?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this study, which was conducted between November 2017 and October 2018 at Gökçeada Island, the specific growth rate (SGR), a length–weight correlation with a regression to determine the growth pattern of mussel spat, and survival rates of Mediterranean mussel spat were determined. Mediterranean mussels collected from the wild (N = 120) were grouped on each net (N = 40). Then, chlorophyll-a, water temperature, salinity, pH and particulate matter values were collected on a monthly basis, in triplicate. The mean chlorophyll-a was 0.21 ± 0.12 μg L−1, mean water temperature was 18.32 ± 5.46°C, mean salinity was 34.65 ± 2.38‰, and mean pH was 8.12 ± 0.04, while the composite of total particulate matter (TPM) was 17.88 ± 11.25 mg L−1. Length–weight correlation analysis indicated a strong correlation between the two parameters (r = 0.91). According to the results of the regression analysis performed between length and weight, a growth of negative allometry was detected in Mediterranean mussels (W = 0.0003L2.7254). Although the mussel survival rate was 77.5% in August 2018, it decreased to 13.3% by the end of the study in October 2018, due to the increase in the quantity of fouling organisms that gathered on the nets. At the beginning of the study, in November 2017, the average length was 19.58 ± 0.29 mm; 12 months later, in October 2018, it had increased to 43.77 ± 1.02 mm. These data indicate that according to the general linear model analysis, temperature was the only parameter affecting Mediterranean mussel growth among the environmental variables TPM, particulate organic matter, particulate inorganic matter, temperature, salinity, chlorophyll-a and pH. Since chlorophyll-a, which is an important parameter for the growth of mussels, remained at low levels in this study, its effect on growth has not been determined statistically.
1. Introduction
Aquaculture is the industry involved in cultivation of aquatic animals such as finfish (e.g. salmon, trout, sea bass, carp and tuna), molluscs (e.g. mussels, oysters and clams), crustaceans (e.g. shrimp, crab and lobster), plants (e.g. algae) and other organisms (e.g. frogs, pearls and aquatic mammals). In 2014, finfish and molluscs made up 98.2% of all aquaculture production (by weight) in the EU (EUROSTAT Citation2018). In total, 17.139 million tons of mollusc cultivation including 1.1 million tons of Mytilidae culture contributed to the total 110.2 million tons of aquaculture production in the world (FAO Citation2018). Considering the total production of Mediterranean mussels in the world, the top three producers are China (845.038 tons), Spain (225.308 tons) and Italy (63.700 tons) (Smaal et al. 2019). In Turkey, aquaculture production was 276.502 tons in 2017, and mussel cultivation contributed 489 tons of this total production (TURKSTAT Citation2018). Although total mussel production in Turkey is low nowadays, mussel farms established for commercial production are increasing day by day. Therefore, it is estimated that the total production amount will increase in the future (Serdar & Yıldırım Citation2018). In Turkey, the cultivation method used in established mussel farms is based on longline culture. Likewise, longline is the most used culture method for mussel cultivation in the world. It is applied by hanging the raft culture or by releasing the collectors into the water (Kamermans & Capelle Citation2019). The nets are usually used on the longline for the settlement of the mussel spat. Additionally, there are some studies on mussel cultivation that are performed using nets (Yıldız & Lök Citation2005; Yıldız et al. Citation2013).
Mytilus galloprovincialis is a filter feeding organism that feeds on inorganic materials, phytoplankton, organic detritus and bacteria from the sea (Karayücel et al. Citation2003). The mussels’ growth rates depend on endogenous and environmental factors such as population density, salinity, food availability, temperature and the amount of nutrients in the water (Bayne & Newell Citation1983). Some researchers have determined that barotropic tide and internal wave motion, which depend on the periodic tidal nature of water, changes the quantity of chlorophyll-a in the sea (Sangrà et al. Citation2001). Therefore, site selection for mussel culture is one of the most important factors in the mussels’ growth and survival rates (Dickie et al. Citation1984; Asokan et al. Citation2011).
Since mussels are filter-feeding organisms, they reduce the nutrient load in the environment. Therefore, farmers have recently begun using the integrated multi-trophic aquaculture (IMTA) method to make greater use of marine areas and to increase product variety and quantity. IMTA, which is a poly-culture of bivalves, fish and seaweed, has become a popular strategy as an eco-farming mode (Smaal et al. 2019). The most important reason for using this new cultivation technology is to limit the effects of climate change. The algae-grown environment is rich in chlorophyll-a, which is beneficial for the filter-feeding mussels, while at the same time it provides richness in organic matter for fish feces. The applicability of IMTA will be evaluated according to the results of environmental variables that are obtained during this study.
The aim of this study is to determine the growth conditions of mussels on nets, which are intensely cultivated on longlines worldwide. Mussel culture activities have been very popular in Turkey (Serdar & Yıldırım Citation2018). Previous production studies concerning Mediterranean mussel have been conducted in the Marmara Sea (Serdar et al. Citation2017), Black Sea (Aral Citation1999), Mediterranean (Lök et al. Citation2007) and Dardanelles (Yıldız et al. Citation2010), and the cultivation potential of Mediterranean mussel at Gökçeada Island has not been studied yet. For reasons such as global warming, pollution and the urbanization of sea coasts, the areas to be reserved for cultivation are constantly restricted. This leads scientists and investors to search for new locations for production. Accordingly, an evaluation of mussel farming was carried out around Gökçeada Island in Turkey for the first time by the method of mussel cultivation on nets. While the latter is the second aim of this study, the final aim is to examine which environmental variables – chlorophyll-a, total particular matter (TPM), particular organic matter (POM), particulate inorganic matter (PIM), salinity, water temperature and pH – have an impact on the growth of mussels cultivated on nets.
This study was supported by the master’s thesis project number 25357 of the Executive Secretary of the Istanbul University Scientific Research Projects, and it includes a part of the aforementioned master’s thesis.
2. Materials and methods
2.1. Study area
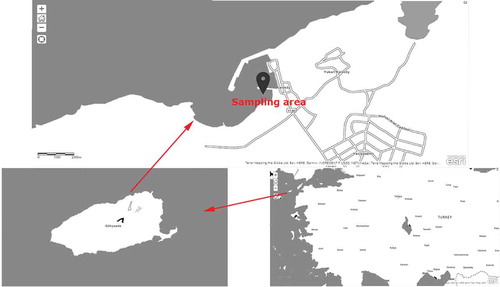
Although it is called Gökçeada Island nowadays, the island was known as Imroz Island until 1970. It is one of the two Turkish islands located in the northern region of the Aegean Sea. Gökçeada is the largest island of Turkey, with a surface area of 289.5 km2 and a coastline length of 95 km. It is located in the northwest of the Dardanelles. While the Mediterranean climate mostly dominates on the southern shores of Gökçeada, the Marmara climate dominates the northern coasts. The prevailing wind direction on the island is northeast, causing an average of 300 windy days throughout the year. The temperature varies between 7°C in the summer and 25°C in the winter. Additionally, The Turkish Marine Research Foundation (TÜDAV) has established a preliminary fauna–flora inventory for the sea and land area of the island and identified 180 marine species (Anonymous, Citation2020).
2.2. Sampling and analyses
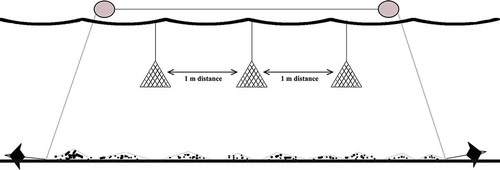
The mussel experimental system was established at Kaleköy-Gökçeada Island at the coordinates 40°13ʹ53.5794ʹʹN, 25°53ʹ38.2452ʹʹE (). The culture system comprised four pieces, each having an anchor with a weight of 7.5 kg, galvanized chain with a diameter of 8 mm and length of 65 m, a roda rope for connection points with a diameter of 8 mm, and two buoys with a volume of 200 L each. Then, a total of 120 mussels with an average length of 19.58 ± 0.29 mm and weight of 0.98 ± 0.07 g, that were picked from the natural stocks, were added on three nets. Each net has a mesh width of 4 mm and N = 40 mussels were placed on each of the three nets. Finally, the nets were hung to the main system ().
The water temperature, salinity and pH variables were measured on a monthly basis in triplicate in the sampling area, using a multi-parameter (HQ40d) device provided by the Gökçeada Marine Research Unit of the Faculty of Aquatic Sciences of Istanbul University.
The first step of the analysis was to filter a 1-L water sample through Whatman GF/C filter paper, using a vacuum pump and protecting the water from the effects of light throughout the process (APHA Citation2000). Samples were kept in acetone in the dark overnight and afterward were centrifuged at 3000 rpm for 20 minutes. Then, the supernatant layer was removed and the acetone was driven in the spectrophotometer as a blank; then the supernatant layer, which was added to the quartz cuvette, was measured at wavelengths of 630, 647, 664 and 750 nm and the results were recorded (APHA Citation2000). In the following equation of the parameters, v is the acetone volume, V is the volume of the water sample filtered at the beginning of the process and I is the quartz cuvette length:
The analysis of water samples for total particulate matter was performed by burning the used filter papers in a muffle furnace for 6 hours at 450°C. Afterward, the filter papers were cooled in a desiccator to remove the humidity. All weights of TPM, POM and PIM were calculated using a precision laboratory scale and initial weights were detected. The water filtration process was carried out using filter papers, which were dried at 60°C for 38 hours and then weighed again. Once more, final weights were recorded after the muffle furnace step (which took 6 hours at 450°C) and calculations of total particulate matter were carried out based on the following equation, in mg L−1 (Strickland & Parsons Citation1972):
In EquationEquation (2(2)
(2) ), W2 is the total weight of the filter paper and dry matter, W1 is the pre-weight of the filter paper, V is the volume of the filtered water sample and X is the blank correction constant.
In the specific growth rate measurements, the nets were taken out of the study area every month and dried using drying papers for approximately 5 to 10 minutes. As the next step, mussels’ lengths were assessed using digital calipers (Bts) and their weights were recorded using a precision laboratory scale. The estimates were calculated using the following equation (Malouf & Bricelj Citation1989):
where L1 is the first length and L2 is final length. The difference between t2 and t1 is 30 days (Chatterji et al. Citation1984).
The length–weight correlation was computed using the following regression equation (Ricker Citation1975) and the logarithm of both sides was linearized:
W = aLb(4)
To determine the growth pattern of Mytilus galloprovincialis, length and weight values were subjected to regression analysis, and then “a” and “b” values were computed.
2.3. Statistics
A general linear model (GLM) multivariate analysis was used to identify the correlation between the environmental variables and length–weight growth of Mediterranean mussels. Spearman’s correlation analysis was used to determine the strength in the two sets of data. All analyses were conducted using the SPSS 21 program. A correlation matrix was created for the length and weight of the Mediterranean mussels. Also, chlorophyll-a, seston quantities, a length–weight graph of the nets, survival rate and specific growth rate were computed using Microsoft Office Excel 2013.
3. Results
In this study, the mean water temperature throughout the study period was recorded as 18.32 ± 5.46°C, the highest water temperature (26.7°C) was obtained in August 2018 and the lowest water temperature (11.8°C) was obtained in February 2018. Salinity was measured as 34.65 ± 2.38‰. The lowest value of salinity was 31.1‰, taken in January 2018, and the highest value was 38.6‰, taken in February 2018. Despite this, January and February 2018 were the months when the highest increase in salinity was recorded. There were no important changes in the pH; its mean value was 8.12 ± 0.04 (minimum 8.1 and maximum 8.2; see ).
Table I. Values (± standard error, SE) of environmental variables from November 2017 to October 2018 in the Gökçeada Island, Aegean Sea.
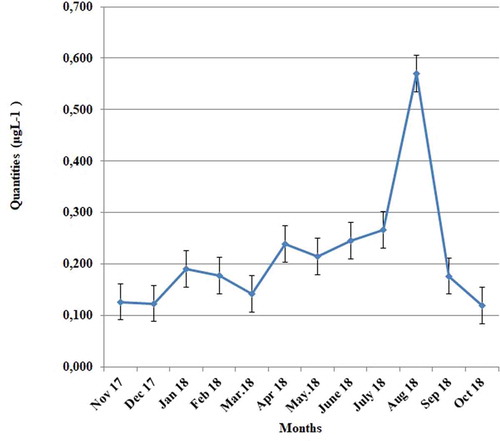
The mean chlorophyll-a value over 12 months was 0.21 ± 0.12 μg L−1; the highest value was 0.57 μg L−1, obtained in August 2018, and the lowest was 0.11 μg L−1, obtained in October 2018 ().
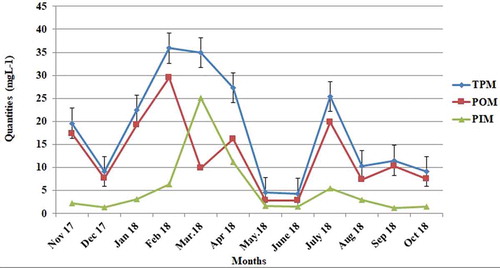
The mean value obtained for TPM, encompassing both PIM and POM, was 17.88 ± 11.25 mg L−1. Significant differences and fluctuations were detected by the monthly calculations. The highest value for TPM, 35.90 mg L−1, was obtained in February 2018, and the lowest value, 4.36 mgL was obtained in June 2018. In the winter months, the value of POM reached its highest level in February (29.53 mg L−1), whereas in the summer season the highest value was detected in July (19.83 mg L−1). PIM reached its highest value in March 2018 (25.10 mg L−1) for the winter months and in July 2018 (5.53 mg L−1) for the summer months ().
It was determined, based on the outcomes of the GLM multivariate analysis concerning monthly length–weight growth of the Mediterranean mussels and the environmental variables, that the length–weight growth of mussels was strongly related to the water temperature (p < 0.01) (). According to the Spearman’s correlation analysis, while a positive correlation was detected between chlorophyll-a and PIM (p < 0.05), a strong negative correlation was detected between water temperature, TPM and POM values (p < 0.01) (). In terms of other findings obtained with Spearman’s correlation, a positive correlation was determined between salinity and TPM, and a similar correlation was recorded between TPM, POM and PIM (p < 0.01).
Table II. Outcomes of the general linear model (GLM) multivariate analysis of mussels’ growth and environmental variables.
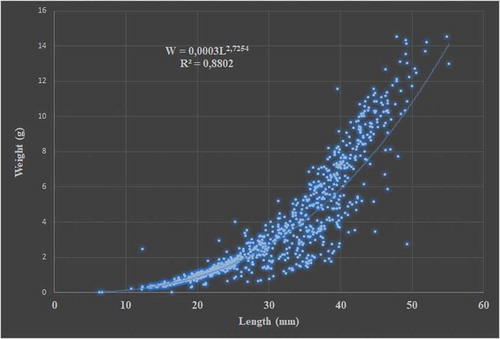
The correlation analysis conducted using mussels’ lengths and weights during the experiment also indicated that there was a strong positive correlation between length and weight (r = 0.91) ().
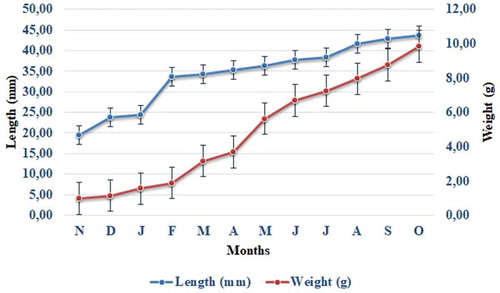
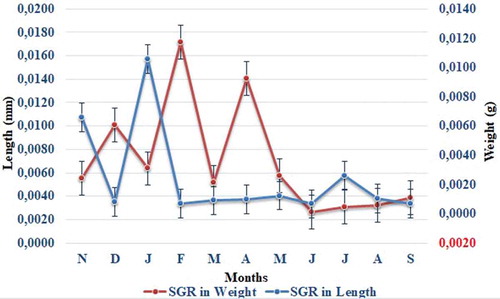
In the mussels (N = 120), at the beginning of the study, the mean length was 19.58 ± 0.29 mm and the mean weight was 0.98 ± 0.07 g. At the end of the study, lengths were recorded as 43.77±1.02mm and weights as 9.84±0.73g (). The specific growth rate was based on the increase in length. According to the measurements carried out based on the length increase, the highest winter growth of the mussels was detected in January–February, while the highest summer growth occurred in July–August. After November 2017, a sudden increase in growth was seen in the months of January and February, followed by a steady increase in the spring and summer periods (). The findings suggest the extreme increase witnessed in January and February was associated with the rapid growth of small-sized mussels. The second reason for the sudden increase is that the high quantity of TPM recorded in the water during these months is related to the changes in temperature. The third reason is that the lowest salinity level, which also occurred at this time, affected the growth of the mussels in a positive way.
No significant difference in specific growth rate was determined throughout the study months (p > 0.05).
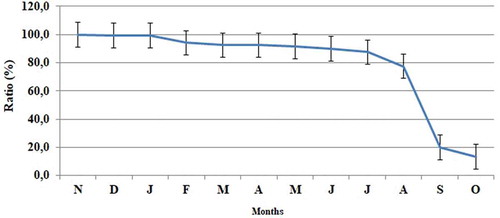
The percentage survival rate of the mussels was 80% at the end of the 10th month, although this decreased to approximately 13.3% in the last 2 months of the study due to fouling organisms such as tunicates, nudibranchs, crabs and gastropods which covered nets in the region during the autumn months (). This fact is considered the main reason for this decrease.
In this study, length–weight correlations for Mediterranean mussels were examined and the value of W was determined to be 0.0003L2.7254 (R2 = 0.88) (). Since the obtained value for b was less than 3 (b = 2.7254), the growth was determined to represent negative allometry.
4. Discussion
This study, conducted between November 2017 and October 2018, examined the scope of environmental variables such as salinity, temperature and pH as well as chlorophyll-a TPM, POM and PIM to affect the growth of Mediterranean mussels. This study assessed the environmental variables in the Gökçeada Island region in terms of suitability for mussel farming on nets. Based on the findings, it was determined that water temperature is the most significant factor affecting length–weight growth in mussels. These outcomes confirm the similar results obtained by numerous researchers (Seed Citation1976; Sukhotin & Maximovich Citation1994). In terms of chlorophyll-a, which is an important parameter in mussel growth along with temperature, growth performance similar to that obtained in other studies did not occur, because of the low levels of chlorophyll-a present throughout this study. This situation can be interpreted as an outcome of the fresh water inlet to the region where the study was performed. Fields et al. (Citation2015) compared surface chlorophyll-a, primary production and satellite images of different hydrographical sounds in New England and determined that the quantity of phytoplankton was not significantly different between Block Island Sound and Rhode Island Sound, but the quantity of primary production and satellite surface chlorophyll at one station was higher (50%) than at the other. They did not describe the mechanism underlying this difference, but argued that differences in water column stratification led to limited production at the station where chlorophyll is low, thus limiting surface nutrients. These findings, and this theory, of Fields et al. (Citation2015) support the results of our study.
During winter and summer months when the length growth and specific growth rates reached their highest values, salinity remained at approximately the same level (34.5‰). These salinity findings are similar to those from many other studies (Camacho et al. Citation1995; Yıldırım Citation1997; Lök Citation2001; Parisi et al. Citation2005). Additionally, the pH remained in an optimum range (8.1–8.2) for mussel growth.
As per the findings of this study, and according to the GLM analysis, the water temperature (p < 0.01) affected the growth of the Mediterranean mussels. From February to September 2018, length growth values increased gradually, in parallel with the temperature which increased from 13°C to 26.7°C, remaining within the optimum range. Many researchers have found that the optimum range is within these values (Figueras Citation1990; Yıldız & Lök Citation2005; Özkahya Citation2015). The water temperature started to decrease during the last 2 months of the study, when the number of fouling organisms increased; the survival rate decreased inversely. This study shows that the temperature was inversely correlated (p < 0.01) with TPM and POM according to Spearman’s correlation. In February, when mussels’ growth peaked but the water temperature was at its lowest levels during the whole study, TPM and POM levels were found to be at their highest levels. This situation is similar to that discussed by Karayücel et al. (Citation2003).
According to the examination of the mussels’ growth in winter, the extreme increase witnessed during January–February occurred for three reasons. One of the most important reasons is that small mussels demonstrate faster growth acceleration compared to other size groups. Yıldız and Lök (Citation2005) reported that the small-sized mussel group has great potential growth capability compared to larger mussels. Additionally, Seed (Citation1976) stated that in large mussels, growth decreases due to decreased metabolic activity compared with juvenile mussels. The second reason is that TPM and PIM values were at their highest level in the specified months. Although the water temperature at Gökçeada Island region is low in winter months, nutrition availability such as TPM and POM is maintained in the sea; hence, growth of the Mediterranean mussels continues. The last reason is that during the 12 months of the study, the lowest salinity was detected in January and February, compared to the other months. Some researchers have suggested that decreased salinity levels may have a positive effect on the growth of mussels (Fischer Citation1986; Segnini de Bravo Citation2003).
At the beginning of the study the mussels had a mean length of 19.58 ± 0.29 mm and a mean weight of 0.98 ± 0.07 g. At the end of the 12 months of study, they had reached a mean length of 43.77 ± 1.02 mm and a mean weight of 9.84 ± 0.73 g. An acceptable increase in growth was observed. These results showed strong similarity to those of other studies carried out all around the world. For instance, Lök (Citation2001) observed that the growth in the length of the mussels from first group was ranging from 22.73 ± 0.10 mm to 39.02 ± 1.01 mm, the second group was from 25.12 ± 0.07 to 43.50 ± 0.87 mm, the third group was from 30.41 ± 0.07 to 45.41 ± 0.72 mm and the other three groups were approximately the same, in Urla (in the Aegean region) between August 1996 and July 1997. The mussels studied by Yıldız et al. (Citation2006) in Çanakkale Strait had a mean length of 20.72 ± 0.13 mm at the beginning of their study, whereas 12 months later they had reached a mean length of 56.75 ± 0.83 mm. According to Yıldırım (Citation1997), mussels were 15.49 ± 0.19 mm long at the beginning of the study, in May 1996, whereas by the end of the study, in May 1997, they had reached a length of 57.4 ± 0.52 mm. Finally, Karayücel and Karayücel (Citation1999) observed through different results that mussel lengths ranged from 24.14 ± 0.40 mm to 52.92 ± 0.72 mm and from 24.14 ± 0.40 mm to 51.03 ± 0.60 mm on lantern nets in Loch Kishorn, Scotland as contrary our study area. Despite being carried out in different regions, the values obtained in these studies are similar. Given that studies of Mediterranean mussel cultivation on nets are limited, only four studies are compared in .
Table III. Spearman’s correlation results between environmental variables and mussel growth.
Table IV. Length and weight correlation in the Mediterranean mussel.
Table V. Length growth results for some studies of Mediterranean mussel cultivation on nets.
Many researchers have determined, using regression analysis, that mussels in different regions have negative allometry (< 3) growth (Bayne & Worrall Citation1980; Ceccherelli & Rossi Citation1984; Balcioğlu & Gönülal Citation2017). Mussels were similarly observed to have negative allometry growth in this study.
Yıldız and Lök (Citation2005) indicated that losses reached peak levels on Mediterranean mussel nets during two periods, between May and June 2002 and between September and October 2002. The greatest decrease occurred in the second period, and the survival rate was found to be 68.75% on seven different Mediterranean mussel net systems. In the present study, large numbers of mussel deaths occurred starting from the 10th month of the study, caused by fouling organisms such as tunicates, nudibranchs, crabs and gastropods that were increasing on the nets. In this case, it was observed that the water change on the nets is low and the nutrient intake decreases directly.
Consequently, temperature was the most important parameter affecting the growth of Mediterranean mussels on nets. The temperature, which regularly increased in the optimum range from February 2018 to the end of the study, has an impact on the growth of mussels. Due to the low chlorophyll-a value in the study area, mussels normally expected to reach a length of 70–80 mm and a weight of 10–11 g in a 12-month period had a length of 43.77 ± 1.02 mm and a weight of 9.84 ± 0.73 g. Salinity and pH, as determined during the study, remained within the appropriate range for Mediterranean mussel cultivation. The fact that mussels continue to grow during the winter months, when the water temperature can be considered low, indicates that the sea water in the region is rich in terms of TPM and POM. Although the mussels have a large salinity tolerance, it is an important finding that no mussels were observed at 38‰ salinity in the natural stocks at Gökçeada Island. Furthermore, mussels lived and grew in this study in water of approximately 34‰ salinity.
This study is the first conducted around Gökçeada Island, Turkey, in terms of Mediterranean mussels’ growth on nets. Findings of this study showed that net cultivation is suitable for mussels’ growth performance and survival. However, some suggestions are required. To increase the survival rate of mussels on mesh nets, it is necessary to clean the nets regularly or to develop new methods to increase the water exchange on nets. Another suggestion would be to enable Mediterranean mussels’ growth on nets by harvesting at the end of the 10th month at Gökçeada Island. As in our study, the IMTA cultivation method can be tried in the study regions, where chlorophyll-a levels are low, with the aim of causing the mussels to reach market size in a shorter time.
Acknowledgements
We thank Assoc. Prof. Dr. Onur Gönülal and employees in the Gökçeada Marine Research Unit of Istanbul University for their help and support during this study. We thank Assist. Prof. Dr. Ferhat Çağiltay for providing assistance and different points of view. We also thank Assist. Prof. Dr. Benal Gül for helping us in the sampling area and laboratory, as well as Prof. Dr. Mustafa Yildiz and Assist. Prof. Dr. Esra Billur Balcioğlu, from Istanbul University Faculty of Aquatic Sciences for their assistance in the laboratory.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Anonymous. 2020. http://tudav.org/en/our-fields/marine-protected-areas/gokceada-marine-park/gokceada-marine-park/ Accessed on December 2019 10
- APHA. 2000. Standard methods for the examination of water and wastewater. Washington. DC: American Public Health Association. Inc.
- Aral O. 1999. Growth of the Mediterranean mussel (Mytilus galloprovincialis lam., 1819) on ropers in the Black Sea Turkey. Turkish Journal of Veterinary and Animal Sciences 23(2):183–190.
- Asokan PK, Laxmilatha P, Surendranath VG, Sivadasan MP. 2011. Site selection for Mussel culture.
- Balcioğlu EB, Gönülal O. 2017. A study on biometry of Mussels (Mytilus galloprovincialis, Lamarck,1819) collected from various regions of Marmara Sea. Süleyman Demirel University. Journal of Natural and Applied Sciences 21(2):397–400. (In Turkish).
- Bayne BL, Newell RC. 1983. Physiological energetics of marine molluscs. UK-Cambridge: The mollusca Academic Press. pp. 407–515.
- Bayne BL, Worrall CM. 1980. Growth and production of mussels Mytilus edulis from two populations. Marine Ecology Progress Series 3:317–328. DOI:10.3354/meps003317.
- Camacho AP, Labarta U, Beiras R. 1995. Growth of mussels (Mytilus edulis galloprovincialis) on cultivation rafts: Influence of seed source cultivation site and phytoplanton availability. Aquaculture 138(1–4):349–362. DOI:10.1016/0044-8486(95)01139-0.
- Ceccherelli VU, Rossi R. 1984. Settlement, growth and production of the mussel Mytilus galloprovincialis. Marine ecology progress series. Oldendorf 16(1):173–184. DOI:10.3354/meps016173.
- Chatterji A, Ansari ZA, Ingole BS, Parulekar AH. 1984. Growth of the green mussel, Perna viridis L. in a sea water circulating system. Aquaculture 40(1):47–55. DOI:10.1016/0044-8486(84)90215-1.
- Dickie LM, Boudreau PR, Freeman KR. 1984. Influences of stock and site on growth and mortality in the blue mussel (Mytilus edulis). Canadian Journal of Fisheries and Aquatic Sciences 41(1):134–140. DOI:10.1139/f84-013.
- EUROSTAT. 2018. Fishery statistics. Available: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Fishery_statistics#Aquaculture_statistics. Accessed May 2019 5.
- FAO. 2018. Available: http://www.fao.org/3/I9540EN/i9540en.pdf. Accessed September 2018 19.
- Fields L, Mercer J, Hyde KJ, Brush M, Nixon SW, Oviatt C, Codiga D. 2015. Comparison of surface chlorophyll, primary production, and satellite imagery in hydrographically different sounds off southern New England. Marine Ecology Progress Series 535:29–45. DOI:10.3354/meps11386.
- Figueras AL. 1990. Mussel culture in Spain. Marine Behaviour and Physiology 16(3):177–207. DOI:10.1080/10236249009378748.
- Fischer H. 1986. Influence of temperature, salinity, and oxygen on the cadmium balance of mussels Mytilus edulis. Marine Ecology Progress Series 32(2–3):265. DOI:10.3354/meps032265.
- Kamermans P, Capelle JJ. 2019. Provisioning of mussel seed and its efficient use in culture. In: Smaal A., Ferreira J., Grant J., Petersen J., Strand Ø. (eds) Goods and Services of Marine Bivalves. Springer, Cham.
- Karayücel S, Karayücel I. 1999. Growth and mortality of Mussels (Mytilus edulis L.) reared in Lantern nets in Loch Kishorn, Scotland. Turkish Journal of Veterinary and Animal Sciences 23(5):397–402.
- Karayücel S, Karayücel I, Erdem M, Saygun S, Uyan O. 2003. Growth and production in long-line cultivated Mediterranean mussel (Mytilus galloprovincialis) in Sinop, Black Sea. The Israeli Journal of Aquaculture-Bamidgeh 55(3):169–178.
- Lök A. 2001. Growth rate of different size groups of mussels, Mytilus galloprovincialis Lamarck, 1819 in Iskele-Urla (Bay of Izmir). Ege Journal of Fisheries and Aquatic Sciences 18(1):141–147. (In Turkish).
- Lök A, Acarlι S, Serdar S, Köse A, Yιldιz H. 2007. Growth and mortality of Mediterranean mussel Mytilus galloprovincialis Lam., 1819, in relation to size on longline in Mersin Bay, Izmir (Turkey–Aegean Sea). Aquaculture Research 38(8):819–826. DOI:10.1111/j.1365-2109.2007.01717.x.
- Özkahya P 2015. Determination of bioecological features of Mytilus galloprovincialis in aquaculture areas at Güllük Bay. PhD, Mugla Sıtkı Kocman University Graduate School of Natural and Applied Sciences. Bornova-İzmir. (In Turkish).
- Malouf RE, Bricelj VM. 1989. Comparative biology of clams: Environmental tolerances, feeding and growth. In: Manzi JJ, Castagna M, editors. Clam mariculture in North America. Amsterdam, The Netherlands: Elsevier. pp. 23–73.
- Parisi G, Giorgi G, Messini A, Poli BM. 2005. Growth performance and quality traits of mussel (Mytilus galloprovincialis Lamarck) reared in two different sites in Tuscany. Italian Journal of Animal Science 4(sup2):612–614. DOI:10.4081/ijas.2005.2s.612.
- Ricker WE. 1975. Computation and interpretation of biological statistics of fish populations. Bulletin of Fish Resident 191:1–382.
- Sangrà P, Basterretxea Oyarzabal G, Llopart P, Luis J, Arístegui J. 2001. Chlorophyll increase due to internal waves on the shelf break of Gran Canaria (Canary Islands). Scientia Marina. DOI: 10.3989/scimar.2001.65s189.
- Seed R. 1976. Ecology. In Marine Mussels: Their Ecology and Physiology (ed. Bayne, B. L.), pp. 13–65. Cambridge University Press.
- Segnini de Bravo MI. 2003. Influence of salinity on the physiological conditions in mussels, Perna perna and Perna viridis (Bivalvia: Mytilidae). Revista De Biologia Tropical 51:4.
- Serdar S, Ş Y, Ertan A, Gökvardar A, Sabancı F 2017. Mussel culture in the net-bags in Erdek Bay Marmara Sea. A paper presented at this conference. 19th National Fisheries Symposium, 12-15 September 2017, Sinop. pp.147. (Sinop-Turkish).
- Serdar S, Yıldırım Ş 2018. An increasing trend in Turkey: Mussel culture. A paper presented at this conference. The 2nd International Fisheries Symposium, 4-8 November 2018, Girne-Turkish Republic of Northern Cyprus, Kyrenia. pp.106–107.
- Smaal AC, Ferreira JG, Grant J, Petersen JK, Strand Ø. 2019. Goods and services of marine bivalves. Cham, Switzerland: Springer. p. 591.
- Strickland JDH, Parsons TR. 1972. A practical handbook of seawater analysis. Bulletin Fishery Research Board, Canada, 167, 310.
- Sukhotin AA, Maximovich NV. 1994. Variability of growth rate in Mytilus edulis L. from the Chupa Inlet (the White Sea). Journal of Experimental Marine Biology and Ecology 176(1):15–26. DOI:10.1016/0022-0981(94)90194-5.
- TURKSTAT. 2018. Fishery statistics. Ankara Turkey: Turkish Statistical Institute.
- Yıldırım Ş 1997. Mussel (Mytilus galloprovincialis, Lamarck 1819) culture in floating cages in two different stations. MSc, Ege University Graduate School of Natural and Applied Science, Bornova- İzmir. (In Turkish).
- Yıldız H, Acarlı S, Berber S, Vural P, Gündüz F. 2013. A preliminary study on Mediterranean Mussel (Mytilus galloprovincialis Lamarck, 1819) culture in integrated multitrofik aquaculture systems in Canakkale Strait. Alinteri Journal of Agricultural Sciences, 25(B); 38–44. (Turkey). (In Turkish).
- Yıldız H, Lök A. 2005. Growth and survival rates of different size classes of black mussel (Mytilus galloprovincialis Lamarck, 1819) at two culture systems in Dardanelles. Ege Journal of Fisheries and Aquatic Sciences 22(1);69–74. (In Turkish).
- Yıldız H, Lök A, Acarlı S, Serdar S, Köse A. 2010. A preliminary survey on settlement and recruitment patterns of Mediterranean mussel (Mytilus galloprovincialis) in Dardanelles, Turkey. Journal of the Faculty of Veterinary Medicine Kafkas University 16:319–324.
- Yıldız H, Lök A, Köse A, Serdar S, Acarlı S. 2006. Rope culture of mediterranean mussels (Mytilus galloprovincialis L. 1819) in the Dardanelles. Ege Journal of Fisheries and Aquatic Sciences 23:319–322. (In Turkish).