Abstract
American mink Neovison vison is one of the most harmful non-indigenous species in Iceland and has been proven to be a useful indirect bioindicator and biomonitor for numerous environmental pollutants. Therefore, the main objective of the study was to determine the total nickel concentration in the spleen of 35 females and 30 males obtained from Brokey archipelago and the south coast of Hvammsfjörður (Dalabyggð, Iceland) using graphite furnace atomic absorption spectroscopy. We also assessed the correlation between nickel concentration and selected anatomical and morphological parameters, hypothesising that invasive alien N. vison is a promising candidate species for biomonitoring the deposition of this trace element. The results indicated a substantial variation in nickel concentration in the spleen tissue of examined animals. For males, the maximum concentration exceeded the average level by more than 16 times, and for females by more than 7 times. The correlation coefficient between morphometric features and the level of nickel concentration in the spleen did not show a significant relationship in any of the tested combinations, for all tested animals or for each sex separately. In conclusion, American mink in Iceland can be considered a promising species for qualitative and quantitative assessment of ecosystems in terms of nickel pollution.
1. Introduction
Invasive alien species often generate risks to human health, have a negative impact on ecosystem services and, consequently, are responsible for significant economic losses (Scalera et al. Citation2012). The problem of biological invasions is particularly important for insular ecosystems, characterised by high ecological vulnerability and sensitivity to deleterious effects of alien species (Bellard et al. Citation2016; Russell et al. Citation2017). This is well illustrated by the case of Iceland, aggregating all characteristics of subpolar regions related to the introduction of invasive non-native species, i.e. relatively low native species richness, availability of unoccupied ecological niches, simplified terrestrial trophic networks, low temperatures reducing the effectiveness of ecological homeostatic mechanisms and considerable anthropoppression (Bennett et al. Citation2015; Stefansson et al. Citation2016; CAFF and PAME Citation2017). Currently, 390 non-indigenous species are recorded in the country, of which seven are invasive and 25 potentially invasive (NOBANIS Citation2020).
Hankard et al. (Citation1993) defined “biomonitoring” as the measurement of the typical response of living organisms to a particular change in their environment, revealing changes over space and time. Markert et al. (Citation1999) differentiated “bioindication” and “biomonitoring” by pointing out the qualitative approach of the first and the quantitative approach of the latter. Consequently, a “bioindicator” can be defined as an organism that provides information on the state (quality) of the environment, while a “biomonitor” additionally allows the quantification of this information (Markert et al. Citation1999). According to Hopkin (Citation1993), organisms used for in situ biomonitoring should meet the following basic conditions: be ecologically meaningful (relevant), be common and widely distributed (reliable), be relatively resistant to xenobiotics (robust), exhibit characteristic and measurable (responsive), as well as repeatable in different sites (reproducible) response to xenobiotic.
At least one invasive alien species in Iceland may be considered a candidate bioindicator and biomonitor, in respect to the above criteria: the American mink Neovison vison, recognised as one of the most harmful non-indigenous species on the island, for which eradication activities have been undertaken (von Schmalensee Citation2010; Stefansson et al. Citation2016; von Schmalensee & Stefánsson Citation2017). It has been proven to be a sensitive and useful indirect bioindicator and biomonitor for assessing and screening for exposure and effects of heavy metals (Capodagli & Parker Citation2007; Kalisinska et al. Citation2016), polychlorinated biphenyls (PCBs) (Luxon et al. Citation2014), polybrominated biphenyls (PBBs) (Bleavins et al. Citation1981), dioxins/furans (Haynes et al. Citation2002), and dichlorodiphenyltrichloroethane (DDT)/metabolites (Haynes et al. Citation2002), as well as, more generally, a sentinel species in environmental monitoring (Nowakowicz-Dębek et al. Citation2013). American mink is a semi-aquatic, territorial, year-round active, crepuscular and nocturnal, solitary mesocarnivore mustelid (Ray Citation2000). Native to North America, the mink is a well-known invasive mammalian species that, due to considerable ecological and phenotypic plasticity, has successfully invaded many territories across the world (Long Citation2003; Melero et al. Citation2012). It was introduced in Iceland in 1931 for fur-farming and, as a result of escapes, soon established a permanent feral population and had spread throughout the country by 1975 (Skírnisson et al. Citation2004). Currently, N. vison occupies almost all Icelandic marine coastline, areas along rivers and lakes in lowlands, as well as most islands up to approx. 10 km from the coast (Bjornsson & Hersteinsson Citation1991; Skírnisson et al. Citation2004). The country’s population size is unknown and seems to fluctuate considerably but could, from local studies, be estimated at roughly somewhere between 5000 and 10,000 animals (Sidorovich Citation1993). Neovison vison is an opportunistic predator and food generalist which, depending on the area and availability of prey, primarily hunts for small mammals, birds and their eggs, fish, frogs, crustaceans and insects (Bonesi & MacDonald Citation2004). Due to the negative consequences of the presence of American mink for the natural environment of Iceland, already in the late 1930s the government started paying bounties for killed mink – a procedure still ongoing to limit its negative ecological effects (Hannesson Citation1956; Stefansson et al. Citation2016). Being widespread game is a great advantage of the candidate species for bioindication and biomonitoring of numerous environmental pollutants, including various elements (Persson et al. Citation2012; Kalisinska et al. Citation2016).
One of the main elements commonly found in terrestrial and aquatic environments is nickel (Ni). It is considered an ultra-trace element, and has been proven to be essential, at very low concentrations, for various physiological and biochemical functions in many species (Phipps et al. Citation2002; Muyssen et al. Citation2004). Deficiency of nickel may result in impaired activity of several enzymes, delayed gestation period and reduction in the number of offspring, anaemia, contact allergy and skin eruptions, and reduced haemoglobin and haematocrit values (WHO Citation1991). At large doses Ni has toxic, mutagenic and carcinogenic effects, confirmed in both plants and animals (WHO Citation1991; Kasprzak & Salnikow Citation2007; DeForesty & Schlekat Citation2013). The main physiological clearance route for Ni, in mammals, is urinary excretion, although this element is also found in sweat, saliva and hair (WHO Citation2000). In mammalian species nickel is detected in kidneys, testes, brain, lung, spleen, liver, heart and hair (Obone et al. Citation1999; Pereira et al. Citation2006).
In this study we determined the total nickel concentration in spleens of American mink from Brokey archipelago and the south coast of Hvammsfjörður, Breiðafjörður Bay, West Iceland (). We also assessed the correlation between nickel concentration and selected anatomical and morphological parameters, hypothesising that invasive alien N. vison is a promising candidate species for biomonitoring of the deposition of this trace element in the country.
2. Materials and methods
The research area covered Brokey archipelago, located east of the village of Stykkishólmur, in the mouth of Hvammsfjörður, the largest fjord extending inland from Breiðafjörður Bay. Breiðafjörður is the second largest bay in Iceland (2874 km2), characterised by shallow waters surrounding over 3000 islands, islets and skerries (Petersen et al. Citation1998; Carlsen et al. Citation2018). Human activity in the area is limited to fishing, tourism, eiderdown collecting, hunting and algal harvest (Petersen et al. Citation1998). The area’s diverse and rich natural and cultural heritage resulted in the establishment of the legally protected Breiðafjörður Conservation Area (Pagnan & Legare Citation2002). Brokey archipelago includes five main islands – Brokey (the largest island of Breiðafjörður), Öxney, Suðurey, Ólafsey and Norðurey – and several dozen smaller islets. These islands are relatively flat, uninhabited and washed by strong tidal currents. Tides of up to 5 m form a vast intertidal zone, offering favourable conditions for high biodiversity and productivity. Extensive algal canopies offer optimal conditions for invertebrates and fish, and as a result also for bird communities and pinnipeds. Breiðafjörður islands are vegetated, with local flora represented by 230 vascular plant species; however, dominant species and species richness vary substantially from island to island. Breiðafjörður Bay is well known as the main habitat of macroalgae around Iceland and has important spawning and nursery grounds for many fish, crustaceans and other invertebrates. It has large seabird colonies, e.g. shag Phalacrocorax aristotelis, cormorant Phalacrocorax carbo, common eider (Somateria mollissima), black guillemot (Cepphus grylle), Atlantic puffin (Fratercula arctica), lesser black-backed gull Larus fuscus, black-legged kittiwake Rissa tridactyla, Arctic tern Sterna paradisaea and great black-backed gull Larus marinus, and is also an important stopover for High-Arctic nesting brent goose Branta bernicla and knot Calidris canutus (Petersen et al. Citation1998; Carlsen et al. Citation2018). In total, over 70% of Icelandic regular breeding bird species have been recorded in the region (Petersen et al. Citation1998). Rich food sources are also the basis for a well-established population of American mink in the Breiðafjörður region, predating on fish, birds, invertebrates and wood mouse Apodemus sylvaticus. The only potential predators of or competitors with American mink in the area are white-tailed eagle Haliaeetus albicilla and the Arctic fox (Vulpes lagopus) (Salo et al. Citation2008; Magnusdottir et al. Citation2012, Citation2014a, Citationb; Unnsteinsdóttir et al. Citation2016).
A total of 65 American mink (30 females and 35 males) were hunted with dogs, leghold and death traps during seven hunting seasons in 2012–2018 in the municipality of Dalabyggð, West Iceland (). Immediately after the hunting day carcasses were sent to the West Iceland Nature Research Centre and frozen at −20°C. During post-mortem examination, body weight and body length (without tail) were measured, and the spleen was removed, weighed to the nearest 0.01 g and frozen again at −20°C. Next, frozen spleens were sent to the Faculty of Food Sciences and Fisheries of the West Pomeranian University of Technology, Szczecin (Poland). Processing of spleen samples was carried out using high-pressure microwave digestion (Speedwave Xpert, Germany); 1 ± 0.01 g of wet weight tissue was dissolved in 6 mL of a mixture of concentrated nitric acid (HNO3) and perchloric acid (HClO4) (ultra-pure, Merck, Germany; acid ratio 5:1). After digestion, samples were diluted with Milli-Q (18.2 MΩ) water to a total volume of 25 mL. Digestions were performed in three replicates.
Element concentrations were determined using a Hitachi ZA3000 Series Polarized Zeeman Atomic Absorption Spectrophotometer (Hitachi High-Technologies Corporation, Japan). Ni concentration was determined by graphite furnace atomic absorption spectroscopy (GFAAS). Calibration curves were established using certified standard solutions (1000 mg/L) from Merck (Germany). The accuracy of the analytical method was tested with reference material fish muscle ERM-BB422 (European Reference Materials, European Commission – Joint Research Centre, Institute for Reference Materials and Measurements, Belgium).
The obtained results were subjected to statistical analysis. The average concentration of nickel and its level of variation in the spleen of all individuals, as well as of individual sexes, was determined. For each of the above combinations, compliance with the normal distribution was tested with the Shapiro–Wilk test. Mann–Whitney U test was used to investigate the level of significance of the difference in nickel concentration in females’ vs. males’ spleens.
Correlations were determined and tested for significance using the nonparametric Spearman method or parametric Pearson analysis. All analyses were performed using the Statistica v. 12.0 (StatSoft, Poland) software. Geographic Information System (GIS) analyses and visualisations were performed using MapInfo v. 11.0 (Pitney Bowes Software, USA) tool set.
3.Results
Because the nickel concentrations in the examined spleens did not display a normal distribution for all animals (W = 0.294, p < 0.0001), nor for males (W = 0.252, p < 0.001) and females (W = 0.539, p < 0.001) separately, in addition to the mean value and standard deviation, the median and range values were also determined ().
Table I. Concentrations of nickel (ppm) in the spleens of Neovison vison in Breiðafjörður Bay, West Iceland
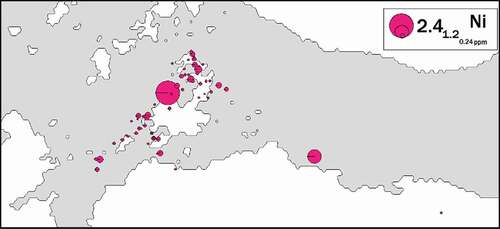
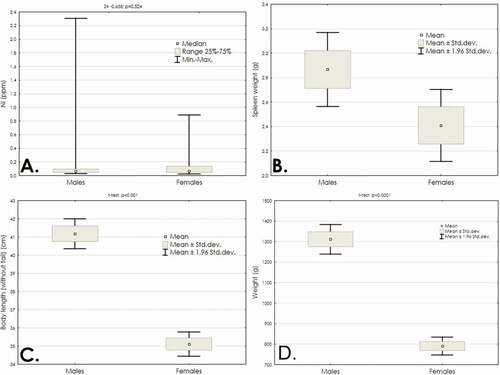
The results presented in indicate a very large variation in nickel concentration in the spleen tissue of the examined animals. Male spleens had a particularly high level of nickel variability. The specificity of this variability is the occurrence of single individuals with an extremely high nickel content in their spleen. For males, the maximum concentration exceeded the average level by more than 16 times, and for females by more than 7 times. Presumably, it was this high variability that did not allow us to determine the significance of the differences (). At the same time, a comparison of spleen weight, tailless body length and body weight indicates that the harvested males were generally larger than females, and these differences were statistically significant (for all indicated traits p < 0.001), as shown in ).
Figure 2. Comparison between male and female mink in terms of (a) nickel concentration in the spleen (ppm), (b) spleen weight (g), (c) tailless body length (cm) and (d) body weight (g)
All tested morphometric features were positively correlated. Examination of the correlation coefficient between morphometric features and the level of nickel concentration in the spleen did not show a significant relationship in any of the tested combinations, for all tested animals or for each sex separately ().
Table II. Correlation coefficient between nickel concentration in the spleen and morphometric traits of all captured mink
An analysis of identified correlations in females () basically confirms the relationships described above. There were no significant correlations between nickel concentration in the spleens and their mass, or with the body weight and tailless body length of individuals. Notably, there is no significant correlation between the latter and body weight in females.
The expected strong positive correlation between body length and weight was found for males (). However, the correlation between spleen weight and body weight was definitely weaker than that of females, and its significance was confirmed only after the application (justified in this case) of the parametric Pearson analysis. However, no correlations between the concentration of nickel in the spleen and the morphometric traits studied were confirmed in males. In fact, there is no such relationship here because the calculated values of the correlation coefficient were close to zero.
The results of the correlation analysis show that the Ni concentration in the spleen of tested animals is to some extent determined by the sex and body condition – which, therefore, is not clearly conditioned by the content of this element in the spleen. The above findings show that the nickel concentration in the body of American mink occurring in the study area depends on the individual exposure of the particular animal.
This is confirmed by the results of the spatial analysis of the sampling site locations and the Ni concentrations in the spleens of individuals caught in these sites (). This analysis found a random distribution of nickel concentration in the spleen of the tested animals. There was no significant trend of dependence indicating a gradient of Ni concentration in the bodies of tested animals, and thus in the study area. A good illustration justifying such a statement is the presence of individuals with extremely different nickel contents in their spleens in directly adjacent locations.
4. Discussion
The global annual release of nickel to the environment by natural processes is about 150,000 Mg, while human activity is responsible for the release of an additional 180,000 Mg annually (Merian Citation1984). Ni redistribution from both natural and anthropogenic sources has commonly been reported in Iceland (Harasim Citation2019). The research area is located outside the natural sources of airborne nickel (i.e. volcanic activity), but at the same time, Iceland is known for rocks containing olivine and geothermal waters, characterised by considerably high Ni content (Wetang’ula Citation2004; Herzberg et al. Citation2016). For example, Wetang’ula (Citation2004) reported the following Ni concentrations in a hot-water spring near Lake Þingvallavatn in south-western Iceland: 9.9–13.2 mg × kg−1 in sediments, 1.52–2.72 mg × kg−1 in mosses (dry weight), 1.0 mg × kg−1 in the alternate water-milfoil Myriophyllum alterniflorum (dry weight), 1.96–1.97 mg × kg−1 in the wandering snail Lymnaea peregra (dry weight), <0.02–0.0327 mg × kg−1 in the three-spined stickleback Gasterosteus aculeatus (dry weight) and <0.02–0.0453 mg × kg−1 in the Arctic charr Salvelinus alpinus (dry weight).
The results obtained for N. vison in the present study, namely 0.134 mg × kg−1 (wet weight), were higher than those found for the abovementioned species. Such elevated values are unlikely to result from the specificity of natural sources of nickel in the study area. The concentration of nickel found in moss in a location in the immediate vicinity of the study area was very low (2.23 mg × kg−1 dry weight in 2015) in comparison with other areas; on the other hand the dispersion of volcanic fumes and dust results in their presence throughout the entire island (Arnalds et al. Citation2014; Magnússon Citation2018). Soil erosion affects the study area only slightly, but this source of Ni in biocoenoses may play some role in shaping Ni concentrations in the tested animals (Arnalds et al. Citation2001). The impact of two of the three aluminium smelters in Iceland, i.e. Straumsvík and Grundartangi, located in the south part of Faxaflói Bay and representing the sector responsible for the largest nickel emissions, on the content of this element in American mink spleens needs to be further investigated. However, it can be assumed that because of their location about 100 km south of our study area, the possible transfer of contaminants in water masses via the Icelandic Coastal Current, which runs in a clockwise direction around the island, should not be omitted (Stefánsson & Ólafsson Citation1991; Valdimarsson & Malmberg Citation1999; Logemann et al. Citation2013). The huge inter-individual variance in nickel content in the spleen of the examined American mink may be connected to localised pollution from four shipwrecks, local harbours and old dumpsites in the study area, or individual diet specialisation (Stefánsson Citation2006). Individual feeding specificity has been described in case of N. vison in Belarus (Sidorovich et al. Citation2001). In addition, particularly strong tidal currents in Breiðafjörður Bay allow full penetration and random flow of waters between islands of Hvammsfjörður (Petersen et al. Citation1998).
It is interesting to compare the concentration of nickel in American mink in Iceland with the results obtained by other authors. Brzeziński et al. (Citation2014) reported an average content of this element in kidneys and livers of N. vison from Poland (Drawa National Park) of 0.29 and 0.27 mg × kg−1 dry weight, respectively. Ni concentration in livers of animals from the vicinity of Sudbury (Canada), home to the world’s largest nickel smelting operation, ranges from 0.43 to 0.7 mg × kg−1, while in kidneys it ranges from 0.5 to 0.74 mg × kg−1 (all values for wet weight; Wren et al. Citation1988; von Schmalensee Citation2010; Parker & Capodagli Citation2011). Even higher values were found in American mink in the USA (wet weight); in Illinois it was 1.1 mg × kg−1 in kidney, 0.9 mg × kg−1 in liver and 0.7 mg × kg−1 in muscle tissue (Halbrook et al. Citation1996). The observed differences in nickel concentration in animals originating in Iceland vs. those from Poland and North America are most likely due to the different types of tissues examined – Ni concentration in renal tissue is the highest among all organs, which is associated with the dominant role of urinary secretion of this organ. The difference between hepatic tissue and spleen is usually smaller, but, as confirmed for many mammalian species, it is the liver that has a higher nickel content (WHO Citation1991). The median concentration obtained in this study is within the physiological Ni content range specified for human spleen (wet weight) – <0.30–0.007 mg × kg−1 (Sumino et al. Citation1975; Rezuke et al. Citation1987).
The absence of the expected, positive correlation between tailless body length and body weight of tested animals can be explained by the very diverse condition of the examined females – which, in turn, can be explained by the high energetic cost of reproduction and maternal care for offspring (McNab Citation2006; Heldstab et al. Citation2017). Thus, an interesting result is the occurrence of a positive, statistically significant correlation between the spleen mass and the body weight of females, as shown in . What is more, the absence of a correlation between nickel concentration in spleen and morphometric features of American mink in the present study indicates the animal’s considerable resistance to a broad spectrum of nickel concentration (0.026–2.305 mg × kg−1). Taking into account the lack of such correlation also for sex indicates the possibility of using all individuals of this species, regardless of gender or physiognomic condition, to monitor the concentration of nickel.
Although many authors argue against the potential of nickel for biomagnification in terrestrial ecosystems, analysis of the available literature, regarding its concentration in tissues of organisms of different trophic levels in Iceland, allows us to plot a certain trend of increasing concentration consistent with ascending trophic levels of food chains (WHO Citation1991; Wetang’ula Citation2004; Knox et al. Citation2019). The ability of nickel to be transferred along aquatic food chains was described by Dumas and Hare (Citation2008), and its bioaccumulation is well documented (Palermo et al. Citation2015). Regardless of whether nickel is subject to biomagnification and bioaccumulation, the high availability of American mink hunted due to its control in Iceland allows for ongoing monitoring of the level of Ni pollution of both aquatic and terrestrial ecosystems on the island. Regarding the question of whether American mink is a suitable monitoring species for nickel in Iceland, one should refer to the criteria that a good biomonitor should meet ().
Table III. A list of conditions for an “ideal” biomonitor and the corresponding features of American mink in Iceland [Stefánsson et al., Citation2016; Dunstone, Citation1993; Tataruch and Kierdorf, Citation2003; Ellenberg, Citation1991; Rosenberg and Resh, Citation1993; Hilty and Merenlender, Citation2000; Füreder and Reynolds, Citation2003]
In conclusion, American mink in Iceland can be considered a promising indirect and passive bioindicative and biomonitoring species for nickel. It is especially favoured by specific ecological conditions, enabling the use of this non-indigenous species for not only a qualitative, but above all a quantitative assessment of ecosystems in terms of Ni pollution. Biomonitoring should in this case be defined, following Markert et al. (Citation1999), as “a method of observing the impact of external factors on ecosystems and their development over a period, or of ascertaining differences between one location and another” (Markert et al. Citation1999). The only apparent downside to using N. vison for biomonitoring is its mobility. Despite being a territorial species, individuals will occasionally travel long distances(up to several kilometres), e.g. as part of the dispersal of young or the roaming of males during the mating season (Dunstone Citation1993). This can potentially limit localised conclusions for small-scale landscapes but will not distort the overall picture. Information obtained directly from the analysis of nickel concentration in N. vison organs is valuable due to the semi-aquatic lifestyle of this species and the top predator position it occupies in the trophic network. This potentially allows extrapolating an assessment based on it to other elements of the ecological system in which the species functions in Iceland. What is more, it is recommended to further research the biomonitoring potential of this species in relation to other pollutants and to determine the formal and legal possibility of including American mink in the national biological monitoring system in Iceland.
Author contributions
Conceptualisation, Jakub Skorupski and Przemysław Śmietana; data curation, Jakub Skorupski and Przemysław Śmietana; formal analysis, Przemysław Śmietana; funding acquisition, Jakub Skorupski; investigation, Jakub Skorupski, Przemysław Śmietana and Arkadiusz Nędzarek; methodology, Jakub Skorupski, Przemysław Śmietana, Remigiusz Panicz and Piotr Eljasik; project administration, Jakub Skorupski and Remigiusz Panicz; Resources, Jakub Skorupski, Przemysław Śmietana, Róbert Stefánsson, Menja von Schmalensee and Arkadiusz Nędzarek; supervision, Jakub Skorupski; Validation, Jakub Skorupski, Przemysław Śmietana and Arkadiusz Nędzarek; visualisation, Jakub Skorupski and Przemysław Śmietana; writing – original draft, Jakub Skorupski, Przemysław Śmietana, Róbert Stefánsson, Menja von Schmalensee, Remigiusz Panicz and Magdalena Szenejko; writing – review and editing, Jakub Skorupski, Przemysław Śmietana, Róbert Stefánsson, Menja von Schmalensee, Remigiusz Panicz and Magdalena Szenejko. All authors have read and agreed to the published version of the manuscript.
Acknowledgements
The authors express their gratitude to the hunters who voluntarily provided hunted animals for the purposes of this research project.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Arnalds O, Olafsson H, Dagsson-Waldhauserova P. 2014. Quantification of iron-rich volcanogenic dust emissions and deposition over the ocean from Icelandic dust sources. Biogeosciences 11(23):6623–6632. DOI:10.5194/bg-11-6623-2014.
- Arnalds O, Thorarinsdottir EF, Metusalemsson S, Jonsson A, Gretarsson E, Arnason A. 2001. Soil erosion in Iceland. Reykjavík: Soil Conservation Service, Agricultural Research Institute.
- Bellard C, Cassey P, Blackburn TM. 2016. Alien species as a driver of recent extinctions. Biology Letters 12(2):20150623. DOI:10.1098/rsbl.2015.0623.
- Bennett JR, Shaw JD, Terauds A, Smol JP, Aerts R, Bergstrom DM, Blais JM, Cheung WWL, Chown SL, Lea MA, Nielsen UN, Pauly D, Reimer KJ, Riddle MJ, Snape I, Stark JS, Tulloch VJ, Possingham HP. 2015. Polar lessons learned: Long-term management based on shared threats in Arctic and Antarctic environments. Frontiers in Ecology and the Environment 13(6):316–324. DOI:10.1890/140315.
- Bjornsson T, Hersteinsson P. 1991. Minkar vid sunnanverdan Breidafjord [Mink in southern Breidafjordur Bay, W-Iceland]. Wildlife Management News 7:3–12.
- Bleavins M, Aulerich R, Ringer R 1981. Placental and mammary transfer of polychlorinated and polybrominated biphenyls in the mink and ferret, In: Kenaga E, Lamb D, editors. Avian and mammalian wildlife toxicology: Second conference, West Conshohocken, PA: ASTM International, Philadelphia, USA. pp.121–131. DOI:10.1520/STP28377S.
- Bonesi L, MacDonald DW. 2004. Differential habitat use promotes sustainable coexistence between the specialist otter and the generalist mink. OIKOS 106(3):509–519. DOI:10.1111/j.0030-1299.2004.13034.x.
- Brzeziński M, Zalewski A, Niemczynowicz A, Jarzyna I, Suska-Malawska M. 2014. The use of chemical markers for the identification of farm escapees in feral mink populations. Ecotoxicology 23(5):767–778. DOI:10.1007/s10646-014-1213-y.
- Capodagli L, Parker GH. 2007. Accumulation and tissue distribution of toxic metals with accompanying effects on body condition measures, in mink (Mustela vison) and muskrat (Ondatra zibethicus) living near mining/smelting operations. In: Gore RB, editor. Environmental research at the leading edge. Hauppauge, NY: Nova Science Publishers. pp. 75–112.
- Carlsen TH, Ásgeirsson Á, Jónsson JE. 2018. Vegetation mapping of islands in Breiðafjörður, West-Iceland. Norsk Institutt for Bioøkonomi Rapport 4(21):1–72.
- Conservation of Arctic Flora and Fauna (CAFF) and Protection of the Arctic Marine Environment (PAME). 2017. Arctic invasive alien species: Strategy and action plan. The Conservation of Arctic Flora and Fauna (CAFF) and Protection of the Arctic Marine Environment (PAME) are working groups of the Arctic council. Akureyri, Iceland. pp. 1–20.
- DeForesty DK, Schlekat CE. 2013. Nickel toxicity to marine organisms. Integrated Environmental Assessment and Management 9(4):580–589. DOI:10.1002/ieam.1419.
- Dumas J, Hare L. 2008. The internal distribution of nickel and thallium in two freshwater invertebrates and its relevance to trophic transfer. Environmental Science & Technology 42(14):5144–5149. DOI:10.1021/es800378j.
- Dunstone N. 1993. The mink. London, UK: Poyser.
- Ellenberg, H. 1991. Bioindicators and Biological Monitoring. In: Ellenberg H, Arndt U, Bretthauer R, Ruthsatz B, Steubing L, editors. Biological Monitoring: Signals from the Environment. Braunschweig: Vieweg. pp. 13–74.
- Füreder L, Reynolds JD. 2003. Is Austropotamobius pallipes a good bioindicator? Bulletin Français de la Pêche et de la Pisciculture 370–371:157–163. DOI:10.1051/kmae:2003011
- Halbrook RS, Woolf A, Hubert Jr. GF, Ross S, Braselton WE. 1996. Contaminant concentrations in Illinois mink and otter. Ecotoxicology 5(2):103–114. DOI:10.1007/BF00119049.
- Hankard P, Osborn D, Brown M, Callaway J, Shore RF, George D, Dighton J 1993. Biomonitoring methodologies. Report to Her majesty’s inspectorate of pollution. Huntingdon: Institute of Terrestrial Ecology.
- Hannesson E. 1956. Islenski villiminkurinn. Nokkur atridi ur aldarfjordungssogu minkanna her á landi. Visir 6(6):1956.
- Harasim P. 2019. Nickel resources and sources. In: Tsadilas CD, Rinklebe J, Selim HM, editors. Nickel in soils and plants. Boca Raton: Taylor & Francis Group. pp. 87–104.
- Haynes JM, Wellman ST, Pagano JJ. 2002. RAP progress in the rochester embayment of lake Ontario: Population monitoring, trophic relationships, and levels of bioaccumulative chemicals of concern in mink, a sentinel species. New York: State Great Lakes Protection Fund.
- Heldstab SA, van Schaik KP, Isler K. 2017. Getting fat or getting help? How female mammals cope with energetic constraints on reproduction. Frontiers in Zoology 14(1):29. DOI:10.1186/s12983-017-0214-0.
- Herzberg C, Vidito C, Starkey NA. 2016. Nickel–cobalt contents of olivine record origins of mantle peridotite and related rocks. American Mineralogist 101(9):1952–1966. DOI:10.2138/am-2016-5538.
- Hilty J, Merenlender A. 2000. Faunal indicator taxa selection for monitoring ecosystem health. Biological Conservation 92:185–197. DOI:10.1016/S0006-3207(99)00052-X
- Hopkin SP. 1993. In situ biological monitoring of pollution in terrestrial and aquatic ecosystems. In: Calow P, editor. Handbook of ecotoxicology. Oxford, UK: Blackwell Scientific Publications. pp. 397–427.
- Kalisinska E, Lanocha-Arendarczyk N, Kosik-Bogacka D, Budis H, Podlasinska J, Popiolek M, Pirog A, Jedrzejewska E. 2016. Brains of native and alien mesocarnivores in biomonitoring of toxic metals in Europe. PLoS One 11(8):e0159935. DOI:10.1371/journal.pone.0159935.
- Kasprzak KS, Salnikow K. 2007. Nickel toxicity and carcinogenesis. In: Sigel A, Sigel H, Sigel RKO, editors. Nickel and its surprising impact in nature. Vol. 2. Hoboken, NJ, USA: John Wiley & Sons. pp. 621–645.
- Knox AS, Paller MH, Li L. 2019. A review of nickel in sediments. In: Tsadilas CD, Rinklebe J, Selim HM, editors. Nickel in soils and plants. Boca Raton: Taylor & Francis Group. pp. 143–180.
- Logemann K, Ólafsson J, Snorrason Á, Valdimarsson H, Marteinsdóttir G. 2013. The circulation of Icelandic waters – A modelling study. Ocean Science 9:931–955.
- Long JL. 2003. Introduced mammals of the world: Their history, distribution and influence. Wallingford, UK: CABI Publishing. pp. 60–82.
- Luxon M, Toll J, Hanson C. 2014. Assessing effects of PCB exposure on American mink (Mustela vison) abundance in portland harbor. Integrated Environmental Assessment and Management 10(1):60–68. DOI:10.1002/ieam.1498.
- Magnusdottir R, Stefansson RA, von Schmalensee M, Macdonald DW, Hersteinsson P. 2012. Habitat- and sex-related differences in a small carnivore’s diet in a competitor-free environment. European Journal of Wildlife Research 58(4):669–676. DOI:10.1007/s10344-012-0615-5.
- Magnusdottir R, von Schmalensee M, Stefánsson R, Macdonald D, Hersteinsson P. 2014a. A foe in woe: American mink (Neovison vison) diet changes during a population decrease. Mammalian Biology – Zeitschrift für Säugetierkunde 79(1):58–63. DOI:10.1016/j.mambio.2013.08.002.
- Magnusdottir R, von Schmalensee M, Stefansson RA, Macdonald DW, Hersteinsson P. 2014b. A foe in woe: American mink (Neovison vison) diet changes during a population decrease. Mammalian Biology 79(1):58–63. DOI:10.1016/j.mambio.
- Magnússon SH. 2018. Vöktun þungmálma og brennisteins í mosa á Íslandi 1990-2015. Garðabær, Iceland: Áhrif frá iðjuverum og eldvirkni, Náttúrufræðistofnun Íslands.
- Markert B, Wappelhorst O, Weckert V, Herpin U, Siewers U, Friese K, Breulmann G. 1999. The use of bioindicators for monitoring the heavy metal status of the environment. Journal of Radioanalytical and Nuclear Chemistry 240:425–429. DOI:10.1007/BF02349387.
- McNab BK. 2006. The energetics of reproduction in endotherms and its implication for their conservation. Integrative and Comparative Biology 46(6):1159–1168. Epub 2006 Jul 11. DOI:10.1093/icb/icl016.
- Melero Y, Santulli G, Gómez A, Gosàlbez J, Rodriguez-Refojos C, Palazón S. 2012. Morphological variation of introduced species: The case of American mink (Neovison vison) in Spain. Mammalian Biology 77(5):345–350. DOI:10.1016/j.mambio.2012.02.001.
- Merian E. 1984. Introduction on environmental chemistry and global cycles of chromium, nickel, cobalt, beryllium, arsenic, cadmium, and selenium, and their derivates. Society of Environmental Toxicology and Chemistry 8(1):9–38. DOI:10.1080/02772248409357038.
- Muyssen BTA, Brix KV, DeForest DK, Janssen CR. 2004. Nickel essentiality and homeostasis in aquatic organisms. Environmental Reviews 12(2):113–131. DOI:10.1139/A04-004.
- NOBANIS. 2020. Available http://www.NOBANIS.org. Accessed Mar 2020 24.
- Nowakowicz-Dębek B, Zoń A, Jakubczak A, Wnuk W. 2013. Hematological parameters of wild and farm mink, red fox and raccoon dog. Medycyna Weterynaryjna 69(1):40–42.
- Obone É, Chakrabarti SK, Bai C, Malick MA, Lamontagne L, Subramanian KS. 1999. Toxicity and bioaccumulation of nickel sulfate in Sprague-Dawley rats following 13 weeks of subchronic exposure. Journal of Toxicology and Environmental Health Part A 57(6):379–401. DOI:10.1080/009841099157593.
- Pagnan J, Legare G. 2002. CAFF (Conservation of Arctic Flora and Fauna), protected areas of the arctic: Conserving a full range of values. Ottawa, Canada: CAFF. pp. 1–55.
- Palermo FF, Risso WE, Simonato JD, Martinez CBR. 2015. Bioaccumulation of nickel and its biochemical and genotoxic effects on juveniles of the neotropical fish Prochilodus lineatus. Ecotoxicology and Environmental Safety 116:19–28. DOI:10.1016/j.ecoenv.2015.02.032.
- Parker GH, Capodagli L. 2011. On the redistribution of tissue metal (cadmium, nickel and lead) loads in mink accompanying parasitic infection by the giant kidney worm (Dioctophyme renale). In: Daniels JA, editor. Advances in environmental research. Vol. 13. New York: Nova Science Publishers, Inc. pp. 187–217.
- Pereira R, Pereira ML, Ribeiro R, Gonçalves F. 2006. Tissues and hair residues and histopathology in wild rats (Rattus rattus L.) and Algerian mice (Mus spretus Lataste) from an abandoned mine area (Southeast Portugal). Environmental Pollution (Barking, Essex: 1987) 139(3):561–575. DOI:10.1016/j.envpol.2005.04.038.
- Persson S, Brunström B, Bäcklin BM, Kindahl H, Magnusson U. 2012. Wild mink (Neovison vison) as sentinels in environmental monitoring. Acta veterinaria Scandinavica 54(1):1–9. DOI10.1186/1751-0147-54-S1-S9.
- Petersen Æ, Þorvarðardóttir G, Pagnan J, Einarsson S. 1998. Breiðafjörður West-Iceland: An Arctic marine protected area. PARKS the International Journal for Protected Area Managers 8(2):23–28.
- Phipps T, Tank SL, Wirtz J, Brewer L, Coyner A, Ortego LS, Fairbrother A. 2002. Essentiality of nickel and homeostatic mechanisms for its regulation in terrestrial organisms. Environmental Reviews 10(4):209–261. DOI:10.1139/a02-009.
- Ray JC. 2000. Mesocarnivores of northeastern North America: Status and conservation issues. Wildlife Conservation Society Working Papers 15:1–82.
- Rezuke WN, Knight JA, Sunderman Jr. FW. 1987. Reference values for nickel concentrations in human tissue and bile. Amerixan Journal of Industrial Medicine 11(4):419–426. DOI:10.1002/ajim.4700110404.
- Rosenberg DM, Resh VH. 1993. Freshwater Biomonitoring and Benthic Macroinvertebrates. New York: Chapman and Hall. pp. 1–488.
- Russell JC, Meyer JY, Holmes ND, Pagad S. 2017. Invasive alien species on islands: Impacts, distribution, interactions and management. Environmental Conservation 44(4):359–370. DOI:10.1017/S0376892917000297.
- Salo P, Nordström M, Thomson R, Korpimäki E. 2008. Risk induced by a native top predator reduces alien mink movements. The Journal of Animal Ecology 77:1092–1098. DOI:10.1111/j.1365-2656.2008.01430.x.
- Scalera R, Genovesi P, Essl F, Rabitsch W. 2012. The impacts of invasive alien species in Europe. European Environment Agency Technical Report 16:114. DOI:10.2800/65864.
- Sidorovich V. 1993. Reproductive plasticity of the American mink Mustela vison in Belarus. Acta theriologica 38:175–183. DOI:10.4098/AT.arch.93-16.
- Sidorovich V, Macdonald D, Pikulik MM, Kruuk H. 2001. Individual feeding specialization in the European mink, Mustela lutreola and the American mink, M. vison in north-eastern Belarus. Folia Zoologica Praha 50:27–42.
- Skírnisson K, Stefansson RA, von Schmalensee M. 2004. Minkur. In: Hersteinsson P, editor. Islensk spendyr. Iceland: Vaka-Helgafell. pp. 88–97.
- Stefánsson RA. 2006. Skipsflök á landi í nágrenni Stykkishólms. Iceland: West Iceland Nature Research Center, Stykkishólmur.
- Stefansson RA, von Schmalensee M, Skorupski J. 2016. A tale of conquest and crisis: Invasion history and status of the American mink (Neovison vison) in Iceland. Acta Biologica 23:87–100. DOI:10.18276/ab.2016.23-08.
- Stefánsson U, Ólafsson J. 1991. Nutrients and fertility of Iclandic waters. Rit Fiskideildar 7(3):1–56.
- Sumino K, Hayakawa K, Shibata T, Kitamura S. 1975. Heavy metals in normal Japanese tissues. Archives of Environmental Health 30(10):487–494. DOI:10.1080/00039896.1975.10666759.
- Tataruch F, Kierdorf H. 2003. Mammals as biomonitors. In: Markert BA, Breure AM, Zechmeister HG, editors. Bioindicators and Biomonitors: Principles, Concepts and Applications. Amsterdam, Netherlands: Elsevier Science. pp. 737–772. DOI:10.1016/S0927-5215(03)80150-9.
- Unnsteinsdóttir ER, Hersteinsson P, Pálsson S, Angerbjörn A. 2016. The fall and rise of the Icelandic arctic fox (Vulpes lagopus): A 50-year demographic study on a non-cyclic arctic fox population. Oecologia 181(4):1129–1138. DOI:10.1007/s0044.
- Valdimarsson H, Malmberg S-A. 1999. Near-surface circulation in Icelandic waters derived from satellite tracked drifters. Rit Fiskideildar 16:23–39.
- von Schmalensee M. 2010. Vágestir í vistkerfum – Seinni hluti Framandi og ágengar tegundir á Íslandi. Natt ´urufræðingurinn 80:84–102.
- von Schmalensee M, Stefánsson RA. 2017. Invasive alien species in Iceland – Overview, management and public influence. In: Skorupski J, editor. Book of abstracts of the “science in the service of nature – Focus on the conservation genetics and combating invasive alien species. Szczecin, Poland: University of Szczecin. p. 25.
- Wetang’ula GN. 2004. Assessment of geothermal wastewater disposal effects case studies: Nesjavellir (Iceland) and Olkaria (Kenya) fields. Reykjavík, Iceland: University of Iceland.
- WHO. 1991. Nickel. Environmental health criteria 108. Geneva: World Health Organisation.
- WHO. 2000. WHO air quality guidelines for Europe. 2nd ed. Geneva: World Health Organisation.
- Wren CD, Fischer KL, Stokes PM. 1988. Levels of lead, cadmium and other elements in mink and otter from Ontario, Canada. Environmental Pollution 52(3):193–202. DOI:10.1016/0269-7491(88)90003-6.