Abstract
Specimens of common smooth-hound, Mustelus mustelus, fished as bycatch in the Northwestern Adriatic Sea, were subjected to a complete parasitological examination. Parasites were processed for morphological and morphometric analyses aimed at identifying them to the species level. Parasite identity was confirmed through amplification and sequencing of 28S rDNA. The nematode Acanthocheilus rotundatus was recovered from the stomach and the proximal intestine, while the cestode Calliobothrium verticillatum was collected from the spiral valve. Our results confirm the occurrence of C. verticillatum in M. mustelus from the Mediterranean Sea and provide the first sequence data of A. rotundatus from the same area. Updated knowledge on the parasite fauna of M. mustelus from Adriatic Sea contributes to elucidating aspects of the ecology, biology and health status of elasmobranchs from the Mediterranean Sea, providing useful information for their proper management and conservation.
Introduction
The correct management of elasmobranch populations for conservation purposes requires the acquisition of detailed information concerning their biology and ecology. Currently, in the Mediterranean Sea, important gaps of knowledge occur even for the most studied elasmobranch species.
Sharks are definitive hosts of a variety of trophically transmitted helminth parasites (Caira & Healy Citation2004). The analysis of the parasite fauna provides useful insights on diet and habitat preferences of their shark host (Klimpel et al. Citation2003). Moreover, the potential use of parasites as biological tags for the identification of different shark stocks has been explored successfully, highlighting a greater potential of intestinal cestodes (Yamaguchi et al. Citation2003).
Parasitological investigations focused on elasmobranchs from the Mediterranean Sea are scarce; the compilation of accurate and updated checklists is further complicated by the occurrence of old records of infection and the doubtful identity of the shark host.
The common smooth-hound Mustelus mustelus (Linnaeus, 1758) occurs throughout the Eastern Atlantic and Mediterranean Sea, where it represents the target or bycatch of different fishing techniques (Marino et al. Citation2018). Parasitological data from Mediterranean M. mustelus are scarce and, in several instances, represented by old literature (Rudolphi Citation1819; Euzet Citation1959). In particular, the cestode fauna of this species is poorly known (Bernot et al. Citation2016). Recently, M. mustelus collected off Croatia were investigated with the aim of linking the parasite assemblage in the gastrointestinal tract with the trophic ecology of the species and providing preliminary insights on the role of M. mustelus in the life cycle of different groups of parasites (Gračan et al. Citation2014). This species shows a non-specialised feeding behaviour, which varies in relation to members’ geographic distribution and growth stage (Costantini et al. Citation2000); therefore, their parasite assemblage is expected to vary accordingly.
The conservation of M. mustelus is threatened by the high percentage of sexually immature individuals caught and landed; therefore, this species is currently classified as Endangered (EN) by the Italian Committee of the International Union for Conservation of Nature (IUCN), with the priority of setting an appropriate minimum size for commercial fisheries (www.iucn.it/scheda.php?id=1856264254); similarly, the number of individuals captured for scientific research should be limited. In this context, the use of dead bycatch samples is a useful way to obtain biological and ecological data from those specimens that would otherwise have been discarded.
The present work provides a morphological and molecular characterisation of gastrointestinal parasites in M. mustelus collected off the Italian coasts of the Northern Adriatic Sea, updating information about the parasite fauna of this shark species; the integration of our data with previously published parasitological records highlights the possible occurrence of distinct parasite assemblages in smooth-hounds from different areas of the Northern Adriatic Sea.
Materials and methods
Study area and parasite collection
Three specimens of M. mustelus, fished as bycatch in the Northwestern Adriatic Sea (FAO 37.2.1) from June to November 2019, were subjected to a complete parasitological examination: the skin, buccal and nasal cavities, gill chamber and visceral organs were inspected for the presence of metazoan parasites. All collected parasites were washed in saline and preserved in 70% ethanol for subsequent morphological and molecular analyses for species identification. Two taxa, one belonging to nematodes and one to cestode parasites, were macroscopically distinguished.
Morphological analyses
After measuring the total length, nematode and cestode parasites were subjected to microscopical observation following clarification in Amman’s lactophenol to study their morphology (Khalil et al. Citation1994; Gibbons Citation2010). Before clearing the worms, a little piece of the nematodes’ body devoid of taxonomic features and one cestode proglottid were excised with a sterile scalpel and stored at −20°C for further molecular analysis.
Nematodes were additionally characterised by scanning electron microscopy; samples stored in 70% ethanol were dehydrated through a graded ethanol series, critical point dried, sputter-coated with gold, and observed using a JEOL JSM-5410 scanning electron microscope operating at 15 kV.
Measurements were taken with the imaging software NIS-Elements (Nikon, Campi Bisenzio [FI], Italy), and are given in micrometres (µm; mean followed by the range in parentheses).
Molecular analysis
For molecular analysis, genomic DNA was extracted from four cestodes and six nematodes using a PureLink® Genomic DNA Kit (Life Technologies, Carlsbad, California) following the manufacturer’s instructions.
For cestodes, the amplification of the 28S rDNA region was performed with primers U178_f (5ʹ-GCACCCGCTGAAYTTAAG-3ʹ) and L1642_r (5ʹ-CCAGCGCCATCCATTTTCA-3ʹ) (Lockyer et al. Citation2003). For sequencing, the internal primers 900 F (5ʹ-CCGTCTTGAAACACGGACCAAG-3ʹ) and EDC2 (5ʹ-CCTTGGTCCGTGTTTCAAGACGGG-3ʹ) of Lockyer et al. (Citation2003) were used. For nematodes, the amplification of the complete ITS rDNA region was performed with primers NC5_f (50-GTAGGTGAACCTGCGGAAGGATCATT-30) and NC2_r (50-TTAGTTTCTTCCTCCGCT-30) (Zhu et al. Citation1998).
The polymerase chain reaction (PCR) products were electrophoresed on 1% agarose gel stained with SYBR® Safe DNA Gel Stain (Thermo Fisher Scientific, Carlsbad, California) in 0.5X TBE. Amplicons were purified with Nucleo-Spin Gel and PCR Cleanup (Mackerey-Nagel, Düren, Germany). All samples were sequenced with an ABI 3730 DNA analyser (StarSEQ, Mainz Germany). The trace files were assembled with ContigExpress (VectorNTI Advance 11 software, Invitrogen, Carlsbad, California), and the consensus sequences were compared with previously published data using BLAST tools (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The sequences generated in this study were deposited in GenBank under the accession numbers MT967225–MT967230 and MT981401–MT981404.
Results
The examined M. mustelus measured 44, 46 and 66 cm in total length. Parasitic helminths were found in the digestive tract of a single specimen (total length 66 cm); in particular, larval and adult nematodes belonging to the species Acanthocheilus rotundatus (Rudolphi, Citation1819) (15 specimens) were recovered from the stomach and the proximal intestine, while adult cestodes belonging to the species Calliobothrium verticillatum (Rudolphi, Citation1819) (five specimens) were collected from the spiral valve.
Of these, morphometric data for eight A. rotundatus (one fourth-stage larva, five adult males and two adult females) and four C. verticillatum are reported below.
Morphological analyses
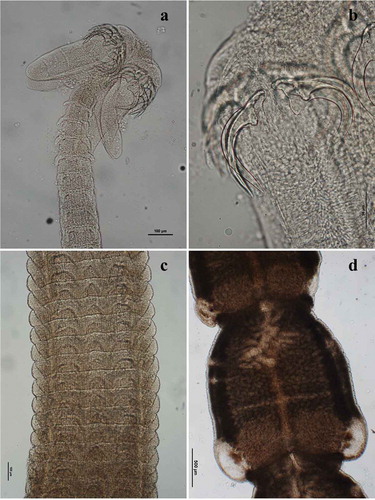
Calliobothrium verticillatum (Rudolphi, Citation1819)()
Adult morphology: Body 66,809 (62,612–75,629) long; scolex 453 (385–509) long × 326 (263–374) wide (), with four bothridia 430 (465–396) long × 121 (100–133) wide, each divided into three loculi by two transverse septa; anterior loculus 172 (136–189) long; middle loculus 71 (60–77) long; posterior loculus 73 (67–84) long; apical region of each bothridium with anterior muscular pad 48 (40–52) long × 115 wide (93–129), bearing three apical suckers arranged in horizontal pattern; two pairs of articulated, unipronged hooks (): lateral axial hook 128 (117–140) long; medial axial hook 124 (112–143) long; lateral abaxial hook 141 (124–173) long; medial abaxial hook 128 (114–148) long. Strobila with 259 (168–316) segments, craspedote, laciniated, euapolytic. Pattern of proglottid laciniation variable along length of strobila (); immature proglottids 155 (105–240) long × 565 (457–621) wide; mature proglottids 1497 (1137–2171) long × 1262 (888–1885) wide; genital pore lateral, irregularly alternating; testes numerous, in two lateral columns; postvaginal testes present on poral side; ovary at posterior end of proglottid; vagina anterior to cirrus sac; vitelline follicles lateral; uterus sacciform, medioventral; uterine pore medioventral.
Figure 1. Calliobothrium verticillatum adult: (a) scolex; (b) detail of hooks; (c) immature proglottids; (d) mature proglottids
Acanthocheilus rotundatus (Rudolphi, Citation1819)()
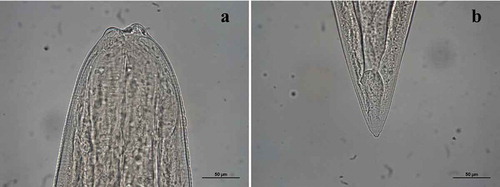
Fourth-stage larva morphology (one specimen): body 15,426 long × 421 wide; anterior end () with three small labia each provided with four small sclerified pieces with rounded tips; dorsal lip with two double papillae, latero-ventral lips each showing a double papilla and an amphid; oesophagus 1374 long becoming narrower at anterior end; ventriculus rounded, 137 long × 171 wide; nerve ring 466 from anterior end; excretory pore slightly posterior to nerve ring, 554 from anterior end; deirids 503 from anterior end; lateral alae extending slightly posteriorly from anterior end to mid-length of the tail; tail conical, 91 long with small terminal mucron ().
Adult general morphology (): body large, becoming narrower towards the anterior extremity (); lateral alae absent. Three small, semicircular lips each provided on its internal face with two bifid teeth (), ). Dorsal lip with two double papillae, latero-ventral lips each presenting a double papilla, a single latero-ventral papilla and an amphid; interlabia absent. Esophagus expanded at the anterior end. Nerve ring at around one-third to one-quarter of esophagus length. Deirids small, rounded, slightly posterior to the nerve ring; excretory pore slightly posterior to nerve ring (). Ventriculus small, rounded, wider than long (). Tail conical, with small terminal mucron.
Figure 3. Acanthocheilus rotundatus adults: (a) male specimen, anterior end; (b) male specimen, detail of ventriculus (arrow); (c) male specimen, anterior end with detail of bifid teeth (arrows) and double papilla (arrowhead); (d) male specimen, detail of excretory pore (arrow); (e) female specimen, detail of vulva (arrow); male specimen, caudal end
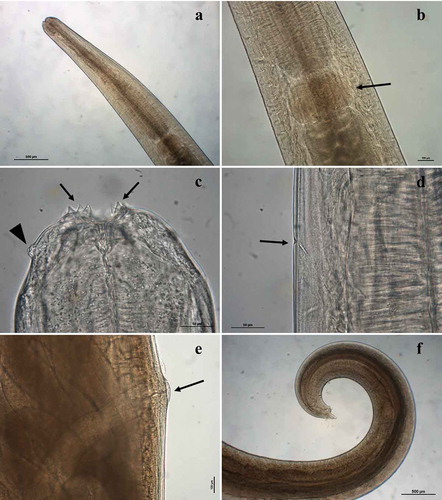
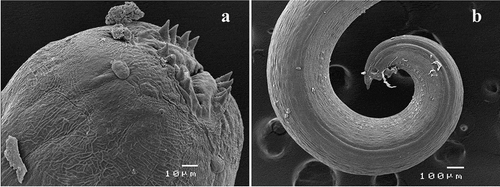
Figure 4. Acanthocheilus rotundatus adult male, scanning electron micrographs: (a) detail of anterior end; (b) caudal end
Adult male morphometry (five specimens): body 31,922 (25,051–36,095) long × 802 (650–1060) wide; esophagus 2460 (2259–2912) long; ventriculus 268 (248–292) long × 363 (310–401) wide; nerve ring, excretory pore and deirids 821 (693–925), 848 (695–908) and 816 (731–905) from anterior end, respectively; spicules short, robust, and similar in length, pointed at distal end, 380 (263–523) long; gubernaculum absent; 41 (40–42) precloacal papillae (); tail 166 (126–204) long ().
Adult female morphometry (two specimens): body 39,709 (30,305–49,113) long × 1120 (947–1293) wide; esophagus 3062 (2723–3401) long; ventriculus 331 (318–344) long × 440 (411–467) wide; nerve ring, excretory pore and deirids 1053 (814–1293), 1171 (976–1365) and 1108 (907–1308), respectively, from anterior end; vulva 12,610 (12,045–13,174) from anterior end (); vagina muscular, directed posteriorly from vulva; two long uteri; tail 533 (520–546) long.
Molecular analyses
The 28S rDNA of four C. verticillatum were successfully sequenced and were identical to each other. The BLAST search gave 99.9% identity with C. verticillatum (KF685753) of Caira et al. (Citation2014). The ITS rDNA of six A. rotundatus were successfully sequenced and identical to each other. The BLAST analysis gave 98.6% identity with Acanthocheilus rotundatus (MF061679) of Li et al. (Citation2018).
Discussion
Parasitological examination of M. mustelus from the Northwestern Adriatic Sea allowed us to identify the cestode species C. verticillatum and the nematode species A. rotundatus.
Calliobothrium verticillatum, the type species of the genus Calliobothrium, was originally described by Rudolphi (Citation1819) from an undetermined shark host referred to Squali galei collected off Italy. Calliobothrium verticillatum has been reported in Mustelus vulgaris (synonymised with M. mustelus) from the Northeastern Atlantic (van Beneden Citation1850) and subsequently reported by Euzet (Citation1959) infecting Mediterranean Mustelus, namely M. mustelus and Mustelus canis (Mitchill, 1815); however, the latter species is now known not to occur in the Mediterranean area (Bernot et al. Citation2016). Our results would therefore confirm the occurrence of this host–parasite association in Mediterranean waters.
Euzet (Citation1959) reported the presence of a highly diverse cestode fauna in M. mustelus from French coasts, including the phyllobothriideans Phyllobothrium lactuca Van Beneden, Citation1850, Scyphophyllidium triacis (Yamaguti, 1952) Caira, Jensen and Ruhnke in Caira, Jensen, Hayes and Ruhnke, 2020 and Orygmatobothrium musteli (Van Beneden, 1849) Diesing, 1863; the tetraphyllideans C. verticillatum (Rudolphi, Citation1819), Symcallio eschrichti (van Beneden, Citation1850) Bernot, Caira and Pickering, 2015, and Symcallio lintoni (Euzet, 1954) Bernot, Caira and Pickering, 2015; the onchoproteocephalidean Acanthobothrium mathiasi Euzet, Citation1959; the diphyllidean Coronocestus musteli (Pintner, 1889) Caira, Marques, Jensen, Kuchta and Ivanov, 2013; and the trypanorhynchs Eutetrarhynchus ruficollis (Eysenhardt, 1829) Pintner, 1913 and Lacistorhynchus tenuis (Van Beneden, 1858) Pintner, 1913.
Regarding C. verticillatum, the total length of the specimens analysed in the present study is slightly shorter compared to the specimens analysed by Euzet (Citation1959); however, the morphometry of the scolex features (hooks, bothridia, accessory suckers) and the number and size of segments in the strobila are in accordance with those reported by Euzet. Scolex morphology is also in accordance with the description provided in Caira and Ruhnke (Citation1991).
Different species of Mustelus act as definitive hosts in the life cycle of Calliobothrium spp. Adults of C. verticillatum mature in the shark spiral intestine and produce hexacanth embryos that are released from gravid proglottids; hermit crabs act as intermediate hosts after ingesting hexacanths, and within their body the embryo develops firstly into a procercoid and subsequently into a plerocercoid larva; the life cycle is completed when infected hermit crabs are ingested by a shark (McDermott et al. Citation2010). Plerocercoids of C. verticillatum are found in the lumen of the anterior midgut caeca of hermit crab Pagurus pollicaris Say, 1817 [in Say, 1817–1818] from extra-Mediterranean areas (Caira & Ruhnke Citation1991), where remarkable prevalence values (over 95%; Cherry et al. Citation1991) have been reported.
A. rotundatus was originally described by Rudolphi (Citation1819) infecting Galeorhinus galeus (Linnaeus, 1758) from the Adriatic Sea and was subsequently redescribed by Moravec and Nagasawa (Citation2000) infecting Mustelus griseus Pietschmann, 1908 and Mustelus manazo Bleeker, 1855 from Japanese waters. Acanthocheilus rotundatus was reported infecting different shark species worldwide (Moravec & Nagasawa Citation2000, and references therein); in particular it was reported in M. mustelus from the Adriatic Sea, where it was identified with morphological methods (Petter et al. Citation1991). Our work provides the first sequence data of A. rotundatus from the Mediterranean Sea, since only sequences of specimens collected in extra-Mediterranean areas (Li et al. Citation2018) were previously available. Furthermore, the caudal end of male A. rotundatus is here characterised by scanning electron microscopy for the first time.
With respect to the morphometric data of A. rotundatus generated in this study, our measurements of male and female specimens are in accordance with morphometric data reported in previous studies (Diaz Citation1971; Petter et al. Citation1991; Moravec and Nagasawa Citation2000). In particular, the morphometric data of our adult females fall between those reported by these authors. Adult males examined are within the range of measurements reported by the same authors; however, we observed more numerous pairs of precloacal papillae (40–42 pairs) than those reported by Petter et al. (Citation1991) (30–36 pairs) and by Moravec and Nagasawa (Citation2000) (25–29 pairs); nevertheless, as pointed out by the latter authors, in ascaridoids the number of precloacal papillae is subject to a considerable degree of intraspecific variability. With respect to the fourth-stage larva, it was not possible to perform a full comparison due to the scarcity of morphometric data available in the literature for this larval stage. This work therefore represents the first detailed morphometric characterisation of A. rotundatus fourth-stage larva.
The life cycle of Acanthocheilus quadridentatus (syn. A. rotundatus) has been described by Diaz (Citation1971): eggs are produced by gravid adult females in the intestine of the elasmobranchs and are released into the environment with the host’s faeces; eggs hatch in seawater and release first-stage larvae, which undergo their first moult to second-stage larvae; the latter are found in the pyloric caeca of the hermit crab Pagurus prideaux Leach, 1815 [in Leach, 1815–1875], where they develop and moult into third-stage larvae, infective to the definitive elasmobranch host; in the stomach and proximal intestine of different elasmobranch species (i.e. M. mustelus, Squalus acanthias, Raja asterias), third-stage larvae moult into fourth-stage larvae, which undergo further growth and sexual maturation. Data on the distribution of A. rotundatus in elasmobranchs are scarce. In the intermediate host P. prideaux, remarkable prevalence (50%) and intensity (1–15) values of infection with A. rotundatus were reported (Diaz Citation1971); it is currently unknown whether other invertebrate or vertebrate hosts are involved in the transmission of the parasite to its definitive elasmobranch host.
Mustelus mustelus from the Northeastern Adriatic have been recently examined for gastrointestinal parasites by Gračan et al. (Citation2014); these authors reported the presence of the trematode Ptychogonimus megastomum (Rudolphi, Citation1819) Lühe, 1900, the cestodes Eutetrarhynchus sp. and Phyllobothrium sp. and the nematode Cucullanus micropapillatus Tornquist, 1931 as the most prevalent and dominant species, and linked this information to the feeding habits of M. mustelus inferred from the analysis of gastrointestinal contents. Indeed, no prey items belonging to the genus Pagurus were reported in the gastrointestinal tract of the M. mustelus analysed in that study (Gračan et al. Citation2014).
Our findings, reporting the presence of different species of trophically transmitted parasites, would therefore suggest the possible occurrence of distinct parasite assemblages, arguably associated to different prey composition, in M. mustelus from different areas of the Northern Adriatic Sea.
Further screenings are needed to determine the parasite diversity of M. mustelus in different parts of the Mediterranean Sea. Moreover, additional information concerning the life cycle of the parasites identified in the present study would allow researchers to elucidate the full range of hosts potentially involved in parasite transmission to M. mustelus and provide valuable insights into its feeding ecology.
Acknowledgements
The authors are grateful to Letizia Modeo and Simone Gabrielli (Centro Interdipartimentale di Microscopia Elettronica – Università di Pisa) for their technical assistance in the preparation of samples for scanning electron microscopy. Thanks are also due to the staff of CESTHA (Experimental Centre for Habitat Conservation) for their help in the collection of some of the examined specimens.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bernot JP, Caira JN, Pickering M. 2016. Diversity, phylogenetic relationships and host associations of Calliobothrium and Symcallio (Cestoda:‘Tetraphyllidea’) parasitising triakid sharks. Invertebrate Systematics 30:616–634. DOI: 10.1071/IS15040.
- Caira JN, Healy CJ. 2004. Elasmobranchs as hosts of metazoan parasites. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of sharks and their relatives. Boca Raton: CRC Press. pp. 523–551.
- Caira JN, Jensen K, Waeschenbach A, Olson PD, Littlewood DTJ. 2014. Orders out of chaos–molecular phylogenetics reveals the complexity of shark and stingray tapeworm relationships. International Journal for Parasitology 44:55–73. DOI: 10.1016/j.ijpara.2013.10.004.
- Caira JN, Ruhnke TR. 1991. A comparison of scolex morphology between the plerocercoid and the adult of Calliobothrium verticillatum (Tetraphyllidea: Onchobothriidae). Canadian Journal of Zoology 69:1484–1488. DOI: 10.1139/z91-207.
- Cherry B, Neese AS, Bullis RA, Schad GA. 1991. Investigations into the life cycle of Calliobothrium verticillatum, a tapeworm of Mustelus canis. The Biological Bulletin 181:358. DOI: 10.1086/BBLv181n2p358.
- Costantini M, Bernardini M, Cordone P, Guilianini PG, Orel G. 2000. Observations on fishery, feeding habits and reproductive biology of Mustelus mustelus (Chondrichthyes, Triakidae) in Northern Adriatic Sea. Biologia Marina Mediterranea 7:427–432.
- Diaz JP. 1971. Cycle évolutif d’Acanthoceilus quadridentatus Molin, 1858 (Nematoda). Vie et Milieu 22:289–304.
- Euzet L. 1959. Recherches sur les cestodes tétraphyllides des sélaciens des côtes de France. PhD Thesis, Université de Montpellier, Montpellier, France.
- Gibbons LM. 2010. Keys to the nematode parasites of vertebrates, supplementary volume. Wallingford, UK: CAB International. pp. 416.
- Gračan R, Mladineo I, Lazar B. 2014. Insight into the diet composition and gastrointestinal parasite community of the common smooth-hound, Mustelus mustelus (Carcharhiniformes: Triakidae), in the northern Adriatic Sea. Natura Croatica 23:35–44.
- Khalil LF, Jones A, Bray RA. 1994. Keys to the cestode parasites of vertebrates. Wallingford, Oxon: CAB INTERNATIONAL. 768 pp.
- Klimpel S, Palm HW, Seehagen A. 2003. Metazoan parasites and food composition of juvenile Etmopterus spinax (L., 1758) (Dalatiidae, Squaliformes) from the Norwegian Deep. Parasitology Research 89:245–251. DOI: 10.1007/s00436-002-0741-1.
- Li L, Lü L, Nadler SA, Gibson DI, Zhang LP, Chen HX, Zhao WT, Guo YN. 2018. Molecular phylogeny and dating reveal a terrestrial origin in the early carboniferous for ascaridoid nematodes. Systematic Biology 67:888–900. DOI: 10.1093/sysbio/syy018.
- Lockyer AE, Olson PD, Littlewood DTJ. 2003. Utility of complete large and small subunit rRNA genes in resolving the phylogeny of the Neodermata (Platyhelminthes): Implications and a review of the cercomer theory. Biological Journal of the Linnean Society 78:155–171. DOI: 10.1046/j.1095-8312.2003.00141.x.
- Marino IAM, Finotto L, Colloca F, Di Lorenzo M, Gristina M, Farrell ED, Zane L, Mazzoldi C. 2018. Resolving the ambiguities in the identification of two smooth-hound sharks (Mustelus mustelus and Mustelus punctulatus) using genetics and morphology. Marine Biodiversity 48:1551–1562. DOI: 10.1007/s12526-017-0701-8.
- McDermott JJ, Williams JD, Boyko CB. 2010. The unwanted guests of hermits: A global review of the diversity and natural history of hermit crab parasites. Journal of Experimental Marine Biology and Ecology 394:2–44. DOI: 10.1016/J.JEMBE.2010.06.022.
- Moravec F, Nagasawa K. 2000. Two remarkable nematodes from sharks in Japan. Journal of Natural History 34:1–13. DOI: 10.1080/002229300299660.
- Petter AJ, Paradiznik V, Radujkovic BM, Cassone J. 1991. Redescription d’Acanthocheilus rotundatus (Ascaridoidea, Nematoda), parasite de requins (Pleurotremata, Selachii). Considérations sur les affinités du genre Acanthocheilus et conclusions taxonomiques. Annales de Parasitologie Humaine et Comparée 66:187–194. DOI: 10.1051/parasite/1991665187.
- Rudolphi CA. 1819. Entozoorum Synopsis Cui Accedunt Mantissa Duplex et Indices Locupletissimi. Berlin: Sumtibus Augusti Rücker.
- van Beneden PJ. 1850. Recherches sur la faune littorale de Belgique. Memoires de l’academie royale des sciences, des lettres et des beaux-arts de Belgique 25: 1-1. www.iucn.it/scheda.php?id=1856264254. Accessed Jun 2020.
- Yamaguchi A, Yokoyama H, Ogawa K, Taniuchi T. 2003. Use of parasites as biological tags for separating stocks of the starspotted dogfish Mustelus manazo in Japan and Taiwan. Fisheries Science 69:337–342. DOI: 10.1046/j.1444-2906.2003.00626.x.
- Zhu X, Gasser RB, Podolska M, Chilton NB. 1998. Characterisation of anisakid nematodes with zoonotic potential by nuclear ribosomal DNA sequences. International Journal for Parasitology 28:1911–1921. DOI: 10.1016/S0020-7519(98)00150-7.