Abstract
The aim of the present work was to compare the oxidative system in the fourth-instar larvae of four species of Diptera belonging to the family Chironomidae: Chironomus riparius, Paratanytarsus sp., Prodiamesa olivacea and Conchapelopia melanops. These species were selected based on their ecological and biological differences and were assessed in relation to their ecological and biological characteristics. The following parameters of oxidative stress were determined: the levels of total antioxidant capacity (TAC) and reduced glutathione; the activity of the antioxidant enzyme catalase (CAT); the level of malondialdehyde (MDA), a product of lipid peroxidation; and glutathione S-transferase (GST) activity. Results demonstrated significant differences among taxa in the specific activity of most investigated parameters. Prodiamesa olivacea showed the greatest antioxidant protection (activity of CAT, level of reduced glutathione (GSH)) and the highest detoxification activities (activity of GST and level of GSH). Predatory C. melanops was shown to be the most promiscuous biomarker for assessing the total antioxidant activity. We demonstrate that the four chironomid taxa, occupying the same ecosystem, share an important antioxidant enzymatic and non-enzymatic pool, but with oxidative stress parameters differing among the four species.
1. Introduction
Numerous environmental pollutants induce oxidative stress. A balance between intracellular pro-oxidants (including extracellular stimuli like pollutants) and antioxidant systems may be used to assess environmental stress. For this reason, antioxidant enzymes have been considered sensitive biomarkers of oxidative stress, particularly in aquatic organisms (Valavanidis et al. Citation2006). Consequently, the persistence of a population exposed to a range of stressors depends on the protection provided by detoxifying and antioxidant mechanisms, whose function is the restoration of internal cellular homeostasis (Bonnefoy et al. Citation2002; Vertuani et al. Citation2004). Methods for studying oxidative stress in living organisms include evaluation of the activity of antioxidant enzymes, the level of low-molecular antioxidants, analysis of lipid peroxidation products and evaluation of the total systemic antioxidant potential. Oxidative stress resulting from exposure to chemicals is associated with changes in glutathione S-transferase activity, which catalyzes the conjugation of glutathione with xenobiotics in the second phase of their metabolism (Bukowska Citation2005). Levels of glutathione are also informative because this antioxidant plays an important role both in neutralizing reactive oxygen species (ROS) and in decreasing xenobiotic effects.
The use of bioindicators for the assessment of risks posed by pollution has grown significantly over the past few years. Bioindicators are useful markers of pollution because they are particularly sensitive and are the first organisms to express detectable changes that demonstrate a stress response to toxins (Huggett et al. Citation1992). The freshwater aquatic organisms used as bioindicators include some fish species, as well as invertebrates, including representatives of Crustacea, Annelida, Plecoptera, Trichoptera, Ephemeroptera, Diptera, Odonata and Mollusca (de Lafontaine et al. Citation2000; Berra et al. Citation2004; Bonnail et al. Citation2016; Mohanty & Luna Citation2016). The Chironomidae (Diptera) in particular are considered to be effective indicators of water quality (Saether Citation1979; Mousavi et al. Citation2003). They show a broad range of ecological characteristics and can tolerate a wide range of environmental conditions (Battarbee Citation2000; Cañedo-Argüelles et al. Citation2016). In addition, they comprise a large number of species and occur in high abundance in all freshwater aquatic ecosystems, and have been successfully used in a variety of biomonitoring-based approaches (Rosenberg Citation1992). Chironomids are good bioindicators of the aquatic environment because they spend most of their life in bottom sediments, where they are exposed to toxic substances such as heavy metals, radioactive substances, pesticides and other xenobiotics, originating from sources such as industrial waste and agricultural runoff. Chironomids have been shown to be valuable models for research on morphological deformity, trophic classification of lakes and paleolimnology, and in toxicity studies (Meregalli et al. Citation2000; De Bisthoven et al. Citation2005; Lee & Choi Citation2009; Weltje et al. Citation2010; Rebechi & Navarro-Silva Citation2012). Studies of their behavior and life history, as well as biomonitoring studies, have been carried out at population and ecosystem levels. In recent years, they have been increasingly used as biochemical and physiological indicators of environmental stress.
The aim of the present study was to investigate parameters of oxidative stress, including total antioxidant capacity (TAC), glutathione, lipid peroxidation and catalase (CAT), in chironomid larvae. We also investigated the ability of chironomid species to metabolize xenobiotics by determining the activity of glutathione S-transferase (GST) and reduced glutathione levels. Four species of Chironomidae – Chironomus riparius (Meigen, 1804) (subfamily Chiromoninae), Paratanytarsus sp. (subfamily Chiromoninae), Prodiamesa olivacea (Meigen, 1818) (subfamily Prodiamesinae) and Conchapelopia melanops (Meigen, 1818) (subfamily Tanypodinae) – were selected for investigation, based on their contrasting ecological and biological characteristics, such as mode of feeding and diet.
The most widely used chironomid species used as bioindicators belong to the genus Chironomus: C. riparius (in Europe and North America), C. dilutus (in North America) and C. yoshimatsui (in Japan). However, laboratory-bred individuals often demonstrate genetic impoverishment (Nowak et al. Citation2007), limiting their similarity to the wild specimens which they are intended to represent (Brown et al. Citation2011). In addition, these taxa are not cosmopolitan species, which creates difficulties in comparing results obtained in different geographical regions. Particular attention has been focused on C. riparius, which is becoming increasingly popular as a model organism for aquatic bioassays. This species is easy to culture in the laboratory, is widely distributed in the northern hemisphere in temperate latitudes and is found in both lentic and lotic waters, often at high densities and usually in waters enriched with sediment (Armitage et al. Citation1995; Péry & Garric Citation2006). Its life cycle is typically completed within a period of 3 to 4 weeks at 20°C. This species possesses hemoglobin (Hb) with a high affinity for oxygen, which results in a high tolerance of the larvae to a range of contaminants (Osmulski & Leyko Citation1986; Choi et al. Citation1999; Choi & Ha Citation2009; Al-Shami et al. Citation2010). Therefore, the species prefers eutrophic and organically enriched waters (Armitage et al. Citation1995). It is an opportunistic feeder on detritus (collector-gatherer feeding on sediment-deposited detritus), building tubes in the substrate and with the larvae growing to a large size (Rasmussen Citation1985; De Haas et al. Citation2006).
Paratanytarsus spp. are eurytopic forms with medium-sized larvae, which are adapted to a wide range of ecological conditions. Larvae are filter feeders, of smaller body size than C. riparius, but also build tubes that protect the larvae from the negative effects of chemical contaminants (Winner et al. Citation1980; Halpern et al. Citation2002). Notably, P. grimmii, a cosmopolitan chironomid belonging to this family, is considered a pest because of its capacity to breed in water distribution systems. Paratanytarsus grimmii is able to reproduce asexually through aphonic parthenogenesis, reducing the risk of crossbreeding with related individuals and inbreeding depression (Porter Citation1971). A case has been made for the utilization of this species in routine ecotoxicological studies (Gagliardi et al. Citation2015).
Prodiamesa olivacea was selected for the present study because it possesses a large larva and occurs in both lotic and lentic systems. It is associated with packets of leaves, i.e. large-grained allochthonic organic matter (De Bisthoven et al. Citation1992). This species is classified as a picker (collector-gatherer, feeding on leaves), although it shows mobility and contributes to the mechanical fragmentation of the foreign matter. This species, together with C. riparius, is used in biomonitoring studies based on the appearance of deformities (Servia et al. Citation1998).
The final taxon used in the study was Conchapelopia sp., which is ubiquitous in Europe in both lenitic and lotic ecosystems (Vallenduuk & Moller Pillot Citation2007). Conchapelopia melanops, the species used here, is a ubiquitous species living in large stagnant waters and diverse running waters and can comprise from 10 to 20% of the total benthos density along a longitudinal river profile (Vallenduuk & Moller Pillot Citation2007). It belongs to the subfamily Tanypodinae, consisting mainly of predators. The Tanypodinae exhibit well-defined food preferences, feeding mainly on the larval forms of other Chironomidae species (Baker & McLachlan Citation1979).
2. Material and methods
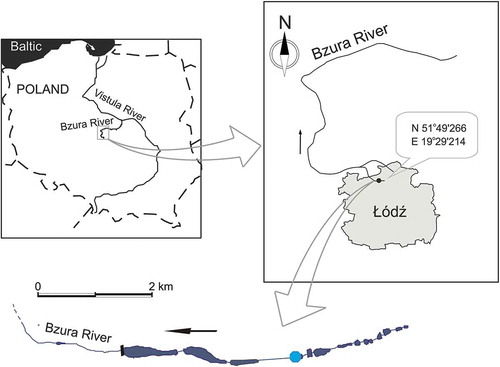
Fourth-instar chironomid larvae were collected using a net in the upstream part of the River Bzura in autumn 2017 (three sampling occasions). The organisms were stored in polypropylene bottles containing river water and quickly transferred to the laboratory where they were identified under a stereoscopic microscope, using the keys of Wiederholm (Citation1983, Citation1986, Citation1989), and frozen. Depth and velocity of the river flow and the area of the river bottom (where the samples were collected) were measured. The physico-chemical parameters analyzed were conductivity, dissolved oxygen, temperature and acidity.
At each site, three samples (0.1 × 0.1 m) of river bottom were collected using a tubular sampler 0.01 m2 in cross-sectional area. The size classification of inorganic particles and inorganic substrate index, SI (Cummins Citation1962; Quinn & Hickey Citation1990), were calculated. In addition, the amount of benthic particulate organic matter (BPOM) was evaluated. Using sieves and filters, the organic matter was divided into two fractions – coarse (BCPOM > 1 mm) and fine (BFPOM < 1 mm) particulate organic matter – according to Petersen et al. (Citation1989). Periphyton was measured as chlorophyll a concentration using the method of Goltermann et al. (Citation1978).
2.1. Study area
The study was conducted in a low-order stretch of the River Bzura in central Poland. The River Bzura is a tributary of the River Vistula, debouching into the Vistula 587 km from its source. It is 166 km long and its drainage is ca. 7788 km2. The Bzura flows close to the city of Łódź. The study sites are located in a first-order stream section (). This habitat is characterized by a sandy-bottom substrate with large amounts of allochthonous organic matter, especially tree leaves (Alnus glutinosa (L.)), covering the stream bed over the whole year.
2.2. Cell-free extract preparation and enzyme activity measurements
Chironomidae of a very similar size were selected from each sample for biochemical analyses. Samples (wet mass 0.046–0.083 g) were placed in 100 mM sodium phosphate buffer (pH 7.4, 100 mM KCl and 1 mM EDTA) and maintained on ice until homogenization. Homogenization using a CAT X-120 knife homogenizer was performed on ice at 2000 rpm for 2 min, and the homogenates were then centrifuged at 10,000 rpm for 10 min (4 °C) (Sigma 3–16 kL separator). The supernatants were immediately used for estimations. For each sampling date, three replicates were performed to determine individual parameters.
The protein concentration was determined using a spectrophotometric method with Folin reagent (Lowry et al. Citation1951).
2.3. Activity of catalase
Determination of catalase activity was performed according to the method of Aebi (Citation1984). The method is based on the decomposition of hydrogen peroxide, which is indicated by a decrease in absorbance at 240 nm. The assay mixture (volume 3 mL) consisted of 2 µL or 3 µL of homogenate (depending on the kind of homogenate), 50 mM potassium phosphate buffer (pH 7.0) and 54 mM hydrogen peroxide. One unit of CAT activity was defined as the enzyme activity that degraded 1 μmol of H2O2 in 1 min. Activity of catalase was expressed as (μmol × min-1) × mg-1 protein in homogenate.
2.4. Activity of glutathione S-transferase
GST activity was assayed spectrophotometrically by monitoring the conjugation of 1-chloro-2,4-dinitrobenzene (CDNB) with GSH at λmax = 340 nm at 37 °C. We used a volume of 15 µL of homogenate (50 µL of 20 mM GSH, 100 µL of 20 mM CDNB and 835 µL of 0.1 M potassium phosphate buffer (pH 6.5)). The rate of increase of product concentration was monitored by measuring absorbance at 340 nm at 25 °C for 3 min in a SPECORD 250 plus spectrophotometer (Analytik Jena). Within this period, the rate of reaction was linear with time. Activity of GST was calculated using an extinction coefficient of 9.6 mM–1 × cm–1 and was expressed as nmol/min/mg protein in homogenate (Habig et al. Citation1974).
2.5. The level of reduced glutathione
The level of reduced GSH in homogenate was determined by a modified method of Ellman (Citation1959). Homogenate was deproteinated by addition of 20% trichloroacetic acid (TCA) to a final concentration of 2%. Than a volume of 5 µL of 10 mM 5,5ʹ-dithiobis(2-nitrobenzoic acid) (DTNB) was added to 50 µL of supernatants cleared by centrifugation (10 min, 15,000 rpm/min). The formation of yellow anion 5-thio-2-nitrobenzoate, which is proportional to the total glutathione concentration, was monitored at 412 nm and 25 °C against reagent controls. The concentration of GSH in the samples was calculated from a millimolar absorption coefficient for DTNB (ε = 13.6 mM−1 × cm−1) and was expressed as µmol/mg protein in homogenate.
2.6. Determination of lipid peroxidation (malondialdehyde level)
Lipid peroxidation (LPO) was measured using thiobarbituric acid (TBA) according to the method of Stocks and Dormandy (Citation1971). The concentration of the end product of LPO, malondialdehyde (MDA; a thiobarbituric acid-reactive substance), is an index of lipid peroxidation and oxidative stress. The level of MDA was monitored at 532 nm and calculated using the MDA absorption coefficient (156 mM−1 × cm−1). The level of MDA was expressed as µmol/mg protein in homogenate.
2.7. Total antioxidant capacity
The total antioxidant capacity in the homogenate was determined by means of reduction of 2,2ʹ-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS). Absorbance measurement was performed at 414 nm. Considering that one Trolox molecule reacts with two ABTS+ molecules, the calculated values were multiplied by two (unit per µmol of Trolox equivalent L–1, which is used as a standard in antioxidant capacity measurements) (Bartosz Citation2003).
2.8. Statistical analysis
All statistical analyses were carried out using Statistica 10.0 (StatSoft Inc.). One-way analysis of variance (ANOVA) was used to examine differences among investigated parameters.
3. Results
River characteristics and water physico-chemical parameters measured in the River Bzura during the study are shown in .
Table I. Physico-chemical parameters for the sampling site. SI - inorganic substrate index, pH – acidity. SD- standard deviation.
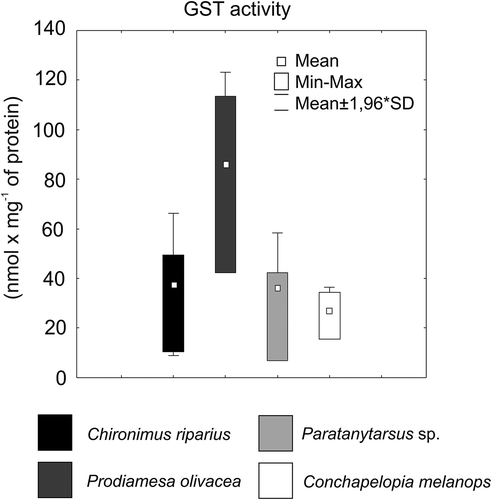
Data for oxidative stress parameters of chironomid species are shown in . C. riparius shows a low activity of CAT, and the lowest levels of GSH, LPO and TAC. In contrast, the highest enzyme activity for CAT and highest levels of GSH and LPO were observed for P. olivacea. Conchapelopia melanops shows the lowest activity of CAT (). Prodiamesa olivacea shows the highest detoxycation activity (GST, ). Larvae of Paratanytarsus sp. showed average parameter values ( and ).
Figure 2. Catalase (CAT) activity and level of reduced glutathione (GSH), lipid peroxidation (LPO) and total antioxidant capacity (TAC) in the four studied chironomid species.
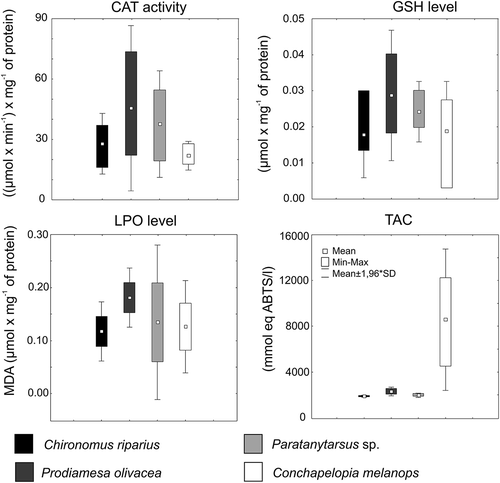
There were significant differences (, ANOVA) in whole-body CAT and GST activities as well as in levels of GSH, MDA and TAC. Differences were observed among the chironomid taxa in GSH content and CAT activity. Prodiamesa olivacea was characterized by significantly (p < 0.05) higher CAT activity and GSH level, with respect to C. riparius and C. melanops. GST levels for P. olivacea (p < 0.001) differed from those of all other investigated taxa. For LPO levels, significant differences were found between P. olivacea and C. riparius. Total antioxidant capacity was different between C. melanops and the other chironomid taxa (post-hoc Tukey test).
Table II. Differences (ANOVA) between taxa studied for parameters examined; df (3,32) Post hoc differences: 1 – Chironomus riparius, 2 – Prodiamesa olivacea, 3 – Paratanytarsus sp. 4 – Conchapelopia melanops df – number of degrees of freedom CAT – activity of catalase GSH – the level of reduced glutathione GST – activity of glutathione S-transferase MDA – level of malondialdehyde TAC – total antioxidant capacity.
For GSH levels, a statistically significant negative correlation was recorded for size of inorganic substrate (r = −0.359, p = 0.032), and a positive correlation was found for BCPOM (r = 0.372, p = 0.025). Among the enzymes studied, CAT activity showed a positive correlation with dissolved oxygen (r = 0.338, p = 0.044) and a negative correlation with temperature (r = 0.384, p = 0.021) and conductivity (r = −0.361, p = 0.031).
In C. riparius, total antioxidant activity was positively correlated with CAT activity, while in P. olivacea this parameter was positively correlated with GSH level. For TAC only negative correlations, with CAT activity (C. melanops) and GSH level (P. olivacea and Paratanytarsus sp.), were found. LPO was positively correlated with GST activity in C. riparius and negatively correlated with GST activity in Paratanytarsus sp. ().
Table III. Pearson correlaton coefficients between selected chironomidae taxa and investigated parameters. C. rip. – Chironomus riparius, P. oli. – Prodiamesa olivacea, Para. – Paratanytarsus sp., C. mel. – Conchapelopia melanops CAT – activity of catalase GSH – the level of reduced glutathione GST – activity of glutathione S-transferase MDA – level of malondialdehyde TAC – total antioxidant capacity.
4. Discussion
Many hydrophobic pollutants that exist in the aquatic environment accumulate in sediment. As a consequence, benthic organisms risk exposure to their effects. Exposure to xenobiotics in sediments may cause adverse effects at lower trophic levels, potentially leading to biomagnification of these toxicants, and thus more serious toxic effects, at higher trophic levels (Newman Citation1998). Regardless of feeding strategy and quality of ingested food, chironomids are unable to avoid toxic substances contained in water and sediments (De Haas et al. Citation2002) and, consequently, are considered to be good biomarkers of the condition of the environment. We detected significant differences between taxa with reference to the specific activity of most investigated parameters. Our results emphasize that four chironomid taxa, living in the same ecosystem, share an important antioxidant (enzymatic and nonenzymatic) pool, as well as detoxifying properties, and the values of the investigated parameters are different in each of the taxa.
LPO plays a significant role as a marker of oxidative stress, associated with accelerated aging of the cells (Guéraud et al. Citation2010; Negre-Salvayre et al. Citation2010). It involves oxidation of lipids, which is associated with formation of peroxide that is neutralized by utilization of glutathione. LPO is most frequently initiated by the hydroxyl radical. It is the most reactive form of oxygen species, formed as a result of reduction of oxygen. It is created by the interaction of chemical substances (electron donors) and oxygen present in the environment (Bartosz Citation2003). The primary factor here is oxygen, which transforms into a hydroxyl radical that initiates the peroxidation process. LPO was found to be at a low level in the red larvae of C. riparius and Paratanytarsus sp., while it achieved the highest value in the white larvae of P. olivacea. It is conceivable that the red forms of Hb demonstrate higher peroxidase activity than other types of Hb in the Chironomidae. In the peroxidation reaction, red forms of Hb degrade hydrogen peroxide to water and oxygen, thus preventing the formation of hydroxyl radicals, the initiator of LPO. In studies of chironomids, LPO within 0.05 nmol mg−1 was observed (Campos et al. Citation2016). Our results are approximately an order of magnitude higher (from 0.12 nmol mg−1 in C. riparius to 0.18 nmol mg−1 in P. olivacea). Comparable results were obtained in studies of a contaminated eutrophic reservoir, where the sensitivity of daphnids (Wojtal-Frankiewicz et al. Citation2013) and the response of the antioxidant system of zebra mussels, dependent on the environmental characteristics of the ecosystem, were investigated (Wojtal-Frankiewicz et al. Citation2017). In this study, despite the LPO values differing significantly within the same species among seasons, temperature was strongly, negatively correlated with LPO, with a high level of oxidative stress generated at lower temperatures. In our study, as in the studies of zebra mussels, the temperature in the River Bzura was relatively low, and that may have been the reason for inhibition of the growth of Chironomidae taxa, while LPO values were observed to be significantly higher in P. olivacea, and this taxon has been described as a cold stenothermal species in the profundal zone of lakes (Marziali & Rossaro Citation2013). Other field and experimental studies on molluscs have demonstrated that a decrease in antioxidant enzyme activity and peroxidase pool causes an increase in LPO (Doyotte et al. Citation1997; Choi et al. Citation2001). Oxidative stress may cause partial damage to the internal mitochondrial membranes by LPO, which interferes with the function of respiratory electron transport system (ETS) units and reduces their activity (Cossu et al. Citation2000).
Superoxide dismutase (decomposing the superoxide anion radical), as well as catalase and glutathione peroxidase, which consume hydrogen peroxide, play an important role in protection against LPO. These enzymes, therefore, prevent the formation of the hydroxyl radical in Fenton’s reaction, which initiates LPO. In our study, no correlation between LPO and CAT activity was observed in any of the studied taxa, similarly to the case in molluscs (Bielen et al. Citation2016) where prooxidative conditions upon exposure to ZnCl2 were not evidenced by an increase in CAT activity. CAT is a haem-based protein, which catalyzes decomposition of hydrogen peroxide to water and oxygen. The effectiveness of antioxidant catalase varies depending on the organism, the type of stressor and the time of exposure, and is used as a biomarker of oxidative stress (Halliwell & Gutteridge Citation2007; Osman et al. Citation2007). Our results indicate differences in the activity of catalase in the studied taxa. It is noteworthy that CAT exhibits a typical bell-shaped response to toxic chemicals (Viarengo et al. Citation2007); the initial increase in enzyme activity (reflecting the induction due to pro-oxidant conditions) leads to a decrease, which may be the consequence of a variety of factors (increased catabolic rate, direct inhibition of CAT by toxic chemicals, problems with compensation of oxidative stress). This bell-shaped response is further changed with the concentration of a chemical substance, and with exposure time (Viarengo et al. Citation2007); both increases and decreases in CAT activity are reported. Our results indicate that P. olivacea and Paratanytarsus sp. exhibit wide ranges of CAT activity. Additionally, P. olivacea, which has the highest CAT activity, shows significant differences in this parameter from all other taxa studied with the exception of Paratanytarsus sp. High CAT activity is associated with high energy expenditure during the transformation of chironomids, which causes a periodic increase in the content of hydrogen peroxide in the mitochondria and the cytosol, due to active adenosine-5-triphosphoric acid (ATP) synthesis (Ahmad Citation1995). Another reason is the need to compensate for the deficit of peroxidase activity (Ahmad Citation1992) in the process of hydrogen peroxide decomposition.
The overall CAT activity in bivalves collected from Oxbow Lake in the lower course of the River Drawa was between 9 and 18 μmol min−1 mg−1 protein (Bielen et al. Citation2016). The studies conducted in two rivers in Italy estimated the activity of this enzyme in winter at 9.4–11.5 μmol min−1 mg−1 protein, whereas in summer its activity reached 13.6 μmol min−1 mg−1 protein for the whole chironomid family. There were seasonal variations, which apparently were independent of the environmental parameters. Interestingly, it was found that the Plecoptera, which includes species requiring well-oxygenated water, shows the highest activity of CAT, suggesting a protective role of this enzyme against oxidative stress. This finding explains the low CAT values in chironomids which have Hb (C. riparius and Paratanytarsus sp.); they are the taxa with optimum development in warm ecosystems that are rich in nutrients, and possess a strong ability to adjust their oxy-regulatory capacity; i.e. the ability to maintain high oxygen exchange (respiration rate) with low oxygen availability (oxy-regulators). It should be emphasized that these larvae have 13 types of Hb (Osmulski & Leyko Citation1986). Chironomus riparius, which inhabits bottom sediments, is exposed to anaerobic conditions, and thus is adapted to low oxygen concentrations in the environment by production of a high content of Hb. Hb metabolizes xenobiotics (Ha & Choi Citation2008), which enables this species to survive polluted environments. This capability is associated with oxygenous metabolism, but auto-oxidation of heme in respiratory fluids of this taxon leads to the formation of high amounts of ROS. Consequently, antioxidative enzymes, which neutralize free radicals, play a crucial role in this species (Choi et al. Citation1999). Other taxa, such as P. olivacea, which sometimes occupy the same sites as C. riparius, have no Hb. High CAT activity was observed in P. olivacea, which has a low temperature optimum and is classified as an oxy-conformer from poor nutrient conditions (Brodersen et al. Citation2004, Citation2008; Marziali et al. Citation2006; Marziali & Rossaro Citation2013).
During the lipid peroxidation process, ROS and fatty acid peroxides increase and GSH is involved in the degradation of peroxides with glutathione peroxidase. GSH is a tripeptide, which has various functions in living organisms. It belongs to the most important antioxidants, which react with ROS, thus protecting thiol groups of proteins before irreversible inactivation (Bartosz Citation2003). It plays a crucial role in cellular protection against reactive species and electrophiles, and it is involved in regulation and maintenance of the cellular redox status and activity of specific enzymes (Lushchak Citation2012). In aquatic ecosystems, GSH enables effective detoxification of microcystins (Wiegand & Plussmacher Citation2005). A decrease in GSH content is usually associated with intensive detoxification and/or with the presence of oxidative stress, and also limits LPO (Doyotte et al. Citation1997; Choi et al. Citation2001). Increased oxidation reactions contribute to increased LPO and decrease in GSH, which stimulates an increase in the activity of the arachidonic acid cascade enzymes: cyclooxygenase and lipoxygenase, and causes an influx of calcium ions into the cells leading to damage to the cellular membrane and its receptors. A consequence of oxidative stress is cytoplasmic membrane permeability, causing depolarization. In our study, a correlation between GSH level and amount of coarse particulate organic matter was noted. The lowest GSH level was determined in the tissues of the predatory species C. melanops. Because of its diet, it is particularly vulnerable to toxic chemicals, which must be detoxified in the presence of GSH. This is associated with the fact that predators are susceptible to biomagnification of pollutants and toxins accumulated in their prey.
GST is the primary enzyme responsible for detoxification of xenobiotics (Bukowska Citation2005). GST activity has been well recognized, in many biomonitoring programs, as a biomarker of contamination of aquatic environments (Livingstone Citation1993; Van der Oost et al. Citation1997; Cajaraville et al. Citation2000). The activity of GST is an indicator of exposure to organic pollutants and metals (McLoughlin et al. Citation2000). This indicator has been used in studies of environmental pollution, including exposure to sediment-related carcinogens in fish (Gallagher et al. Citation1998; Melgar Riol et al. Citation2001), as well as in studies using molluscs (Sheehan & Power Citation1999). The application of GST activity as an indicator associated with pollution or potential resistance to pesticides has been assessed in the case of many species of insects (Hodge et al. Citation2000). The Paratanytarsus sp. chironomids are, similarly to molluscs, filtering invertebrates able to accumulate pollutants at analytical levels in their soft tissues (Goldberg & Bertine Citation2000). However, in our study the activity of GST was high in P. olivacea, inhabiting leaf packets, and was even higher than the maximum standard value reported for non-exposed C. riparius (Domingues et al. Citation2007). Nevertheless, these values were not as high as those obtained by Palacio-Cortes et al. (Citation2017) in a study of C. sancticaroli; the detoxification of xenobiotics is presumably efficiently performed in this genus (Palacio-Cortes et al. Citation2017). In contrast, red chironomid larvae, both of filtering Paratanytarsus sp. and of pickers C. riparius, showed average GST values comparable to those of previous studies (Choi et al. Citation2000; Domingues et al. Citation2007).
We also examined TAC as an essential biomarker of oxidative stress (Słowińska et al. Citation2016). High TAC values were obtained for predatory C. melanops, while this species generally showed relatively low enzymatic activity. Probably a high level of TAC; e.g. the presence of a large number of low-molecular antioxidants (vitamins A, C, E and other compounds), compensates for enzymatic activity, which is at a moderate level.
On the basis of our research, it can be concluded that chironomids are a diverse and flexible group of insects, reflecting the morphological, physiological and environmental characteristics of these species as well as their resilience to anthropogenic changes to the environment. The efficient defense mechanisms of these insects appear to have contributed significantly to their evolutionary success (Cytryńska et al. Citation2016). The results obtained in the present study indicate a range of defense mechanisms, including antioxidant defense and detoxification.
The usefulness of species, or groups of species, whose behavior can serve as biological indicators that reflect changes in pollution is increasingly recognized (Dziock et al. Citation2006). Depending on the sensitivity of the organism, various responses are observed, from changes in behavior and physiology, through changes in morphology and biochemistry, and ultimately reflected in changes in mortality rates. Pander and Geist (Citation2013) showed the role of bioindication in the analysis of the status of water pools and the methods to rehabilitate water resources. The organisms most commonly used are crustaceans, molluscs, fish, protozoa and algae (Schneider & Lindstrøm Citation2009; Sasikumar & Krishnakumar Citation2011; Negishi et al. Citation2013). Among chironomids, the most commonly used species is C. riparius. Our studies indicate, however, that each of the taxa examined reacted to the quality of the environment in a different way. The predator C. melanops appeared to have the greatest utility for bioindication. Therefore, it is emphasized that owing to the effects of biomagnification, ecotoxicological studies should be verified under natural conditions, through biological and chemical monitoring of aquatic ecosystems, in order to assess the exposure of ecosystems to toxic substances and their effects on the organisms in the food chain, as well their ultimate bioaccumulation and toxicity.
5. Conclusion
The organisms studied expressed a variety of feeding strategies, which was reflected in their metabolism and capacity for antioxidant defense, detoxification and neurological response.
We have observed that, in spite of a low activity of the biomarkers studied, which are characteristic for predators, C. melanops has the highest TAC activity (sum of the various antioxidants) which shows an excellent adjustment of metabolism of this taxon, due to food pull from which the valuable components may be used to neutralize xenobiotics and alleviate oxidative stress. The way of nutrients achievement makes it possible to tight oxidative stress; the low activity of the studied biomarkers is compensated by substances having the ability to scavenge free radicals.
Another strategy is characteristic of P. olivacea, which inhabits leaf packets and is exposed to chlorophyll a metabolites and an elevated level of ROS, and is thus characterized by high CAT and GST activity and a high GSH level.
Supplemental Material
Download TIFF Image (2 MB)Acknowledgements
The authors wish to thank Professor Bożena Bukowska for her expert support and assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
Supplemental data for this article can be accessed here.
References
- Aebi H. 1984. Catalase in vitro. Methods in Enzymology 105:121–126.
- Ahmad S. 1992. Biochemical defense of pro-oxidant plant allelochemicals by herbivorous insects. Biochemical Systematics and Ecology 20:269–296. DOI: 10.1016/0305-1978(92)90040-K.
- Ahmad S. 1995. Oxidative stress from environmental pollutants. Archives of Insect Biochemistry and Physiology 29:135–157. DOI: 10.1002/arch.940290205.
- Al-Shami SA, Rawi CSM, Hassanahmad A, Nor SAM. 2010. Distribution of Chironomidae (Insecta: Diptera) in polluted rivers of the Juru River Basin, Penang, Malaysia. Journal of Environmental Sciences 22(11):1718–1727. DOI: 10.1016/S1001-0742(09)60311-9.
- Armitage PD, Cranston PS, Pinder LCV. 1995. The Chironomidae: Biology and Ecology of non-biting Midges. London: Chapman & Hall.
- Baker AS, McLachlan AJ. 1979. Food preferences of Tanypodinae larvae (Diptera: Chironomidea). Hydrobiologia 62(3):283–288. DOI: 10.1007/BF00043546.
- Bartosz G. 2003. Cookbook for novice researchers, reactive oxygen species. In: Second face oxygen: Free radical in nature. 2nd ed. Warsaw: Wydawnictwo Naukowe PWN.
- Battarbee RW. 2000. Palaeolimnological approaches to climate change, with special regard to the biological record. Quaternary Science Reviews 19:107–124. DOI: 10.1016/S0277-3791(99)00057-8.
- Berra E, Forcella M, Giacchini R, Marziali L, Rossaro B, Parenti P 2004. Evaluation of enzyme biomarkers in freshwater invertebrates from Taro and Ticino river, Italy. Annales De Limnologie - International Journal of Limnology 40:169–180. DOI: 10.1051/limn/2004015.
- Bielen A, Bosnjak I, Sepcic K, Jaklic M, Cvitanic M, Lusic J, et al. 2016. Differences in tolerance to anthropogenic stress between invasive and native bivalves. The Science of the Total Environment 543:449–459. DOI: 10.1016/j.scitotenv.2015.11.049.
- Bonnail E, Buruaem LM, Araujo GS, Abessa DMS, Del Valls TA. 2016. Multiple Biomarker Responses in Corbicula fluminea Exposed to Copper in Laboratory Toxicity. Archives of Environmental Contamination and Toxicology 71:278–285. DOI: 10.1007/s00244-016-0281-9.
- Bonnefoy M, Drai J, Kostka T. 2002. Antioxidants to slow aging, facts and perspectives. La Presse medicale 25:1174–1184.
- Brodersen KP, Pedersen O, Lindegaard C, Chironomids HK. 2004. (Diptera) and oxy-regulatory capacity: An experimental approach to paleolimnological interpretation. Limnology and Oceanography 49:1549–1559. DOI: 10.4319/lo.2004.49.5.1549.
- Brodersen KP, Pedersen O, Walker IR, Jensen MT. 2008. Respiration of midges (Diptera; Chironomidae) in British Columbian lakes: Oxy-regulation, temperature and their role as palaeo-indicators. Freshwater Biology 53:593–602. DOI: 10.1111/j.1365-2427.2007.01922.x.
- Brown AR, Bickley LK, Le Page G, Hosken DJ, Paull GC, Hamilton PB, et al. 2011. Are toxicological responses in laboratory (inbred) zebrafish representative of those in outbred (wild) populations?—A case study with an endocrine disrupting chemical. Environmental Science & Technology 45:4166–4172. DOI: 10.1021/es200122r.
- Bukowska B. 2005. Glutation: Funkcje i czynniki zmniejszające jego stężenie. Medycyna Pracy 56:69–80.
- Cajaraville MP, Bebianno MJ, Blasco J, Porte C, Sarasquete C, Viarengo A. 2000. The use of biomarkers to assess the impact of pollution in coastal environments of the Iberian Peninsula: A practical approach. The Science of the Total Environment 247:295–311. DOI: 10.1016/S0048-9697(99)00499-4.
- Campos D, Gravato C,, Quintaneiro C, Soares AMVM, Pestana JLT. 2016. Responses of the aquatic midge Chironomus riparius to DEET exposure. Aquatic Toxicology (Amsterdam, Netherlands) 172:80–85. DOI: 10.1016/j.aquatox.2015.12.020.
- Cañedo-Argüelles M, Bogan M, Lytle DA, Are Chironomidae PN. 2016. (Diptera) good indicators of water scarcity? Dryland streams as a case study. Ecological Indicators 71:155–162. DOI: 10.1016/j.ecolind.2016.07.002.
- Choi J, Ha MH. 2009. Effect of cadmium exposure on the globin protein expression in 4th instar larvae of Chironomus riparius Mg. (Diptera: Chironomidae): An ecotoxicoproteomics approach. Proteomics 9:31–39. DOI: 10.1002/pmic.200701197.
- Choi J, Roche H, Caquet T. 1999. Characterization of superoxide dismutase activity in Chironomus riparius Mg. (Diptera. Chironomidae) larvae, a potential biomarker. Comparative Biochemistry and Physiology - Part C: Toxicology 124:73–81.
- Choi J, Roche H, Caquet T. 2000. Effects of physical (hypoxia, hyperoxia) and chemical (potassium dichromate, fenitrothion) stress on antioxidant enzyme activities in Chironomus riparius Mg. (Diptera, Chironomidae) larvae: Potential biomarkers. Environmental Toxicology And Chemistry / SETAC 19:495–500.
- Choi J, Roche H, Caquet T. 2001. Hypoxia, hyperoxia and exposure to potassium dichromate or fenitrothion alter the energy metabolism in Chironomus riparius Mg. (Diptera: Chironomidae) larvae. Comparative Biochemistry and Physiology - Part C: Toxicology 130:11–17.
- Cossu C, Doyotte A, Babut M, Exinger A, Vasseur P. 2000. Antioxidant biomarkers in freshwater bivalves, Unio tumidus, in response to different contamination profiles of aquatic sediments. Ecotoxicology and Environmental Safety 45:106–121. DOI: 10.1006/eesa.1999.1842.
- Cummins KW. 1962. An evaluation of some techniques for the collection and analysis of benthic samples with special emphasis on lotic waters. The American Midland Naturalist 67:477–504. DOI: 10.2307/2422722.
- Cytryńska M, Wojda I, Jakubowicz T. 2016. How insects combat infections. In: Ballarin L, Cammarata M, editors. Lessons in immunity: From single-cell organisms to mammals. Amsterdam-Boston-Heidelberg-London-New York-Oxford-Paris-San Diego-San Francisco-Singapore-Sydney-Tokyo:.Academic Press/ Elsevier. pp. 117–128.
- De Bisthoven LJ, Gerhardt A, Soares AMVM. 2005. Chironomidae larvae as bioindicators of an acid mine drainage in Portugal. Hydrobiologia 532:181–191. DOI: 10.1007/s10750-004-1387-z.
- De Bisthoven LJ, Van Looy E, Ceusters R. 1992. Gullentrop,s F., Ollevier, F. Densities of Prodiamesa olivacea (Meigen) (Diptera: Chironomidae) in a second order stream, the Laan (Belgium): Relation to river dynamics. Netherlands Journal of Aquatic Ecology 26:485–490. DOI: 10.1007/BF02255279.
- De Haas EM, Reuvers B, Moermond CTA, Koelmans AA, Kraak MHS. 2002. Responses of benthic invertebrates to combined toxicant and food input in floodplain lake sediment. Environmental Toxicology And Chemistry / SETAC 21:2165–2171. DOI: 10.1002/etc.5620211020.
- De Haas EM, Wagner C, Koelmans AA, Kraak MHS, Admiraal W. 2006. Habitat selection by chironomid larvae: Fast growth requires fast food. Journal of Animal Research 75:148–155. DOI: 10.1111/j.1365-2656.2005.01030.x.
- de Lafontaine Y, Gagne´ F, Blaise C, Costan G, Gagnon P, Chan HM. 2000. Biomarkers in zebra mussels (Dreissena polymorpha) for the assessment and monitoring of water quality of the St Lawrence River (Canada). Aquatic Toxicology (Amsterdam, Netherlands) 50:51–71. DOI: 10.1016/S0166-445X(99)00094-6.
- Domingues I, Guilhermino L, Soares AMVM, Nogueira AJA. 2007. Assessing dimethoate contamination in temperate and tropical climates: Potential use of biomarkers in bioassays with two chironomid species. Chemosphere 69:145–154. DOI: 10.1016/j.chemosphere.2007.04.013.
- Doyotte A, Cossu C, Jacquin MC, Babut M, Vasseur P. 1997. Antioxidant enzymes, glutathione and lipid peroxidation as relevant biomarkers of experimental or field exposure in the gills and the digestive gland of the freshwater bivalve Unio tumidus. Aquatic Toxicology (Amsterdam, Netherlands) 39:93–110. DOI: 10.1016/S0166-445X(97)00024-6.
- Dziock F, Henle K, Foeckler F, Follner K, Scholz M 2006. Biological indicator systems in floodplains - a review. International Review of Hydrobiology 4:271–291. DOI: 10.1002/iroh.200510885.
- Ellman G. 1959. Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics 82:70–77. DOI: 10.1016/0003-9861(59)90090-6.
- Gagliardi BS, Long SM, Pettigrove VJ, Hoffmann AA. 2015. The Parthenogenetic Cosmopolitan Chironomid, Paratanytarsus grimmii, as a New Standard Test Species for Ecotoxicology: Culturing Methodology and Sensitivity to Aqueous Pollutants. Bulletin of Environmental Contamination and Toxicology 95:350–356. DOI: 10.1007/s00128-015-1578-5.
- Gallagher EP, Sheehy KM, Janssen PL, Eaton DL, Collier TK. 1998. Isolation and cloning of homologous glutathione S-transferase cDNAs from English sole and starry flounder liver. Aquatic Toxicology (Amsterdam, Netherlands) 44:171–182. DOI: 10.1016/S0166-445X(98)00077-0.
- Goldberg ED, Bertine KK. 2000. Beyond the Mussel Watch - new directions for monitoring marine pollution. The Science of the Total Environment 247:165–174. DOI: 10.1016/S0048-9697(99)00488-X.
- Goltermann HL, Clymos RS, Ohmstad MAM. 1978. Methods for Physical and Chemical Analysis of Fresh Water. Oxford: Blackwell Scientific Publication.
- Guéraud F, Atalay M, Bresgen N, Cipak A, Eckl PM, Huc L, Jouanin I, Siems W, Uchida K. 2010. Chemistry and biochemistry of lipid peroxidation products. Free Radical Research 44:1098–1124. DOI: 10.3109/10715762.2010.498477.
- Ha MH, Choi J. 2008. Effect of environmental contaminants on hemoglobin of larvae of aquatic midge. Chironomus riparius (Diptera: Chironomidae); a potential biomarker for ecotoxicity monitoring. Chemosphere 71:1928–1936. DOI: 10.1016/j.chemosphere.2008.01.018.
- Habig WH, Pabst MJ, Jakoby WB. 1974. Glutathione S-Transferases. The first enzymatic step in mercapturic acid formation. The Journal of Biological Chemistry 249:7130–7139.
- Halliwell B, Gutteridge J. 2007. Free radicals in biology and medicine. New York: Oxford University Press. p. 704.
- Halpern M, Gasith A, Broza M. 2002. Does the tube of a benthic chironomid larva play a role in protecting its dweller against chemical toxicants? Hydrobiologia 470:49–55. DOI: 10.1023/A:1015665027535.
- Hodge S, Longley M, Booth L, Heppelthwaite V, O’Halloran K. 2000. An Evaluation of Glutathione S-Transferase activity in the Tasmanian Lacewing (Micromus tasmaniae) as a Biomarker of Organophosphate Contamination. Bulletin of Environmental Contamination and Toxicology 65:8–15. DOI: 10.1007/s0012800087.
- Huggett RJ, Kimerle RA, Mehrle PM, Bergman HL. 1992. Biomarkers. Biochemical, physiological, and histological markers of anthropogenic stress. Boca Raton, FL: Lewis Publishers.
- Lee SW, Choi J. 2009. Multi-level ecotoxicity assay on the aquatic midge, Chironomus tentans (Diptera, Chironomidae) exposed to octachlorostyrene. Environmental Toxicology and Pharmacology 28:269–274. DOI: 10.1016/j.etap.2009.05.004.
- Livingstone DR. 1993. Biotechnology and pollution monitoring: Use of molecular biomarkers in the aquatic environment. Journal of Chemical Technology & Biotechnology 57:195–211. DOI: 10.1002/jctb.280570302.
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. 1951. Protein measurement with the Folin phenol reagent. The Journal of Biological Chemistry 193:265–275.
- Lushchak VI. 2012. Glutathione homeostasis and functions: Potential targets for medical interventions. Journal of Amino Acids 2012(Article ID 736837):26. DOI: 10.1155/2012/736837.
- Marziali L, Lencioni V, Rossaro B. 2006. Adaptations of pupae of Chironomidae (Insecta: Diptera) to oxygen-poor habitats. Polish Journal of Ecology 54:687–693.
- Marziali L, Rossaro B. 2013. Response of chironomid species (Diptera, Chironomidae) to water temperature: Effects on species distribution in specific habitats. Journal of Entomological and Acarological Research 45:73–89. DOI: 10.4081/jear.2013.e14.
- McLoughlin N, Yin D, Maltby L, Wood RM, Yu H. 2000. Evaluation of sensitivity and specificity of two crustacean biochemical biomarkers. Environmental Toxicology And Chemistry / SETAC 19:2085–2092. DOI: 10.1002/etc.5620190818.
- Melgar Riol MJ, Novoa Valinas MC, Garcia Fernandez MA, Perez Lopez M. 2001. Glutathione S-transferases from rainbow trout liver and freshly isolated hepatocytes: Purification and characterization. Comparative Biochemistry and Physiology - Part C: Toxicology 128:227–235. DOI: 10.1016/s1532-0456(00)00196-4.
- Meregalli G, Vermeulen ACV, Ollevier F. 2000. The use of chironomid deformation in an in situ test for sediment toxicity. Ecotoxicology and Environmental Safety 47:231–238. DOI: 10.1006/eesa.2000.1981.
- Mohanty D, Luna S. 2016. Multivariate analysis of potential biomarkers of oxidative stress in Notopterus notopterus tissues from Mahanadi River as a function of concentration of heavy metals. Chemosphere 155:28–38. DOI: 10.1016/j.chemosphere.2016.04.035.
- Mousavi SK, Primicerio R, Amundsen PA. 2003. Diversity and structure of Chironomidae (Diptera) communities along a gradient of heavy metal contamination in a subarctic watercourse. The Science of the Total Environment 307:93–110. DOI: 10.1016/S0048-9697(02)00465-5.
- Negishi JN, Nagayama S, Kume M. 2013. Unionoid mussels as an indicator of fish communities: A conceptual framework and empirical evidence. Ecological Indicators 24:127–137. DOI: 10.1016/j.ecolind.2012.05.029.
- Negre-Salvayre A, Auge N, Ayala V, Basaga H, Boada J, Brenke R, et al. 2010. Pathological aspects of lipid peroxidation. Free Radical Research 44:1125–1171. DOI: 10.3109/10715762.2010.498478.
- Newman MC. 1998. Fundamentals of ecotoxicology. Chelsea, MI: Sleeping Bear/Ann Arbor Press.
- Nowak C, Vogt C, Diogo JB, Schwenk K. 2007. Genetic impoverishment in laboratory cultures of the test organism Chironomus riparius. Environmental Toxicology and Chemistry 26:1018–1022. DOI: 10.1897/06-349R.1.
- Osman A, Wuertz S, Mekkawy I, Exner H, Kirschbaum F. 2007. Lead Induced Malformations in Embryos of the African Catfish Clarias gariepinus (Burchell, 1822). Environmental Toxicology 22:375–389. DOI: 10.1002/tox.20272.
- Osmulski PA, Leyko W. 1986. Structure, function and physiological role of Chironomus haemoglobin. Comparative Biochemistry and Physiology B 85B:701–722. DOI: 10.1016/0305-0491(86)90166-5.
- Palacio-Cortes AM, Signorini-Souza IDL, Hara ELY, Disner RG, Rebechi D, Grassi MT, et al. 2017. Polybrominated diphenyl ethers (PBDEs) effects on Chironomus sancticaroli larvae after short-term exposure. Ecotoxicology and Environmental Safety 139:308–315. DOI: 10.1016/j.ecoenv.2017.01.052.
- Pander J, Geist J. 2013. Ecological indicators for stream restoration success. Ecological Indicators 30:106–118. DOI: 10.1016/j.ecolind.2013.01.039.
- Péry ARR, Garric J. 2006. Modelling effects of temperature and feeding level on the life cycle of the midge Chironomus riparius: An energy-based modelling approach. Hydrobiologia 553:59–66. DOI: 10.1007/s10750-005-1284-0.
- Petersen RC, Cummins KW, Ward GM. 1989. Microbial and animal processing of detritus in a woodland stream. Ecological Monographs 59:21–39. DOI: 10.2307/2937290.
- Porter DL. 1971. Oogenesis and chromosomal heterozygosity in the thelytokous midge, Lundstroemia parthenogenetica (Diptera, Chironomidae). Chromosoma 32:332–342. DOI: 10.1007/BF00284841.
- Quinn JM, Hickey CW. 1990. Characterization and classification of benthic invertebrate communities in 88 New Zealand rivers in relation to environmental factors. New Zealand Journal of Marine and Freshwater Research 24:387–409. DOI: 10.1080/00288330.1990.9516432.
- Rasmussen JB. 1985. Effects of density and microdetritus enrichment on the growth of chironomid larvae in a small pond. Canadian JJournal of Fisheries and Aquatic Sciences 42:1418–1422. DOI: 10.1139/f85-177.
- Rebechi D, Navarro-Silva MA. 2012. Setting the reference for the use of Chironomus sancticaroli (Diptera: Chironomidae) as bioindicator: Ontogenetic pattern of larval head structures. Zoologia (Curitiba) 29:167–171.
- Rosenberg DM. 1992. Freshwater biomonitoring and Chironomidae. Netherlands Journal of Aquatic Ecology 26:101–122. DOI: 10.1007/BF02255231.
- Saether OA. 1979. Chironomid communities as water quality indicators. Holarctic Ecology 2:65–74.
- Sasikumar G, Krishnakumar PK. 2011. Aquaculture planning for suspended bivalve farming systems: The integration of physiological response of green mussel with environmental variability in site selection. Ecological Indicators 11:734–740. DOI: 10.1016/j.ecolind.2010.06.008.
- Schneider S, Lindstrøm EA. 2009. Bioindication in Norwegian rivers using non-diatomaceous benthic algae: The acidification index periphyton (AIP). Ecological Indicators 9:1206–1211. DOI: 10.1016/j.ecolind.2009.02.008.
- Servia MJ, Cobo F, Gonzalez MA. 1998. Deformities in larval Prodiamesa olivacea (Meigen, 1818) (Diptera, Chironomidae) and their use as bioindicators of toxic sediment stress. Hydrobiology 385:153–162. DOI: 10.1023/A:1003466012110.
- Sheehan D, Power A. 1999. Effects of seasonality on xenobiotic and antioxidant defence mechanisms of bivalve molluscs. Comparative Biochemistry and Physiology - Part C: Toxicology 123:193–199. DOI: 10.1016/s0742-8413(99)00033-x.
- Słowińska M, Nynca J, Wilde J, Bąk B, Siuda M, Ciereszko A. 2016. Total antioxidant capacity of honeybee haemolymph in relation to age and exposure to pesticide, and comparison to antioxidant capacity of seminal plasma. Apidologie 47:227–236. DOI: 10.1007/s13592-015-0391-9.
- StatSoft Inc. Statistica (data analysis software system), version 10. Avaiable at: http://www.statsoft.com (2011).
- Stocks J, Dormandy T. 1971. The Autoxidation of Human Red Cell Lipids Induced by Hydrogen Peroxide. British Journal of Haematology 20:95–111. DOI: 10.1111/j.1365-2141.1971.tb00790.x.
- Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M. 2006. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicology and Environmental Safety 64(9):178–189. DOI: 10.1016/j.ecoenv.2005.03.013.
- Vallenduuk HJ, Moller Pillot HKM. 2007. Chironomidae Larvae of the Netherlands and Adjacent Lowlands. General Ecology and Tanypodinae. Zeist: KNNV Publishing.
- Van der Oost R, Vindimian E, van den Brink P, Satumalay K, Heida H, Vermeulen NPE. 1997. Biomonitoring aquatic pollution with feral eel (Anguilla anguilla): III. Statistical analyses of relationships between contaminant exposure and biomarkers. Aquatic Toxicology (Amsterdam, Netherlands) 39:45–75. DOI: 10.1016/S0166-445X(96)00851-X.
- Vertuani S, Angusti A, Manfredini S. 2004. The antioxidants and pro-antioxidants network: An overview. Current Pharmaceutical 10:1677–1694. DOI: 10.2174/1381612043384655.
- Viarengo A, Lowe D, Bolognesi C, Fabbri E, Koehler A. 2007. The use of biomarkers In biomonitoring: A 2-tier approach assessing the level of pollutant-induced stress syndrome in sentinel organisms. Home Journals Comparative Biochemistry and Physiology - Part C: Toxicology & Pharmacology 146:281–300.
- Weltje L, Rufli H, Heimbach F, Wheeler J, Vervliet Scheebaum M, Hamer M. 2010. The chironomid acute toxicity test: Development of a new test system. Integrated Environmental Assessment and Management 6:301–307. DOI: 10.1897/IEAM_2009-069.1.
- Wiederholm T, ed. 1983. The larvae of Chironomidae (Diptera) of the Holartcic region - Keys and diagnoses. Scandinavian Entomology 19:1–457.
- Wiederholm T, ed. 1986. The pupae of Chironomidae (Diptera) of the Holarctic region - Keys and diagnoses. Scandinavian Entomology 28:1–482.
- Wiederholm T, ed. 1989. The adult males of Chironomidae (Diptera) of the Holarctic region - Keys and diagnoses. Scandinavian Entomology 34:1–532.
- Wiegand C, Plussmacher S. 2005. Ecological effects of selected cyanobacterial secondary metabolites: A short review. Toxicology and Applied Pharmacology 203:201–218. DOI: 10.1016/j.taap.2004.11.002.
- Winner RW, Boesel MW, Farrell MP. 1980. Insect community structure as an index of heavy-metal pollution in lotic ecosystems. Canadian JJournal of Fisheries and Aquatic Sciences 37:647–655. DOI: 10.1139/f80-081.
- Wojtal-Frankiewicz A, Bernasinska J, Frankiewicz P, Gwoździnski K, Jurczak T. 2017. The role of environmental factors in the induction of oxidative stress in zebra mussel (Dreissena polymorpha). Aquatic Ecology 51:289–306. DOI: 10.1007/s10452-017-9617-4.
- Wojtal-Frankiewicz A, Bernasinska J, Jurczak T, Gwoździnski K, Frankiewicz P, Wielanek M. 2013. Microcystin assimilation and detoxification by Daphnia spp. in two ecosystems of different cyanotoxin concentrations. Journal of Limnology 72:154–171. DOI: 10.4081/jlimnol.2013.e13.