Abstract
Recent evidence show that the classical distinction between innate and acquired immunity can no longer be employed to thoroughly characterize immune defenses. As an example, both insects and vertebrates possess non-canonical defense activities that differ from those identified so far; namely, insects display immune memory and mammals show innate-like lymphocytes. At the base of these observations, it can be speculated that multicellular eukaryotes share immune cells deriving from ancestral granular and non-granular immunocytes as well as sets of soluble effectors and signaling immune-related molecules. On one hand, evolutionary pressure has selected genes coding for group-specific defense molecules; on the other, some cell types and gene products can be presently considered as being enriched with group-specific adaptations deriving from most ancient protostomes and deuterostomes. The immune defenses of more recent extant animal groups could then be seen as overlying layers constituted by both ancient cells retaining broad features for non-self defenses and more specialized cells and immunomodulatory molecules for group-specific capabilities.
1. Introduction
Each specimen belonging to any animal species should be regarded to as a super-organism living in equilibrium with internal and environmental microbiomes. These, together with the hosting genome, form a hologenome that is able to shape the evolution of species (Rosenberg & Zilber-Rosenberg Citation2016) or be of help to explain certain behavioral features (Zwollo Citation2012). Harmful microbial species instead may induce infectious pathologies resulting in the death of the animal or in the organism being more prone to predation. Physiological defense mechanisms must therefore be very efficient against viruses, bacteria, and parasites in all eukaryotes (e.g. Kumar et al. Citation2013), including plants (e.g. Fujii et al. Citation2016), and available knowledge shows that every eukaryotic species investigated can discriminate non-self and produce antimicrobial substances (König et al. Citation2015; Destoumieux-Garzón et al. Citation2016; Mylonakis et al. Citation2016; He et al. Citation2017; Buonocore et al. Citation2019).
The importance of immune defenses in maintaining the fitness of individuals and ensuring species survival can be also appreciated by the boosting of speciation rate in fish (Malmstrøm et al. Citation2016) and positive selection in birds and mammals (Shultz & Sackton Citation2019). In all eukaryotic phyla immune defense activities are carried out by molecules and cells, some of which display a remarkable degree of conservation. As an example, clear similarities have been reported among hormone-like molecules present from protists to mammals, such as the ciliates MAT2 (Mating pheromone Er-2/Er-9) and mammalian IL-2 (Interleukin-2), thus suggesting a conservation in humoral communications as old as a billion years (Vallesi et al. Citation1998). Also, the genes coding for the main non-self recognition and effector proteins with immunoglobulin (Ig) domains and leucine-rich repeats (LRRs) domains already existed before the divergence between choanoflagellate and animal ancestor (Gauthier et al. Citation2010).
Main cell populations involved in self/non-self recognition, which must be as ancient as multicellular organisms, discriminate isogeneic cells (i.e. cells sharing the exact same genome) from allogeneic (i.e. same genome but distinct alleles) and xenogeneic (i.e. diverse species) cells and kill the latter by secreting cytotoxic factors. Phagocytosis is a very ancient activity present since unicellular Eukaryotes: it is a process of particle internalization enacted by professional phagocytes either for nutrition or clearance of harmful bacteria from the site of infection. Some granule-containing defense cells, upon non-self recognition, secrete granules, and liberate families of proteases and/or signaling factors in the extracellular environment, which activate cascade responses leading to the elimination of microbes. Together, these briefly described activities can collectively be regarded as “inflammatory-like.” All bilateria species investigated contain such cells and mechanisms: evolution has either added novel functions, which can be typical of and restricted to certain phyla, or specialized the products of existing genes. Genetic changes led to extant animal groups, where investigations on the mechanisms of immune defenses suggest that ancient cell population and gene products form a sort of common “lower layer.” Subsequent evolutionary steps added further “layers,” either shared or group-specific, with added defense capabilities against non-self and microbes. Extant species display several levels of immune responses and recent data show that canonical attributes related to innate and acquired immunity are no longer valid, with invertebrates showing immune memory and vertebrates possessing innate-type lymphocytes. We will describe here a brief summary on the available knowledge on principal animal internal defense mechanisms and of the cells performing immune activities, reinforcing the hypothesis on the origin and presence of “immune layers” that accumulated during evolution and contributed to the immune system of vertebrates as we know it.
2. Sensor and effector peptides
2.1. Leucine-rich repeats domains
Peptides containing Leucine-Rich Repeats (LRR) are among the most ancient non-self detector system in eukaryotes. They are present from protists and plants onwards in the molecular form of toll-like receptors (TLRs), so-called because of the homology with Drosophila Toll gene. TLRs are well-conserved classes of secreted, transmembrane and cytosolic PRRs that have differentiated along the various phyla to sense group-specific pathogens typical of the microbiome to which the species are exposed. As a parallel form of co-evolution, agnathans LRR-based lymphocytes act both as cell-associated sensor for pathogens and soluble effector molecules in the form of Variable Lymphocyte Receptor diversity region peptides (VLR gene) (Alder et al. Citation2005). From a minimum of one TLR gene in Porifera and nematodes (Nie et al. Citation2018) to the incredible known maximum of 222 in echinoderms (Hibino et al. Citation2006), the number of expressed TLR receptors is quite variable among groups: a TLR-like in Porifera (Wiens et al. Citation2007), between 16 and 105 in Annelida (Davidson et al. Citation2008), up to 56 in mollusks (Adema et al. Citation2017) 12 of which only in bivalves (Ren et al. Citation2020), at least 20 in amphibians (Ishii et al. Citation2007), 9 in Drosophila (Bilak et al. Citation2003), 48 in amphioxus (Huang et al. Citation2008), 21 in fish and 13 in mammals (Nie et al. Citation2018).
2.2. Immunoglobulin domain-containing peptides
Genes coding for Ig domain-containing peptides (IgDPs) which, as the name implies, contain immunoglobulin (Ig)-like domains, are as ancient as prokaryotes (Mei et al. Citation2015) and have been shaped in their canonical form since unicellular eukaryotes (King et al. Citation2008). They span through the genomes of all animal phyla and have the singular feature of permitting a high degree of recombination in certain Ig such as TCR and BCR. This makes IgDPs the perfect candidates to interact with a potential high number of counterparts in immune responses. From an evolutionary immune perspective, it is as if Ig genes have tested all possibilities to achieve a high degree of recombination and serve as detectors/effectors for the non-self. Scanning the eukaryotic genomes, unicellular protists have a low and undetermined number of genes coding for IgDPs (King et al. Citation2008): 2 genes have been identified in Porifera (Pancer et al. Citation1996), at least 3 in Cnidaria (Miller et al. Citation2007), 2 in Nematoda (Bénard et al. Citation2012), more than 850 in the human genome (Barrow & Trowsdale Citation2008). Molluscan IgDPs are represented by the FREP family described below (see section 1.4). IgDPs are known to sense and respond to non-self in crustaceans (Niu et al. Citation2019) and, starting from exapodes, are clearly associated with immune responses because Drosophila and other insect hemocytes produce Down syndrome cell-adhesion molecule (Dscam) peptides employed in immune defense upon in vivo infection (Smith et al. Citation2011). Dscam peptides are non-antibody IgDPs produced by differential exon usage variants of the same multiexonic gene, generating more than 38.000 different peptides in Drosophila. The Dscam represent a paradigm on the high plasticity of IgDPs because even in the absence of a recombination-activating genes (RAG) machinery, which is at the base of vertebrate Ig and T cell receptor molecules V-D-J regions recombination, Drosophila hemocytes are able to produce with Dscam a true immune-related molecular diversity against non-self. Interestingly, chelicerates also have Dscam genes, even though some canonical Ig domains lack from the sequence (Yue et al. Citation2016), whereas in crustaceans the Dscam gene produces many immune-related peptides (Niu et al. Citation2019). The similarities between crustacea and insect in the Dscam gene usage reinforce the hypothesis of a Pancrustacea superphylum.
2.3. Peptidoglycan recognition proteins
Peptidoglycan recognition proteins (PGRPs) are antimicrobial molecules that recognize conserved peptidoglycans of the outer cell wall of both Gram-positive and Gram-negative bacteria. PGRPs have been found in mollusks, insects, echinoderms, and vertebrates but not in other investigated eukaryotes such as plants and nematodes (Kang et al. Citation1998). The number of predicted or identified PGRP genes differ among phyla: 9 in mollusks (Wang et al. Citation2018), 19 in insects (Dziarski & Gupta Citation2006b), 2 in echinoderms (Coteur et al. Citation2007), 17 in amphioxus (Huang & Xu Citation2016), 3 in zebrafish (Chang et al. Citation2007) and 4 in mammals (Dziarski & Gupta Citation2006a). In invertebrates, PGRPs exert their activities as soluble or cell-surface pattern recognition receptors by hydrolyzing peptidoglycans or inducing antibacterial peptides. In vertebrates, as in zebrafish and mammals, they are secreted and involved in direct killing of bacteria (Li et al. Citation2007; Kashyap et al. Citation2014). In mammals, PGRPs are also involved in the maintenance of intestinal microbial community (Royet et al. Citation2011).
2.4. Fibrinogen-related proteins
Fibrinogen-related proteins (FREPs), coded by genes expressing IgDPs and fibrinogen-like (FBG) domains, are emerging as important defenses of invertebrates innate immunity (Hanington & Zhang Citation2011). FREPs have recently been studied from a functional perspective in arthropod and mollusk models, and their importance is evidenced by the large number of genes identified in investigated species (approximately 190) (Huang et al. Citation2015). They appear to be fundamental in molluscan immunity and were identified in mussels (Romero et al. Citation2011), scallops (Zhang et al. Citation2009), oysters (Huang et al. Citation2015), and gastropods (Adema et al. Citation1997). Gastropods, in particular, are characterized by unique FREPs structures and recombination, possibly driven by a parasite-host retrotransposon transfer (Vasta & Ahmed Citation2008): despite the underlying mechanism are still unknown (Random diversification? Clonal expansion of selected hemocytes populations?), the extreme diversification found in Biomphalaria glabrata FREPs allows the organism to combat the 200 million-year long parasitic relationship with digenean trematods (Al-Khalaifah & Al-Nasser Citation2019). FREPs are IgDP with a molecular machinery comparable to that present in arthropods for Dscam (see section 1.2), and thus display a high degree of sequence polymorphism through recombination and point mutations (Adema Citation2015).
2.5. Cysteine-rich scavenger receptors
The Scavenger Receptor Cysteine-Rich (SRCR) domain is a module that defines a superfamily (SRCR-SF) coded by conserved genes in most animal eukaryotic phyla, and employed in innate immunity defenses at embryonic and adult stages (Hohenester et al. Citation1999). The SRCR motif is 100–110 amino acid long and expressed in both hematopoietic and non-hematopoietic cells. Corresponding proteins can be divided into two families, distributed among different animal species/phyla (Sarrias et al. Citation2004). SRCR are principally involved in recognizing non-self in innate reactions and promote phagocytosis by immunocytes. SRCR have been identified in Porifera (Pancer et al. Citation1997), in nematodes (Liu et al. Citation2015), and then in all subsequent animal phyla investigated, with a high degree of structural and phylogenetic conservation (Sarrias et al. Citation2004). Worth of note, the sea urchin Strongylocentrotus purpuratus genome expresses 1200 SRCR domains (Sodergren et al. Citation2006).
2.6. Lectins
Lectins are glycoproteins that bind sugar groups of other molecules in a very specific way and in presence of Ca2+. Lectins play a role in immune recognition of cell populations, carbohydrates, proteins, and may bind bacteria and viruses to assist immune cells in their removal. Lectins are ubiquitous among eukaryotes, and in animal species are involved in innate defenses in all phyla investigated (Vasta et al. Citation2007), from Porifera (Gardères et al. Citation2015) onwards to Arthropoda (Marques & Barracco Citation2000) and Chordata (Parrinello et al. Citation2015). In particular, mannose-binding lectins play a major role in the front line of defenses toward elimination of invading pathogens (Vasta et al. Citation1994).
3. Proteases cascades
Genes coding for protease cascade reactions (PRCRs) that modify soluble peptides and lead to a more dense physical state of body fluids are widespread among animal species and are generally triggered by non-self molecules (e.g. bacteria, bacterial cell wall components and parasites) (e.g. Lu et al. Citation2014; Nonaka Citation2014; Perdomo-Morales et al. Citation2019). Lipopolysaccharide (LPS) from Gram-negative bacteria is a prime stimulant for PRCRs in animal eukaryotes, which sense the substance through toll-like receptors (TLRs) and trigger serum clotting, encapsulation, and coagulation. The protease cascades involved in non-self defense among animal species lead to serum/hemolymph clotting, encapsulation, and wound repair.
3.1. Hemolymph/blood clotting
In Arthropods, protease cascades take part in fast antimicrobial defense. Granule-containing immunocytes circulating in the hemocoel of the chelicerate Limulus species have TLR receptors that bind LPS (Inamori et al. Citation2004) and trigger intracellular reactions leading to degranulation of granulocytes (i.e. the only type of hemocyte in the systemic circulation of the adult animal). Large granules contain about 20 protein components including clotting and antimicrobial factors (i.e. factors B/C, proclotting enzyme, anti-LPS factor), whereas small granules exclusively contain an additional antimicrobial substance (i.e. Tachyplesin) and 6 major protein components (Iwanaga et al. Citation1992). All these lead to a gelified hemolymph into which invading bacteria are delayed in their proliferation and cleared by phagocytosis and phagocytes. Interestingly, a biotechnological application developed from such a feature of Limulus amoebocyte lysate is an assay (i.e. LAL test) employed worldwide for the detection of LPS-contaminated sanitary equipment since 1977 (Nakamura et al. Citation1977) and later enhanced in its sensitivity by kinetic chromogenic variants (Lindsay et al. Citation1989).
Hemolymph clotting by protease cascades is also present in crustaceans and insects. In most of the crustacean species, hemolymph coagulation is mediated by the phenoloxidase (PO) cascade and transglutaminase released from hemocytes and catalyzes the polymerization of a clotting protein (Perdomo-Morales et al. Citation2019). In insects, serine proteases containing the arthropod exclusive Clip domain promote pathogen-induced hemolymph clotting.
In vertebrates, two more specialized mechanisms based on protease cascades are present in blood, namely the fibrin-based clotting system for wound healing (Laurens et al. Citation2006) and the complement system for innate immune defense (Rus et al. Citation2005).
3.2. Encapsulation
Another defense mechanism triggered by TLRs detection of non-self and protease cascades in arthropods (Vazquez et al. Citation2009; Lu et al. Citation2014), echinoderms (Clow et al. Citation2004), and tunicates (Franchi & Ballarin Citation2017) is the encapsulation of foreign or invading particles. Proteases cascade in encapsulation have evolved in a peculiar pathway in which the enzyme phenoloxidase, either dependently or independently of dopamine as substrate, leads to the oxidation of polyphenols to encapsulate non-self within a rigid structure formed of melanin (González-Santoyo & Córdoba-Aguilar Citation2012). Because of the severe implications that may arise from a phenoloxidase cascade, these reactions have evolved under the strict regulation of the prophenoloxidase (proPO) activation system (Amparyup et al. Citation2013). Multicellular organisms that succeed in penetrating the crustacean/insect cuticle trigger the secretion of granules that contain process-specific enzymes from granulocytes and start the encapsulation cascade (Gallo et al. Citation2011; Zhang et al. Citation2014). A paradigm in insects can be found in parasitoid wasps that inoculate early-stage embryos into the body of a host species, which in turn responds by activating the phenoloxidase cascade to encapsulate and kill them. Generally speaking, also the production of pearls can be considered an encapsulation-based defense system performed by some bivalve Mollusks (Southgate & Lucas Citation2008).
3.3. Complement system
The complement system, also known as complement cascade, is an essential element of humoral innate defenses. It improves the efficiency of other immune players such as antibodies and phagocytes in eliminating invading microorganisms and induces inflammatory processes (Janeway et al. Citation2001). It is composed of many proteins, of which the C3 molecule is the more conserved among Metazoa, being present in different forms since 500 million years from Porifera onwards in all animal phyla, and reaching the maximal specialization in mammals (Nonaka Citation2014; Elvington et al. Citation2016). Three activation pathways exist, namely the classical pathway, the MB-lectin pathway and the alternative pathway. They differ on the basis of the molecules that initiate the cascade: the pathogen- or antibody/antigen complex-binding C1q protein, the mannan-binding lectin protein or the C3, following its spontaneous hydrolysis, respectively. Regardless of the activation pathway, the system converges at the effector molecules level: C3 is cleaved into C3a and C3b by C3 convertase. As a result, the complement system produces soluble factors that have effects on immunocyte chemotaxis, opsonization of pathogens, phagocytosis, inflammation, and also induces vasodilation and vascular permeability in vertebrates. Incremental layers of the complement system can be evidenced by the adding of new genes to the classical and lytic pathways coding for membrane attack components, and the induction of Vertebrate-specific pro-inflammatory components.
4. Phagocytes
Phagocytes are cells capable of engulfing extracellular particles by enclosing them into cellular projections, with the phagocytosed material then being intracellularly destroyed through lytic enzymes or into lysosomes. Phagocytes migrate towards the stimulus thanks to signals mediated by receptors associated with plasma membrane, mainly TLRs and chemotactic peptides receptors. From unicellular eukaryotes included and onward, all animal phyla display cell populations performing active phagocytosis with the main scope of eliminating foreign invading organisms through their destruction by intracellular lysosomes. Along evolution, the phagocytic activity has been enriched with additional features: from the basal layer of basic food capture in protists (Verni & Gualtieri Citation1997) to the upper complex layer of induction of immune memory in mammals by phagocytic dendritic cells (Hole et al. Citation2019). Basal phagocytosis of non-self particles also show incremental layers of complexity with regards to the intracellular pathways followed by the phagocytosed material. In more ancient processes, phagocytosed lytic products combine with MHC class I to define a self/non-self condition. In Vertebrates, the phagocytosed lytic products coming from microbial pathogens follow additional intracellular pathways to bind to MHC class II and induce activities of more specialized immune cells such as lymphocytes and professional antigen-presenting cells. The main cell populations performing phagocytosis can be defined as granulocytes (section 3.1), macrophages, and dendritic cells (section 3.2), as well as B1 and γδ-T lymphocytes (section 4.3) (Gao et al. Citation2012; Silva & Correia-Neves Citation2012; Scapigliati et al. Citation2018).
4.1. Granulocytes
Granules-containing cells of Metazoa belong to several subclasses that can be found along with animal clades and include, for instance, mollusk hemocytes, earthworm coelomocytes, chelicerate amoebocytes, insect granulocytes, vertebrate neutrophils, basophils, eosinophils (Hartenstein Citation2006 and references therein). From an evolutionary point of view and for the purpose of this review it is proposed that all granules-containing cell subpopulations of Metazoa could be collectively termed granulocytes.
The main function of granulocytes is to secrete the granules containing lytic enzymes, coagulation/clotting/encapsulation factors, soluble mediators of innate responses, and inflammatory factors upon detection of pathogen signatures by TLRs, the most studied PRRs family (Akira et al. Citation2006; Hartenstein Citation2006; Mogensen Citation2009). Some granulocytes such as vertebrate neutrophils are also directly involved in pathogen elimination by phagocytosis.
4.2. Macrophages and dendritic cells
Immunocytes without or with few granules described in Metazoa are cell types able to i) migrate both in vivo (Barros-Becker et al. Citation2017) and in vitro (Green et al. Citation2012), ii) perform phagocytosis (Hartenstein Citation2006), and iii) secrete soluble factors modulating innate and acquired immune responses (Silva Citation2010). These include hemocytes/plasmatocytes/coelomocytes present in coelomic fluid of mollusks, annelids, arthropods, echinoderms, tunicates (Wright Citation1981; Cheng Citation1984; Canicatti & D’Ancona Citation1989). In vertebrates, hemocytes/plasmatocytes are identified as macrophages, which are cells endowed with additional features than invertebrates such as the presentation of the antigen/MHC complex to T cells (Hartenstein Citation2006). An important activity of vertebrate macrophages is the phagocytic process: the pathogen is phagocytosed and processed by lysosomes, its molecular components exposed on the plasma membrane in the form of antigens presented to the immune system and rapidly detected by immune receptors present in lymphocytes. This, in turn, activates/regulates the transcription of genes involved in innate responses (Xu et al. Citation2019) and metabolic responses linked to innate responses, such as those triggered by nitric oxide (Bayne et al. Citation2001).
An evolutionary-specialized macrophage-like cell population present in vertebrates is represented by dendritic cells, whose activities include phagocytosis of pathogens, expression of antigens on the plasma membrane and migration in tissues where they represent a store of antigenic memory for lymphocytes (Permanyer et al. Citation2018). DC can phagocytose infected dead cells, process PAMPs/antigens in the phagosome, send processed product to both MHC-I and MHC-II, and thus activate acquired responses through the expression of costimulatory molecules such as CD80 and CD86, and secretion of cytokines (Iwasaki & Medzhitov Citation2010). It should be noted that, at present, the equivalent of dendritic cells has yet not been found in invertebrates. This observation allows to speculate that dendritic cells of Vertebrates could represent the extant upper layer of phagocytic processes.
5. Lymphocytes
Lymphocytes of vertebrates are an immunocyte type displaying the unique feature, except for gamete precursors during meiosis, of somatically recombining Ig genes. Somatic recombination in lymphocytes is restricted to B and T cell receptors (BcR and TcR), and is the result of the plasticity of ancient genes coding for peptides containing Ig-domains that along evolution have met the domestication of transposons by chordates (Carmona & Schatz Citation2017) and the possibilities offered by whole genome duplications (WGD) that originated vertebrates (Dehal & Boore Citation2005). Cellular/functional features of lymphocytes (e.g. antigen-specific clonality, somatic recombination) appear to be restricted to vertebrates and a limited knowledge is available on lymphocyte-like cells in Metazoa. Cells performing direct cell-mediated immunity can be found in some invertebrate animal phyla such as mollusks (Grandiosa et al. Citation2016) and amphioxus (Huang et al. Citation2007). The recognition and processing of non-self by cytotoxic activities mediated by cells secreting lytic factors, similarly to those performed by vertebrate cytotoxic T lymphocytes, is present from Porifera onwards as a result of compulsory innate defenses (Nappi & Ottaviani Citation2000 and references therein).
5.1. Classical lymphocytes
Vertebrate lymphocytes are classified as leukocytes responsible of modulating acquired immunity against pathogenic infections, to which they respond with both the production of soluble factors and cell-mediated activities. The latter response aims at rearranging antibody and TcR molecules by pathogen-driven somatic recombination, increasing their tissutal abundance through clonal expansion and ultimately acquiring an extraordinary specificity against the pathogen antigenic determinants. Very importantly, the specificity for the pathogen(s) is maintained during lifespan through memory mechanisms. Lymphocytes are classified as B cells, which produce and secrete antibodies, and T cells, which are responsible for producing anti-inflammatory soluble mediators and killing cells displaying antigens that have been recognized as foreign and dangerous. These two classes express peculiar surface-associated Ig-based antigen receptors together with associated co-receptors: the B cell receptor (BcR) is constituted by a single type immunoglobulin molecule (IgD, IgM, IgA, IgG, or IgE) associated with co-receptors (mainly CD79 and CD19); the TcR is constituted by two heterodimers (TCRαβ, TcRγδ) expressed in two different T cell subclasses and associated with two main co-receptors (CD4, CD8). The first studies describing lymphocyte activities outside mammals were performed about 80 years ago in fish (Duff Citation1942).
5.2. Innate lymphoid cells
Besides macrophages, granulocytes, and dendritic cells, additional types of innate immune cells known as innate lymphoid cells (ILCs) have been described in mammals (Vivier et al. Citation2016). ILCs are lymphocytes lacking BcR and TcR that are classified into three groups based on the expression of defined transcription factors, functional characteristics, and phenotype (Spits et al. Citation2013). Overall, they serve as the first line of defense against microorganisms, representing the major players during inflammatory activities (Vivier et al. Citation2018). Interestingly, ILCs are present in all tissues with a prevalent concentration at mucosal surfaces. The cell population constituting ILCs is mostly composed by cell lacking granules, with the exclusion of granule-containing natural killer (NK) cells. As discussed below, ILCs of mammals display many features in common with invertebrate immunocytes and may represent a “low layer” of immune cells in vertebrates (Vivier et al. Citation2016).
5.3. Innate-like lymphocytes
Despite lymphocytes are described as the players of acquired responses, a growing body of research has recently established the presence of subpopulation of cells with innate-like capabilities (Verykokakis et al. Citation2014). This additional group of mammalian lymphocytes may be regarded to as a bridge between innate and adaptive responses in that they display a functional BcR/TcR but are endowed with unconventional or innate-like properties. The mammalian innate-like lymphocytes (mILL) have been mainly identified as subpopulations of T cells (i.e. mainly γδ-T cells but also natural killer T cells -NKT- and mucosa-associated invariant T cells -MAIT) and a subpopulation of B cells (i.e. B1-B cells) (Fagarasan et al. Citation2000; Carding & Egan Citation2002; Godfrey et al. Citation2004; Le Bourhis et al. Citation2011). mILL are mainly located at mucosal tissues, produce peculiar cytokine patterns, and their functions and origin are a subject of much investigations due to both their unusual features and involvement in severe pathologies (Bennett et al. Citation2015; Zou et al. Citation2017). Previous investigations pointed out the hypothesis that populations of mILL might represent an “immune lower layer” in mammals, since their features appear to be similar to those present in lymphocytes of teleost fish, the oldest living vertebrate ancestors of mammals (Scapigliati et al. Citation2018).
6. Discussion
6.1. Similarities between invertebrate and vertebrate immunocytes and the origin of inflammation
Given that all animal species must enact defense strategies against pathogenic organisms, an evolutionary convergence on common basic cellular and molecular features can be observed among Metazoa, and an evolutionary hypothesis can be drawn. For instance, an evident similarity in the morphology of a granule-containing immunocyte can be observed among representatives of the animal kingdom, e.g. a mollusk (Cueto et al. Citation2015), a crustacean (Wu et al. Citation2019), a protochordate (Hirose et al. Citation2003) and a fish (Chaves-Pozo et al. Citation2004). This holds true also for fast-acting granulocytes between much distant clades, as in Limulus amoebocytes (Ding et al. Citation1993) and mammal mast cells (Akin Citation2014).
A peculiar innate and fast antibacterial response of vertebrate granulocytes is the formation of neutrophil extracellular traps (NETs), where the activated cell extrudes chromatin to form a fibrous network that entraps bacteria and triggers downstream immune stimuli (Jorch & Kubes Citation2017). The formation of NETs in response to pathogens has been recently observed in mollusks (Lange et al. Citation2017), in annelids (Homa Citation2018), and in tunicates (Franchi et al. Citation2019), thus demonstrating additional conserved relationships between invertebrate and vertebrate granulocyte activities.
Gene products with activities similar to pro-inflammatory cytokines (interleukins, tumor necrosis factor), cytokine-dependent signal transduction pathways (MyD88, IRAK, NFkB/IkB) and inflammatory factors (C-reactive protein) have counterparts in invertebrates (Black et al. Citation2004; Scapigliati et al. Citation2006; Steelman & Connors Citation2009; Ottaviani et al. Citation2011). The described activities, although not being exhaustive of many others reported, clearly suggest that innate inflammation-like activities are present throughout the animal phyla. In vertebrates, the cellular and humoral innate activities inherited by invertebrates have been enriched with new gene products, and are classically defined as inflammation (Sheehan et al. Citation2018).
6.2. Evolutionary plasticity of LRR and Ig domains
Along with conserved pathways, many genes coding for molecules that sense and fight pathogens have independently evolved in animal lineages as group-specific, and some of these important products are listed above. However, two lineages of gene products are constantly present in any animal group investigated from unicellular eukaryotes to mammals, namely those containing LRRs and Ig domains (Halaby & Mornon Citation1998; Du Pasquier Citation2009).
LRRs confer a peculiar conserved tri-dimensionality to peptides (Kajava et al. Citation1995) and their structure is employed as a molecular palette capable of exquisite specificities when sensing different forms of pathogenic viruses, bacteria, and parasites. TLR surface receptors are the main peptides to present LRRs; while also present in plants, they were identified in a variety of phyla and abundances, e.g. from Cnidaria in a single gene copy to Echinodermata with up to 222 TLR genes (Hibino et al. Citation2006; van der Burg et al. Citation2016). The gene copy numbers of TLR can be employed as a tool to investigate the relationships between the biology of a species and its habitat, as for instance in mussels living in a location characterized by a high microbial load (Toubiana et al. Citation2013; Ren et al. Citation2020).
The evolutionary plasticity of LRR is further confirmed in agnathans, in which LRR-based peptides are employed for sensing pathogens and producing antibody-like soluble LRR-based peptides with high specificity for pathogens. The agnathan vertebrate Petromyzon genus shows immune defenses based almost entirely on LRR gene products, with an acquired immunity system composed of an almost complete set of B-like cells, T killer/helper-like cells, and memory cells similar to those present in jawed vertebrates (Pancer et al. Citation2004). This feature clearly represents a convergent and parallel paraphyletic evolution in immune defenses among vertebrates.
IgDPs-coding genes are present in prokaryotes (Mei et al. Citation2015) and started diversifying in eukaryotes from protists onwards (King et al. Citation2008). Common traits in IgDPs include their size (about 70–100 amino acids), structure (beta-sheets linked by disulfide bridges) and functionality (receptors for extracellular substances on the cell surface of invertebrate and vertebrate immunocytes and other cell types). The IgDP main gene products among animal eukaryotes are related to cell-adhesion (Albelda & Buck Citation1990), leukocyte homing during infection (Yong & Khwaja Citation1990), non-self recognition (Scapigliati et al. Citation2018). Related to immune functions, a topic of this review, IgDPs are present in major histocompatibility complex (MHC), B lymphocytes either in the form of membrane-associated BcR or secreted antibody, T lymphocytes as membrane-associated TcR, and lymphocyte co-receptors.
The plasticity of IgDP-coding genes is clearly evident in MHC and the Dscam gene in Drosophila. The former is one of the most polymorphic known genes in animals, the latter is involved in axon physiology and is the dipteran homolog of a human neural cell-adhesion IgDP (see section 1.2) (Brites et al. Citation2011). In hemocytes the Dscam gene may be present in more than 38.000 isoforms through differential exon usage mechanisms, and evolution selected this high variability as a molecular mechanism to recognize and fight pathogens, as shown upon in vivo infection (Smith et al. Citation2011).
6.3. Long-living invertebrates and trained immunity
With the available knowledge, it is generally assumed that invertebrates do not show reactions attributable to acquired immunity, mainly for the lack of RAG genes and lymphocytes. However, several species may even have a lifespan of some centuries, such as members of the Hyalospongiae family (Porifera), the genus Hydra (Cnidaria), Arctica islandica and Margaritifera margaritifera (Mollusca), Homarus americanus (Arthropoda) and Mesocentrotus franciscanus (Echinodermata). It is likely that these organisms encounter the same pathogen multiple times over the course of their life and it can be therefore speculated that they could be protected by an innate-based mechanism enriched with memory.
On the other hand, recent evidences have shown that some cells of the vertebrate innate system, mainly macrophages and innate-like lymphocytes, are able to “remember” a pathogenic attack through mechanisms known as trained immunity (Netea et al. Citation2016; Petit et al. Citation2019) prompted by epigenetic and metabolic processes (Arts et al. Citation2016; Van Der Heijden et al. Citation2018). Taking into account the similarities occurring in innate immunity cells among animal phyla (this review), it is conceivable to speculate that trained immunity is common to invertebrates and vertebrates and that was shaped by convergent evolution, as recently theorized (Penkov et al. Citation2019). Further research will better clarify this important argument.
6.4. Specializations of vertebrates
Vertebrates represent roughly the 2% of all animal species, yet peculiar specializations related to many aspects of animal physiology and behavior, particularly immune defenses, were developed. Immune features of vertebrates include the so-called acquired immunity, which is characterized by the presence of lymphocytes able to somatically rearrange LRR-based and, mostly, Ig-based genes, together with MHC class II surface molecules, and an exquisite specificity for antigens. Lymphocytes are driven by pathogens to expand clonally, possibly maintaining a lifelong immune memory. Such an acquired system is restricted to vertebrates due to two main evolutionary reasons: i) a taming of ancient transposase enzymes in invertebrate chordates (Zhang et al. Citation2019) and ii) the whole genome duplication (WGD) events that originated in vertebrates (Dehal & Boore Citation2005) and added new genetic tools to the immune gene machinery. WGDs allowed the origin of true RAG enzymes and their encounter with a perfect partner, the IgDP genes coding for antibodies and lymphocyte receptors, thus producing an almost endless set of possibilities for non-self recognition and elimination. In agnathan vertebrates, a similar and paralogous evolutionary process took place, where WGD likely induced the cytidine deaminase enzyme (CDA) to interact with LRR-coding VLR genes and originate sequence diversities and cell populations similar to that of Ig and TcR (Alder et al. Citation2005; Rogozin et al. Citation2007).
6.5. Immune layers
Extant animal species must rely on efficient immune defenses to guarantee their reproductive fitness, and defense mechanisms are, in turn, dependent on the animals’ genomes. The evolution of immune defenses can be depicted as layers of gene products overlying on top of each other; the lower the layer, the more primitive and shared the activities performed by the defense mechanism in question. Such layers initially originated in ancestors and only became established in following groups by acquiring specializations on the base of gene evolution and environmental microbial pressure. In this view, a phylogenetic tree based on common established features that specialized along evolutionary times producing branches with group-specific antimicrobial defenses can be drawn. The presence of immune layers is predominantly discussed in mammals due to the extensive knowledge related to human health (Herzenberg & Herzenberg Citation1989; Hornef & Torow Citation2020). Indeed, the lower layer of mammals innate immune humoral include features inherited from invertebrates, namely TLRs, AMPs, PGRPs, protease cascades, lectins, together with granule-containing cell populations, phagocytes, and natural cytotoxic cells. These represent the basis of inflammatory activities as they are known in vertebrates. The above layer could be represented by innate lymphoid cells (ILCs) and sub-populations of lymphocytes with an innate-like behavior, namely γδ-T lymphocytes and B1-B cells, collectively known as mILL. Despite lymphocytes are classically defined as actors of the acquired immune system, it appears rather evident that mammalian lymphocytes must be included into two diverse layers because of their different anatomical locations, origin, and anti-pathogen activities. The presence of mILL as heritage from teleost fish, as well as the possible origin of vertebrate lymphocytes from invertebrate immunocytes through evolutionary changes, has been recently discussed (Scapigliati et al. Citation2018). Several immune activities common to most Metazoa became more specialized in vertebrates/mammals, and thus could be attributed to upper and more recent layers, like dendritic cells deriving from phagocytes, γδ-T cells deriving from thymus, B2-B cells deriving from bone marrow, antigen-sampling intestinal M-cells deriving (likely) from intestinal leukocytes. As discussed above, the great immune specializations of jawed vertebrates derive from the possibilities offered by whole-genome duplication events and plasticity of genes related to non-self recognition/elimination, with the Dscam gene as a paradigm. The powerfulness of an evolutionary match between WGD and RAGs/CDA with LRR is also shown by agnathans acquired immune system, which represents a parallel and convergent evolution within vertebrates in the fight against pathogens. A tentative and brief summary of immune activities organized as layers is reported in .
Figure 1. A summary of animal main immune activities as incremental layers. The figure shows main gene products and cellular activities involved in internal defenses as they have been described in this work, related to reported animal groups and arranged as layers
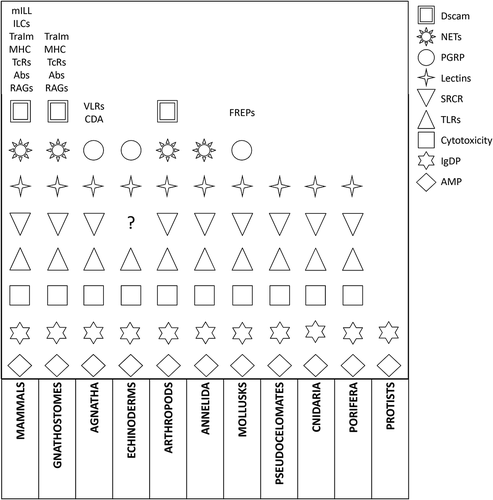
7. Conclusion
Immune defenses play a major role in the evolution of animal species. Some behaviors such as physical competition for mating could be interpreted as a display of immune wellness to be transmitted to progeny. Within the zoological field, new lines of research at the gene-environment level such as reproductive immunology (Zwollo Citation2012), behavioral immunology (Boltana et al. Citation2018) and the interaction between animal immunology and environmental pollutants (Della Torre et al. Citation2015) have emerged in recent years.
In a zoological context, the aim of this review was to supply the reader with novel arguments that could help in the understanding of animal evolution by leveraging on the fundamental parameter of internal defenses for survival to pathogens. Also, with regards to immune responses depicted as incremental layers, it may simplify the complex network of cellular populations and sensor/effector molecules that have been described in Metazoa and, principally, in mammals. At last, knowledge of evolution and biodiversity of immune defenses may be exploited by the steadily expanding biotechnologies sector with the ultimate aim of developing novel environmental-friendlier cross-pathogen therapeutics (Wibowo & Zhao Citation2019).
Disclosure statement
The authors declare that no financial interest or benefit arise from the direct application of this research.
Additional information
Funding
References
- Adema CM. 2015. Fibrinogen-related proteins (Freps) in mollusks. In: Results and problems in cell differentiation. 111–129. DOI:10.1007/978-3-319-20819-0_5.
- Adema CM, Hertel LA, Miller RD, Loker ES. 1997. A family of fibrinogen-related proteins that precipitates parasite-derived molecules is produced by an invertebrate after infection. Proceedings of the National Academy of Sciences of the United States of America 94(16):8691–8696. DOI:10.1073/pnas.94.16.8691.
- Adema CM, Hillier LW, Jones CS, Loker ES, Knight M, et al. 2017. Whole genome analysis of a schistosomiasis-transmitting freshwater snail. Nature Communications 8(1):15451. DOI: 10.1038/ncomms15451.
- Akin C. 2014. Morphology and histologic identification of mast cells. In: Encyclopedia of medical immunology. pp. 503–506. DOI:10.1007/978-1-4614-9194-1_82.
- Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell. DOI: 10.1016/j.cell.2006.02.015.
- Albelda SM, Buck CA. 1990. Integrins and other cell adhesion molecules. The FASEB Journal 4:2868–2880. DOI: 10.1096/fasebj.4.11.2199285.
- Alder MN, Rogozin IB, Iyer LM, Glazko GV, Cooper MD, et al. 2005. Immunology: Diversity and function of adaptive immune receptors in a jawless vertebrate. Science 310:1970–1973. DOI: 10.1126/science.1119420.
- Al-Khalaifah H, Al-Nasser A. 2019. Immune response of molluscs. In: Diarte-Plata G, Escamilla-Montes R, editors. Molluscs. IntechOpen. DOI:10.5772/intechopen.81778.
- Amparyup P, Charoensapsri W, Tassanakajon A. 2013. Prophenoloxidase system and its role in shrimp immune responses against major pathogens. Fish and Shellfish Immunology 34:990–1001. DOI: 10.1016/j.fsi.2012.08.019.
- Arts RJW, Joosten LAB, Netea MG. 2016. Immunometabolic circuits in trained immunity. Seminars in Immunology 28:425–430. DOI: 10.1016/j.smim.2016.09.002.
- Barros-Becker F, Lam PY, Fisher R, Huttenlocher A. 2017. Live imaging reveals distinct modes of neutrophil and macrophage migration within interstitial tissues. Journal of Cell Science. DOI: 10.1242/jcs.206128.
- Barrow AD, Trowsdale J. 2008. The extended human leukocyte receptor complex: Diverse ways of modulating immune responses. Immunological Reviews 224:98–123. DOI: 10.1111/j.1600-065X.2008.00653.x.
- Bayne CJ, Hahn UK, Bender RC. 2001. Mechanisms of molluscan host resistance and of parasite strategies for survival. Parasitology 123:159–167. DOI: 10.1017/s0031182001008137.
- Bénard CY, Blanchette C, Recio J, Hobert O. 2012. The secreted immunoglobulin domain proteins ZIG-5 and ZIG-8 cooperate with L1CAM/SAX-7 to maintain nervous system integrity. PLoS Genetics 8. DOI: 10.1371/journal.pgen.1002819.
- Bennett MS, Round JL, Leung DT. 2015. Innate-like lymphocytes in intestinal infections. Current Opinion in Infectious Diseases 28:457–463. DOI: 10.1097/QCO.0000000000000189.
- Bilak H, Tauszig-Delamasure S, Imler JL. 2003. Toll and Toll-like receptors in Drosophila. Biochemical Society Transactions 31:648–651. DOI: 10.1042/BST0310648.
- Black S, Kushner I, Samols D. 2004. C-reactive protein. Journal of Biological Chemistry 279:48487–48490. DOI: 10.1074/jbc.R400025200.
- Boltana S, Sanhueza N, Donoso A, Aguilar A, Crespo D, et al. 2018. The expression of TRPV channels, prostaglandin E2 and pro-inflammatory cytokines during behavioural fever in fish. Brain, Behavior, and Immunity 71:169–181. DOI: 10.1016/j.bbi.2018.03.023.
- Brites D, Encinas-Viso F, Ebert D, Pasquier L, Haag CR. 2011. Population genetics of duplicated alternatively spliced exons of the Dscam gene in Daphnia and Drosophila. PLoS ONE 6:e27947. DOI: 10.1371/journal.pone.0027947.
- Buonocore F, Picchietti S, Porcelli F, Della Pelle G, Olivieri C, et al. 2019. Fish-derived antimicrobial peptides: Activity of a chionodracine mutant against bacterial models and human bacterial pathogens. Developmental & Comparative Immunology 96:9–17. DOI: 10.1016/j.dci.2019.02.012.
- Canicatti C, D’Ancona G. 1989. Cellular aspects of Holothuria polii immune response. Journal of Invertebrate Pathology 53:152–158. DOI: 10.1016/0022-2011(89)90002-5.
- Carding SR, Egan PJ. 2002. γδ T cells: Functional plasticity and heterogeneity. Nature Reviews Immunology 2:336–345. DOI: 10.1038/nri797.
- Carmona LM, Schatz DG. 2017. New insights into the evolutionary origins of the recombination‐activating gene proteins and V(D)J recombination. The FEBS Journal 284:1590–1605. DOI: 10.1111/febs.13990.
- CDCC VDH, Noz MP, Joosten LAB, Netea MG, Riksen NP, et al. 2018. Epigenetics and Trained Immunity. Antioxidants and Redox Signaling 29:1023–1040. DOI: 10.1089/ars.2017.7310.
- Chang MX, Nie P, Wei LL. 2007. Short and long peptidoglycan recognition proteins (PGRPs) in zebrafish, with findings of multiple PGRP homologs in teleost fish. Molecular Immunology 44:3005–3023. DOI: 10.1016/j.molimm.2006.12.029.
- Chaves-Pozo E, Mulero V, Meseguer J, García Ayala A. 2004. Flow cytometry based techniques to study testicular acidophilic granulocytes from the protandrous fish gilthead seabream (Sparus aurata L.). Biological Procedures Online 6:129–136. DOI: 10.1251/bpo81.
- Cheng TC, ed. 1984. Comparative pathobiology, volume 6: invertebrate blood - cells and serum factors. Springer US: Boston, MA. DOI:10.1007/978-1-4684-4766-8.
- Clow LA, Raftos DA, Gross PS, Smith LC. 2004. The sea urchin complement homologue, SpC3, functions as an opsonin. Journal of Experimental Biology 207:2147–2155. DOI: 10.1242/jeb.01001.
- Coteur G, Mellroth P, Lefortery CD, Gillan D, Dubois P, et al. 2007. Peptidoglycan recognition proteins with amidase activity in early deuterostomes (Echinodermata). Developmental & Comparative Immunology 31:790–804. DOI: 10.1016/j.dci.2006.11.006.
- Cueto JA, Rodriguez C, Vega IA, Castro-Vazquez A. 2015. Immune defenses of the invasive apple snail Pomacea canaliculata (Caenogastropoda, Ampullariidae): Phagocytic hemocytes in the circulation and the kidney. PLoS ONE 10. DOI: 10.1371/journal.pone.0123964.
- Davidson CR, Best NM, Francis JW, Cooper EL, Wood TC. 2008. Toll-like receptor genes (TLRs) from Capitella capitata and Helobdella robusta (Annelida). Developmental and Comparative Immunology 32:608–612. DOI: 10.1016/j.dci.2007.11.004.
- Dehal P, Boore JL. 2005. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biology 3. DOI: 10.1371/journal.pbio.0030314.
- Della Torre C, Buonocore F, Frenzilli G, Corsolini S, Brunelli A, et al. 2015. Influence of titanium dioxide nanoparticles on 2,3,7,8-tetrachlorodibenzo-p-dioxin bioconcentration and toxicity in the marine fish European sea bass (Dicentrarchus labrax). Environmental Pollution 196:185–193. DOI: 10.1016/j.envpol.2014.09.020.
- Destoumieux-Garzón D, Rosa RD, Schmitt P, Barreto C, Vidal-Dupiol J, et al. 2016. Antimicrobial peptides in marine invertebrate health and disease. Philosophical Transactions of the Royal Society B: Biological Sciences 371:20150300. DOI: 10.1098/rstb.2015.0300.
- Ding JL, Sababathy TK, Ho B. 1993. Morphological changes of Carcinoscorpius rotundicauda amoebocyte and Escherichia coli during their interaction. Cytobios 75:21–32.
- Du Pasquier L. 2009. Diversification des immunorécepteurs au cours de l’évolution des métazoaires. Medicine/Sciences 25:273–280. DOI: 10.1051/medsci/2009253273.
- Duff DCB. 1942. The Oral Immunization of Trout Against Bacterium Salmonicida. The Journal of Immunology 44:87–94.
- Dziarski R, Gupta D. 2006a. Mammalian PGRPs: Novel antibacterial proteins. Cellular Microbiology 8:1059–1069. DOI: 10.1111/j.1462-5822.2006.00726.x.
- Dziarski R, Gupta D. 2006b. The peptidoglycan recognition proteins (PGRPs). Genome Biology 7. DOI: 10.1186/gb-2006-7-8-232.
- Elvington M, Liszewski MK, Atkinson JP. 2016. Evolution of the complement system: From defense of the single cell to guardian of the intravascular space. Immunological Reviews 274:9–15. DOI: 10.1111/imr.12474.
- Fagarasan S, Shinkura R, Kamata T, Nogaki F, Ikuta K, et al. 2000. Mechanism of B1 cell differentiation and migration in GALT. Current Topics in Microbiology and Immunology 252:221–229. DOI: 10.1007/978-3-642-57284-5_23.
- Franchi N, Ballarin L. 2017. Immunity in protochordates: The tunicate perspective. Frontiers in Immunology 8. DOI: 10.3389/fimmu.2017.00674.
- Franchi N, Ballarin L, Peronato A, Cima F, Grimaldi A, et al. 2019. Functional amyloidogenesis in immunocytes from the colonial ascidian Botryllus schlosseri: Evolutionary perspective. Developmental and Comparative Immunology 90:108–120. DOI: 10.1016/j.dci.2018.09.010.
- Fujii S, Kubo KI, Takayama S. 2016. Non-self- and self-recognition models in plant self-incompatibility. Nature Plants 2. DOI: 10.1038/nplants.2016.130.
- Gallo C, Schiavon F, Ballarin L. 2011. Insight on cellular and humoral components of innate immunity in Squilla mantis (Crustacea, Stomatopoda). Fish & Shellfish Immunology 31:423–431. DOI: 10.1016/j.fsi.2011.06.013.
- Gao J, Ma X, Gu W, Fu M, An J, et al. 2012. Novel functions of murine B1 cells: Active phagocytic and microbicidal abilities. European Journal of Immunology 42:982–992. DOI: 10.1002/eji.201141519.
- Gardères J, Bourguet-Kondracki ML, Hamer B, Batel R, Schröder HC, et al. 2015. Porifera lectins: Diversity, physiological roles and biotechnological potential. Marine Drugs 13:5059–5101. DOI: 10.3390/md13085059.
- Gauthier MEA, Du Pasquier L, Degnan BM. 2010. The genome of the sponge Amphimedon queenslandica provides new perspectives into the origin of Toll-like and interleukin 1 receptor pathways. Evolution & Development 12:519–533. DOI: 10.1111/j.1525-142X.2010.00436.x.
- Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. 2004. NKT cells. What’s in a Name? Nature Reviews Immunology 4:231–237. DOI: 10.1038/nri1309.
- González-Santoyo I, Córdoba-Aguilar A. 2012. Phenoloxidase: A key component of the insect immune system. Entomologia Experimentalis Et Applicata 142:1–16. DOI: 10.1111/j.1570-7458.2011.01187.x.
- Grandiosa R, Mérien F, Pillay K, Alfaro A. 2016. Innovative application of classic and newer techniques for the characterization of haemocytes in the New Zealand black-footed abalone (Haliotis iris). Fish & Shellfish Immunology 48:175–184. DOI: 10.1016/j.fsi.2015.11.039.
- Green TD, Park J, Yin Q, Fang S, Crews AL, et al. 2012. Directed migration of mouse macrophages in vitro involves myristoylated alanine-rich C-kinase substrate (MARCKS) protein. Journal of Leukocyte Biology. DOI:10.1189/jlb.1211604.
- Halaby DM, Mornon JPE. 1998. The immunoglobulin superfamily: An insight on its tissular, species, and functional diversity. Journal of Molecular Evolution 46:389–400. DOI: 10.1007/PL00006318.
- Hanington PC, Zhang SM. 2011. The primary role of fibrinogen-related proteins in invertebrates is defense, not coagulation. Journal of Innate Immunity 3:17–27. DOI: 10.1159/000321882.
- Hartenstein V. 2006. Blood cells and blood cell development in the animal kingdom. Annual Review of Cell and Developmental Biology. DOI: 10.1146/annurev.cellbio.22.010605.093317.
- He F, Mai LH, Gardères J, Hussain A, Erakovic Haber V, et al. 2017. Major antimicrobial representatives from marine sponges and/or their associated bacteria. Progress in Molecular and Subcellular Biology 55:35–89. DOI: 10.1007/978-3-319-51284-6_2.
- Herzenberg Leonore A, Herzenberg Leonard A. 1989. Toward a layered immune system. Cell 59:953–954. DOI: 10.1016/0092-8674(89)90748-4.
- Hibino T, Loza-Coll M, Messier C, Majeske AJ, Cohen AH, et al. 2006. The immune gene repertoire encoded in the purple sea urchin genome. Developmental Biology 300:349–365. DOI: 10.1016/j.ydbio.2006.08.065.
- Hirose E, Shirae M, Saito Y. 2003. Ultrastructures and Classification of Circulating Hemocytes in 9 Botryllid Ascidians (Chordata: Ascidiacea). Zoological Science 20:647–656. DOI: 10.2108/zsj.20.647.
- Hohenester E, Sasaki T, Timpl R. 1999. Crystal structure of a scavenger receptor cysteine-rich domain sheds light on an ancient superfamily. Nature Structural Biology 6:228–232. DOI: 10.1038/6669.
- Hole CR, Wager CML, Castro-Lopez N, Campuzano A, Cai H, et al. 2019. Induction of memory-like dendritic cell responses in vivo. Nature Communications 10. DOI:10.1038/s41467-019-10486-5.
- Homa J. 2018. Earthworm coelomocyte extracellular traps: Structural and functional similarities with neutrophil NETs. Cell and Tissue Research 371:407–414. DOI: 10.1007/s00441-018-2787-0.
- Hornef MW, Torow N. 2020. “Layered immunity” and the “neonatal window of opportunity” – Timed succession of non-redundant phases to establish mucosal host–microbial homeostasis after birth. Immunology 159:15–25. DOI: 10.1111/imm.13149.
- Huang B, Zhang L, Li L, Tang X, Zhang G. 2015. Highly diverse fibrinogen-related proteins in the Pacific oyster Crassostrea gigas. Fish and Shellfish Immunology 43:485–490. DOI: 10.1016/j.fsi.2015.01.021.
- Huang G, Xie X, Han Y, Fan L, Chen J, et al. 2007. The identification of lymphocyte-like cells and lymphoid-related genes in amphioxus indicates the twilight for the emergency of adaptive immune system Unutmaz D [ed.]. PLoS ONE 2:e206. DOI: 10.1371/journal.pone.0000206.
- Huang S, Xu A. 2016. Genomic and transcriptomic view of amphioxus immunity. In: Amphioxus immunity: tracing the origins of human immunity. pp. 57–84. DOI:10.1016/B978-0-12-849903-0.00004-X.
- Huang S, Yuan S, Guo L, Yanhong Y, Li J, et al. 2008. Genomic analysis of the immune gene repertoire of amphioxus reveals extraordinary innate complexity and diversity. Genome Research 18:1112–1126. DOI: 10.1101/gr.069674.107.
- Inamori K, Ariki S, Kawabata S. 2004. A Toll-like receptor in horseshoe crabs. Immunological Reviews 198:106–115. DOI: 10.1111/j.0105-2896.2004.0131.x.
- Ishii A, Kawasaki M, Matsumoto M, Tochinai S, Seya T. 2007. Phylogenetic and expression analysis of amphibian Xenopus Toll-like receptors. Immunogenetics 59:281–293. DOI: 10.1007/s00251-007-0193-y.
- Iwanaga S, Miyata T, Tokunaga F, Muta T. 1992. Molecular mechanism of hemolymph clotting system in Limulus. Thrombosis Research 68:1–32. DOI: 10.1016/0049-3848(92)90124-S.
- Iwasaki A, Medzhitov R. 2010. Regulation of adaptive immunity by the innate immune system. Science 327:291–295. DOI: 10.1126/science.1183021.
- Janeway CA, Travers P, Walport M, Shlomchik MJ. 2001. The complement system and innate immunity. In: Janeway CA, Travers P, Walport M, Shlomchik MJ, editors. Immunobiology: the immune system in health and disease. New York:Garland Science ISBN:0815341016
- Jorch SK, Kubes P. 2017. An emerging role for neutrophil extracellular traps in noninfectious disease. Nature Medicine 23:279–287. DOI: 10.1038/nm.4294.
- Kajava AV, Vassart G, Wodak SJ. 1995. Modeling of the three-dimensional structure of proteins with the typical leucine-rich repeats. Structure 3:867–877. DOI: 10.1016/S0969-2126(01)00222-2.
- Kang D, Liu G, Lundström A, Gelius E, Steiner H. 1998. A peptidoglycan recognition protein in innate immunity conserved from insects to humans. Proceedings of the National Academy of Sciences of the United States of America 95:10078–10082. DOI: 10.1073/pnas.95.17.10078.
- Kashyap DR, Rompca A, Gaballa A, Helmann JD, Chan J, et al. 2014. Peptidoglycan recognition proteins kill bacteria by inducing oxidative, thiol, and metal stress. PLoS Pathogens 10. DOI:10.1371/journal.ppat.1004280.
- King N, Westbrook MJ, Young SL, Kuo A, Abedin M, et al. 2008. The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature 451:783–788. DOI: 10.1038/nature06617.
- König E, Bininda-Emonds ORP, Shaw C. 2015. The diversity and evolution of anuran skin peptides. Peptides 63:96–117. DOI: 10.1016/j.peptides.2014.11.003.
- Kumar S, Ingle H, Prasad DVR, Kumar H. 2013. Recognition of bacterial infection by innate immune sensors. Critical Reviews in Microbiology 39:229–246. DOI: 10.3109/1040841X.2012.706249.
- Lange MK, Penagos-Tabares F, Muñoz-Caro T, Gärtner U, Mejer H, et al. 2017. Gastropod-derived haemocyte extracellular traps entrap metastrongyloid larval stages of Angiostrongylus vasorum, Aelurostrongylus abstrusus and Troglostrongylus brevior. Parasites and Vectors 10:1–12. DOI: 10.1186/s13071-016-1961-z.
- Laurens N, Koolwijk P, de Maat MP. 2006. Fibrin structure and wound healing. Journal of Thrombosis and Haemostasis: JTH 4:932–939. DOI: 10.1111/j.1538-7836.2006.01861.x.
- Le Bourhis L, Guerri L, Dusseaux M, Martin E, Soudais C, et al. 2011. Mucosal-associated invariant T cells: Unconventional development and function. Trends in Immunology 32:212–218. DOI: 10.1016/j.it.2011.02.005.
- Li X, Wang S, Qi J, Echtenkamp SF, Chatterjee R, et al. 2007. Zebrafish Peptidoglycan Recognition Proteins Are Bactericidal Amidases Essential for Defense against Bacterial Infections. Immunity 27:518–529. DOI: 10.1016/j.immuni.2007.07.020.
- Lindsay GK, Roslansky PF, Novitsky TJ. 1989. Single-step, chromogenic Limulus amebocyte lysate assay for endotoxin. Journal of Clinical Microbiology 27:947–951. DOI: 10.1128/jcm.27.5.947-951.1989.
- Liu RD, Cui J, Liu XL, Jiang P, Sun GG, et al. 2015. Comparative proteomic analysis of surface proteins of Trichinella spiralis muscle larvae and intestinal infective larvae. Acta Tropica. DOI:10.1016/j.actatropica.2015.07.002.
- Lu A, Zhang Q, Zhang J, Yang B, Wu K, et al. 2014. Insect prophenoloxidase: The view beyond immunity. Frontiers in Physiology 5(JUL). DOI:10.3389/fphys.2014.00252.
- Malmstrøm M, Matschiner M, Tørresen OK, Star B, Snipen LG, et al. 2016. Evolution of the immune system influences speciation rates in teleost fishes. Nature Genetics 48:1204–1210. DOI: 10.1038/ng.3645.
- Marques MRF, Barracco MA. 2000. Lectins, as non-self-recognition factors, in crustaceans. Aquaculture 191:23–44. DOI: 10.1016/S0044-8486(00)00417-8.
- Mei S, Zhang J, Zhang X, Tu X. 2015. Solution structure of a bacterial immunoglobulin-like domain of the outer membrane protein (LigB) from Leptospira. Proteins: Structure, Function and Bioinformatics 83:195–200. DOI: 10.1002/prot.24723.
- Miller DJ, Hemmrich G, Ball EE, Hayward DC, Khalturin K, et al. 2007. The innate immune repertoire in Cnidaria - Ancestral complexity and stochastic gene loss. Genome Biology 8. DOI:10.1186/gb-2007-8-4-r59.
- Mogensen TH. 2009. Pathogen recognition and inflammatory signaling in innate immune defenses. Clinical Microbiology Reviews. DOI: 10.1128/CMR.00046-08.
- Mylonakis E, Podsiadlowski L, Muhammed M, Vilcinskas A. 2016. Diversity, evolution and medical applications of insect antimicrobial peptides. Philosophical Transactions of the Royal Society B: Biological Sciences 371. DOI: 10.1098/rstb.2015.0290.
- Nakamura S, Morita T, Iwanaga S, Miwa M, Takahashi K. 1977. A sensitive substrate for the clotting enzyme in horseshoe crab hemocytes. Journal of Biochemistry. DOI: 10.1093/oxfordjournals.jbchem.a131612.
- Nappi AJ, Ottaviani E. 2000. Cytotoxicity and cytotoxic molecules in invertebrates. BioEssays 22:469–480. DOI: 10.1002/(SICI)1521-1878(200005)22:5<469::AID-BIES9>3.0.CO;2-4.
- Netea MG, Joosten LAB, Latz E, Mills KHG, Natoli G, et al. 2016. Trained immunity: A program of innate immune memory in health and disease. Science 352:427. DOI: 10.1126/science.aaf1098.
- Nie L, Cai S-Y, Shao J-Z CJ. 2018. Toll-Like Receptors, Associated Biological Roles, and Signaling Networks in Non-Mammals. Frontiers in Immunology 9. DOI: 10.3389/fimmu.2018.01523.
- Niu GJ, Wang S, Xu JD, Yang MC, Sun JJ, et al. 2019. The polymeric immunoglobulin receptor-like protein from Marsupenaeus japonicus is a receptor for white spot syndrome virus infection Liu S-L [ed.]. PLoS Pathogens 15:e1007558. DOI: 10.1371/journal.ppat.1007558.
- Nonaka M. 2014. Evolution of the complement system. Sub-Cellular Biochemistry 80:31–43. DOI: 10.1007/978-94-017-8881-6_3.
- Ottaviani E, Franchini A, Malagoli D. 2011. Inflammatory response in molluscs: cross-taxa and evolutionary considerations. Current Pharmaceutical Design 16:4160–4165. DOI: 10.2174/138161210794519084.
- Pancer Z, Amemiya CT, Ehrhardt GRA, Ceitlin J, Larry Gartland G, et al. 2004. Somatic diversification of variable lymphocyte receptors in the agnathan sea lamprey. Nature 430:174–180. DOI: 10.1038/nature02740.
- Pancer Z, Kruse M, Schäcke H, Scheffer U, Steffen R, et al. 1996. Polymorphism in the immunoglobulin-like domains of the receptor tyrosine kinase from the sponge Geodia cydonium. Cell Communication and Adhesion 4:327–339. DOI: 10.3109/15419069609010776.
- Pancer Z, Münkner J, Müller I, Müller WEG. 1997. A novel member of an ancient superfamily: Sponge (Geodia cydonium, Porifera) putative protein that features scavenger receptor cysteine-rich repeats. Gene 193:211–218. DOI: 10.1016/S0378-1119(97)00135-2.
- Parrinello D, Sanfratello MA, Vizzini A, Parrinello N, Cammarata M. 2015. Ciona intestinalis galectin (CiLgals-a and CiLgals-b) genes are differentially expressed in endostyle zones and challenged by LPS. Fish and Shellfish Immunology 42:171–176. DOI: 10.1016/j.fsi.2014.10.026.
- Penkov S, Mitroulis I, Hajishengallis G, Chavakis T. 2019. Immunometabolic crosstalk. an ancestral principle of trained immunity? Trends in Immunology 40:1–11. DOI: 10.1016/j.it.2018.11.002.
- Perdomo-Morales R, Montero-Alejo V, Perera E. 2019. The clotting system in decapod crustaceans: History, current knowledge and what we need to know beyond the models. Fish & Shellfish Immunology 84:204–212. DOI: 10.1016/j.fsi.2018.09.060.
- Permanyer M, Bošnjak B, Förster R. 2018. Dendritic cells, T cells and lymphatics: Dialogues in migration and beyond. Current Opinion in Immunology 53:173–179. DOI: 10.1016/j.coi.2018.05.004.
- Petit J, Embregts CWE, Forlenza M, Wiegertjes GF. 2019. Evidence of trained immunity in a fish: conserved features in carp macrophages. The Journal of Immunology 203:216–224. DOI: 10.4049/jimmunol.1900137.
- Ren Y, Dong W, Yang Y, Pan B, Bu W. 2020. Molecular and expression characterization of Toll-like receptor family genes from the Anadara sativa (Bivalvia, Arcidae) transcriptome. Developmental & Comparative Immunology 106:103630. DOI: 10.1016/j.dci.2020.103630.
- Rogozin IB, Iyer LM, Liang L, Glazko GV, Liston VG, et al. 2007. Evolution and diversification of lamprey antigen receptors: Evidence for involvement of an AID-APOBEC family cytosine deaminase. Nature Immunology 8:647–656. DOI: 10.1038/ni1463.
- Romero A, Dios S, Poisa-Beiro L, Costa MM, Posada D, et al. 2011. Individual sequence variability and functional activities of fibrinogen-related proteins (FREPs) in the Mediterranean mussel (Mytilus galloprovincialis) suggest ancient and complex immune recognition models in invertebrates. Developmental and Comparative Immunology 35:334–344. DOI: 10.1016/j.dci.2010.10.007.
- Rosenberg E, Zilber-Rosenberg I. 2016. Microbes drive evolution of animals and plants: the hologenome concept. mBio 7. DOI: 10.1128/mBio.01395-15.
- Royet J, Gupta D, Dziarski R. 2011. Peptidoglycan recognition proteins: Modulators of the microbiome and inflammation. Nature Reviews Immunology 11:837–851. DOI: 10.1038/nri3089.
- Rus H, Cudrici C, Niculescu F. 2005. The role of the complement system in innate immunity. Immunologic Research 33:103–112. DOI: 10.1385/IR:33:2:103.
- Sarrias MR, Grønlund J, Padilla O, Madsen J, Holmskov U, et al. 2004. The Scavenger Receptor Cysteine-Rich (SRCR) domain: An ancient and highly conserved protein module of the innate immune system. Critical Reviews in Immunology 24:1–37. DOI: 10.1615/critrevimmunol.v24.i1.10.
- Scapigliati G, Buonocore F, Mazzini M. 2006. Biological activity of cytokines: an evolutionary perspective. Current Pharmaceutical Design 12:3071–3081. DOI: 10.2174/138161206777947489.
- Scapigliati G, Fausto AM, Picchietti S. 2018. Fish lymphocytes: an evolutionary equivalent of mammalian innate-like lymphocytes? Frontiers in Immunology 9:1–8. DOI: 10.3389/fimmu.2018.00971.
- Sheehan G, Garvey A, Croke M, Kavanagh K. 2018. Innate humoral immune defences in mammals and insects: The same, with differences? Virulence 9:1625–1639. DOI: 10.1080/21505594.2018.1526531.
- Shultz AJ, Sackton TB. 2019. Immune genes are hotspots of shared positive selection across birds and mammals. eLife 8. DOI: 10.7554/eLife.41815.
- Silva MT. 2010. When two is better than one: Macrophages and neutrophils work in concert in innate immunity as complementary and cooperative partners of a myeloid phagocyte system. Journal of Leukocyte Biology. DOI: 10.1189/jlb.0809549.
- Silva MT, Correia-Neves M. 2012. Neutrophils and macrophages: The main partners of phagocyte cell systems. Frontiers in Immunology 3. DOI: 10.3389/fimmu.2012.00174.
- Smith PH, Mwangi JM, Afrane YA, Yan G, Obbard DJ, et al. 2011. Alternative splicing of the Anopheles gambiae Dscam gene in diverse Plasmodium falciparum infections. Malaria Journal 10. DOI:10.1186/1475-2875-10-156.
- Sodergren E, Weinstock GM, Davidson EH, Cameron RA, Gibbs RA, et al. 2006. The genome of the sea urchin Strongylocentrotus purpuratus. Science 314:941–952. DOI: 10.1126/science.1133609.
- Southgate PC, Lucas JS. 2008. The Pearl Oyster. Elsevier. DOI: 10.1016/B978-0-444-52976-3.X0001-0.
- Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, et al. 2013. Innate lymphoid cells-a proposal for uniform nomenclature. Nature Reviews Immunology 13:145–149. DOI: 10.1038/nri3365.
- Steelman BN, Connors VA. 2009. Chemokinetic Effect of Interleukin-1β on Cultured Biomphalaria glabrata Embryonic Cells. Journal of Parasitology 95:772–774. DOI: 10.1645/ge-1867.1.
- Toubiana M, Gerdol M, Rosani U, Pallavicini A, Venier P, et al. 2013. Toll-like receptors and MyD88 adaptors in Mytilus: Complete cds and gene expression levels. Developmental and Comparative Immunology 40:158–166. DOI: 10.1016/j.dci.2013.02.006.
- Vallesi A, Giuli G, Ghiara P, Scapigliati G, Luporini P. 1998. Structure-function relationships of pheromones of the ciliate Euplotes raikovi with mammalian growth factors: Cross-reactivity between Er-1 and interleukin-2 systems. Experimental Cell Research 241:253–259. DOI: 10.1006/excr.1998.4056.
- van der Burg CA, Prentis PJ, Surm JM, Pavasovic A. 2016. Insights into the innate immunome of actiniarians using a comparative genomic approach. BMC Genomics 17. DOI: 10.1186/s12864-016-3204-2.
- Vasta GR, Ahmed H. 2008. Animal Lectins: A functional view. CRC Press. DOI: 10.1201/9781420006971.
- Vasta GR, Ahmed H, Fink NE, Elola MT, Marsh AG, et al. 1994. Animal lectins as self/non‐self recognition molecules: biochemical and genetic approaches to understanding their biological roles and evolution. Annals of the New York Academy of Sciences 712:55–73. DOI: 10.1111/j.1749-6632.1994.tb33562.x.
- Vasta GR, Ahmed H, Tasumi S, Odom EW, Saito K. 2007. Biological roles of lectins in innate immunity: Molecular and structural basis for diversity in self/non-self recognition. In: Lambris JD, editor. Advances in experimental medicine and biology. Springer: New York. pp. 389–406. DOI:10.1007/978-0-387-71767-8_27.
- Vazquez L, Alpuche J, Maldonado G, Agundis C, Pereyra-Morales A, et al. 2009. Immunity mechanisms in crustaceans. Innate Immunity 15:179–188. DOI: 10.1177/1753425909102876.
- Verni F, Gualtieri P. 1997. Feeding behaviour in ciliated protists. Micron 28:487–504. DOI: 10.1016/S0968-4328(97)00028-0.
- Verykokakis M, Zook EC, Kee BL. 2014. ID’ing innate and innate-like lymphoid cells. Immunological Reviews 261:177–197. DOI: 10.1111/imr.12203.
- Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, et al. 2018. Innate lymphoid cells: 10 years on. Cell 174:1054–1066. DOI: 10.1016/j.cell.2018.07.017.
- Vivier E, Van De Pavert SA, Cooper MD, Belz GT. 2016. The evolution of innate lymphoid cells. Nature Immunology 17:790–794. DOI: 10.1038/ni.3459.
- Wang L, Song X, Song L. 2018. The oyster immunity. Developmental & Comparative Immunology 80:99–118. DOI: 10.1016/j.dci.2017.05.025.
- Wibowo D, Zhao CX. 2019. Recent achievements and perspectives for large-scale recombinant production of antimicrobial peptides. Applied Microbiology and Biotechnology 103:659–671. DOI: 10.1007/s00253-018-9524-1.
- Wiens M, Korzhev M, Perović-Ottstadt S, Luthringer B, Brandt D, et al. 2007. Toll-like receptors are part of the innate immune defense system of sponges (Demospongiae: Porifera). Molecular Biology and Evolution 24:792–804. DOI: 10.1093/molbev/msl208.
- Wright RK. 1981. Urochordates. In: Ratcliffe NA, Rowley AF, editors. Invertebrate blood cells, Vol. 2. London: Academic Press, 565–626. ISBN:0125821026.
- Wu F, Xie Z, Yan M, Li Q, Song J, et al. 2019. Classification and characterization of hemocytes from two Asian horseshoe crab species Tachypleus tridentatus and Carcinoscorpius rotundicauda. Scientific Reports 9. DOI:10.1038/s41598-019-43630-8.
- Xu K, Zhang Z, Xu Z, Tang Z, Liu L, et al. 2019. A novel invertebrate toll-like receptor is involved in TLR mediated signal pathway of thick shell mussel Mytilus coruscus. Developmental and Comparative Immunology 97:11–19. DOI: 10.1016/j.dci.2019.03.012.
- Yong K, Khwaja A. 1990. Leucocyte cellular adhesion molecules. Blood Reviews 4:211–225. DOI: 10.1016/0268-960X(90)90001-9.
- Yue Y, Meng Y, Ma H, Hou S, Cao G, et al. 2016. A large family of Dscam genes with tandemly arrayed 5′ cassettes in Chelicerata. Nature Communications 7. DOI:10.1038/ncomms11252.
- Zhang H, Wang L, Song L, Song X, Wang B, et al. 2009. A fibrinogen-related protein from bay scallop Argopecten irradians involved in innate immunity as pattern recognition receptor. Fish and Shellfish Immunology 26:56–64. DOI: 10.1016/j.fsi.2008.07.019.
- Zhang K, Tan J, Xu M, Su J, Hu R, et al. 2014. A novel granulocyte-specific α integrin is essential for cellular immunity in the silkworm Bombyx mori. Journal of Insect Physiology 71:61–67. DOI: 10.1016/j.jinsphys.2014.10.007.
- Zhang Y, Cheng TC, Huang G, Lu Q, Surleac MD, et al. 2019. Transposon molecular domestication and the evolution of the RAG recombinase. Nature 569:79–84. DOI: 10.1038/s41586-019-1093-7.
- Zou C, Zhao P, Xiao Z, Han X, Fu F, et al. 2017. γδ T cells in cancer immunotherapy. Oncotarget 8:8900–8909. DOI: 10.18632/oncotarget.13051.
- Zwollo P. 2012. Why spawning salmon return to their natal stream: The immunological imprinting hypothesis. Developmental and Comparative Immunology 38:27–29. DOI: 10.1016/j.dci.2012.03.011.
