. London: Harper Collins Publishing Ltd.. pp. 368.
- Corrales X, Coll M, Ofr E, Heymans JJ, Steenbeek J, Goren M, Edelist D, Gal G. 2019. Future scenarios of marine resources and ecosystem conditions in the Eastern Mediterranean under the impacts of fshing, alien species and sea warming. Scientific REportS 8(1):14284. DOI:10.1038/s41598-018-32666-x.
- Corsini-Foka M, Sioulas A. 2009. On two old specimens of Alopias superciliosus (Chondrichthyes: Alopiidae) from the Aegean waters. Marine Biodiversity Records 2:e72. DOI: 10.1017/S175526720900044X.
- COUNCIL REGULATION. 1997. (EC) No 894/97 of 29 April 1997. Laying down certain technical measures for the conservation of fishery resources.
- Couturier LIE, Marshall AD, Jaine FRA, Kashiwagi T, Pierce SJ, Townsend KA, Weeks SJ, Bennett MB, Richardson AJ. 2012. Biology, ecology and conservation of the Mobulidae. Journal of Fish Biology 80(5):1075–1119 10.1111/j.1095-8649.2012.03264.X PMID: 22497374.
- D’Ancona U. 1949. Rilievi statistici sulla pesca nell’alto Adriatico. Atti Ist. Ven. Science Letter Art. 108:41–53.
- Damalas D, Megalofonou P. 2012. Occurrences of large sharks in the open waters of the southeastern Mediterranean Sea. Journal of Natural History 46(43–44):2701–2723. DOI:10.1080/00222933.2012.716864.
- Damalas D, Vassilopoulou V. 2011. Chondrichthyan by-catch and discards in the demersal trawl fishery of the central Aegean Sea (Eastern Mediterranean). Fisheries Research 108(1):142–152. DOI:10.1016/j.fishres.2010.12.012.
- de Carvalho MR, Last PR, Séret B. 2016. Torpedo Rays. In: Last PR, et al.,editor. Family Torpedinidae. Rays of the World. CSIRO publishing. p.184–203
- De la Serna JM, Valeiras J, Ortiz JM, Macías D 2002. Large pelagic Sharks as By-catch in the Mediterranean Swordfish Longline Fishery: Some Biological Aspects (Elasmobranch Fisheries – Poster). NAFO SCR Doc. 02/137. Serial No. N4759. Scientific Council Meeting.
- De Maddalena A, Della Rovere G. 2005. First record of the pigeye shark, Carcharhinus amboinensis (Müller & Henle, 1839), in the Mediterranean Sea. Annales, Series Historia Naturalis 15(2):209–212.
- Demidov AB. 2008. Seasonal dynamics and estimation of the annual primary production of phytoplankton in the Black Sea. Oceanology 48(5):664–678. DOI:10.1134/S0001437008050068.
- Derrick DH, Cheok J, Dulvy NK.2020.Spatially congruent sites of importance for global shark and ray biodiversity.PLoS ONE 15(7):e0235559 . PONE.0235559 10.1371/journal.pone.0235559
- Di Natale A, Celona A, Mangano A. 2005. A series of catch records by the harpoon fishery in the Strait of Messina from 1976 to 2003. SCRS/2004/160 Col. Vol. Sci. Pap. ICCAT 58(4):1348–1359.
- Diatta Y, Clotilde-Ba FL, Capapé C. 2001. Rôle trophique du poulpe commun, Octopus vulgaris, chez les Elasmobranches de la côte du Sénégal (Atlantique oriental tropical). Comparaison avec les espèces des côtes tunisiennes (Méditerranée centrale). Acta Adriatica 42(1):77–88.
- DiBattista JD, Choat JH, Gaither MR, Hobbs J-PA, Lozano-Cortes DF, Myers RF, Paulay G, Rocha LA, Toonen RJ, Westneat MW, Berumen ML. 2015. On the origin of endemic species in the Red Sea. Journal of Biogeography 43(1):1–18.
- Dieuzeide R, Novella M, (collab. J. Roland). 1953-55. Catalogue des Poissons des Côtes algériennes. Bull. Stn Aquic. Pêch. Castiglione, I (n.s.), (4), 1952 [1953]: 1-135, 73 fig. n. num.; II (n.s.), (5), 1953 [1954]: 1-258, 135 fig. n. num.; III (n.s.), (6), 1954 [1955]: 1-384, 202 fig. n. num.
- Döderlein P 1879. Manuale Ittiologico del Mediterraneo ossia sinossi metodica delle varie specie di pesci riscontrate sin qui nel Mediterraneo e in particolare nei mari di Sicilia. 5 parts. Palermo: Pt 1, 1879: i–viii + 1–67; pt 2, 1881: 1, –120; pt 3, 1884: 121–258; +.
- Duhamel du Monceau H. 1777. Traité general des peches et histoire des poisons. Vol 4. Paris. pp. 1769–1782.
- Dulčić J, Soldo A, Jardas I. 2005. Adriatic fish biodiversity and review of bibliography related to Croatian small-scale coastal fisheries. In: should be cited as follows: AdriaMed. 2005. Adriatic Sea Small-scale Fisheries. pp.184 Report of the AdriaMed Technical Consultation on Adriatic Sea Small-Scale Fisheries. Split, Croatia, 14th – 15th October 2003. FAO-MiPAF Scientific Cooperation to Support Responsible Fisheries in the Adriatic Sea. GCP/RER/010/ITA/TD15. AdriaMed Technical Documents 15.
- Dulvy NK, Allen DJ, Ralph GM, Walls RHL. 2016. The conservation status of Sharks, Rays and Chimaeras in the Mediterranean Sea [Brochure]. Malaga, Spain: IUCN. pp. 14.
- Dulvy NK, Fowler SL, Musick JA, Cavanagh RD, Kyne PM, Harrison LR, Pollock CM. 2014. Extinction risk and conservation of the world’s sharks and rays. ELife 3:e00590. DOI:10.7554/eLife.00590.
- Ebert DA, Fowler S, Compagno L. 2013. Sharks of the world. Plympton: Wild Nature Press.p p. 528. St. Maurice, Plymouth PL7 1NH.
- Ebert DA, Stehmann M. 2013. Sharks, batoids, and chimaeras of the North Atlantic. Rome: FAO Species Catalogue of fishery Purpose. No. 7. pp. 523.
- Echwikhi K, Saidi B, Bradai MN, Bouain A. 2013. Preliminary data on elasmobranch gillnet fishery in the Gulf of Gabès, Tunisia. Journal of Applied Ichthyology 29(5):1080–1085. DOI:10.1111/JAI.12022.
- Ekman S. 1953. Zoogeography of the Sea. London: Sidgwick and Jackson. 417 pp.
- El Kamel O, Mnasri N, Ben Souissi J, Boumaïza M, Ben Amor MM, Capapé C. 2009. Inventory of elasmobranch species caught in the Lagoon of Bizerte (North-eastern Tunisia, central Mediterranean). Pan-American Journal of Aquatic Sciences 4:382–412.
- El Sayed RS. 1994. Check–List of Egyptian Mediterranean Fishes. Alexandria, Egypt: Institute of Oceanography and Fisheries. p. 77. ix + 77 p.
- Emig C, Geistdoerfer P. 2005. The Mediterranean deep-sea fauna: Historical evolution, bathymetric variations and geographical changes. Carnets de Géologie/Notebooks on Geology, Maintenon, Art.2004/01, CG2004-A01-CCE-PG. DOI.ORG/10.4267/2042/3230.
- Erguden D, Turan C, Gurlek M, Uyan A, Reyhaniye AN. 2014. First record of marbled stingray, Dasyatis marmorata (Elasmobranchii: Myliobatiformes: Dasyatidae), on the coast of Turkey, north-eastern Mediterranean. Acta Ichthyologica Et Piscatoria 44(2):159–161. DOI:10.3750/AIP2014.44.2.11.
- EUROPEAN COMMISSION. 2009. The role of maritime zones in promoting effective governance for protection of the Mediterranean marine environment. Report of the expert group of governance of the Mediterranean Sea.
- FAO. 2007. Sharks and Rays of the Red Sea and the Gulf of Aden. FAO Species Identification Cards. Rome: FAO. pp. 110.
- FAO. 2009. Sharks and Rays of the Mediterranean and Black Sea. FAO Species Identification Cards. Rome: FAO. pp. 113.
- FAO. 2018a. Species Photographic Plates. Mediterranean Sharks, by Monica Barone, Fabrizio Serena and Mark Dimech. Rome: FAO. pp 20
- FAO. 2018b. Species Photographic Plates. Mediterranean skates, rays and chimaeras, by Monica Barone, Fabrizio Serena and Mark Dimech. Rome: FAO. pp 18
- FAO. 2018c. The State of Mediterranean and Black Sea Fisheries. General Fisheries Commission for the Mediterranean. Rome: FAO. pp 172
- FAO. 2019. Report of the forty-second session of the General Fisheries Commission for the Mediterranean (GFCM), FAO headquarters, Rome, Italy, 22–26 October 2018. GFCM Report No.42. Rome: FAO. 146 pp. Licence: CC BY-NC-SA 3.0 IGO.
- Faria VV, Mc Davitt MT, Charvet P, Wiley TR, Simpfendorfer CA, Naylor GJP. 2013. Species delineation and global population structure of Critically Endangered sawfishes (Pristidae). Zoological Journal of the Linnean Society 167(1):136–164. DOI:10.1111/j.1096-3642.2012.00872.x.
- Farrag MMS. 2016. Deep-sea ichthyofauna from Eastern Mediterranean Sea, Egypt: Update and new records. Egyptian Journal of Aquatic Research 42(4):479–489.
- Ferguson IK 1994. Checklist of sharks frequenting the Mediterranean Sea. In: Proceedings of the Second European Shark and Ray Workshop, Natural History Museum, London, February 1994. Tag and Release Schemes and Shark and Ray Management Plans (Fowler S.L. & R.C. Earll, eds), pp. 49–51. Peterborough: Joint Nature and Conservancy Council.
- Fergusson IK, Vacchi M, Serena F. 2002. Note on the declining status of the sand tiger shark Carcharias taurus in the Mediterranean Sea. In: Vacchi M, La Mesa G, Serena F, Séret B, editors. Proceedings of the 4th European Elasmobranch Association Meeting. pp. 73–76. Paris: Association Societe Francaise d’Ichtyologie (SFI).
- Ferretti F, Crowder LB, Micheli F. 2014. Using disparate datasets to reconstruct historical baselines of animal populations. In: Marine historical ecology in conservation. applying the past to manage for the future. University of California Press. pp. 63–85.
- Ferretti F, Morey G, Serena F, Mancusi C, Coelho RP, Seisay M, Litvinov F, Buscher E. 2016a. Squatina oculata. In: The IUCN Red List of Threatened Species. 2016: e.T61418A16570000
- Ferretti F, Morey G, Serena F, Mancusi C, Fowler SL, Dipper F, Ellis J 2016b. Squatina squatina. The IUCN Red List of Threatened Species 2015: e.T39332A48933059. HTTPS://WWW.IUCNRED LIST.ORG/SPECIES/39332/48933059.
- Ferretti F, Morey G, Séret B, Sprem JS, Micheli F. 2015. Falling through the cracks: The fading history of a large iconic predator. Fish and Fisheries. John Wiley & Sons Ltd. 15. DOI: 10.1111/FAF.12108.
- Ferretti F, Myers RA, Serena F, Lotze H. 2008. Loss of large predatory sharks from the Mediterranean Sea. Conservation Biology 22(4):952–964. DOI:10.1111/j.1523-1739.2008.00938.x.
- Ferretti F, Notarbartolo Di Sciara G, Serena F, Ducrocq M. 2016c. Rhinoptera marginata (errata version published in 2016). The IUCN Red List of Threatened Species 2016: E.T161463A97837871.
- Ferretti F, Osio GC, Jenkins CJ, Rosenberg AA, Lotze HK. 2013. Long-term change in a meso-predator community in response to prolonged and heterogeneous human impact. Scientific Reports 3(1):1057. DOI:10.1038/srep01057.
- Ferretti F, Worm B, Britten GL, Heithaus MR, Lotze HK. 2010. Patterns and ecosystem consequences of shark declines in the ocean.. Ecology Letters 13(8):1055–1071. DOI:10.1111/j.1461-0248.2010.01489.x.
- Fischer W, Schneider M, Bauchot ML. 1987. Méditerranée et Mer Noir. Zone de pêches 37. Vol. II. Vertébrés. Fiches FAO d’identification des especes puor les besoins de la pêche. (Revision 1). Rome: FAO. 1529 pp.
- Follesa MC, Cannas R, Cabiddu S, Cau A, Mulas A, Porcu C, Cau A. 2012. Preliminary observations of the reproductive biology and diet of the Norwegian skate Dipturus nidarosiensis (Rajidae) from central western Mediterranean. Cybium 36(3):473–477.
- Follesa MC, Marongiu MF, Zupa W, Bellodi A, Cau A, Cannas R, Colloca F, Djurovic M, Isajlovic I, Jadaud A, Manfredi C, Mulas A, Peristeraki P, Porcu C, Ramirez-Amaro S, Salmerón Jiménez F, Serena F, Sion L, Thasitis I, Cau A, Carbonara P. 2019. Spatial variability of Chondrichthyes in the northern Mediterranean. Sci. Mar. 83S1: 000-000. DOI: ORG/10.3989/SCIMAR.04998.23A.
- Fortibuoni T, Giovanardi O, Pranovi F, Raicevich S, Solidoro C, Libralato S. 2017. Analysis of long-term changes in a Mediterranean Marine Ecosystem based on fishery landings. Frontiers in Marine Science 4:33. DOI:10.3389/fmars.2017.00204
- Fortuna CM, Kell L, Holcer D, Canese S, Jr E F, Mackelworth P, Summer GD. 2014. Summer distribution and abundance of the giant devil ray (Mobula mobular) in the Adriatic Sea: Baseline data for an iterative management framework. Scientia Marina 78(2):227–237. DOI:10.3989/scimar.03920.30D.
- Fredj G, Maurin C. 1987. Les poissons dans la banque de données MEDIFAUNE. Application à l’étude des caractéristiques de la faune ichtyologique Méditerranéenne. Cybium 11(3):218–299.
- Fricke R. Ed. 2020a. Eschmeyer’s Catalog of Fishes: References. California Academy of Science. (http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp). Electronic version accessed 04 May 2020.
- Fricke R, Bi̇lecenoğlu M, Sari HM. 2007. Annotated checklist of fish and lamprey species (Gnathostomata and Petromyzon tomorphi) of Turkey, including a Red List of threatened and declining Species. Stuttgarter Beiträge zur Naturkunde 706:1–168.
- Fricke R, Eschmeyer WN, van der Laan R. (Eds). 2020b. Eschmeyer’s Catalog of Fishes: Genera, species, references. Electronic version. California Accademy of Science. accessed http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp
- Frodella N, Cannas R, Velonà A, Carbonara P, Farrell ED, Fiorentino F, Follesa MC, Garofalo G, Hemida F, Mancusi C, Stagioni M, Ungaro N, Serena F, Tinti F, Cariani A. 2016. Population connectivity and phylogeography of the Mediterranean endemic skate Raja polystigma and evidence of its hybridization with the parapatric sibling R. montagui. Marine Ecology Progress Series 554:99–113. DOI: 10.3354/MEPS11799.
- Froese R, Pauly D. Eds. 2019. FishBase. World Wide Web electronic publication www.fishbase.org, (08/2019).
- Froese R, Pauly D. Eds. 2020a. FishBase. Carcharhinus acarenatus Moreno & Hoyos 1983. Accessed through: World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=298418on2020-05-30
- Froese R, Pauly D. Eds. 2020b. FishBase. Torpedo alexandrinsis Mazhar 1987. Accessed through: World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=271677on2020-05-12
- Fromentin J, Farrugio H. 2005. Results on the 2003 observer program on board the French purse seiner targeting Atlantic bluefin tuna in the Mediterranean Sea. Col. Vol. Science Paper ICCAT 58(2):779–782.
- Garcia SM. 2011. Long‐term trends in small pelagic and bottom fisheries in the Mediterranean: 1950–2008. Valbonne, France: Plan Bleu.
- Garibaldi F, Orsi-Relini L. 2012. Record of Carcharhinus falciformis (Bibron in Mueller & Henler, 1839) in Italian waters (Ligurian Sea, Northernwestern Mediterranean). Cybium 36(2):399–400.
- Garibaldi L, Caddy JF. 1998. Biogeographic characterization of Mediterranean and Black Seas faunal provinces using GIS procedures. Ocean & Coastal Management 39(3):211–227. DOI:10.1016/S0964-5691(98)00008-8.
- Garman S 1885. The generic name of the pastinacas or sting rays, Proc.U.S. Nat. mus. VIII.
- Garman S. 1913. The Plagiostomia (Sharks, Skates, and Rays). Memoirs of the Museum of Comparative Zoology at Harvard College 36:1–528. DOI: 10.5962/BHL.TITLE.43732.
- Garofalo G, Gristina M, Fiorentino F, Cigala Fulgosi F, Norrito G, Sinacori G. 2003. Distribution pattern of rays (Pisces, Rajidae) in the Strait of Sicily in relation to fishing pressure. Hydrobiologia 503(1–3):245–250. DOI:10.1023/B:HYDR.0000008487.25578.d4.
- Gàrrick JAF. 1982. Sharks of the genus Carcharhinus. NOAA Technology Reports NMFS Circuit (445:194.
- Gàrrick JAF. 1985. Additions to a revision of the shark genus Carcharhinus: Synonymy of Aprionodon and Hypoprion, and description of a new species of Carcharhinus (Carcharhinidae). Department of Commerce, National Oceanic and Atmospheric Administration, National Marine Fisheries Service. NOAA Tech. Rep. NMFS 34. 26 pp.
- Geldiay R. 1969. Izmir Körfezinin başlıca balıkları ve muhtemel invasionları. Izmir: Ege Üniversitesi Fen Fakültesi Monogra leri. pp. 135. (in Turkish).
- Geraci ML, Di Lorenzo M, Falsone F, Scannella D, Di Maio F, Colloca F, Vitale S, Serena F. 2019. the occurrence of norwegian skate, Dipturus nidarosiensis (elasmobranchii: Rajiformes: Rajidae), in the Strait of Sicily, central Mediterranean. Acta Ichthyologica Et Piscatoria 49(2):203–208. DOI:10.3750/AIEP/02566.
- GFCM, 2018a. Recommendation GFCM/42/2018/2 on fisheries management measures for the conservation of sharks and rays in the GFCM area of application, amending Recommendation GFCM/36/2012/3.
- GFCM. 2018b. Working Group on the Black Sea (WGBS). Seventh meeting of the WGBS. Burgas, Bulgaria, 11–13 July 2018. 53 pp.
- Giarruso ME 2019. Indagini morfologiche e genetiche sul genere Squalus nello stretto di Sicilia. Corso di laurea magistrale in biologia marina. Tesi di laurea in struttura e connettività delle popolazioni marine. VI Sessione, Anno Accademico 2017/2018. 31 pp.
- Gil De Sola L. 1994. Ictiofauna demersal de la plataforma continental del mar Alboran (Mediterraneo sudoccidental iberico). Biologie Institute Espanola Oceanography 10(1):63–79.
- Giménez J, Cardador L, Mazor T, Kark S, Bellido JM, Coll M, Navarro J. 2020. Marine protected areas for demersal elasmobranchs in highly exploited Mediterranean ecosystems. Marine Environmental Research. In Press, Journal Pre-proof 160:105033. DOI:10.1016/j.marenvres.2020.105033.
- Golani D. 1996. The Marine Ichthyofauna of the Eastern Levant. History, Inventory and Characterization. Israel Journal of Zoology 42:15–55.
- Golani D, Orsi-Relini L, Massutí E, Quignard JP. 2002. Fishes. In: Briand F, editor. CIESM Atlas of exotic species in the Mediterranean. Vol. 1. CIESM Publications, Monaco. 256 pp.
- Golani D. 2005. Check-list of the Mediterranean Fishes of Israel. Zootaxa 947(1):1–90. DOI:10.11646/ZOOTAXA.947.1.1.
- Golani D 2006. Cartilaginous fishes of the Mediterranean coast of Israel. In: The Proceedings of the International Work- shop on Mediterranean Cartilaginous Fishes with Emphasis on Southern and Eastern Mediterranean (Başusta N., Keskýn Ç., Serena F. & B. Séret, eds): 95–100. Istanbul: Turkish Marine Research Foundation.
- Golani D, Capapé C. 2004. First records of the blue stingray, Dasyatis chrysonota (Smith, 1828) (Chondrichthyes: Dasyatidae), off the coast of Israel (eastern Mediterranean). Acta Adriatica 45(1):107–113.
- Golani D, Öztürk B, Başusta N. 2006. Fishes of the eastern Mediterranean. Istanbul: Turkish Marine Research Foundation.
- Golani D, Fricke R. 2018. Checklist of the Red Sea Fishes with delineation of the Gulf of Suez, Gulf of Aqaba, endemism and Lessepsian migrants. Zootaxa 4509 (1): 001–215. DOI.ORG/10.11646/ZOOTAXA.4509.1.1
- Gordon CA, Hood AR, Al Mabruk SAA, Barker J, Bartolí A, Ben Abdelhamid S, Bradaï MN, Dulvy NK, Fortibuoni T, Giovos I, Jimenez Alvarado D, Meyers EKM, Morey G, Niedermuller S, Pauly A, Serena F, Vacchi M. 2019. Mediterranean Angel Sharks: Regional Action Plan. United Kingdom: The Shark Trust. pp. 36.
- Grace MA 2001. Field guide to requiem sharks (Elasmobranchiomorphi: Carcharhinidae) of the Western North Atlantic. U.S. Dep. Commer., NOAA Tech. Rep. NMFS 153. 32 pp.
- Griffiths AM, Sims DW, Johnson A, Lynghammar A, McHugh M, Bakken T, Genner MJ. 2011. Levels of connectivity between longnose skate (Dipturus oxyrinchus) in the Mediterranean Sea and the north-eastern Atlantic Ocean. Conservation Genetics 12(2):577–582. DOI:10.1007/s10592-010-0127-3.
- Gruber SH, Compagno LJV. 1981. Taxonomic status and biology of the bigeye thresher, Alopias superciliosus (Lowe, 1839). Fishery Bulletin, National Marine Fisheries Service 79:617–640.
- Guijarro B, Quetglas A, Moranta J, Ordines F, Valls M, González N, Massutí E. 2012. Inter- and intra-annual trends and status indicators of nektobenthic elasmobranchs off the Balearic Islands (northwestern Mediterra¬nean). Scientia Marina 76(1):87–96. DOI:10.3989/scimar.03432.22A.
- Guisande C, Patti B, Vaamonde A, Manjarrés-Hernández A, Pelayo-Villamil P, García-Roselló E, González-Dacosta J, Heine J, Granado-Lorencio C. 2013. Factors affecting species richness of marine elasmobranchs. Biodiversity and Conservation 22(8):1703–1714. DOI:10.1007/s10531-013-0507-3.
- Harrison L, Dulvy N. 2014. Sawfish: A Global Strategy for Conservation. Vancouver: IUCN Species Survival Com- mission’s Shark Specialist Group.
- Hassan M. 2013. Occurrence of large-eyed rabbitfish Hydrolagus mirabilis, Chimaeridae, in Syrian waters (Eastern Mediterranean). Marine Biodiversity Records 6(e):7. DOI:10.1017/S175526721200111X.
- Hemida F. 2005. Les sélaciens de la côte algérienne: Biosystématique des Requins et des Raies; Ecologie, Reproduction et Exploitation de quelques populations capturées. Thèse de Doctorat. Algérie: Université des sciences et de la Technologie, Houari Boumediene. pp. 231.
- Hemida F, Capapé C. 2008. On the occurrence of the longfin mako, Isurus paucus (Chondrichthyes: Isuridae) off the Algerian coast (southwestern Mediterranean). Acta Adriat 49(2):185–189.
- Hemida F, Labidi N 2001. Notes on the Carcharhinids of the Algerian Basin. Proc. 4th Meeting of European Elasmobranch Association, Livorno (Italy).
- Hemida F, Seridji R, Labidi N, Bensaci J, Capapé C. 2002. Records of Carcharhinus spp. (Condrichthyes: Carcharhinidae) from off the Algerian coast (southern Mediterranean). Acta Adriat 43(2):83–92.
- Holcer D, Lazar B, Mackelworth P, Fortuna CM. 2012. Rare or just unknown? The occurrence of the giant devil ray (Mobula mobular) in the Adriatic Sea. Journal of Applied Ichthyology 29(1):139–144.
- Hopkins TS. 1985. Physics of the sea. In: Margalef R, editor. Key environments: Western Mediterranean. New York: Pergamon Press. p. 100–125.
- Hosegood J, Humble E, Ogden R, de Bruyn M, Creer S, Stevens G, Abudaya M, Bassos-Hull K, Bonfil R, Fernando DD, Foote A, Hipperson H, Jabado RW, Kaden J, Moazzam M, Peel L, Pollett S, Ponzo A, Poortvliet M, Salah J, Senn H, Stewart J, Wintner S, Carvalho G. 2018. Phylogenomics and species delimitation of mobulid rays reveals cryptic diversity and a new species of manta ray. BioRxiv Preprint First Posted Online Oct 31:2018. DOI: 10.1101/458141.
- Hureau JC, Monod T, Eds. 1979. Check-list of the fishes of the north-eastern Atlantic and of the Mediterranean, Clofnam. Vol. 1. Paris: UNESCO.
- Iglésias SP. 2014. Handbook of the marine fishes of Europe and adjacent waters (A natural classification based on collection specimens, with DNA barcodes and standardized photographs), Volume I (Chondrichthyans and Cyclostomata), Provisional version 08, 01 March 2014. 105p. http://iccanam.mnhn.fr.
- Iglésias SP, Lecointre G, Sellos DY. 2005. Extensive paraphylies within sharks of the order Carcharhiniformes inferred from nuclear and mitochondrial genes. Molecular Phylogenetics and Evolution 34(3):569–583. DOI:10.1016/j.ympev.2004.10.022.
- Iglésias SP, Toulhoat L, Sellos DY. 2010. Taxonomic confusion and market mislabeling of threatened skates: Important consequences for their conservation status. Aquatic Conservation: Marine and Freshwater Ecosystems 20(3):319–333. DOI:10.1002/aqc.1083.
- Iglésias SP. 2014. Handbook of the marine fishes of Europe and adjacent waters (A natural classification based on collection specimens, with DNA barcodes and standardized photographs), Volume I (Chondrichthyans and Cyclostomata), Provisional version 08, 01 March 2014. 105 pp. HTTP://ICCANAM.MNHN.FR.
- International Commission on Zoological Nomenclature. 1999. International Code of Zoological Nomenclature, 4th Edition. The International Trust for Zoological Nomenclature, London, Xxix + 306 Pp DOI.ORG/10.5962/BHL.TITLE.50608
- Jardas I. 1984. Horizontal and vertical distribution of benthos Selachians (Pleurotremata, Hypotremata) in the Adriatic. FAO Fish. Rep. 290:95–108.
- Jukic-Peladic S, Vrgoc N, Krstulovic-Sifner S, Piccinetti C, Piccinetti-Manfrin G, Marano G, Ungaro N. 2001. Long-term changes in demersal resources of the Adriatic Sea: Comparison between trawl surveys carried out in 1948 and 1998. Fisheries Research 53(1):95–104. DOI:10.1016/S0165-7836(00)00232-0.
- Kabasakal H. 1998. Sharks and rays fisheries in Turkey. Shark News 11:8.
- Kabasakal H. 2002. Elasmobranch species of the seas of Turkey. In Annales, Ser. Hist. Nat. 12(1):15–22.
- Kabasakal H, Bilecenoğlu M. 2020. Shark infested internet: An analysis of internet-based media reports on rare and large sharks of Turkey. FishTaxa 16:8–18.
- Kabasakal H, Karhan SU 2007. On the occurrence of the bigeye thresher shark, Alopias superciliosus (Chondrichthyes: Alopiidae), in Turkish waters. JMBA2, Biodiversity Records 5745.
- Katsanevakis S, Coll M, Piroddi C, Steenbeek J, Ben Rais Lasram F, Zenetos A, Cardoso AC. 2014. Invading the Mediterranean Sea: Biodiversity patterns shaped by human activities. Frontiers in Marine Science 1(32). DOI: 10.3389/FMARS.2014.00032.
- Keskin Ç. 2010. A review of fish fauna in the Turkish Black Sea. Journal Black Sea Mediterranean Environment 16(2):195–210.
- Kindsvater HK, Mangel M, Reynolds JD, Dulvy NK. 2016. Ten principles from evolutionary ecology essential for effective marine conservation. Ecology and Evolution 6(7):2125–2138. DOI:10.1002/ECE3.2012.
- Kousteni V, Bakiu R, Benhmida A, Crocetta F, Di Martino V, Dogrammatzi A, Doumpas N, Durmishaj S, Giovos I, Gökoğlu M, Huseyinoglu M, Jimenez C, Kalogirou S, Kleitou P, Lipej L, Macali A, Petani A, Petović S, Prato E, Fernando R, Sghaier Y, Stancanelli B, Teker S, Tiralongo F, Trkov D. 2019. New Mediterranean Biodiversity Records 2019. Mediterranean Marine Science 20(1):230–247. DOI:ORG/10.12681/mms.19609.
- Kousteni V, Kasapidis P, Kotoulas G, Megalofonou P. 2016. Evidence of high genetic connectivity for the long- nose spurdog Squalus blainville in the Mediterranean Sea. Mediterranean Marine Science 17(2):371–383. DOI:10.12681/mms.1222.
- Krefft G. 1968. Knorpelfische (Chondrichthyes) aus dem tropischen Ostatlantik. Atlantide Rep 10:32–80.
- Kroodsma DA, Mayorga J, Hochberg T, Miller NA, Boerder K, Ferretti F, Wilson A, Bergman B, White TD, Block BA, Woods P, Sullivan B, Costello C, Worm B. 2018. Tracking the global footprint of fsheries. Science 359(6378):904–908. DOI:10.1126/science.aao5646.
- Lacépéde E. 1798. Histoire Naturelle de Poissons. Paris. pp. 1798–1804.
- Lapinsky M, Giovos I. 2019. Novi nalazi kritično ugroženog sklata sivca Squatina squatina(Linnaeus, 1758) s otoka Korzike, Francuska. Acta Adriatica 60(2):205–210. DOI:10.32582/aa.60.2.10.
- Last PR, Weigmann S, Yang L. 2016b. Changes to the nomenclature of the skates (Chondrichthyes: Rajiformes). In: Last PR, Yearsley GK, editors. Rays of the World: Supplementary information. Melbourne: CSIRO Special Publication. pp. 11–34.
- Last PR, White WT, de Carvalho MR, Séret B, Stehmann MFW, Naylor GJP. 2016a. Rays of the World. Melbourne: CSIRO Publishing & Cornell University Press. pp. 790.
- Last PR, White WT, Pogonosk JJ 2007. Descriptions of new dogfishes of the genus Squalus (Squaloidea: Squalidae). Series: CSIRO Marine and Atmospheric Research Paper; 14. 130 pp.
- Lauria V, Gristina M, Attrill MJ, Fiorentino F, Garofalo G. 2015. Predictive habitat suitability models to aid conservation of elasmobranch diversity in the central Mediterranean Sea. Scientific Reports 5(1):13245. DOI:10.1038/SREP13245.
- Lawson JM, Pollom RA, Gordon CA, Barker J, Meyers EKM, Zidowitz H, Ellis JR, Bartoli A, Morey G, Fowler SL, Alvarado DJ, Fordham SV, Sharp R, Hood AR, Dulvy NK. 2019. Extinction risk and conservation of critically endangered angel sharks in the Eastern Atlantic and Mediterranean Sea. – ICES Journal of Marine Science. DOI: 10.1093/ICESJMS/FSZ222.
- Leone A, Carlisle A, Serena F, Ruth R, Litvin SY, Ferretti F 2014. Restoring the history of sawfish in the Mediterranean from museum exhibits. Associazione Nazionale Musei Scientifici Congress. Museo di Storia Naturale del Mediterraneo Livorno 11-13 novembre 2014-Italy. Poster.
- Leone A, Puncher GN, Ferretti F, Sperone E, Tripepi S, Micarelli P, Garibaldi F, Bressi N, Dall’Asta A, Minelli D, Cilli E, Vanni S, Serena F, Diaz-Jaimes P, Baele G, Cariani A, Tinti F. 2020. 2020. Pliocene Colonization of the Mediterranean by Great White Shark Inferred from Fossil Records, Historical Jaws, Phylogeographic and Divergence Time Analyses. J Biogeogr. 00:1–11. DOI: 10.1111/jbi.13794.
- Lloris D, Rucabado J. 1998. Guide FAO d’identification des espèces pour les besoins de la pêche. In: FAO, editor. Guide d’Identification des Ressources Marines Vivantes du Maroc. Rome: FAO. pp. 263
- Mahmoud MSF. 2016. Deep-sea ichthyofauna from Eastern Mediterranean Sea, Egypt: Update and new records. Egyptian Journal of Aquatic Research 42(4):479–489.
- Mancini R 1922. L’Arcipelago Toscano. Seconda crociera di pesca marittima. Ministero per l’Agricoltura, Ispettorato Generale della Pesca, Italy.
- Mancusi C, Nicolosi P, Arculeo M, Barbagli F, Carlini R, Costantini M, Doria G, Fabris G, Maio N, Mattioli G, Mizzan L, Podestà M, Salmaso R, Vanni S, Zuffi M, Serena F, Vacchi M. 2000. The presence of Elasmobranchs in the collections of the main Italian Natural History Museums. In: Vacchi M, La Mesa G, Serena F, Séret B, editors. Proc. 4th Europ. Elasm. Assoc. Meet. Livorno (Italy): ICRAM, ARPAT & SFI, 2002. pp. 97–108.
- Mancusi C, Baino R, Fortuna C, De Sola L, Morey G, Bradai MN, Kallianotis A, Soldo A, Hemida F, Saad A, Dimech M, Peristeraki P, Bariche M, Clò S, De Sabata E, Castellano L, Garibaldi F, Lanteri L, Tinti F, Pais A, Sperone E, Micarelli P, Poisson F, Sion L, Carlucci R, Cebrian-Menchero D, Séret B, Ferretti F, El-Far A, Saygu I, Shakman E, Bartoli A, Guallart J, Damalas D, Megalofonou P, Vacchi M, Colloca F, Bottaro M, Notarbartolo Di Sciara G, Follesa M, Cannas R, Kabasakal H, Zava B, Cavlan G, Jung A, Abudaya M, Kolitari J, Barash A, Joksimovic A, Cetkovic I, Marčeta B, Gonzalez VL, Tiralongo F, Giovos I, Bargnesi F, Lelli S, Barone M, Moro S, Mazzoldi C, Charis C, Abella A, Serena F. 2020. MEDLEM database, a data collection on large Elasmobranchs in the Mediterranean and Black seas. Mediterranean Marine Science:276–288. DOI: HTTPS://DOI.ORG/10.12681/MMS.21148.
- Mancusi C, Clò S, Affronte M, Bradaï MN, Hemida F, Serena F, Soldo A, Vacchi M. 2005. On the presence of basking shark (Cetorhinus maximus) in the Mediterranean Sea. Sur la présence du requin-pèlerin (Cetorhinus maximus) en Méditerranée. Cybium 29(4):399–405.
- Manjaji-Matsumoto BM, Last PR. 2008. Himantura leoparda sp. nov., a new whipray (Myliobatoidei: Dasyatidae) from the Indo–Pacific. Descriptions of new Australian Chondrichthyans, CSIRO Mar. Atm. Res. Pap. 22:293–301.
- Maravelias CD, Tserpes G, Pantazi M, Peristeraki P. 2012. Habitat Selection and Temporal Abundance Fluctuations of Demersal Cartilaginous Species in the Aegean Sea (Eastern Mediterranean). PLoS ONE 7(4):e35474. DOI:10.1371/JOURNAL.PONE.0035474.
- Marino IAM, Finotto L, Colloca F, Di Lorenzo M, Gristina M, Farrell ED, Zane L, Mazzoldi C. 2017. Resolving the ambiguities in the identification of two smooth-hound sharks (Mustelus mustelus and Mustelus punctulatus) using genetics and morphology. Marine Biodiversity. DOI: 10.1007/s12526-017-0701-8.
- Marouani S, Chaâba R, Kadri H, Saidi B, Bouain A, Maltagliati F, Last P, Séret B, Bradaï MN. 2012. Taxonomic research on Squalus megalops (Macleay, 1881) and Squalus blainvillei (Risso, 1827) (Chondrichthyes: Squalidae) in Tunisian waters (central Mediterranean Sea). Scientia Marina 76(1):97–109. DOI:10.3989/scimar.03440.22B.
- Marsili S. 2007. Revision of the teeth of the genus Carcharhinus (Elasmobranchii; Carcharhinidae) from Pliocene of Tuscany, Italy. Rivista Italiana di Pleontologia e Stratigrafia 113(1):79–95.
- Massi D, Titone A, Mancusi C, Serena F, Fiorentino F. 2017. First finding of Dipturus nidarosiensis (Storm, 1881) (Chondrichthyes: Elasmobranchii, Rajidae) egg capsule in the Strait of Sicily. Biologia Marina Mediterranea 24(1):194–195.
- Massutí E, Moranta J. 2003. Demersal assemblages and depth distribution of elasmobranchs from the continental shelf and slope trawling grounds off the Balearic Islands (western Mediterranean). ICES Journal of Marine Science 60(4):753–766. DOI:10.1016/S1054-3139(03)00089-4.
- Massutí E, Reñones O. 2005. Demersal resource assemblages in the trawl fishing grounds off the Balearic Islands (western Mediterranean). Scientia Marina 69(1):167–181. DOI:10.3989/scimar.2005.69n1167.
- Mate I, Barrull J, Bueno M. 1999. Observaciones de tiburones (Chondrichtyes Euselachii) en aguas de Catalunya (Mediterráneo NO), con algunos aspectos generales de su ecología. Scientia Gerundensis 24:127–151.
- Mater S, Meriç N, 1996. Türkiye Omurgalılar Tür Listesi: 129-172. In: Deniz Balıkları Pisces. Kence A, Bilgin C, (Eds). Nurol Matbaacılık A.Ş Publishers, Ankara, Turkey. (In Turkish).
- Maurin C, Bonnet M. 1970. Poissons des côtesnord-ouest africaines (campagnes de la Thalassa), (1962 et 1968). Revue des Travaux de l’Institut scientifique et technique des Pêches maritimes 34:125–170.
- Maynou F, Sbrana M, Sartor P, Maravelias C, Kavadas S, Damalas D, Osio G. 2011. Estimating trends of population decline in long‐lived marine species in the Mediterranean Sea based on fishers’ perceptions. PLoS ONE 6(7):e21818. DOI:10.1371/journal.pone.0021818.
- Mazhar FMM. 1982. The elasmobranchs of the Mediterranean. VI. Three torpedos. Bulletin of the Institute of Oceanography and Fisheries 8(2):109–135.
- McClenachan L, Ferretti F, Baum JK. 2012. From archives to conservation: Why historical data are needed to set baselines for marine animals and ecosystems. Conservation Letters 5(5):349–359. DOI:10.1111/j.1755-263X.2012.00253.x.
- McEachran JD, Capapé C. 1984. Rhinopteridae. In: Whitehead PJP, Bauchot M-L, Hureau J-C, Nielsen J, Tortonese E, editors. Fishes of the North-eastern Atlantic and the Mediterranean. Vol. 1. Paris, France: UNESCO. pp. 208–209.
- McEachran JD, Séret B. 1990. Rhinopteridae. In: Quero JC, Hureau JC, Karrer C, Post A, L. S, editors. Check-list of the Fishes of the Eastern Tropical Atlantic (CLOFETA). Paris, France: UNESCO. Vol. 1 pp. 71–72.
- McEachran JD, Dunn KA. 1998. Phylogenetic analysis of skates, a morphologically conservative clade of elasmobranchs (Chondrichthyes: Rajidae). Copeia 1998(2):271–290. DOI:10.2307/1447424.
- McEachran JD, Sèret B. 1987. Allocation of the name Sphyrna tudes (Valenciennes, 1822) and status of the nominal species Sphyrna couardi Cadenat, 1951 (Chondrichthyes, Sphirnidae). Cybium 11(1):39–46.
- Megalofonou P, Damalas D, Yannopoulos C. 2005. Composition and abundance of pelagic shark by-catch in the eastern Mediterranean Sea. Cybium 29:135–140.
- Megalofonou P, Damalas D, Yannopoulos C, De Metrio G, Deforio M, De la Serna JM, Macias D 2000. Bycatches and discards of sharks in the large pelagic fisheries in the Mediterranean Sea. Final Report of the Project No. 97/50 DG XIV/C1. 336 p. and Annex.
- Mejuto JB, Garcia–Cortés B, de la Serna JM. 2002. Preliminary scientific estimations of by–catches landed by the Spanish surface longline fleet in 1999 in the Atlantic Ocean and Mediterranean Sea. Col. Vol. Science Paper ICCAT 54(4):1150–1163.
- Melendez MJ, Báez JC, Serna-Quintero JM, Camiñas JA, IdL F, Real R, Macias D. 2017. Historical and ecological drivers of the spatial pattern of Chondrichthyes species richness in the Mediterranean Sea. PLoS ONE 12(4):e0175699 PONE.0175699 10.1371/journal.pone.0175699
- Menesini E. 1968. Ittiodontoliti Miocenici di Terra d’Otranto (Puglia) Paleont. It 65:1–61.
- Menesini E. 1974. Ittiodontoliti delle formazioni Terziarie dell’Arcipelago Maltese. Paleont It 67:121–162.
- Menni RC, Lucifora LO. 2007. Condrictios de la Argentina y Uruguay. ProBiota, FCNyM, UNLP, Serie Técnica-Didáctica, La Plata, Argentina, 11:1–15.
- Monteiro P, Ribeiro D, Silva JA, Bispo J, Gonçalves JMS. 2008. Ichthyofauna assemblages from two unexplored Atlantic seamounts: Northwest Bank and João Valente Bank (Cape Verde archipelago). Scientia Marina 72(1):133–143.
- Moranta J, Stefanescu C, Massutı E, Morales-Nin B, Lloris D. 1998. Fish community structure and depth-related trends on the continental slope of the Balearic Islands (Algerian basin, western Mediterranean). Marine Ecology Progress Series 171:247–259. DOI:10.3354/meps171247.
- Moreno JA. 1987. Jaquetones. In: Ministerio de Agricoltura, Pesca y Alimentacion, Secretaria General Tecnica, editor. Tiburones del genero Carcharhinus del Atlantico Oriental y Mediterraneo Occidental. Madrid: Ministerio de Agricoltura, Pesca y Alimentacion, Secretaria General Tecnica. pp. 250
- Moreno JA, Hoyos A. 1983. Première capture en eaux espagnoles et en Méditerranée de Carcharhinus altimus (S. Sprinter, 1950). Cybium 71:65–70.
- Moreno JM. 1995. Guia de los tiburones de aguas ibèricas, Atlantico Nororiental y Mediterraneo (Guide of sharks from Iberian waters, northeastern Atlantic and Mediterranean). Madrid: Piramide édit. pp. 310.
- Morey G, Soldo A, Riera F, Serena F. 2008. Records of Carcharhinus limbatus and C. plumbeus (Chondrichthyes: Carcharhinidae) from off the Balearic Islands (NW Mediterranean). Cybium 32(3):195–200.
- Moro S, Jona-Lasinio G, Block B, Micheli F, De Leo G, Serena F, Bottaro M, Scacco U, Ferretti F. 2019. Abundance and distribution of the white shark in the Mediterranean Sea. Fish Fish 00:1–12. DOI: ORG/10.1111/FAF.12432.
- Mouneimne N. 1977. Liste des poissons de la côte du Liban (Méditerranée orientale) (Check-list of fishes from the coast of Lebanon (eastern Mediterranean)). Cybium 1:37–66.
- Muammer Oral M. 2010. Alien fish species in the Mediterranean – Black Sea Basin. Journal Black Sea/Mediterranean Environment 16(1):87–132.
- Mumtaz ET, Başusta N. 2018. Near term embryos and gravid females of Lusitanian cownose ray (Rhinoptera marginata) in Mersin Bay, the eastern Mediterranean Sea. CSIRO PUBLISHING Marine and Freshwater Research. DOI: ORG/10.1071/MF17356.
- Muñoz-Chápuli R, Ortega AP. 1985. Resurrection of Galeus atlanticus (Vaillant, 1888), as a valid species from the NE-Atlantic Ocean and the Mediterranean Sea. Bulletin Musium National Histry Natural, Sér. 4, Sect. A 7(1):219–233.
- Muñoz-Chápuli R, Ramos F. 1989. Morphological comparison of Squalus blainvillei and S. megalops in the eastern Atlantic, with notes on the genus. Japanese Journal of Ichthyology 36(1):6–21. DOI:10.1007/BF02905668.
- Muñoz-Chápuli R, Ramos F, Garcia-Garrido CM. 1984. Squalus megalops (McLeay, 1882) en el Mediterraneo. Notas sobre su diagnosis, sistematica y distribucion. Bulletin Society. Cat. Ictio. Herp 9:16–21.
- Murray JW. 1991. Black Sea oceanography, result from the 1988 Black Sea Expedition. Deep Sea Research Part A. Oceanographic Research Papers 38(2):266 S665-S1 10.1016/S0198-0149(10)80002-0
- Myers RA, Baum JK, Shepherd T, Powers SP, Peterson CH. 2007. Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 315(5820):1846–1850. DOI:10.1126/science.1138657.
- Neifar L, Euzet L, Kalthoum Ben HO. 2000. New species of the Monocotylidae (Monogenea) from the stingray Dasyatis tortonesi Capapé (Euselachii, Dasyatidae) off the Tunisian coast, with comments on host-specificity and the specific identities of Mediterranean stingrays. Systematic Parasitology 47(1):43–50. DOI:10.1023/A:1006354423136.
- Nelson JS, Grande TC, Wilson MVH. 2016. Fishes of the world. 5th ed. New York: John Wiley & Sons, Inc. 707 pp.
- Nicolosi P, Lo Brutto S, Mancusi C, Farina S, Bellia E, Arculeo M, Serena F 2019. The Elasmobranchs collections in the Italian Natural History Museums. Book of Abstracts. EEA Annual Conferences, Rende, Italy. 132 pp.
- Ninni E 1912. Catalogo dei Pesci del Mare Adriatico (Tip. Carlo Bertotti, Venezia).
- Norman JR 1935. Coast Fish. I: The South Atlantic. “Discovery Rep.”, XII.
- Notarbartolo Di Sciara G, Lauriano G, Pierantonio N, Cañadas A, Donovan G, Panigada S. 2015. The Devil We Don’t Know: Investigating Habitat and Abundance of Endangered Giant Devil Rays in the North-Western Mediterranean Sea. PLoS ONE 10(11):e0141189 10.1371/journal.pone.0141189 PONE.0141189
- Oguz T, Latun V, Latif SMA, Vladimirov VV, Sur HI, Markov AA, Özsoy E, Kotvshchikov BB, Eremeev VV, Ünlüata Ü. 1993. Circulation on the surface and intermediate layers of the Black Sea. Deep Sea Research Part I: Oceanographic Research Papers 40(8):1597–1612. DOI:10.1016/0967-0637(93)90018-X.
- Ordines F, Baro J, Ramírez-Amaro S, Serena F, Sobrino I. 2017. First substantiated record of Raja asterias Delaroche, 1809 (Elasmobranchii: Rajiformes: Rajidae) in the Gulf of Cádiz, north-eastern Atlantic. Acta Ichthyologica Et Piscatoria 47(1):101–106. DOI:10.3750/AIEP/02161.
- Orsi Relini L. 1998. Carcharinus brachyurus (Guenther, 1870) nel Museo dell’Istituto di Zoologia, Università di Genova. Bollettino Musium Ist. Biology Universitat Genova 62–63:93–98.
- Otero M, Serena F, Gerovasileiou V, Barone M, Bo M, Arcos JM, Vulcano A, Xavier J. 2019. Identification guide of vulnerable species incidentally caught in Mediterranean fisheries. Malaga, Spain: IUCN. pp. 204.
- Öztürk B. 1999. Black Sea Biological Diversity Turkey. In: United Nation Publications, editor.GEF Black Sea Environmental Programme. New York, USA: United Nations Publications. pp. 228
- Papaconstantinou C, Vassilopoulou V, Petrakis G, Caragitsou E, Mytilinaeou C, Fourtouni A, Politou C–Y. 1994. The demersal fish fauna of the North and West Aegean Sea. Bioscience 2:35–45.Editore. 388 pp.
- Pastore M, Tortonese E. 1985. Prima segnalazione in Mediterraneo dello squalo Rhizoprionodon acutus (Ruppell). Thalassia salentina 14:11–15.
- Peristeraki P, Kypraios N, Lazarakis G, Tserpes G. 2008. By-catches and discards of the Greek swordfish fishery. IC- CAT. Col. Vol. Science Papers 62(4):1070–1073.
- Pinardi N, Arneri E, Crise A, Ravaioli M, Zavatarelli M. 2006. The physical, sedimentary and ecological structure and variability of shelf areas in the Mediterranean Sea (27). In: Robinson AR, Brink KA, editors. The Sea. Cambridge, MA: Harvard University Press. 1245–1331.
- Pinto de la Rosa FJ. 1994. Tiburones del mar de Alboran. Diputacion de Malaga: Servicio publicaciones Centro de Ediciones. pp. 115.
- Poll M. 1951. Poissons I. Généralité. II Sélaciens et chiméres. Résearch Science Expéd. Océanography Belgique Eaux Cot. African Atl. Sud 1948-1949 4(1):1–154.
- Poortvliet M, Olsen JL, Croll DA, Bernardi G, Newton K, Kollias S, Sullivan JO, Fernando D, Stevens G, Galván F, Magaña FG, Séret B, Wintner S, Hoarau G. 2015. A dated molecular phylogeny of manta and devil rays (Mobulidae) based on 830 mitogenome and nuclear sequences. Molecular Phylogenetics and Evolution 83:72–85. DOI:10.1016/j.ympev.2014.10.012.
- Psomadakis PN, Vacchi M, Di Muccio S, Sarà M. 2009. First historical record of Carcharhinus brachyurus (Chondrichthyes, Carcharhiniformes) in the Mediterranean Sea. Italian Journal of Zoology 76(2):201–207. DOI:10.1080/11250000802364673.
- Quèro J-C. 1984. Alopiidae. In: Whitehead PJP, Bauchot M-L, Hureau J-C, Nielsen J, Tortonese E, editors. Fishes of the North-eastern Atlantic and the Mediterranean. Paris, France: UNESCO. Vol. 1, pp. 91–92.
- Quetglas A, Rueda L, Alvarez-Berastegui D, Guijarro B, Massutí E. 2016. Constrasting responses to harves¬ting and environmental drivers of fast and slow life history species. PLoS ONE 11(2):e0148770. DOI:10.1371/journal.pone.0148770.
- Quignard J-P, Ben Othman S. 1978. Les poissons du golfe de Gabès: Situation actuelle et future. Bulletin Institute National Science Technology Océanography Pêche Salammbô 5(1–4):43–52.
- Quignard JP, Capapé C. 1971. Liste commentée des Sélaciens de Tunisie. Bulliten De Institute Natn. Scientist Technology Océanography Pêche, Salammbô 2:131–141.
- Quignard JP, Tomasini JA. 2000. Mediterranean fish biodiversity. Biologia Marina Mediterranea 7(3):1–66.
- Ragonese S, Cigala Fulgosi F, M L B, Norrito G, Sinacori G. 2003. Annotated check list of the skates (Chondrichthyes. Rajidae) in the Strait of Sicily (Central Mediterranean). Biologia Marina Mediterranea 10(2):874–881.
- Ragonese S, Vitale S, Dimech M, Mazzola S. 2013. Abundances of Demersal Sharks and Chimaera from 1994-2009 Scientific Surveys in the Central Mediterranean Sea. PLoS ONE 8(9):e74865. DOI:10.1371/JOURNAL.PONE.0074865.
- Ramírez-Amaro S, Ordines F, Puerto MÁ, García C, Ramon C, Terrasa B, Massutí E. 2017. New morphological and molecular evidence confirm the presence of the Norwegian skate Dipturus nidarosiensis (Storm, 1881) in the Mediterranean Sea and extend its distribution to the western basin. Mediterranean Marine Science 18(2):251–259. DOI:10.12681/MMS.1950.
- Ramírez-Amaro S, Ordines F, Terrasa B, Esteban A, García C, Guijarro B, Massutí E. 2015. Demersal chondrichthyans in the western Mediterranean: Assemblages and biological parameters of their main species. Marine and Freshwater Research 67(5):636–652. DOI:10.1071/MF15093.
- Ratnasingham S, Hebert PDN. 2007. BOLD: the barcode of life data system (http://www. barcodinglife. org). Molecular Ecology Notes. 7(3):355–364.
- Relini G, Biagi F, Serena F, Belluscio A, Spedicato MT, Rinelli P, Follesa MC, Piccinetti C, Ungaro N, Sion L, Levi D. 2000. Selachians fished by otter trawl in the Italian Seas. Biologia Marina Mediterranea 7:347–384.
- Relini G, Mannini A, De Ranieri S, Bitetto I, Follesa MC, Gancitano V, Manfredi C, Casciaro L, Sion L. 2010a. Chondrichthyes caught during the Medits surveys in the Italian waters. Biology Marine Mediterranean 17(1):193–197.
- Relini G, Serena F, Bottaro M. 2010b. Il progetto ELASMOIT. Biology Marine Mediterranean 17(1):205–218.
- Rey J, Coelho R, Lloris D, Séret B, Gil de Sola L. 2010. Distribution pattern of Galeus atlanticus in the Alborán Sea (south western Mediterranean) and some sexual character comparison with Galeus melastomus. Marine Biology Research 6(4):364–372. DOI:10.1080/17451000903042487.
- Ricklefs RE. 1979. Ecology. 2nd ed. New York: Chiron Press. 966 pp.
- Robins CR, Ray GC. 1986. A field guide to Atlantic coast fishes of North America. Houghton Mifflin Company, Boston, U.S.A. 354 pp.
- Risso A, 1810. Ichthyologie de Nice. Nizza.
- Roskov Y, Ower G, Orrell T, Nicolson D, Bailly N, Kirk PM, Bourgoin T, DeWalt RE, Decock W, Nieukerken E, van Zarucchi J, Penev L. eds. 2020. Species 2000 & ITIS Catalogue of Life, 25th March 2019. Digital resource at www.catalogueoflife.org/col. Species 2000: Leiden, the Netherlands: Naturalis. 2405-8858
- Roux C. 1984. Squatinidae. In: Whitehead PJP, Bauchot M-L, Hureau J-C, Nielsen J, Tortonese E, editors. Fishes of the North-eastern Atlantic and the Mediterranean. Vol. 1. Paris, France: (UNESCO. pp. 510.
- Saad A, Séret B, Ali M. 2005. Liste commentée des Chondrichthyens de Syrie [Annotated list of Chondrichthyans from the coast of Syria]. Rapport de la Commission Internationale pour l’Exploration Scientifique de la mer Méditerranée 37:430.
- Saadaoui A. 2010. Recherche sur la systématique des Dasyatidés: Statut de Dasyatis tortonesei (Capapé, 1977) en Tunisie. Master. Tunisie: Faculté des Sciences de Sfax.
- Scharpf C, Lazara KJ 2019. The ETYFish Project Fish Name Etymology Database.
- Schembri T, Fergusson IK, Schembri PJ. 2003. Revision of the records of shark and ray species from the Maltese islands (Chordata: Chondrichtyes). The Central Medierranean Naturalist, Malta 4:71–104.
- Schrynmakers R. 2001. Etude biométrique et méristique des raies pastenagues du genre Dasyatis (Chondrichthyes: Dasyatidae) d’Atlantique Nord et de Méditerranée. In: Muséum National d'Histoire Naturelle, editor. Université Pierre Et Marie Curie Paris VI. Paris: Muséum National d’Histoire Naturelle. pp. 29
- Schwartz FJ, Burgess GH. 1975. Sharks of North Carolina and adjacent waters. Information Series. Morehead City: North Carolina Department of Natural and Economic Resources. pp. 57.
- Serena F, Vacchi M, Notarbartolo Di Sciara G. 2000. Geographical distribution and biological information on the basking shark, C. maximus in the Tyrrhenian and Ligurian Seas. In: Séret B, Sire J-Y, editors. Proc. 3rd Europ. Elasm. Assoc. Meet., Boulogne sur-Mer. Paris: Soc. Fr. Ichtyol. & IRD. pp. 47–56.
- Serena F. 2005. Field identification guide to the sharks and rays of the Mediterranean and Black Sea. In: FAO, editor. FAO Species Identification Guide for Fishery Purposes. Rome: FAO.pp.97 11 colour plates + egg cases
- Serena F. 2007. Analysis of the elasmobranchs data collected in the frame of the MEDITS project and their potential use for stock assessment and management advice. GFCM workshop on trawl survey based monitoring fishery system in the Mediterranean Rome. Italy 26-28(March):2007.
- Serena F, Papacostantinou C, Relini G, Gil De Sola L, Bertrand J. 2009. Distribution and Abundance of Spiny Dogfish in the Mediterranean Sea based on the Mediterranean International Trawl Surveys Program. In: Gallucci VF, McFarlane GA, Bargmann GC, editors. Biology and management of dogfish sharks. Bethesda, Maryland: American Fisheries Society. pp. 139–149.
- Serena F, Abella AJ, Baino R, Cannas R, Carbonara P, Carlucci R, Facchini MT, Ferrari A, Follesa MC, Gancitano V, Garibaldi F, Garofalo G, Giusto GB, Lanteri L, Mancusi C, Manfredi C, Mannini A, Sartor P, Sbrana M, Sinacori G, Sion L, Tinti F 2018. Preliminary considerations of the status of elasmobranchs in the Italian waters. Poster REF.FF2018_TS1-2_ SERENA. In: FAO. 2018. Fish Forum 2018: Book of abstract. Rome, Licence: CC BY-NC-SA 3.0 IGO. 338 pp.
- Serena F, Abella JA, Baino R, Carbonara P, Facchini MT, Ferrari A, Follesa MC, Gancitano V, Garibaldi F, Manfredi C, Mancusi C, Sartor P, Sbrana M, Sion L, Tinti F. 2014. Lo status degli elasmobranchi dei mari italiani (Elasmostat). I Programma Nazionale triennale della pesca e dell’acquacoltura 2007-2009 (prorogato a tutto il 2012). Progetto di ricerca: “7 – Tematica A3”. Rapporto finale, 28 febbraio 321. DOI: 10.13140/2.1.1721.4723.
- Serena F, Ardizzone G, Baino R, Belluscio A, Bertrand J, Carbonara P, Cau A, De Ranieri S, Dimech M, D’onghia G, Follesa C, Garofalo G, Gil de Sola L, Giordano D, Gristina M, Mannini A, Papacostantinou C, Pasolini P, Pastorelli AM, Relini G, Rinelli P, Sartor P, Sion L, Spedicato MT, Tinti F, Ungaro N. 2011. Considerations on the EU Project - Fish/2004/03-41: Status of, ray populations in the Mediterranean Sea and advice for sustainable exploitation of the stocks GFCM-Scientific Advisory Committee (SAC) Workshop on Stock Assessment of Selected Species of Elasmobranchs in the GFCM area Brussels (Belgium), 12-16 December 2011. 36 pp.
- Serena F, Baino R, Rey J, Papacostantinou C, Relini G. 2005a. Catch composition and abundance of deep-sea elasmobranchi based on the MEDITS trawl survey. In: FAO Report on DEEP SEA 2003, an International Conference on Governance and Management of Deep-sea Fisheries. Queenstown, New Zealand: 1-5 December. Rome: FAO. FAO Fisheries Report No. 772. pp. 395–408
- Serena F, Cecchi E, Mancusi C, Pajetta R. 2005b. Contribution to the knowledge of the biology of Etmopterus spinax (Linnaeus, 1758) (Chondrichthyes, Etmopteridae). In: FAO Report on DEEP SEA 2003, an International Conference on Governance and Management of Deep-sea Fisheries. Queenstown, New Zealand: 1-5 December. FAO Fisheries Report No. 772. Rome. pp. 388–394
- Serena F, Silvestri R. 2018. Preliminary observations on juvenile shark catches as by-catch of the Italian fisheries with particular attention to the Tuscany coasts. In Proceedings of 7 Congress of Codice Armonico, 18b/Scientific Section. Rosignano Marittimo (Li): 158–169
- Serena F, Silvestri R, Voliani A. 1999. Su una cattura accidentale di Taeniura grabata (E. Geoffroy Saint-Hilaire, 1817) (Chondrichthyes, Dasyatidae). Biology Marine Mediterranean 6(1):617–618.
- Serena F, Soldo A, Dulvy N. 2019. Proposal for IUCN-SSG discussion inside of EEA Annual Conference 2019. Book of Abstract. Annual Conference of the European Elasmobranch Association 16–18 October 2019- Rende, Italy
- Séret B. 2016. Batoid fishes. Multiple families (Pp. 1337-1369, 1404-1433). In: Carpenter & De Angelis (eds.). 2016. The living marine resources of the Eastern Central Atlantic. Volume 2: Bivalves, gastropods, hagfishes, sharks, batoid fishes, and chimaeras. FAO Species Identification Guide for Fishery Purposes. Rome: FAO. pp. 665–1509.
- Séret B, McEachran JD 1986. Catalogue critique des types de poisons du Muséum national d’Histoire naturelle. Bulletin de Muséum National d’Histoire Naturelle, Paris, 4ème Série, 8, Section A, n°4 (Supplément): 3–50.
- Serghini M, Boutayeb A, Boumâazi A, Srairi A, Mesoui A, Zoubi A, Dridi A. 2008. Stability of the spatial structures of demersal assemblage in the Moroccan southern Atlantic zone. Applied Ecology and Environmental Research 6(1):117–127. DOI:10.15666/aeer/0601_117127.
- Shlyakhov VA, Daskalov GM. 2008. The state of marine living resources/State of the Environment of the Black Sea (2001–2006/7). In: Ogus T, editor. Publication of the Commission on the Protection of the Black Sea Against Pollution (BSC). Istanbul, Turkey: Commission on the Protection of the Black Sea Against Pollution (BSC). pp. 321–364.
- Sims D, Fowler SL, Clò S, Jung A, Soldo A, Bariche M. 2016. Cetorhinus maximus. The IUCN Red List of Threatened Species. 2016: E.T4292A16527877.
- Sion L, Bozzano A, D’Onghia G, Capezzuto F, Panza M. 2004. Chondrichthyes species in deep waters of the Mediterranean Sea. Scientia Marina 68(S3):153–162. DOI:10.3989/scimar.2004.68s3153.
- Soares KDA, De Carvalho MR. 2019. The catshark genus Scyliorhinus (Chondrichthyes: Carcharhiniformes: Scyliorhinidae): Taxonomy, morphology and distribution. Zootaxa 4601(1):001–147. DOI:10.11646/zootaxa.4601.1.1.
- Soldo A, Bariche M. 2016. Squatina aculeata. In: The IUCN Red List of Threatened Species 2016: e.T61417A16569265
- Soldo A, Peirce R. 2005. Shark chumming in the eastern Adriatic. Annales, Series Historia Naturalis 15:203–208.
- Spaet JLY, Cochran JEM, Berumen ML. 2011. First record of the Pigeye Shark, Carcharhinus amboinensis (Müller & Henle, 1839) (Carcharhiniformes: Carcharhinidae), in the Red Sea. Zoology in the Middle East 52(1):118–121. DOI:10.1080/09397140.2011.10638488.
- Spedicato MT, Massutí E, Mérigot B, Tserpes G, Jadaud A, Relini G. 2019. The MEDITS trawl survey specifications in an ecosystem approach to fishery management. Science Marine 83S1 9–20. DOI: ORG/10.3989/SCIMAR.04915.11X.
- Sperone E, Coppola F, Parise G, Bernabò RFR, Micarelli P, Giglio G, Milazzo C. 2018. Confirmation of the presence of the bigeye thresher Alopias superciliosus in the Tyrrhenian Sea, with first parasitological notes for the Mediterranean Sea. Cahiers de Biologie Marine 59(2):181–185. DOI:10.21411/CBM.A.EA9CAF0A.
- Springer S. 1950. A revision of North American sharks allied to the genus Carcharhinus. Am. Musium Novitates 1451:13.
- Stehmann M, Bürkel DL. 1984. Rajidae. In: Whitehead PJP, Bauchot M-L, Hureau J-C, Nielsen J, Tortonese E, editors. Fishes of the North-eastern Atlantic and the Mediterranean. Vol. 1. Paris, France: UNESCO. pp. 163–196.
- Stehmann M. 1995. A record of Raja clavata, the eastern Atlantic thornback skate, from the southern Madagascar Ridge at Walters Shoal (Elasmobranchii, Rajidae). Journal of Ichthyology 35:63–74.
- Tai I, Benchri S, Zoubai A, Ramdani M, Yahyaoui A, Bazairi H. 2010. Contribution à l’étude de la reproduction et de la croissance de la raie étoilée Raja asterias Delaroche, 1809 dans la région nord atlantique marocaine en 2005. Bulletin De l’Institut Scienti Que, Rabat, Section Sciences De La Vie 32(2):73–80.
- Tobuni I, Abdallah AB, Serena F, Shakman E. 2016. First documented presence of Galeocerdo cuvier (Péron & Lesueur, 1822) (ELASMOBRANCHII, CARCHARHINIDAE) in the Mediterranean basin (Libyan waters). Cambridge University Press. Marine Biodiversity Records 9(1):94. DOI:10.1186/s41200-016-0089-3.
- Torchio M 1960. Rinvenimento del primo maschio di Raja melitensis Clark. Natura, Milano 51, V:65-69, 1 fig.
- Tortonese E. 1937–1938. Intorno agli squali del genere Alopias. Bollettino dei Musei di Zoologia e Anatomia Comparata della Università di Torino 78:1–7.
- Tortonese E. 1951. Studio sui Plagiostomi V – Ulteriori considerazioni sulle specie mediterranee dei generi Sphyrna e Carcharhinus. Analele Museum Stonian Natural 20(1):1–8.
- Tortonese E 1956. Leptocardia, Cyclostomata, Selachii. Fauna d’Italia, Vol II. Ed. Calderini, Bologna. 545 pp.
- Tortonese E. 1986. Gli Squali mediterranei del genere Hexanchus (Chondrichthyes). Atti. Society Italiana Sci. Nature 126(3–4):137–140.
- Tortonese E. 1987. Pesci del Mediterraneo; recenti studi intorno alla sistematica e distribuzione. In: Numero speciale dei Quaderni dell’Istituto di Idrobiologia e Acquacoltura “G. Brunelli”. Roma: Cooperativa Editrice Il Ventaglio. pp. 11.
- Tortonese T. 1989. Osservazioni comparative intorno alla ittiofauna del Mediterraneo e dell’Atlantico occidentale (Florida e isole Bahamas). Natura 1:1–20.
- Tserpes G, Maravelias CD, Pantazi M, Peristeraki P. 2013. Distribution of relatively rare demersal elasmobranchs in the eastern Mediterranean. Estuarine, Coastal and Shelf Science 117:48–53. DOI:10.1016/j.ecss.2012.09.020.
- UNEP/MAP SPA/RAC. 2003. Action Plan for the conservation of cartilagineus fishes (Chondrichthyans) in the Mediterraean Sea. Tunis: Ed. SPA/RAC, 56 pp.
- UNEP/MAP SPA/RAC. 2018. Manuel de formation sur les poissons cartilagineux, identifier et reconnaître les raies et requins de Méditerranée. Par Mohamed Nejmeddine Bradaï, Béchir Saidi et Samira Enajjar, laboratoire Biodiversité Marine de l’INSTM. Ed. SPA/RAC & ASCOB-Syrtis, Tunis: 76 pp (52 pp + 3 Annexes).
- UNEP/MAP SPA/RAC. 2020. Action Plan for the Conservation of Cartilaginous Fishes (Chondrichtyans) in the Mediterranean Sea; by: Bradai, M N., Tunis: Ed SPA/RAC. 18 pp.
- Vacchi M, Serena F, Biagi V. 1996. Cattura di Carcharinus brachyurus (Guenther, 1870) (Pisces, Selachii, Carcharhinidae) nel Mar Tirreno Settentrionale. Biology Marine Mediterranean 3(1):389–390.
- Valavanidis A, Vlachogianni T. 2011. Ecosystems and biodiversity hotspots in the Mediterranean basin threats and conservation efforts. Science Advanced Environmental Toxicology (10):1–24.
- Valenciennes A. 1822. Sur le sous-genre Marteau, Zygaena. Memoir MusiumNaturalHistory Nature Paris 9:222–228.
- Vanni S. 1992. Cataloghi del Museo di Storia Naturale dell’Università di Firenze, Sezione di Zoologia “La Specola”. X.I. Chondrichyhyes. Atti Society Tosc Science Natural Memoir Serie B IC:85–114.
- Vasil’eva ED 2007. Ryby Chernogo morya. Opredelitel’morskih, solonovatovodnyh, evrigalinnyh i prohodnyh vidov s cvet- nymi illustraciami, sobrannymi Bogorodskim SV (Fishes of the Black Sea). Key to marine, brakish-water, eurihaline and anadromous species with colour illustrations collected by Bo- gorodsky SV. VNIRO Publishers, Moscow, Russia. 238 pp. (In Russian).
- Vella A, Vella N, Schembri S. 2017. A molecular approach towards taxonomic identification of elasmobranch species from Maltese fisheries landings. Marine Genomics 36:17–23. DOI:10.1016/j.margen.2017.08.008.
- Verissimo A, Cotton CF, Buch RH, Guallart J, Burgess GH. 2014. Species diversity of the deep-water gulper sharks (Squaliformes: Centrophoridae: Centrophorus) in North Atlantic waters - current status and taxonomic issues. Zoological Journal of the Linnean Society 172(4):803–830. DOI:10.1111/zoj.12194.
- Verissimo A, Zaera-Perez D, Leslie R, Iglesias SP, Séret B, Grigoriou P, Sterioti A, Gubili C, Barria C, Duffy C, Hernandez S, Batjakas IE, Griffiths AM. 2016. Molecular diversity and distribution of eastern Atlantic and Mediterranean dogfishes Squalus highlight taxonomic issues in the genus. Zoologica Scripta 46(4):414–428. DOI:10.1111/zsc.12224.
- Viana ST, De Carvalho MR, GOMES UL. 2016. Taxonomy and morphology of species of the genus Squalus Linnaeus, 1758 from the Southwestern Atlantic Ocean (Chondrichthyes: Squaliformes: Squalidae). Zootaxa 4133(1):1–089. DOI:10.11646/zootaxa.4133.1.1.
- Vinciguerra D 1884. Materiali per lo studio della fauna tunisina raccolti da G. et L. Doria. 1. Pesci. Ann. Mus. Civ. Stor. Natur. Genova: 393–445.
- Walker P, Cavanagh RD, Ducrocq M, Fowler SL. 2005. Chapter 7 – Regional Overviews: Northeast Atlantic (including Mediterranean and Black Sea). P86. In: Fowler SL, Cavanagh RD, Camhi M, Burgess GH, Cailliet GM, Fordham SV, Simpfendorfer CA, Musick JA, editors. Sharks, Rays and Chimaeras: The Status of the Chondrichthyan Fishes. Switzerland and Cambridge, UK:IUCN SSC Shark Specialist Group. IUCN, Gland, Switzerland and Cambridge, UK: 71–95.
- Walls RHL, Dulvy NK. 2020. Eliminating the dark matter of data deficiency by predicting the conservation status of Northeast Atlantic and Mediterranean Sea sharks and rays. Biological Conservation 246:108459. DOI:10.1016/j.biocon.2020.108459.
- Weigmann S. 2016. Annotated checklist of the living sharks, batoids and chimaeras (Chondrichthyes) of the world, with a focus on biogeographical diversity. Journal of Fish Biology 88(3):837–1037. DOI:10.1111/jfb.12874.
- White WT. 2014. A revised generic arrangement for the eagle ray family Myliobatidae, with definitions for the valid genera. Zootaxa 3860, N. 2: 149–166.
- White WT, Corrigan S, Yang L, Henderson AC, Bazinet AL, Swofford DL, Naylor GJP. 2017b. Phylogeny of the manta and devilrays (Chondrichthyes: Mobulidae), with an updated taxonomic arrangement for the family. Zoological Journal of the Linnean Society 182(1):50–75. DOI:10.1093/zoolinnean/zlx018.
- White WT, Ebert DA, Naylor GJP. 2017a. Revision of the genus Centrophorus (Squaliformes: Centrophoridae): Part 2—Description of two new species of Centrophorus and clarification of the status of Centrophorus lusitanicus Barbosa du Bocage & de Brito Capello, 1864. Zootaxa 4344(1):86–114. DOI:10.11646/zootaxa.4344.1.3.
- White WT, Ebert DA, Naylor GJP, Ho H-C, Clerkin P, Veríssimo A, Cotton CF. 2013. class=“HeadingRunIn”>Revision of the genus Centrophorus (Squaliformes: Centrophoridae): Part 1—Redescription of Centrophorus granulosus (Bloch & Schneider), a senior synonym of C. acus Garman and C. niaukang Teng. Zootaxa 3752(1):35. DOI:10.11646/ZOOTAXA.3752.1.5.
- Whitehead PJP, Bauchot M-L, Hureau JCV, Nielsen J, Tortonese E. 1984. Fishes of the North-eastern Atlantic and the Mediterranean (FNAM). Paris: UNESCO. pp. 510.
- Williams T, Helle K, Aschan M. 2008. The distribution of chondrichthyans along the northern coast of Norway. ICES Journal of Marine Science 65(7):1161–1174. DOI:10.1093/ICESJMS/FSN103.
- WoRMS Editorial Board (2020). World Register of Marine Species. Available from http://www.marinespecies.org at VLIZ. Accessed 202005-30. DOI:10.14284/170.
- Yankova M, Pavlov D, Ivanova P, Karpova E, Boltachev A, Öztürk B, Bat L, Oral M, Mgeladze M 2014. Marine fishes in the Black Sea: Recent conservation status. Mediterranean Marine Science Indexed in WoS (Web of Science, ISI Thomson) and SCOPUS The journal is available on line at http://www.medit-mar-sc.net. DOI:ORG/10.12681/MMS.700.
- Yucel N, Sakalli A, Karahan A. 2017. First record of the honeycomb stingray Himantura leoparda (Manjaji-Matsumoto & Last, 2008) (Myliobatoidei: Dasyatidae) in the Mediterranean Sea, confirmed by DNA barcoding. Journal of Applied Ichthyology 33(3):530–532. DOI:10.1111/jai.13283.
- Zava B, Ferrantelli V, Castiglione F, Fiorentino F. 2006. First record of the copper shark Carcharhinus brachyurus (Gunther, 1870) in the Tyrrhenian Sea. Biologia Marina Mediterranea 13(2):300–301.
- Zenetos A, Çinar ME, Pancucci-Papdopoulou MA, Harmelin JG, Furnari G, Andaloro F, Bellou N, Streftaris N, Zibrowius H. 2005. Annotated list of marine alien species in the Mediterranean with records of the worst invasive species. Mediterranean Marine Science 6(2):63–118. DOI:10.12681/mms.186.
- Zenetos A, Gofas S, Verlaque M, Cinar M, Garcia Raso J, Bianchi C, Morri C, Azzurro E, Bi̇lecenoğlu M, Froglia C, Siokou I, Violanti D, Sfriso A, San Martin G, Giangrande A, Katagan T, Ballesteros E, Ramos-Espla A, Mastrototaro F, Ocana O, Zingone A, Gambi M, Streftaris N. 2010. Alien species in the Mediterranean Sea by 2010. A contribution to the application of European Union’s Marine Strategy Framework Directive (MSFD). Part I. Spatial distribution. Mediterranean Marine Science 11(2):381. DOI:10.12681/mms.87.
- Zenetos A, Meriç E, Verlaque M, Galli P, Boudouresque C-F, Giangrande A, Çinar ME, Bi̇lecenoğlu M. 2008. Additions to the annotated list of marine alien biota in the Mediterranean with special emphasis on Foraminifera and Parasites. Mediterranean Marine Science 9(1):119–165. DOI:10.12681/mms.146.
