Abstract
Recent years have witnessed a fall in the numbers of bee colonies worldwide. To a large extent, this is due to diseases affecting honeybees. One such disease is nosemosis, which is caused by fungi from the genus Nosema. The antibiotic fumagallin used to be administered to treat nosemosis, but as it was found capable of being transferred to bee products, this practice has ceased. We therefore focused on natural substances for treating this disease, among them, aqueous extracts of the nest carton produced by the jet-black ant (Lasius fuliginosus). We tested the influence of 0.1% and 1% concentrations of such extracts on the incidence of nosemosis in honeybees. The “birch carton 2” extract turned out to be the most effective inhibitor of this disease. The incidence of nosemosis following the administration of this extract, as manifested by the number of spores, fell ca 18-fold in comparison with the control. Moreover, the “birch carton 2” extract was not found to be toxic towards bees.
GA
1. Introduction
The European honeybee (Apis mellifera Linnaeus, 1758) plays a key role in plant pollination and food production (Prasad et al. Citation2015; Aslan et al. Citation2016). Honeybees also produce honey, an economically important product. These insects are thus ecologically and economically invaluable. Recent years have seen the numbers of bee colonies decline (Cepero et al. Citation2014). This may be due to pesticide application, weather conditions and the availability of food resources, but also to the spread of new diseases and parasites (VanEngelsdorp & Meixner Citation2010; Cepero et al. Citation2014; Staveley et al. Citation2014).
One of the main causes of honeybee death is nosemosis. In our climate zone, nosemosis is caused by two species of microsporidians from the genus Nosema, i.e. N. apis and N. ceranae I. Fr. (Gajger et al. Citation2015). Microsporidia are intracellular parasites that attack both vertebrates and invertebrates. The first case of nosemosis was recorded in 1909; at that time, it was caused only by N. apis E. Zander (Zander Citation1909). The infection of the Asiatic honeybee Apis cerana by N. ceranae was reported in 1996. The first natural infection by N. ceranae of honeybees kept in Taiwan was reported in 2005 (Fries et al. Citation1996; Chen & Huang Citation2010; Holt & Grozinger Citation2016). Subsequent infections by N. ceranae took place in Europe (Paxton et al. Citation2007), and the USA. (Chen et al. Citation2007). The disease caused by N. apis is called nosemosis A, while that due to N. ceranae is nosemosis C (Higes et al. Citation2010). The life cycle of Nosema spp. begins when the honeybee swallows this parasite. Spores of Nosema spp. may be present in honey, nectar, pollen and excreta. Osmotic pressure causes the formation of a polar filament, by which the infected material is injected from the spore into the epithelial cells of the midgut, where the parasite multiplies and matures. The newly formed spores are released into the lumen of the midgut, from where they pass to the exterior in excrement (Burnham Citation2019). Digestion is disturbed, malnourishment occurs, and physiological and anatomical changes to the midgut cells take place. Nosema spores create a layer of mature spores on the intestine surface (Ptaszyńska et al. Citation2014), which leads to honeybee malnutrition and changes in the composition of microelements (Ptaszyńska et al. Citation2018a). Furthermore, spores of N. ceranae and N. apis have been found in the salivary glands, fat body, throat glands and Malpighian tubules of honeybees what causes impairments of these glands and tissue functions (Chen & Huang Citation2010; Copley & Jabaji Citation2011; Ptaszyńska et al. Citation2012). These processes weaken the honeybees and ultimately kill them, which can lead to colony collapse.
The antibiotic fumagillin, isolated from Aspergillus fumigatus, used to be administered to treat nosemosis. But in February 2016 this agent was withdrawn by the European Medicines Agency (Regulation 2377/90, European Commission 2009). Research had shown that fumagillin residues in honeybee products posed a risk to consumers (Van den Heever et al. Citation2014). In addition, the long-term administration of this antibiotic to honeybees infected by Nosema spp. could lead to the fungus becoming resistant to it (Huang et al. Citation2013). For this reason, the search began for a natural substance that would be equally effective in treating nosemosis, and yet be consumer-safe.
The results are known of research into the antifungal activity of preparations containing microbiological material derived from ants. A strain of the actinobacteria Streptomyces lasiicapitis, new to science, was isolated from the heads of jet-black ants; it produces kanchanamycin, a substance with antifungal activity (Ye et al. Citation2017). In another experiment, an extract made from the bodies of the same ant species acted selectively on fungi, enhancing the growth of some species, and strongly retarding the development of others (Brinker et al. Citation2018). Furthermore, compounds produced by ants can be good candidates for such antifungal natural substances. Ants have a metapleural gland, which can produce antimicrobial substances to fight infections (Yek & Mueller Citation2011; Tragust Citation2016; Bos et al. Citation2019) and ants from Lasius genus have well-developed metapleural glands (Hölldobler & Engel-Siegel Citation1984). Therefore, we decided to check if such antibiotic substances are also present in nest carton of jet-black ant L. fuliginosus (Latreille, 1798) and if they will be effective in nosemosis treatment.
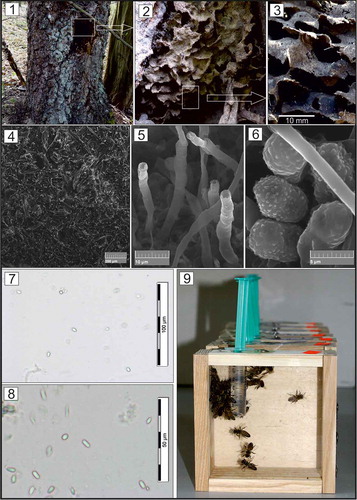
Lasius fuliginosus is an euryecious species of ant that inhabits broad-leaved forests, but may even occur on conifers (Tyler Citation2008; Tăușan Citation2020). Their carton nests are fundamental to the life of the colony and are built from comminuted decayed wood fragments mixed with mineral particles, ants’ own saliva and the honeydew of aphids (). The whole is permeated with fragments of a symbiotic fungi from at least five different Asomycota genera (Maschwitz & Hölldobler Citation1970; Schlick-Steiner et al. Citation2008; Tyler Citation2008; Brinker et al. Citation2018).
Figure 1. The trunk of Betula pendula with hollow inhabited by Lasius fuliginosus ants; figs. 2–3. Carton-nest of L. fuliginosus; fig. 4. Structure of carton-nest observed with SEM; fig. 5. Fungal structures on the surface of carton-nest observed with SEM; fig. 6. Fungal spores on the surface of carton-nest observed with SEM; figs. 7–8 Light micrographs of Nosema spp. spores observed under a bright-field; fig. 9. Cages with honeybees used in the cage tests (authors of photographs: 1–6, B. Staniec, 7–9, A. A. Ptaszyńska)
The aim of the present work was to investigate the influence of aqueous extracts of carton from jet-black ant nests in the treatment of honeybees infected by Nosema spp. fungi.
2. Materials and methods
2.1. Origin of the nest carton and the means of collecting it
Cartoon nest samples were collected as part of a research project in Polesie National Park. The relevant permits for the field studies were issued by the Ministry of the Environment, Poland (Nos. DLP-III.286.148.2016.MGr and DLP -III.286.141.2016.MGr).
The extracts used in the experiment were produced from the nest carton of jet-black ants L. fuliginosus (), specifically, nest fragments taken from different tree species at three localities in different types of habitat:
“oak carton” from an ant nest in a tree hollow in a pedunculate oak (Quercus robur); habitat – dry mixed conifer forest; exact position – [51o23ʹ38.26`N; 22o57ʹ16.09`E.] Rogóźno, 24.06.2018;
“birch carton 1” from a subterranean ant nest under a silver birch (Betula pendula); habitat – mixed woodland; exact position – [51º29ʹ41.5ʹN 23º11`19.8ʹE] Pieszowola, Polesie National Park, 21.06.2018;
“birch carton 2” from an ant nest in a tree hollow in a silver birch (Betula pendula); habitat – alder swamp near Lake Długie; exact position – [51º27ʹ04.12ʹN, 23º10ʹ14.17ʹE] Wola Wereszczyńska, Polesie National Park, 21.06.2018, 16.10.2018.
Ca 500 ml of carton was taken from each nest. This was an optimum volume for research use, and not so much that the ants could not quickly make good the loss. The carton was sampled by a person wearing rubber gloves, cleansed of superfluous material (wood fragments, live and dead ants, other invertebrates), then placed in sterile containers using sterile tweezers. The sealed containers with the research material were then taken to the laboratory and placed in a freezer (−70°C). For the analyses, appropriate portions of the frozen material were placed in sterile test-tubes using sterile tweezers.
2.2. Procedure for obtaining the carton extracts
Portions of nest carton were suspended in MQ redistilled water at a 1:10 weight-to-volume ratio and mechanically pre-treated using a blade homogenizer. The mechanically pre-ground biological material was sonified (80% amplitude, 4-minute cycle – 30s pulse/30s cooling the preparation in ice), filtered through a Miracloth membrane and centrifuged (10 000 g for 15 minutes at 4°C). The supernatant was freeze-dried and used in the subsequent research.
2.3. Collecting the honeybees for the experiment
Honeybees were obtained from the strong, uninfected colony in the Tomaszów district of Lublin province (50°30ʹ29”N 23°42ʹ10E). The health status of the honeybee colony and obtained from its honeybees was checked using standard methods described in 2.5.1.1. To unify the age and caste of honeybees, one-day-old honeybee workers were removed manually from the hive; thereafter, 40 were placed in purpose-built wooden cages of dimensions 12 × 12 × 4 cm. The front wall of each cage was made from glass. Each cage had a vent and also an opening through which a syringe for supplying food was inserted ().
2.4. Experimental research groups
From June to August 2019, two cage experiments in two repetitions were performed using the aqueous extracts. Each experiment consisted of seven research groups and in every research group was five cages, each containing 40 honeybees (total number of honeybees in one group = 200). Each experiment was performed in duplicate; therefore, the total honeybee number was 2800 per one experiment, i.e.: 2.5.1. Experiment I – Assessing the effect of the aqueous extracts on the incidence of nosemosis and 2.5.2. Experiment II – Assessing the effect of the aqueous extracts on healthy honeybees.
The research groups were designated as follows:
Control group: honeybees fed wv 50% sucrose solution without addition of extracts;
Group 1A: honeybees fed with a 0.1% aqueous extract of “oak carton”;
Group 1B: honeybees fed with a 1% aqueous extract of “oak carton”;
Group 2A: honeybees fed with a 0.1% aqueous extract of “birch carton 1”;
Group 2B: honeybees fed with a 1% aqueous extract of “birch carton 1”;
Group 3A: honeybees fed with a 0.1% aqueous extract of “birch carton 2”;
Group 3B: honeybees fed with a 1% aqueous extract of “birch carton 2”.
2.5. Experimental design
The two experiments were conducted, i.e.: I – to assess the effect of the aqueous extracts on the incidence of nosemosis; II – to evaluate the toxicity of the aqueous extracts towards healthy honeybees. From the start of the experiment, fresh food was supplied each day and dead honeybees were removed. Each experiment was conducted through 20 days.
2.5.1. Experiment I – assessing the effect of the aqueous extracts on the incidence of nosemosis
On day 2 of the experiment, the honeybees in all research groups (control, 1A, 1B, 2A, 2B, 3A, 3B) were infected with Nosema spp. spores, 98333,(3) spores per honeybee. On day 7, the following volumes of aqueous extracts were administered to the infected honeybees: 100 μl extract (0.1%) per 100 ml sugar syrup and 1000 μl extract (1%) per 100 ml sugar syrup. The honeybees received the syrup + extract in 5 ml syringes over six consecutive days. After that time, the honeybees were fed with wv 50% sucrose solution without addition of extracts until the last day of the experiment. The control group (C) consisted of infected honeybees that received wv 50% sucrose solution without addition of extracts through all the 20 days of experiment. For proper statistical analysis, the experiment was repeated.
2.5.1.1. Determining the degree of infection with Nosema spp
On days 16 and 20 the number of Nosema spp. spores were counted in accordance with the methodology given by Cantwell (Citation1970), Hornitzky (Citation2008) and Fries et al. (Citation2013). The abdomens of 10 honeybees from each group were pooled and ground in 10 ml of sterile distilled water. Estimation of Nosema spores per honeybee was accomplished using Olympus BX 61 light microscopy and a hemocytometer. For each spore suspension, the averages of two estimates of intensity were used (Cantwell Citation1970; Hornitzky Citation2008; Fries et al. Citation2013). All preparations were conducted in duplicate.
2.5.2. Experiment II – assessing the effect of the aqueous extracts on healthy honeybees
From second to sixth experimental day the honeybees in all research groups (control, 1A, 1B, 2A, 2B, 3A, 3B) were fed with wv 50% sucrose solution without addition of extracts. On day 7, the following quantities of extracts were administered to healthy honeybees in the research groups: groups 1A, 2A and 3A – 100 μl of extract (0.1%) per 100 ml sugar syrup 1:1 (w:v) and groups: 1B, 2B, 3B – 1000 μl extract (1%) per 100 ml sugar syrup 1:1 (w:v). The extracts were administered for six consecutive days. The control group consisted of healthy honeybees that were fed wv 50% sucrose solution without addition of extracts through all the experiment. Dead honeybees were counted and removed every other day until the end of the experiment on the Day 20. For proper statistical analysis, the experiment was repeated.
2.6. Statistical analysis
The experiments: I – to assess the effect of the aqueous extracts on the incidence of nosemosis, and II – to evaluate the toxicity of the aqueous extracts towards healthy honeybees were conducted in duplicate. The data were analysed using TIBCO Statistica 13.3. The results are presented as means with standard deviation. Statistical significance was determined using one-way repeated measures ANOVA with a Greenhouse-Geisser correction, Dunnett’s post-hoc test (data were compared to the control) and Tukey’s pairwise comparison test when applicable.
3. Results
3.1. Experiment I: the effect of aqueous extracts on the incidence of nosemosis
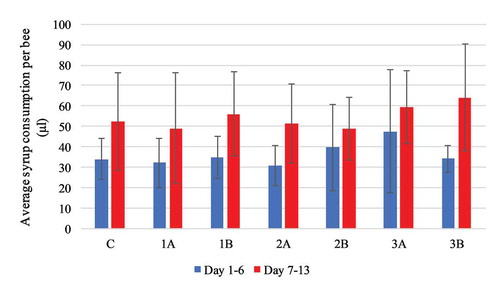
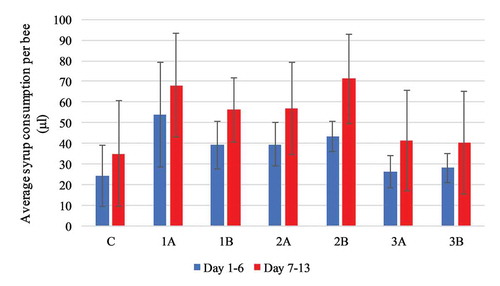
The average daily consumption of syrup per honeybee in all the groups was comparable (): 52.36 μl (± 20.14 μl) in the control group (fed only syrup), and between 49.13 μl (± 22.84 μl) and 60.27 μl (± 12.76 μl) in the experimental groups (1A, 1B, 2A, 2B, 3A, 3B) and presented no statistically significant difference with F(6, 23) = 0.584, p = 0.740 in between subject comparison.
Figure 2. Experiment I: average daily syrup consumption per honeybee infected with Nosema spp. compared with the control group. Values are presented in μl as mean with standard deviation
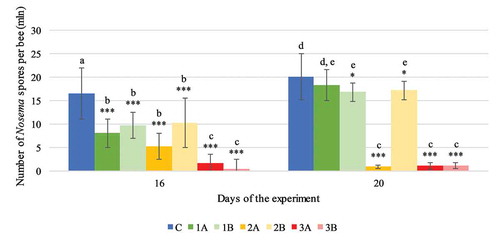
The incidence of nosemosis, expressed as the number of spores, was inhibited in the groups of honeybees receiving sugar syrup with added carton extract. By day 16 of the experiment, the number of Nosema spp. spores in all the experimental groups had fallen significantly compared with the number of spores in the control group (C) (F(6.0) = 239.527, p < 0.001, ). On day 20, the level of infection in all the groups was higher than on day 16, 1A: 18.28 (± 3.26), 1B: 16.83 (± 1.96), 2B: 17.15 (± 1.93), 3B: 1.10 (± 0.64) in contrast to 8.07 (± 3.04), 9.70 (± 2.82), 10.28 (± 5.35), 0.44 (± 0.50) million, respectively, with p < 0,05 in Tukey’s test (F(1.0, 133.0) = 76.149, p < 0.001). The lowest number of spores was observed in groups 3A and 3B where it had fallen to 1.1 million (±0.7), p < 0.001 and 1.1 million (±0.6) p < 0.001 per honeybee by day 20 of the experiment, respectively. By comparison, the number of spores in the control group of infected honeybees (C) oscillated around 20.07 (±4.97) million per honeybee.
Figure 3. Experiment I: number of Nosema spores per honeybee in each experimental group (1A, 1B, 2A, 2B, 3A, 3B) on days 16 and 20 of the experiment. Values are presented as mean with standard deviation. Results with statistically significant differences in the pairwise comparison (Tukey’s test) are marked with different letters of the alphabet (p > 0.05). Statistically significant differences between the control group (C) and the experimental groups on a given day are denoted by asterisks: * p < 0.05, ** p < 0.01, *** p < 0.001 (ANOVA)
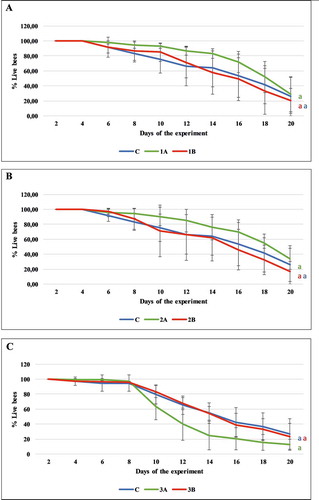
Survivorship was comparable in all the groups of honeybees with respect to the control. The average survival rate reached 93.24 (±2.91), 84.94 (±12.12), 90.39 (±12.51), 71.29 (±34.54), 63.68 (±17.73), 83.41 (±7.92) percent on the tenth day and 28.87 (23.27), 20.22 (16.57), 33.88 (±14.15), 16.92 (±13.27), 12.54 (±7.44), 23.48 (±17.03) on the twentieth day of the experiment for 1A, 1B, 2A, 2B, 3A, 3B, respectively. There was no statistically significant increase in mortality between the honeybees that received the extracts and those fed pure sugar syrup (for 1A, 1B, C: F(2, 12) = 1.218, p = 0.330; 2A, 2B, C: F(2, 11) = 1.098, p = 0.368; 3A, 3B, C: F(2, 7) = 2,052, p = 0.199) ().
Figure 4. Experiment I: mortality in the experimental groups 1A, 1B, 2A, 2B, 3A, 3B. There were no statistically significant differences between the control group (bees fed only syrup) and the experimental groups (1A, 1B, 2A, 2B, 3A, 3B), in which the honeybees were fed syrup containing carton extracts in concentrations of 0.1% (A) and 1% (B), (p > 0.05). Values are presented as mean with standard deviation
3.2. Experiment II: assessing the toxicity of aqueous extracts on healthy honeybees
During the period of extract administration, the average syrup consumption was the highest in group 2B (71.33 ± 21.51 μl per honeybee), and the lowest in group 1B (56.19 ± 15.61 μl per honeybee), the results exhibited no statistical significance in pairwise comparison as well as compared to the control (F(6) = 3.218, p = 0.020) ().
Figure 5. Experiment II: average daily syrup consumption per healthy honeybee compared with the control group. Values are presented in μl as mean with standard deviation
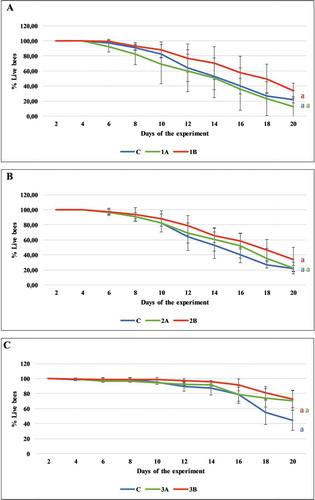
The tested extracts had no statistically significant effect on honeybee longevity in between subject comparison with the control (1A, 1B, C: F(2, 10) = 1.704, p = 0.231; 2A, 2B, C: F(2, 10) = 1.462, p = 0.277; 3A, 3B, C:F(2, 7) = 3.109, p = 0.108). In the middle of the experiment (on the tenth day), the average survival rate reached values of 68.90 (±26.23), 88.28 (±10.55), 82.50 (±12.09), 88.35 (±10.38), 94.33 (±0.88), 98.41 (±2.75) and was the lowest on the twentieth day of the experiment: 12,60 (±21.78), 34.04 (±9.83), 22.57 (±7.81), 34.10 (±15.76), 70.75 (±12.93), 72.80 (±11.63) for 1A, 1B, 2A, 2B, 3A, 3B, respectively ().
Figure 6. Experiment II: mortality of honeybees NOT infected with Nosema spp. in the experimental groups 1A, 1B, 2A, 2B, 3A and 3B. No statistically significant differences were found between the control group (bees fed only syrup) and the experimental groups 1A, 1B, 2A, 2B, 3A and 3B, in which the bees were fed syrup containing carton extracts in concentrations of 0.1% (A) and 1% (B) (p > 0.05). Values are presented as mean with standard deviation
4. Discussion
Numerous substances for controlling nosemosis have been tested, including those with antiseptic properties, like ethanol. But a 10% solution of ethanol in sugar syrup increased the mortality of both healthy honeybees and those infected with Nosema spp. (Ptaszyńska et al. Citation2013). Experiments were performed using an 0.4% solution of acetic acid and an 0.03% solution of benzoic acid, but without any effect on nosemosis in honeybees (Forsgren & Fries Citation2005). Synthetic compounds as porphyrins suppressed the numbers of Nosema spp. five-fold compared with infected honeybees fed with syrup alone (Ptaszyńska et al. Citation2018b). The effect of the enzymatic protein lysozyme added to food on the incidence of nosemosis was studied but in spite of its antibacterial activity, it did not limit the numbers of Nosema spp. spores (Maistrello et al. Citation2008). Natural substances like resveratrol and thymol, isolated from common thyme (Thymus vulgaris), were examined, but in high concentrations were toxic towards honeybees. Essential oils, extracted from such plants as peppermint (Mentha piperita), balm (Melissa officinalis), coriander (Coriander sativum) and summer savory (Satureja hortensis), and also propolis, to some extent retarded the progress of nosemosis and increased honeybee longevity (Dumitru et al. Citation2017; Mura et al. Citation2020). Although a 10% extract of bay laurel (Laurus nobilis) slowed the progress of nosemosis, it killed honeybees in a high concentration (Porrini et al. Citation2011). BeeCleanse, a proprietary blend of herbs, minerals, vitamins and acids, markedly decreased the numbers of Nosema spp. spores, but did not completely eradicate the disease (Araneda et al. Citation2015).
Furthermore, a very promising agent can be extracts from adaptogenic plants, especially obtained from the representatives of Eleutherococcus genus (Ptaszyńska & Załuski Citation2020). Another example is the extract from leaves of Chilean acorn (Cryptocarya alba): this was active against Nosema spp. and not toxic towards honeybees. However, the 8-day duration of this experiment was too short to establish whether longer-term administration of this extract was not in fact toxic (Bravo et al. 2017). In this context, our 20-day experiment on administration of the carton extract had no toxic effect on healthy honeybees. The “birch carton” extract substantially lowered the incidence of nosemosis, reducing the number of spores by as much as 97.97% (p < 0.001) without increasing mortality in comparison with the control group containing infected but untreated honeybees. For comparison, the preparation “Nozevit” reduced the number of spores to 70.91% on day 22 of the experiment performed by Gajger et al. (Citation2009). The level of nosemosis in the groups of honeybees receiving the extracts was always lower than that in the control group, although, the spores’ number fluctuated naturally during the progress of nosemosis, similarly to other studies (Fries et al. Citation2013; Ptaszyńska et al. Citation2016). Furthermore, none of the extracts was toxic towards healthy honeybees.
It is worth stressing that in our study the addition of the extract to the syrup did not reduce food consumption, a factor that significantly influenced the final results of the experiment. The proliferation of the pathogen depends largely on the kind of food consumed by the host (Jack et al. Citation2016). In the laboratory, when food consumption is similar, one can single out the substance most effectively controlling nosemosis. In our experiments, the test honeybees consumed much the same amounts of food with added extracts as their counterparts in the control group, which were fed pure sucrose syrup. One example, showing how important it is for honeybees to consume the food and extract together, is the experiment carried out by Botías et al. (Citation2013), who tested three products: Nosestat®, phenyl salicylate and Vitafeed Gold®. These products turned out to be ineffective in treating nosemosis because the honeybees consumed lower than normal amounts of food when this contained various doses of the test substances.
The “birch carton” extract inhibits the incidence of nosemosis much stronger than the “oak carton” extract. One should therefore formulate the hypothesis that the activity of such a product, and no doubt its composition too, are heavily dependent on the range of local habitat conditions under which the ants construct their carton. The next step in our research into the utility of carton extracts in controlling nosemosis in honeybees will therefore be to elucidate these correlations in detail. Once we have established these dependencies, we will be able to define universal habitat (microhabitat) features that are appropriate for producing a carton with therapeutic properties (with respect to Nosema spp.) comparable to those of “birch carton”. These data will be invaluable during field work, permitting the collection of ant-nest material with a high, more reproducible activity against nosemosis in honeybees. They can also form the basis for determining the optimal conditions for our planned laboratory culture of jet-black ants, which could under controlled conditions produce carton of a specific, high level of biological activity against Nosema spp. Further perspective is to carry out experiments in an apiary to evaluate the effectiveness of the ant nest cartoon extracts under natural conditions and also the efficacy of various methods of administering it to the honeybees, e.g. by spraying it over them. On the basis of such apiary tests, field therapeutic doses, the most effective frequency of extract administration, and the means of food supplementation can be established. One may entertain the hope that an effective natural product based on ant nest cartoon extracts can be produced for controlling this serious disease of honeybees, as nosemosis.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Araneda X, Cumian M, Morales D. 2015. Distribution, epidemiological characteristics and control methods of the pathogen Nosema ceranae Fries in honeybees Apis mellifera L. (Hymenoptera, Apidae). Archivos de Medicina Veterinaria 47:129–138. DOI: 10.4067/S0301-732X2015000200002.
- Aslan CE, Liang C, Galindo B, Kimberly H, Topete W. 2016. The role of honey bees as pollinators in natural areas. Natural Areas Journal 36:478–488. DOI: 10.3375/043.036.0413.
- Bos N, Kankaanpää-Kukkonen V, Freitak D, Stucki D, Sundström L. 2019. Comparison of twelve ant species and their susceptibility to fungal infection. Insects 10:271. DOI: 10.3390/insects10090271.
- Botías C, Martín-Hernández R, Meana A, Higes M. 2013. Screening alternative therapies to control Nosemosis type C in honeybee (Apis mellifera iberiensis) colonies. Research in Veterinary Science 95:1041–1045. DOI: 10.1016/j.rvsc.2013.09.012.
- Brinker P, Weig A, Rambold G, Feldhaar H. 2018. Microbial community composition of nest-carton and adjoining soil of the ant Lasius fuliginosus and the role of host secretions in structuring microbial communities. Fungal Ecology 38:44–53. DOI: 10.1016/j.funeco.2018.08.007.
- Burnham AJ. 2019. Scientific advances in controlling Nosema ceranae (Microsporidia) infections in Honeybees (Apis mellifera). Frontiers in Veterinary Science 6:79. DOI: 10.3389/fvets.2019.00079.
- Cantwell G. 1970. Standard methods for counting Nosema spores. American Bee Journal 110:222–223.
- Cepero A, Ravoet J, Gómez-Moracho T, et al. 2014. Holistic screening of collapsing honey bee colonies in Spain: A case study. BMC Research Notes 7:649. DOI: 10.1186/1756-0500-7-649.
- Chen YP, Evans JD, Smith JB, Pettis JS. 2007. Nosema ceranae is a long-present and widespread microsporidian infection of the European honeybee (Apis mellifera) in the United States. Journal of Invertebrate Pathology 92:152–159. DOI: 10.1016/j.jip.2007.07.010.
- Chen YP, Huang ZY. 2010. Nosema ceranae, a newly identified pathogen of Apis mellifera in the USA and Asia. Apidologie 41:364–374. DOI: 10.1051/apido/2010021.
- Copley TR, Jabaji SH. 2011. Honeybee glands as possible infection reservoirs of Nosema ceranae and Nosema apis in naturally infected forager bees. Journal of Applied Microbiology 112:15–24. DOI: 10.1111/j.1365-2672.2011.05192.x.
- Dumitru A, Chioveanu G, Ionita M, Dobre G, Mitrea IL. 2017. “In Vitro” studies on using natural essential oils in treatment of nosemosis in honeybees: Determination of the therapeutic dose. Scientific Works. Series C. Veterinary Medicine LXIII(2):165–170. ISSN 2065-1295.
- Forsgren E, Fries I. 2005. Acidic-benzoic feed and nosema disease. Journal of Apicultural Science 49:81–88.
- Fries I, Chauzat MP, Chen YP, Doublet V, Generesch E, Gisder S, Higes M, McMahon DP, Martin-Hernandez R, Natsopoulou M, Paxton RJ, Tanner G, Webster TC, Williams GR. 2013. Standard methods for Nosema research. Journal of Apicultural Research 51:1–28. DOI: 10.3896/IBRA.1.52.1.14.
- Fries I, Feng F, Silva AD, Slemenda SB, Pieniazek NJ. 1996. Nosema ceranae n. sp. (Microspora, Nosematidae), morphological and molecular characterization of a microsporidian parasite of the Asian honeybee Apis cerana (Hymenoptera, Apidae). European Journal of Protistology 32:356–365. DOI: 10.1016/S0932-4739(96)80059-9.
- Gajger IT, Petrinec Z, Pinter L, Kozarić Z. 2009. Experimental treatment of Nosema disease with “Nozevit” phytopharmacological preparation. American Bee Journal 149:485–490.
- Gajger IT, Ribaric J, Matak M, Svecnjak L, Kozaric Z, Nejedli S, Smodis-Skerl IM. 2015. Zeolite clinoptilolite as a dietary supplement and remedy for honeybee (Apis mellifera L.) colonies. Veterinary Medicine 60:696–705. DOI: 10.17221/8584-VETMED.
- Higes M, Martín-Hernández R, Meana A. 2010. Nosema ceranae in Europe: An emergent type C nosemosis. Apidologie 41:375–392. DOI: 10.1051/apido/2010019.
- Hölldobler B, Engel-Siegel H. 1984. On the metapleural gland in ants. Psyche 91:201–224. DOI: 10.1155/1984/70141.
- Holt HL, Grozinger CM. 2016. Approaches and challenges to managing Nosema (Microspora: Nosematidae) parasites in honeybee (Hymenoptera: Apidae) colonies. Journal of Economic Entomology 109:1487–1503. DOI: 10.1093/jee/tow103.
- Hornitzky M. 2008. Nosema disease – Literature review and three surveys of beekeepers – Part 2. Rural Industries Research and Development Corporation. Pub. No. 08/006.
- Huang W, Solter L, Yau P, Imai B. 2013. Nosema ceranae escapes fumagillin control in honeybees. PLoS Pathogens 9:e1003185. DOI: 10.1371/journal.ppat.1003185.
- Jack CJ, Uppala SS, Lucas HM, Sagili RR. 2016. Effects of pollen dilution on infection of Nosema ceranae in honeybees. Journal of Insect Physiology 87:12–19. DOI: 10.1016/j.jinsphys.2016.01.004.
- Maistrello L, Lodesani M, Costa C, Leonardi F, Marani G, Caldon M, Mutinelli F, Granato A. 2008. Screening of natural compounds for the control of Nosema disease in honeybees (Apis mellifera). Apidologie 39:436–445. DOI: 10.1051/apido:2008022.
- Maschwitz U, Holldobler B. 1970. Der Kartonnestbau bei Lasius fuliginosus Latr. (Hym. Formicidae). Zeitschrift für vergleichende Physiologie 66:176–189. DOI: 10.1007/BF00297777.
- Mura A, Pusceddu M, Theodorou P, Angioni A, Floris I, Paxton RJ, Satta A. 2020. Propolis consumption reduces nosema ceranae infection of European honey bees (Apis mellifera). Insects 11(2):124. DOI: 10.3390/insects11020124.
- Paxton RJ, Klee J, Korpela S, Fries I. 2007. Nosema ceranae has infected Apis mellifera in Europe since at least 1998 and may be more virulent than Nosema apis. Apidologie 38:558–565. DOI: 10.1051/apido:2007037.
- Porrini MP, Fernández NJ, Garrido PM, Gende LB, Medici SK, Eguaras MJ. 2011. In vivo evaluation of antiparasitic activity of plant extracts on Nosema ceranae (Microsporidia). Apidologie 42:700–707. DOI: 10.1007/s13592-011-0076-y.
- Prasad PY, Mackereth RW, Hanley RS, Qin W. 2015. Honey bees (Apis mellifera L.) and pollination issues: Current status, impacts and potential drivers of decline. The Journal of Agricultural Science 7:93.
- Ptaszyńska AA, Borsuk G, Anusiewicz M, Mułenko W. 2012. Location of Nosema spp. spores within body of honeybee. Medycyna Weterynaryjna 68:618–621.
- Ptaszyńska AA, Borsuk G, Mułenko W, Demetraki-Paleolog J. 2014. Differentiation of Nosema apis and Nosema ceranae spores under Scanning Electron Microscopy (SEM). Journal of Apicultural Research 53:537–544. DOI: 10.3896/IBRA.1.53.5.02.
- Ptaszyńska AA, Borsuk G, Mułenko W, Olszewski K. 2013. Impact of ethanol on Nosema spp. infected bees. Medycyna Weterynaryjna 69:736–740.
- Ptaszyńska AA, Gancarz M, Hurd PJ, Borsuk G, Wiącek D, Nawrocka A, Strachecka A, Załuski D, Paleolog J. 2018a. Changes in the bioelement content of summer and winter western honeybees (Apis mellifera) induced by Nosema ceranae infection. PLoS ONE 13:e0200410. DOI: 10.1371/journal.pone.0200410.
- Ptaszyńska AA, Paleolog J, Borsuk G. 2016. Nosema ceranae infection promotes proliferation of yeasts in honeybee intestines. PLoS One 11:e0164477. DOI: 10.1371/journal.pone.0164477.
- Ptaszyńska AA, Trytek M, Borsuk G, Buczek K, Rybicka-Jasińska K, Gryko D. 2018b. Porphyrins inactivate Nosema spp. Microsporidia. Scientific Reports 8:5523. DOI: 10.1038/s41598-018-23678-8.
- Ptaszyńska AA, Załuski D. 2020. Extracts from Eleutherococcus senticosus (Rupr. et Maxim.) Maxim. roots: A new hope against honeybee death caused by nosemosis. Molecules 25:4452. DOI: 10.3390/molecules25194452.
- Schlick-Steiner BC, Steiner FM, Konrad H, Seifert B, Christian E, Moder K, Stauffer C, Crozier R. 2008. Specificity and transmission mosaic of ant nest-wall fungi. Proceedings of the National Academy of Sciences 105:940–943. DOI: 10.1073/pnas.0708320105.
- Staveley JP, Law SA, Fairbrother A, Menzie CA. 2014. A causal analysis of observed declines in managed honey bees (Apis mellifera). Human and Ecological Risk Assessment 20:566–591. DOI: 10.1080/10807039.2013.831263.
- Tăușan I. 2020. Bernhard Seifert, the ants of Central and North Europe book review. Travaux du Muséum National d’Histoire Naturelle “Grigore Antipa” 63:117–119. DOI: 10.3897/travaux.63.e49981.
- Tragust S. 2016. External immune defence in ant societies (Hymenoptera: Formicidae): The role of antimicrobial venom and metapleural gland secretion. Myrmecological News 23:119–128.
- Tyler J. 2008. The ant, the aphid and the fungus. Field Mycology 9:22–23. DOI: 10.1016/S1468-1641(10)60396-9.
- Van den Heever JP, Thompson TS, Curtis JM, Ibrahim A, Pernal SF. 2014. Fumagillin: An overview of recent scientific advances and their significance for apiculture. Journal of Agricultural and Food Chemistry 62:2728–2737. DOI: 10.1021/jf4055374.
- VanEngelsdorp D, Meixner MD. 2010. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. Journal of Invertebrate Pathology 103:80–95. DOI: 10.1016/j.jip.2009.06.011.
- Ye L, Zhao S, Li Y, Jiang S, Zhao Y, Li J, Yan K, Wang X, Xiang W, Liu C. 2017. Streptomyces lasiicapitis sp. nov., an actinomycete that produces kanchanamycin, isolated from the head of an ant (Lasius fuliginosus L.). International Journal of Systematic and Evolutionary Microbiology 67:1529–1534. DOI: 10.1099/ijsem.0.001756.
- Yek SH, Mueller UG. 2011. The metapleural gland of ants. Biological Reviews 86:774–791. DOI: 10.1111/j.1469-185X.2010.00170.x.
- Zander E. 1909. Tierische Parasiten als Krankenheitserreger bei der Biene. Münchener Bienenzeitung 31:196–204.
