Abstract
Hoatzin, a unique species endemic to Neotropics, has puzzled avian systematists since it was described 244 years ago. Despite its enigmatic origin and unparalleled life cycle, behavior, and mode of life, its parasites only occasionally were investigated. In this study, a new species Calamicoptes hoazinus n. sp. (Laminosioptidae: Fainocoptinae) was described, based on females found in the quill-wall of the primary feather of the hoatzin Opisthocomus hoazin (Statius Muller) (Opisthocomiformes: Opisthocomidae). Following on this we also constructed the key to all known species of the genus Calamicoptes. Importantly, our finding represents the first record of the family Laminosioptidae from the opisthocomiform bird and the first record of this mite family in the Neotropical realm. Scarce records of parasite-host interactions in this group, missing molecular data, and the absence of any phylogenetic study allows only to speculate about evolutionary events leading to observed distribution on the extant Neornithian host tree.
http://zoobank.org/urn:lsid:zoobank.org:pub:F312CDE8-FB1F-4509-85BC-900F534E5EA8
http://zoobank.org/urn:lsid:zoobank.org:act:F1EC0F86-BD68-4340-950E-1E1BB9734B09
Introduction
The hoatzin (Opisthocomus hoazin), the only extant species of the order Opisthocomiformes, is a folivorous bird endemic to riverine floodplains in the Amazonian region (Winkler et al. Citation2020). However, fossils of modern-type Opisthocomiformes are known also from Europe and Africa and the hoatzin lineage may even originate in the Old World (Mayr Citation2014; Mayr & De Pietri Citation2014). The bird presents some unique characteristics, including wing claws in chicks and foregut fermentation, which make its systematic relationships to other birds difficult to determine. Despite hoatzin´s unusual and primitive morphology, genetic studies have shown the species is not as primitive as once thought, and it could be a very derived bird that retains (or reverted to) some plesiomorphic traits (Fain & Houde Citation2004; Hacket et al. Citation2008; Jarvis et al. Citation2014). Its phylogenetic position among Neornithes still constitutes one of the biggest mysteries in the avian systematics (Sibley & Ahlquist Citation1973, Citation1990; Sorenson et al. Citation2003; Ericson et al. Citation2006; Kimball et al. Citation2013; McCormack et al. Citation2013; Prum et al. Citation2015; Dos Santos et al. Citation2018; Houde et al. Citation2019). There have been various attempts to place it in different parts of the bird tree, but phylogenetic analyses mostly have shown low supporting values (Dos Santos et al. Citation2018). However, the most recent molecular phylogeny with robust statistical support placed hoatzin as the sister taxon of the Caprimulgiformes in basal landbirds clade (Kuhl et al. Citation2020); only future researches can confirm this position.
Despite this intriguing systematics and biology, hoatzin parasites and symbionts in general only rarely were studied. As the hoatzin´s ruminant-like digestion is unique in birds, the most attention was paid to its crop microorganism’s community structure and ecology (e.g. Godoy-Vitorino et al. Citation2010, Citation2012; Bardele et al. Citation2017). Haemoparasites were investigated by Renjifo et al. (Citation1952), Gabaldon (Citation1998), and Pacheco et al. (Citation2019); some parasitic nematodes were studied by Renjifo et al. (Citation1952) and Pinto and Gomes (Citation1985). Of ectoparasites, feather lice (Mallophaga) (Kellog Citation1915), and feather mites (Acari) (Atyeo & Gaud Citation1971; Hernandes & Mironov Citation2015; Hernandes et al. Citation2017) have been recorded from this enigmatic host.
During our study of mite fauna of the hoatzin, we found a new species of the rarely collected representatives of the subfamily Fainocoptinae Lukoschus & Lombert Citation1979 (Acariformes: Astigmata: Analgoidea: Laminosioptidae).
Members of the subfamily Fainocoptinae are obligate viviparous parasites inhabiting the follicles of developing wing and contour feather germs. They live directly in the quill-wall or depressions on the outer surface of quills and feed on the outer layers of the non-keratinized base of the quills (Lukoschus & Lombert Citation1979, Citation1980; Skoracki et al. Citation2014). The presence of fainocoptines in the quill wall can cause reactions of surrounding host tissues – the feather sheath of the epidermis of the feather groove becomes activated to hyperkeratosis (Lukoschus & Lombert Citation1980).
The subfamily Fainocoptinae comprises 24 described species grouped in seven genera. Except for Calamicoptes Lukoschus & Lombert, Citation1979 and Fainocoptes Lukoschus & Lombert, Citation1979, all other genera (i.e. Aratingocoptes Fain & Perez, Citation1990a; Colicoptes Lombert & Lukoschus, Citation1981; Podicipedicoptes Lombert, Kethley & Lukoschus, Citation1979; Streetaearus Lukoschus & Lombert, Citation1979; Rallicoptes, Citation1980) are monospecific and known from single host orders (see ).
Table I. Distribution and associations of faincoptine genera with orders and families of avian hosts
Table II. Host associations and distribution of Calamicoptes species of the World
In this paper, we i) describe a new species Calamicoptes hoazinus n. sp.; ii) present a key to all known members of genus Calaminoptes, the most species-rich genus of the family; iii) summarise data on the distribution and host associations of the subfamily Fainocoptinae with orders and families of avian hosts and iv) discuss the known knowledge on hosts and habitats of sister and ancestral phylogenetic lineages related to Laminosioptidae.
Materials and methods
We examined 1 primary, 1 secondary, 2 greater wing coverts, 2 under tail coverts, 10 contour feathers of each of three hoatzin individuals deposited in the Biodiversity Institute & Natural History Museum, The University of Kansas, United States (KUNHM); 5 under- and 5 upper-tail coverts and 10 contour feathers of two individuals deposited in the Bavarian State Collection of Zoology, Munich, Germany (ZSM), and two deposited in the Alexander Koenig Research Museum, Bonn, Germany.
Before mounting, mites were softened and cleared in Nesbitt’s solution at room temperature for three days. Mites were mounted on slides in Hoyer’s medium and investigated using a light microscope (ZEISS Axioscop2+, Germany) with differential interference contrast (DIC) illumination.
In the descriptions below, the idiosomal setation follows Griffiths et al. (Citation1990) with modifications by Norton (Citation1998). The leg setation follows Grandjean (Citation1941). All measurements are in micrometers. Body length was measured from the anterior margin of the gnathosoma to the posterior end. Idiosoma width was measured at the level of setae c2. Measurements of distances between idiosomal setae of different pairs refer to the distance between transverse rows formed by setal pairs.
Specimen depositories and reference numbers are cited using the following abbreviations: AMU – Adam Mickiewicz University, Poznań, Department of Animal Morphology; ZSM – Bavarian State Collection of Zoology, Munich, Germany.
Prevalence and 95% confidence interval (Sterne) were calculated with Quantitative Parasitology 3.0 (Rozsa et al. Citation2000; Reiczigel et al. Citation2019).
Results
One primary feather of one hoatzin from KUNHM was infested by laminosioptids (two females in two separate tunnels in the same quill wall); the prevalence is 33.3% (Sterne´s CI at 95% = 1.7–86.5).
Family: Laminosioptidae Vitzthum, 1931Subfamily: Fainocoptinae Lukoschus & Lombert Citation1979Genus: Calamicoptes Lukoschus & Lombert, Citation1979
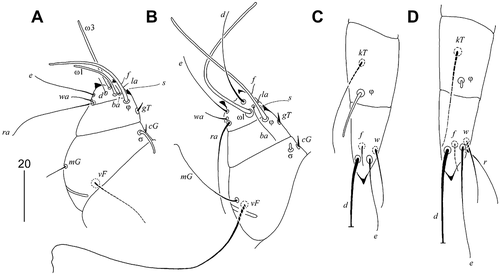
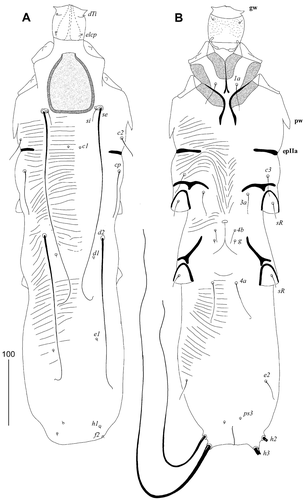
Calamicoptes hoazinus n. sp.()
Figure 1. Calamicoptes hoazinus n. sp., female: (a) – dorsal view; (b) – ventral view. Abbreviations: gw – gnathosomal wing, pw – prodorsal wing, ep – epimerites IIa
Description
Female, holotype
Total body length 680 (650 in 1 paratype), width 165 (165).
Gnathosoma
Gnathosoma ovate, densely granulated ventrally, 55 (55) long, and 55 (55) wide. Lateral extensions of pseudorutellum (gnathosomal wings) present.
Dorsal idiosoma
Dorsal and ventral striations as in ). Propodonotal shield ovate, 90 (95) long, and 70 (75) wide, with heavily sclerotized lateral and posterior margins. Setae se about 16 times longer than si and 4.1–4.6 times longer than setae cp. Short lateral wing-like protrusions (prodorsal wings) situated behind legs II and not reaching epimerites IIa. Cuticle of opisthosoma and region around bases of setae d1 and d2 smooth, intergrading into finely striated regions. Setae d2 long, c1, d1, e1, h1, f2 minute. Setae d2 3.4 times longer than cp.
Ventral idiosoma
Setae c3 and e2 subequal in length and located ventrally. Epimerites I–IV well developed. Epimerites IIa on ventral side longer than on dorsal side. Setae 4a about twice as long as setae 3a. Setae 4b 4–5 times longer than setae g. Setae g and ps3 minute. Oviporus situated at level of anterior part of epimerites III. Terminal extensions of opisthosoma absent. Anus placed subterminally, pseudanal setae ps3 situated near its anterior end. Lengths of idiosomal setae: si 20 (25), se 320 (310), c1 5 (5), c2 35 (30), c3 30 (35), cp 70 (75), d1 5 (5), d2 235 (205), e1 5 (5), e2 30 (35), h1 5 (5), h2 (450), f2 5 (5), 1a 40, 3a 35 (40), 4b 20 (25), 4a 60 (60), ps3 5 (5), g 5 (5). Distances between setal bases: se–se 85 (85), c1–c1 20 (25), c2–c2 155 (135), cp–cp 145 (140), d2–d2 90 (85), d1–d1 60 (60), h1–h1 55 (50), h1–f2 20 (15), e2–e2 120 (110), se–d2 190 (180), c1–d2 140 (125), e2–ps3 75 (70).
Legs
Tarsi I and II with two, and tarsi III and IV with one strongly sclerotized dorso-apical processes. Setae vF of femora II longer than legs II. Chaetotaxy of legs: tarsi I–IV 8–8–4–5, tibiae 1–1–1–1, genua 2–2–0–0, femora 1–1–0–0, trochanters 1–1–1–1. Solenidia ω1 on tarsi II twice as long as ω1I; solenidia σ genua I more than 3 times longer than σII; solenidia φ on tibiae III more than 5 times longer than φIV. Lengths of solenidia: ω1I 20 (20), ω3I 30 (25), φI 40 (35), σI 10 (10), ω1II 60 (60), φII 55 (50), σII short, less than 5, φIII 20 (20), φIV short, less than 5.
Male and immature stages
Not found.
Type material
Female holotype and 1 female paratype from two separate tunnels in the quill-wall of one primary feather of the hoatzin Opisthocomus hoazin (Statius Muller) (Opisthocomiformes: Opisthocomidae); PERU: Loreto Prov., Teniente Lopez, Rio Corrientes, 2°34ʹ60.0”S 76°07ʹ00.0”W, 23 July 1993, coll. D.M. Webb (host reg. no. KUNHM-87,763).
Type material deposition
The holotype is deposited in the AMU (AMU-LAMI-002, paratype in the ZSM (reg. no. ZSMA20190426).
Etymology
The name is derived from the specific name of the host.
Differential diagnosis
Among 11 species of the genus known to date, Calamicoptes hoazinus sp. n. is morphologically most similar to C. fringillae Lombert, Kethley & Lukoschus, Citation1979 described from Pooecetes gramineus (Gmelin) (Passeriformes: Passerellidae) from the United States (Lombert et al. Citation1979). In females of both species, the wing-like protrusions do not extend beyond epimerites IIa; whip-like setae se are long and extend beyond the level of setae d2, and setae d1 and g are present. The new species differs from C. fringillae by the following features: in females of C. hoazinus, the total body length is 650–680 µm; setae e2 are 30–35 µm long; setae 4b are 4–5 times longer than setae g; solenidia φ on tibiae III are more than 5 times longer than φIV. In females of C. fringillae, the total body length is 495–525 µm; setae e2 are 183 µm long; setae 4b and g are subequal in the length; solenidia φ on tibiae III and IV subequal in length.
Setae g absent 2
– Setae g present4
Lateral wing-like protrusions very large, 125–130 µm long C. hymenopterus
– Lateral wing-like protrusions medium-sized, about 50 µm long 3
Bases of setae d1 and d2 situated on the same transverse level C. galli
– Bases of setae d1 situated posterior to level of setal bases d2 C. monedula
Setae se does not reach the level of setal bases d2. Setae d2 very short, 15 µm long C. anatidus
– Setae se reach below the level of setal bases d2. Setae d2 long, more than 100 µm long 5
Distal tip of lateral wing-like protrusions situated behind legs II not extending posteriorly below epimerites IIa 6
– Lateral wing-like protrusions situated behind legs II extending posteriorly below epimerites IIa 7
Setae e2 30–35 µm long; setae 4b 4–5 times longer than setae g. Solenidia φ on tibiae III more than 5 times longer than φIV C. hoazinus n. sp.
– Setae e2 183 µm long. Setae 4b and g subequal in length. Solenidia φ on tibiae III and IV subequal in length C. fringillae
Solenidia φII 60 µm long; φIII 4 times longer than φIV C. meliphagae
– Solenidia φII less than 35 µm long; φIII less than 2.5 times longer than φIV 8
Setae c3 14 µm long C. zumpti
– Setae c3 28–41 µm long 9
Length of setae ps3 14 µm long C. conopophilae
– Setae ps3 as microsetae 3–6 µm long 10
Leg setae vFII 106 µm. Setae 4b and g subequal in length C. arenariae
– Leg setae vFII 67 µm. Setae 4b 1.6 times longer than g C. vanelli
Key to the Calamicoptes species (Females)
Remark
The species C. lomberti Fain & Perez, Citation1990 is known only from males (Fain & Perez Citation1990b) and is not included in the above key. On the other hand, males of this species have a unique feature among Calamicoptes, i.e. lack of setae d1; taking into consideration that fainocoptines are without pronounced sexual dimorphism, this character is highly probably present also in females.
Discussion
To this day, fainocoptines were only known from primaries and wing coverts, but also body feathers of a wide spectrum of neoavian birds (Lukoschus & Lombert Citation1979; Citation1980; Fain Citation1981; Lombert et al. Citation1984; Fain & Perez Citation1990a, b; Skoracki et al. Citation2014).
Members of the genus Calamicoptes are known from the majority of zoogeographic regions (), but our finding from Peru is the first confirmation of the family Laminosioptidae in the Neotropical region. Considering the enormous avian diversity in this region (Jetz et al. Citation2014; Billerman et al. Citation2020), it would be interesting to focus on this ectoparasite group in future research.
The wide spectrum of host lineages occupied by fainocoptines and an even more extensive range of unexplored bird groups indicate that their currently described fauna is only a tip of an iceberg of their real species richness. Taking into account their life-cycle and high host specificity (all known species are monoxenous), it is possible to expect much greater diversity of this group (Costello Citation2016; Okamura et al. Citation2018).
Within the superfamily Analgoidea, the family Laminosioptidae is phylogenetically closely related to the family Dermoglyphidae (Klimov & OConnor Citation2013), living in cavities of feather quills of a large spectrum of bird orders - the paleognath order Tinamiformes, as well as neognath orders Anseriformes, Galliformes (= Galloanserae), Columbiformes, Coraciiformes, Passeriformes, Piciformes (= Neoaves). Dabert & Mironov (Citation1999) suggested that both families probably originated from the ancestor living on down feathers.
It seems that the more ancestral families, e.g. Analgidae (recorded from paleognathous Apterygiformes and Tinamiformes, as well as Galloanserae and Neoaves), and Psoroptoididae (known from paleognathous Casuariformes and neognathous Neoaves) remain on down feathers while the more advanced families entered into the quills (Dermoglyphidae), or moved to the feather follicles (Laminosioptidae: Fainocoptinae) and subcutaneous layers of skin (Laminosioptidae: Laminosioptinae) during the co-evolution with their hosts (Dabert & Mironov 1999).
Considering the distribution of fainocoptines on the phylogenetic tree of their avian hosts, it is possible to expect that these mites parasitized at least the ancestor of Neognathae, as Calamicoptes, the most diversified genus (), is well established in their both clades, Galloanserae (both extant orders, Anseri- as well as Galliformes) and different neoavian orders (Charadriiformes, Columbiformes, Coliiformes, Gruiformes, Passeriformes, Podicipediformes, and Psittaciformes). It could mean that their origin is Mesozoic, as Neoaves split from Galloanseres is suggested to happen in Mid-Cretaceous, before the C-Pg boundary (Chiappe & Dyke Citation2002; Brusatte et al. Citation2015; Worthy et al. Citation2017).
Taken together, while morphologically more derived Fainocoptinae and Laminosioptinae are confirmed only on Neognatha, the sister clade, Dermoglyphidae, and other ancestral and more primitive analgoids like Analgidae and Psoroptoididae are associated with both extant Paleo- and Neognatha (Gaud et al. Citation1973; Mironov Citation2007; Dabert Citation2014). Moreover, considering a diverse assemblage of extinct Neornithes, bushy tree of Euornithes with many vanished lineages and a long evolutionary history of the feather itself (Prum Citation1999; Chuong et al. Citation2000; Sumida & Brochu Citation2000; Gauthier & de Queiroz Citation2001; Clarke et al. Zhang 2006; Xu Citation2006; Livezey & Zusi Citation2007; Hu et al. Citation2009; Xu et al. Citation2009; Zheng et al. Citation2009; Dimond et al. Citation2011; Xing et al. Citation2016; Kundrát et al. Citation2020), it seems premature to hypothesize that Laminosioptidae, including quill wall mites, evolved as late as with Neornithes.
Since we do not have any fossil evidence on Laminosioptidae and Analgoidea in general, we can base our conclusions only on indirect data. Based on the data of host associations, we can more or less assuredly state that Laminosioptidae originated on the ancestors of Neognathae. Moreover, the split into two subfamilies took place on these ancestors. The test of hypothesis of earlier laminosioptid origin, i.e. on Neornithes (meaning all extant birds), needs careful investigations of Paleognathae.
Acknowledgements
We want to thank Mark Blair Robbins and Andrew Townsend Peterson from the Biodiversity Institute & Natural History Museum, Kansas University for the access to Birds’ collections. We also thank two anonymous reviewers for their comments which improved the manuscript substantially.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Atyeo WT, Gaud J. 1971. Feather mites (Analgoidea: Pterolichidae) of the Hoatzin (Aves: Galliformes). The American Midland Naturalist 86(1):152–159. DOI:10.2307/2423695.
- Bardele CF, Schultheiss S, Lynn DH, Wright AG, Dominguez-Bello MG, Obispo NE. 2017. Aviisotricha hoazini n. gen., n. sp., the morphology and molecular phylogeny of an anaerobic ciliate from the crop of the Hoatzin (Opisthocomus hoazin), the cow among the birds. Protist 168(3):3351. DOI:10.1016/j.protis.2017.02.002.
- Billerman SM, Keeney BK, Rodewald PG, Schulenberg TS, Editors. 2020. Birds of the World. Ithaca, NY, USA: Cornell Laboratory of Ornithology. https://birdsoftheworld.org/bow/home
- Brusatte SL, Connor O, Jarvis JK. 2015. The origin and diversification of birds. Current Biology 25:8881–8898. DOI:10.1016/j.cub.2015.08.003.
- Chiappe LM, Dyke GJ. 2002. The Mesozoic radiation of birds. Annual Review of Ecology and Systematics 33(1):91–124. DOI:10.1146/annurev.ecolsys.33.010802.150517.
- Chuong CM, Chodankar R, Widelitz RB, Jiang TX. 2000. Evo-devo of feathers and scales: Building complex epithelial appendages. Current Opinion in Genetics & Development 10(4):449–456. DOI:10.1016/S0959-437X(00)00111-8.
- Clarke JA, Zhou Z, Zhang F. 2006. Insight into the evolution of avian flight from a new clade of Early Cretaceous ornithurines from China and the morphology of Yixianornis grabaui. Journal of Anatomy 208(3):287308. DOI:10.1111/j.1469-7580.2006.00534.x.
- Costello MJ. 2016. Parasite rates of discovery, global species richness and host specificity. Integrative and Comparative Biology 56(4):588–599. DOI:10.1093/icb/icw084.
- Dabert J. 2014. A new feather mite species of the genus Neumannella Trouessart, 1916 (Analgoidea, Dermoglyphidae) from the Red-winged Tinamou Rhynchotus rufescens (Temminck, 1815) (Aves, Tinamiformes) with remarks to the evolution of host-parasite associations of the genus. Acta Parasitologica 59:197–205. DOI: 10.2478/s11686-014-0229-z.
- Dabert J, Mironov SV. 1999. Origin and evolution of feather mites (Astigmata). Experimental and Applied Acarology 23:437–454. DOI: 10.1023/A:1006180705101
- Dimond CC, Cabin RJ, Brooks JS. 2011. Feathers, dinosaurs, and behavioral cues: Defining the visual display hypothesis for the adaptive function of feathers in non-avian theropods. Bios 82(3):58–63. DOI:10.2307/23033900.
- Dos Santos MS, Furo IO, Tagliarini MM, Kretschmer R, OBrien PCM, Ferguson-Smith MA, de Oliveira EHC. 2018. The Karyotype of the Hoatzin (Opisthocomus hoazin) - A Phylogenetic Enigma of the Neornithes. Cytogenetic and Genome Research 156(3):158–164. DOI:10.1159/000494707.
- Ericson PG, Anderson CL, Britton T, Elzanowski A, Johansson US, Källersjö M, Ohlson JI, Parsons TJ, Zuccon D, Mayr G. 2006. Diversification of Neoaves: Integration of molecular sequence data and fossils. Biol Letters 2(4):543–547. DOI:10.1098/rsbl.2006.0523.
- Fain A. 1981. Notes on the genus Laminosioptes Megnin, 1880 (Acari, Astigmata) with description of three new species. Systematic Parasitology 2(2):123–132. DOI:10.1007/BF00009900.
- Fain A, Perez TM. 1990a. A new genus and species of quill wall mites (Acari, Laminosioptidae, Fainocoptinae) from Aratinga holochlora (Aves, Psittacidae) in Mexico. Acarologia 31:195–198. http://www1.montpellier.inra.fr/CBGP/acarologia/article.php?id=2474.
- Fain A, Pérez TM. 1990b. Calamicoptes lomberti n. sp. (Acari, Laminosioptidae) from the quill walls of the Willow Ptarmigan, Lagopus lagopus (L.) (Galliformes, Tetraonidae) from Siberia. Bulletin L’Institut Royal Sciences Naturelles Belgique, Entomogy 60:113–114.
- Fain MG, Houde P. 2004. Parallel radiations in the primary clades of birds. Evolution 58(11):2558–2573. DOI:10.1111/j.0014-3820.2004.tb00884.x.
- Gabaldon A. 1998. Malaria aviaria en un país de la región neotropical Venezuela. Caracas, Venezuela: Fundación Venezolana para la Salud. Caracas, Venezuela. pp. 343.
- Gaud J, Atyeo WT, Berla HF. 1973. Acariens Sarcoptiformes plumicoles parasites des tinamous. Acarologia 14:393–453.
- Gauthier J, de Queiroz K 2001. Feathered dinosaurs, flying dinosaurs, crown dinosaurs, and the name Aves. New perspectives on the origin and early evolution of birds: Proceedings of the International Symposium in Honor of John H. Ostrom, Peabody Museum of Natural History, Yale University, New Haven, USA. pp.7–41.
- Godoy-Vitorino F, Goldfarb KC, Brodie EL, Garcia-Amado MA, Michelangeli F, Domínguez-Bello MG. 2010. Developmental microbial ecology of the crop of the folivorous hoatzin. ISME Journal 4(5):611–620. DOI:10.1038/ismej.2009.147.
- Godoy-Vitorino F, Leal SJ, Díaz WA, Rosales J, Goldfarb KC, García-Amado MA, Michelangeli F, Brodie EL, Domínguez-Bello MG. 2012. Differences in crop bacterial community structure between hoatzins from different geographical locations. Research in Microbiology 163(3):211–220. DOI:10.1016/j.resmic.2012.01.001.
- Grandjean F. 1941. La сhaetotaxie comparee des pattes chez las oribates. Bulletin De La Societe Zoologique De France 66:33–50.
- Griffiths DA, Atyeo WT, Norton RA, Lynch CA. 1990. The idiosomal chaetotaxy of astigmatid mites. Journal of Zoology (London) 220(1):1–32. DOI:10.1111/j.1469-7998.1990.tb04291.x.
- Hackett SJ, Kimball RT, Reddy S, Bowie RCK, Braun EL, Braun MJ, Chojnowski JL, Cox WA, Han K-L, Harshman J, Huddleston CJ, Marks BD, Miglia KJ, Moore WS, Sheldon FH, Steadman DW, Witt CC, Yuri T. 2008. A phylogenomic study of birds reveals their evolutionary history. Science 320(5884):1763–1768. DOI:10.1126/science.1157704.
- Hernandes FA, Bauchan GR, Ochoa R. 2017. New and little known feather mites (Acariformes: Astigmata) analysed with low-temperature scanning electron microscopy. International Journal of Acarology 43(7):499–517. DOI:10.1080/01647954.2017.1367032.
- Hernandes FA, Mironov SV. 2015. The feather mites of the hoatzin Opisthocomus hoazin (Müller) (Aves: Opisthocomiformes), with the description of two new genera and six new species (Acari: Analgoidea, Pterolichoidea). Zootaxa 4034(3):401–444. DOI:10.11646/zootaxa.4034.3.1.
- Houde P, Braun EL, Narula N, Minjares U, Mirarab S. 2019. Phylogenetic signal of indels and the neoavian radiation. Diversity 11:108. DOI: 10.3390/d11070108.
- Hu D, Hou L, Zhang L, Xu X. 2009. A pre-Archaeopteryx troodontid theropod from China with long feathers on the metatarsus. Nature 461(7264):640–643. DOI:10.1038/nature08322.
- Jarvis ED, Mirarab S, Aberer AJ, Li B, Houde P, Li C, Ho SYW, Faircloth BC, Nabholz B, Howard JT, Suh A, Weber CC, da Fonseca RR, Li J, Zhang F, Li H, Zhou L, Narula N, Liu L, Ganapathy G, Boussau B, Bayzid MS, Zavidovych V, Subramanian S, Gabaldun T, Capella-Gutierrez S, Huerta-Cepas J, Rekepalli B, Munch K, Schierup M, Lindow B, Warren WC, Ray D, Green RE, Bruford MW, Zhan X, Dixon A, Li S, Li N, Huang Y, Derryberry EP, Bertelsen MF, Sheldon FH, Brumfield RT, Mello CV, Lovell PV, Writhlin M, Schneider MPC, Prosdocimi F, Samaniego JA, Velazquez AMV, Alfaro-Nunez A, Campos PF, Petersen B, Sicheritz-Ponten T, Pas A, Bailey T, Scofield P, Bunce M, Lambert DM, Zhou Q, Perelman P, Driskell AC, Shapiro B, Xiong Z, Zeng Y, Liu S, Li Z, Liu B, Wu K, Xiao J, Yingi X, Zheng Q, Zhang Y, Yang H, Wang J, Smeds L, Rheindt FE, Braun M, Fjeldsa J, Orlando L, Barker FK, Jonsson KA, Johnson W, Koepfli KP, O’Brien S, Haussler D, Ryder OA, Rahbek C, Willerslev E, Graves GR, Glenn TC, McCormack J, Burt D, Ellegren H, Alstrom P, Edwards SV, Stamatakis A, Mindell DP, Cracraft J, Braun EL, Warnow T, Jun W, Gilbert MTP, Zhang G. 2014. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science 346:320–1331. DOI: 10.1126/science.1253451.
- Jetz W, Thomas GH, Joy JB, Redding DW, Hartmann K, Mooers AO. 2014. Global distribution and conservation of evolutionary distinctness in birds. Current Biology 24(9):919–930. DOI:10.1016/j.cub.2014.03.011.
- Jones J, Gaud J. 1962. The description of Laminosioptes hymenopterus n. sp. (Sarcoptiformes) from the American Crow. Acarologia 4:391–395. https://www1.montpellier.inra.fr/CBGP/acarologia/article.php?id=3930.
- Kellogg VL. 1915. A fourth mallophagan species from the hoatzin. Science New Series 41(1053):365–367.
- Kimball RT, Wang N, Heimer-McGinn V, Ferguson ELB, Braun EL. 2013. Identifying localized biases in large datasets: A case study using the avian tree of life. Molecular Phylogenetics and Evolution 69:1021–1032. DOI: 10.1016/j.ympev.2013.05.029.
- Klimov PB, OConnor B. 2013. Is permanent parasitism reversible? – Critical evidence from early evolution of house dust mites. Systematic Biology 62(3):411–423. DOI:10.1093/sysbio/syt008.
- Kuhl H, Frankl-Vilches C, Bakker A, Mayr G, Nikolaus G, Boerno ST, Klages S, Timmermann B, Gahr M. 2020. An unbiased molecular approach using 3ʹUTRs resolves the avian family-level tree of life. Molecular Biology and Evolution msaa191. DOI: 10.1093/molbev/msaa191.
- Kundrát M, Rich TH, Lindgren J, Sjövall P, Vickers-Rich P, Chiappe LM, Kear BP. 2020. A polar dinosaur feather assemblage from Australia. Gondwana Research 80:1–11.
- Livezey BC, Zusi RL. 2007. Higher-order phylogeny of modern birds (Theropoda, Aves: Neornithes) based on comparative anatomy. II. Analysis and Discussion. Zoological Journal of the Linnean Society 149:1–95. DOI: 10.1111/j.1096-3642.2006.00293.x.
- Lombert HAPM, Gaud J, Lukoschus FS. 1984. Six new species of quill wall mites (Astigmata: Laminosioptidae: Fainocoptinae) from African birds. Acarologia 25:55–66. https://www1.montpellier.inra.fr/CBGP/acarologia/article.php?id=2732.
- Lombert HAPM, Kethley JB, Lukoschus FS. 1979. Observations on quill wall mites from American birds (Astigmata: Laminosioptidae: Fainocoptinae). International Journal of Acarology 5:103–110. DOI: 10.1080/01647957908683132.
- Lombert HAPM, Lukoschus FS. 1981. A new genus and species of quill wall mites (Acarina: Laminosioptidae: Fainocoptinae) from Colius colius (Aves: Coliiformes: Coliidae). Bulletin de l'Institut Royal des Sciences Naturelles de Belgique 53:1–7.
- Lukoschus FS, Lombert HAPM. 1979. Parasites of Western Australia. VII Observations on quill wall mites (Acarina, Astigmata). Records Western Australian Museum 7:265–286.
- Lukoschus FS, Lombert HAPM. 1980. Five new species of quill wall mites from European birds (Astigmata: Laminosioptidae: Fainocoptinae). International Journal of Acarology 6:63–78. DOI: 10.1080/01647958008683196.
- Mayr G. 2014. A hoatzin fossil from the middle Miocene of Kenya documents the past occurrence of modern-type Opisthocomiformes in Africa. Auk 131(1):55–60. DOI:10.1642/AUK-13-134.1.
- Mayr G, De Pietri VL. 2014. Earliest and first Northern Hemispheric hoatzin fossils substantiate Old World origin of a “Neotropic endemic”. Naturwissenschaften 101:143–148. DOI: 10.1007/s00114-014-1144-8.
- McCormack JE, Harvey MG, Faircloth BC, Crawford NG, Glenn TC, Brumfield RT. 2013. A phylogeny of birds based on over 1,500 loci collected by target enrichment and high-throughput sequencing. PLoS One 8:e54848. DOI: 10.1371/journal.pone.0054848.
- Mironov SV. 2007. Systematic notes on two genera of the feather mite family Psoroptoididae (Astigmata: Analgoidea). Acarina 15:135–141.
- Norton R. 1998. Morphological evidence for the evolutionary origin of Astigmata (Acari: Acariformes). Experimental and Applied Acarology 22:559–594. DOI: 10.1023/A:1006135509248.
- Okamura B, Hartigan A, Naldoni J. 2018. Extensive uncharted biodiversity: The parasite dimension. Integrative and Comparative Biology 58:1132–1145. DOI: 10.1093/icb/icy039.
- Pacheco MA, García-Amado MA, Manzano J, Matta NE, Escalante AA. 2019. Blood parasites infecting the Hoatzin (Opisthocomus hoazin), a unique neotropical folivorous bird. PeerJ 7:e6361. DOI: 10.7717/peerj.6361.
- Pinto RM, Gomes DC. 1985. Nematodes of Amazonian birds, with a description of Hoazinstrongylus amazonensis n. gen. n. sp. (Trichostrongylidae, Libyostrongylinae). Memórias Do Instituto Oswaldo Cruz 80(2):213–217.
- Prum RO. 1999. Development and evolutionary origin of feathers. The Journal of Experimental Zoology 285:291–306. DOI: 10.1002/(SICI)1097-010X(19991215)285:4<291::AID-JEZ1>3.0.CO;2-9.
- Prum RO, Berv JS, Dornburg A, Field DJ, Townsend JP, Lemmon EM, Lemmon ER. 2015. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature 526:569–573. DOI: 10.1038/nature15697.
- Reiczigel J, Marozzi M, Fábián I, Rózsa L. 2019. Biostatistics for parasitologists – A primer to Quantitative Parasitology. Trends in Parasitology 35(4):277–281. DOI:10.1016/j.pt.2019.01.003.
- Renjifo S, Sanmartin C, De Zuleta J. 1952. A survey of the blood parasites of vertebrates in eastern Colombia. Acta Tropica 9:121–169.
- Rozsa L, Reiczigel J, Majoros G. 2000. Quantifying parasites in samples of hosts. Journal of Parasitology 86:228–232.
- Sibley CG, Ahlquist JE. 1973. The relationships of the Hoatzin. Auk 90:1–13.
- Sibley CG, Ahlquist JE. 1990. Phylogeny and classification of birds. New Haven, Connecticut,: Yale University Press. pp. xxiii + 976.
- Skoracki M, Kavetska K, Ozminski M, Zawierucha K. 2014. Calamicoptes anatidus sp. nov., a new quill wall mite (Acari: Laminosioptidae) from the Greater Scaup Aythya marila (L.) (Aves: Anseriformes). Acta Parasitologica 59:426–432. DOI: 10.2478/s11686-014-0263-x.
- Sorenson MD, Oneal E, García-Moreno J, Mindell DP. 2003. More taxa, more characters: The hoatzin problem is still unresolved. Molecular Biology and Evolution 20:1484–1498. DOI: 10.1093/molbev/msg157.
- Sumida SS, Brochu CA. 2000. Phylogenetic context for the origin of feathers. Integrative and Comparative Biology 40:486–503. DOI: 10.1093/icb/40.4.486.
- Winkler DW, Billerman SM, Lovette IJ. 2020. Hoatzin (Opisthocomidae), version 1.0. In birds of the world. In: Billerman SM, Keeney BK, Rodewald PG, Schulenberg TS, editors. Cornell lab of ornithology. Ithaca, NY, USA. DOI:10.2173/bow.opisth1.01
- Worthy TH, Degrange FJ, Handley WD, Lee MSY. 2017. The evolution of giant flightless birds and novel phylogenetic relationships for extinct fowl (Aves, Galloanseres). Royal Society Open Science 4(10):170975. DOI:10.1098/rsos.170975.
- Xing L, McKellar RC, Xu X, Li G, Bai M, Iv Ws P, Miyashita T, Benton MJ, Zhang J, Wolfe AP, Yi Q, Tseng K, Ran H, Currie PJ. 2016. A feathered dinosaur tail with primitive plumage trapped in Mid-Cretaceous amber. Current Biology 26:3352–3360. DOI: 10.1016/j.cub.2016.10.008.
- Xu X. 2006. Feathered dinosaurs from China and the evolution of major avian characters. Integrative Zoology 1:4–11. DOI: 10.1111/j.1749-4877.2006.00004.x.
- Xu X, Zheng X, You H 2009. A new feather type in a nonavian theropod and the early evolution of feathers. Proceedings of the National Academy of Sciences of the United States of America 106:832–834. DOI: 10.1073/pnas.0810055106.
- Zheng X-T, You H-L, Xu X, Dong Z-M. 2009. An Early Cretaceous heterodontosaurid dinosaur with filamentous integumentary structures. Nature 458:333–336. DOI: 10.1038/nature07856.