Abstract
Acorn ants mostly inhabit cavities in fallen twigs and hollow acorns. Such places, e.g., dead wood, provide an attractive living resource for many groups of microorganisms, like fungi and bacteria, which can be important for ants. However, during experiments in laboratories, acorn ant colonies are typically kept without dead wood. During laboratory experiments, the preferences of the ant Temnothorax crassispinus for nest sites with pieces of dead wood were checked, and whether the presence of such wood influenced productivity. In binary choice tests, colonies had to choose a nest site when presented with two potential nest sites, one empty, or two cavities with different contents. The ant colonies preferred nest cavities with pieces of dead wood versus empty nest cavities. When cavities were filled with pieces of wood or with pieces of wood previously sterilized (72 hours, 70°C), colonies preferred the unsterilized zones. During a three-month laboratory experiment, colonies were kept in the Petri dishes containing pieces of dead wood, containing “sterilised” pieces of dead wood, or without access to wood. In the experiment, no influence was discovered on the availability of dead wood in either colony growth or colony per capita productivity. Thus, the ants prefer cavities with pieces of wood, but the lack of availability of dead wood during a multi-month experiment has no influence on life history parameters.
Introduction
Wood is an important habitat for many species including ants, termites, beetles, and many other arthropods (Ulyshen Citation2016; King et al. Citation2018; Ulyshen & Sobotnik Citation2018). Wood – both living and dead – can provide suitable places for nest sites (Blüthgen & Feldhaar Citation2010; King et al. Citation2018; Ulyshen & Sobotnik Citation2018; Fernandes et al. Citation2020), and is a source of food, e.g., for some termites and beetles (Ulyshen Citation2016; Ulyshen & Sobotnik Citation2018). Decaying wood is also a good habitat for microorganisms such as fungi and bacteria (Ulyshen Citation2016; Ulyshen & Sobotnik Citation2018; Lupala et al. Citation2019). Microbes can affect wood decomposition (Ulyshen Citation2016; Johnston et al. Citation2016; King et al. Citation2018), wood structure, and thus (indirectly) invertebrates. But the presence of pathogenic microbes can also be a challenge for animals (Dillon & Dillon Citation2004), especially for social insects due to their high densities and frequent interactions (Cremer et al. Citation2007; Liu et al. Citation2019). Therefore, social insects can produce antimicrobial compounds to kill microorganisms or to slow microbial growth (Stow et al. Citation2007; Hoggard et al. Citation2011; Tragust Citation2016; Pereira & Detrain Citation2020), or they can avoid habitats rich with microorganisms (Karlik et al. Citation2016). But microorganisms and animal-bacterial interactions are also important for the biology of animals.
Well known are the mutualistic interactions between ants of the tribe Attini, fungi, and bacteria. These ants obligatory depend on the cultivation of fungus for food, and both ants and fungi depend on mutualistic actinomycete bacteria: the bacteria produce antibiotics that suppress the growth of fungal pathogen, and may protect ants from pathogens (Currie Citation2001; Mehdiabadi & Schultz Citation2010; Ness et al. Citation2010). Furthermore, many microorganisms live in the guts of animals, and gut microbiota affects host physiology and the behaviour of animals (e.g., Ezenwa et al. Citation2012; Engel & Moran Citation2013; McFall-Ngai et al. Citation2013; Teseo et al. Citation2019). This is also true also for ants. For example, it was shown that in the leaf-cutting ants Acromyrmex echinatior, microbiota affects cuticular chemical profiles and thus social interactions (Teseo et al. Citation2019). Gut microbiota can vary among colonies and sampling groups. Also, seasons (Segers et al. Citation2019) and diet changes can result in the remodelling of the gut microbiota (McFall-Ngai et al. Citation2013; Ibarra-Juarez et al. Citation2018; Obadia et al. Citation2018; Teseo et al. Citation2019). It has been shown that colonies of the acorn ant Temnothorax nylanderi, having more diverse gut communities, produce more brood, and relocation from the field to the laboratory changes gut microbiota (Segers et al. Citation2019).
Acorn ants from the genus Temnothorax dwell in pre-existing cavities in wood and acorns (Foitzik et al. Citation2004; Seifert Citation2007; Czechowski et al. Citation2012). They belong to the myrmicine tribe Formicoxenini. Their colonies are small – between several dozen to several hundred individuals. Because of colony size and well-established protocols for rearing them in the laboratory, these ants are frequently used to study life history evolution in social insects (cf. Heinze Citation2006). In laboratory experiments, the ant colonies are typically kept in nests made of plastic, glass and paper. These are kept in plastic Petri dishes, with gypsum base (see, e.g., Modlmeier et al. Citation2013; Lichtenstein et al. Citation2016; Mitrus Citation2016). Such conditions – lacking microbes, which could be potentially harmful – can be favourable for ants. However, dead wood is probably a preferred nesting substrate. If pieces of wood are available in an arena, workers often walk on such pieces of dead wood, moving these pieces, and ant colonies can also relocate from artificial nest sites to cavities in pieces of wood in an arena (S. Mitrus, personal observations). Nevertheless, they prefer bigger nest cavities (Mitrus Citation2015), the ants can relocate to much smaller cavities available to pieces of wood left on the area (S. Mitrus, personal observations). For short-term laboratory experiments, the lack of wood does not influence the results, but sometimes experiments are of greater duration. Thus, for the invertebrates typically connected with habitats rich with dead wood, access to wood can be an important factor influencing fitness.
The objective of this study was to determine whether access to dead wood impacts colonies of the acorn ant Temnothorax crassispinus (Karawajew, 1926). This is a widely distributed species, one that typically inhabits cavities in dead wood and acorns where larvae of other insects have bored cavities. This study posed the following questions: (1) Do the ants prefer nest sites containing dead wood? (2) Does the presence of dead wood influence productivity of colonies? For this study, laboratory experiments were performed including preference tests (i.e., binary choice tests) and a multi-month laboratory experiment.
Materials and methods
This study was performed using acorn ant Temnothorax crassispinus colonies. The ant is present throughout Central and Eastern Europe, and it is widely distributed in Poland. Colonies are small, typically numbering from a few dozen to about 200 workers. The ant lives in light coniferous and mixed forests (Seifert Citation2007; Czechowski et al. Citation2012). Their nests are located in the litter layer, mostly in cavities in acorns and small fallen sticks (Seifert Citation2007; Białas et al. Citation2011; Czechowski et al. Citation2012). Workers thus have permanent contact with dead wood: just inside nest sites, as well as during foraging.
Preference tests
During the study, three binary choice tests were conducted. During these tests, each ant colony was placed in a square Petri dish (10.2 cm × 10.2 cm × 1.9 cm) having a thin plaster base, and two artificial nest sites placed on top. This artificial nest site was a cavity between a piece of cardboard and one-half microscope slide, separated by a Plexiglas frame (3 mm thick), and coated with a piece of a red translucent filter (). The volume of the cavity was approximately 850 mm3. These ants prefer cavities with narrower entrances (Pratt & Pierce Citation2001; Franks et al. Citation2003; Pratt Citation2010); thus, a small piece of Plexiglas was placed at the entrance. During the preference tests, the distances between entries to the nest were approximately 1.5 cm (see ). The Petri dishes with ant colonies were kept in a thermostatic cabinet. A daily cycle of 12 h: 12 h (light, 20°C: dark, 10°C) was maintained throughout the tests, the same as the artificial spring conditions previously used in experiments on T. nylanderi (Foitzik, Strätz, and Heinze Citation2003).
Figure 1. Scheme of preference tests. Two artificial nests were placed in a square Petri dish: one empty, or two cavities with different contents (see Materials and methods section for details of the three preference tests). Colonies of acorn ant Temnothorax crassispinus were released close to the opposite edge of the Petri dish. On the right nest design used during the tests: a cavity between a piece of cardboard and half of a microscope slide (38 mm × 26 mm) kept apart by a Plexiglas frame (3 mm thick), coated with a piece of a red translucent filter. A small piece of Plexiglas reduced the entrance size, as cavities with narrower entrances are inhabited faster
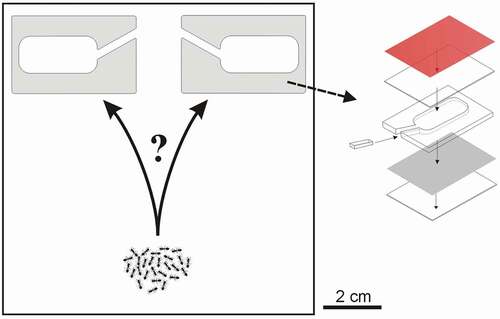
Each ant colony was placed into a separate Petri dish, at a similar distance (approximately 7 cm) from the entrance to two artificial nest cavities (). The position (i.e., left or right) of the nest cavities with different contents (see below) was systematically varied to eliminate any chance of directional biases (see, e.g., Hunt et al. Citation2014). Ant colonies were randomly assigned to these Petri dishes. After 48 hours, it was noted which nest cavity was inhabited by an ant colony.
Preference test 1: empty cavity vs. cavity with pieces of wood
For the test, 28 colonies were used: 13 queenless; 14 containing one queen, and one with two queens (workers: 10–57, mean 28.4, median: 26; brood was present in each colony). Nest sites with ant colonies were collected on 8 May 2018 near Opole, Poland. The sites were opened in the laboratory, and ants were captured with an aspirator, then counted. The colonies were transferred to Petri dishes with artificial nest cavities. Each Petri dish contained two artificial nest cavities: one of the cavities was empty, the other contained approximately 0.5 ml small pieces of fresh wood made of dry twigs without ant colonies. The twigs were collected in the forest, near the area where nest sites with ant colonies were collected. Prior to the experiment, the pieces of wood and open artificial nest cavities were kept in the laboratory at room temperature. The humidity and temperature of the nest sites – with and without pieces of wood – were thus similar.
Preference test 2: empty cavity vs. cavity with sand
A previous field study showed that Temnothorax ant colonies prefer larger nest cavities (Mitrus Citation2015). However, the situation could be more complicated, and colonies can prefer medium to small or small to extra-large nest sites (cf. Franks et al. Citation2006). Pieces of wood inside one cavity (see above: Preference test 1) reduced the volume available for ants. What is more, the Temnothorax ant is able to decrease entrances using soil and leaf litter (Pratt & Pierce Citation2001; Mitrus Citation2019). Thus, the volume of the cavity, as well as available material inside the cavity, can be important for ants and may affect nest site preference.
During the preference test, it was desirable to check if the presence of material filling the cavity (thus reducing the volume) affected the nest choice made by ant colonies. The design of the test was similar to Preference test 1. One cavity was empty, while the second contained 0.5 ml material; however, in this preference test, the cavities contained no pieces of wood like in the preference test 1, but grains of sand (size of grains: 0.4–0.8 mm). A total of 29 ant colonies were used. They were collected on 15 May 2018: 4 queenless and 25 containing one queen (workers: 11–58, mean 30.4, median: 28; brood was present in each colony).
Preference test 3: cavity with pieces of “fresh” wood vs. cavity with pieces of “sterilized” wood
Dead wood contains microflora, thus preference nest sites containing dead wood could be the result of the presence of wood or/and the presence of microorganisms connected with dead wood. In the test, the choice was given between cavities containing dead wood versus cavities with dead wood previously “sterilised”. First, in the forest near the area where nest sites with ant colonies were collected, dry twigs without ant colonies were collected, then made into small pieces, and finally, the pieces were mixed. Next, half the pieces were “sterilised” – kept for 72 hours at 70°C, so most microorganisms were probably killed. The wood – both “sterilised” and “non-sterilised” – was then kept at room temperature; next, approximately 0.5 ml of pieces of wood were placed into each nest cavity. Thus, the humidity and temperature of the nest sites were similar. Ant colonies thus had the ability to choose a cavity with either 0.5 ml of pieces of dead wood or 0.5 ml pieces of dead wood previously “sterilised”. During the preference test, 30 colonies of the ant (collected on 15 May 2018) were used: 11 queenless, 18 containing one queen and one with three queens (workers: 11–56, mean 30.0, median: 27.5; brood was present in each colony).
Long-term experiment: rearing ant colonies with dead wood
In laboratory experiments, acorn ant colonies are typically kept in Petri dishes with a plaster base and artificial nest sites made of plastic, paper and glass (e.g., Modlmeier et al. Citation2013; Lichtenstein et al. Citation2016; Mitrus Citation2016). Such an environment probably lacks microorganisms; however, under natural conditions, they are typically part of an ant’s environment.
For the experiment, the effect of the presence of dead wood on a colony’s development was analysed. On 10 and 17 April 2018, near Opole, 82 nest sites with ant colonies containing workers and brood were collected: 15 queenless, 66 with one queen, and one with two queens (workers: 9–220, median 59.5). They were transferred to Petri dishes containing artificial nest sites. An artificial nest site was a cavity between a piece of cardboard and microscope slide, separated by a Plexiglas frame (3 mm thick), and coated with a piece of a red translucent filter (). The volume of the cavity was approximately 1760 mm3; there was a small piece of Plexiglas in the entrance (see ). After acclimatisation to artificial conditions, 57 colonies were chosen on 24 April 2018 for the experiment: each contained one queen, 21–217 workers and brood (similar number of brood items to number of workers). The 57 colonies were randomly divided into three groups:
Figure 2. Scheme of the long-term experiment: rearing ant colonies with dead wood. Acorn ant Temnothorax crassispinus colonies were reared in square Petri dishes. Colonies were randomly divided into three groups: (a) the “fresh wood group” = the Petri dishes contained several pieces of “fresh” dead wood (in total approximately 1.5 g, changed every 3.5 weeks); (b) the “sterilised wood group” = the Petri dishes contained several pieces of dead wood (in total approximately 1.5 g, changed every 3.5 weeks. However, the pieces of wood were previously “sterilised”, i.e., previously kept 72 hours, at 70°C). Finally, (c) the “control group” (with no wood pieces in the Petri dishes). After three months, worker mortality, number of workers (% of the initial number), and productivity per capita, in relation to experimental treatments were analysed. Above left, nest design used during the experiment: a cavity between a piece of cardboard and a microscope slide (76 mm × 26 mm) kept apart by a Plexiglas frame (3 mm thick), coated with a piece of a red translucent filter
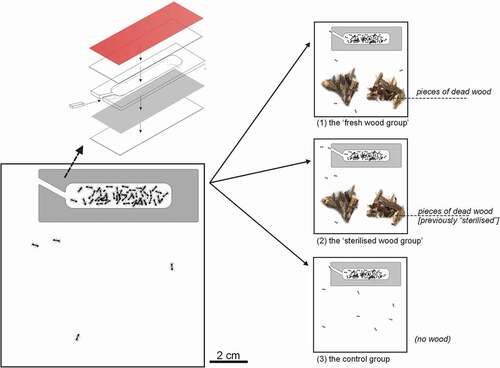
the “fresh wood group” = the Petri dishes additionally contained several pieces of “fresh” dead wood (in total, approximately 1.5 g);
the “sterilised wood group” = the Petri dishes additionally contained several pieces of dead wood (in total, approximately 1.5 g); however, the pieces of wood were previously “sterilised” (i.e., kept 72 hours, at 70°C);
the “control group” (with no wood pieces in the Petri dishes).
For the experiment, wood in the forest was collected. First, dry twigs without ant colonies were collected. Next, in the laboratory, the collected wood was divided into portions containing several pieces of wood approximately 1.5 g (very small sticks collected in the forest, or part of bigger sticks). The portions were then randomly divided into two groups, and one was “sterilised” – kept 72 hours, at 70°C, so most microorganisms were probably killed. The wood was added to Petri dishes with the first two groups. The wood pieces were added on 24 April and then changed approximately every 3.5 weeks (i.e., three times: on 14 May, 7 June, and 25 June). Each time, new wood pieces were collected in the forest several days earlier. The wood was exchanged, as sometimes when such wood pieces are kept in Petri dishes for several weeks, they are covered with mould.
The Petri dishes with colonies were kept in a thermostatic cabinet maintaining a daily cycle of 12: 12 h (light, 20°C: dark, 10°C) starting on 10 April to 20 June, and 14:10 h (light, 27°C: dark, 17°C) for the second part of the experiment, i.e., starting 21 June. This set up the conditions used in previous experiments mimicking spring and summer conditions for T. nylanderi and T. crassispinus ants (Foitzik et al. Citation2003; Mitrus Citation2015). Ants were fed twice a week: once with frozen Dubia roach (Blaptica dubia; length: approximately 12–15 mm) and honey; the next time using approximately 3 mm × 3 mm × 3 mm jelly-like food prepared according to the Bhatkar diet (Hölldobler & Wilson Citation1990). The ants were provided ad libitum water. During feeding, dead workers were collected and counted.
On 23 July, the final number of workers in each colony and the number of sexual individuals produced by each colony were counted. The first males and young queens were observed on, respectively, 9 and 13 July. On 19 July there were no more pupae of sexual individuals in nests, and the experiment was completed.
Data analysis
A chi-square test was performed on the data obtained in the binary choice tests; split colonies were not included in the analyses. For the data obtained during the long-term experiment, the general linear models were used to determine whether worker mortality, number of workers (% of the initial number), and productivity per capita were impacted by treatments. The GLM models included the initial number of workers in a colony (i.e., number of workers during beginning of the experiment) as a continuous predictor. Skewed data (the initial number of workers in a colony, the increase of number of workers) were log-transformed; for data on worker mortality, for transformation, the log10(x + 1) function was used.
In order to estimate the cost of production of sexual individuals, and thus per capita productivity of any colony, the number of produced males and females and adopted literature data for the ant T. nylanderi (a sibling species of T. crassispinus) were used. According to the data, the dry mass and hence the cost of a young queen being produced are 3.02 times higher than that of a male; the dry mass of a worker does not differ from male dry mass (Foitzik & Heinze Citation2000; Foitzik et al. Citation2003). Thus, the relative cost of production of individuals by colony was calculated as: number of produced females × 3.02 + number of produced males + number of new workers (or, for the sexual individuals: number of produced females × 3.02 + number of produced males).
For the long-term experiment, one colony (from the “fresh wood group”) was removed from analysis because of a mistake during the laboratory procedures. All statistical analyses were conducted using the software package Statistica, ver. 13 (Dell Inc Citation2016). All probability values shown are two-tailed.
Results
Preference test 1: empty cavity vs. cavity with pieces of wood
After 48 hours, all colonies used during the experiment inhabited the artificial nest cavities: 25 colonies inhabited one cavity, while three colonies split into two available cavities. Colonies preferred cavities containing pieces of dead wood: 19 colonies chose cavities with wood; 6, empty cavities (χ2 = 6.76, df = 1, p = 0.0093; analyse using the 25 colonies which chose one cavity). One of the colonies removed all pieces of wood from the cavity; nine colonies removed the majority of them; the next three colonies, part of the pieces previously placed into the cavities.
Preference test 2: empty cavity vs. cavity with sand
After 48 hours, 27 of the 29 colonies used in the experiment inhabited nest cavities. A similar number of colonies chose an empty cavity (12) and cavities with grains of sand (11) (χ2 = 0.043, df = 1, p = 0.83). Workers of four colonies split between two available cavities, and ants from next two colonies stayed out of the nest cavities. A total of five of the 11 colonies that chose cavities with sand removed part of the material. They only removed a small volume of the material filling the cavity: two colonies removed two and three grains of sand, respectively; next one a dozen or so grains of sand; the other two colonies removed more, but definitely less than a half of the volume.
Preference test 3: cavity with pieces of “fresh” wood vs. cavity with pieces of “sterilized” wood
After 48 hours, 25 of the 30 colonies used in the preference test inhabited nest cavities. There was weak evidence that colonies may prefer cavities with pieces of “fresh” dead wood (17) over cavities with pieces of dead wood previously “sterilised” (8) (χ2 = 3.24, df = 1, p = 0.072). Workers of the remaining five colonies split between the two available nest cavities. Most colonies (14 of 17 that chose a cavity with “fresh” dead wood; 6 of 8 that chose a cavity with previously “sterilised” dead wood) removed all or a significant part of pieces of wood.
Long-term experiment: rearing ant colonies with dead wood
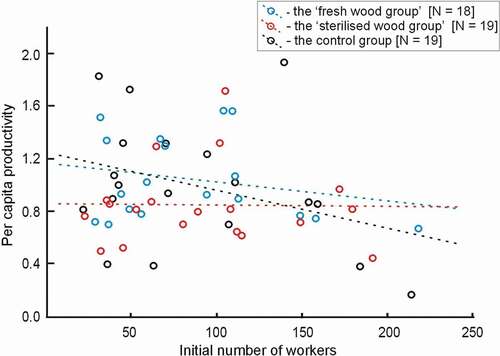
During the experiment, the number of workers in colonies increased from 0 to 218 (0–183% of the initial number of workers). There was no difference in the percentage increase in colony size between the three groups (GLM: F2,52 = 1.36, p = 0.27). However, the growth rate was affected by the initial number of workers (GLM: F1,52 = 6.18, p = 0.016) – the percentage increase of smaller colonies was bigger. Per capita productivity was independent of the experimental group (GLM: F2,52 = 1.13, p = 0.33), and the initial size of the colony was not influenced per capita productivity (GLM: F1,52 = 1.18, p = 0.28) ().
Figure 3. Per capita productivity of acorn ant Temnothorax crassispinus colonies reared in laboratory conditions in relation to the initial number of workers. During a three-month experiment, colonies of the ant were reared in three different regimes: (a) the “fresh wood group” = the Petri dishes contained several pieces of “fresh” dead wood (in total approximately 1.5 g, changed every 3.5 weeks); (b) the “sterilised wood group” = the Petri dishes contained several pieces of dead wood (in total approximately 1.5 g, changed every 3.5 weeks. However, the pieces of wood were previously “sterilised”, i.e., previously kept 72 hours, at 70°C). Finally, (c) the “control group” (with no wood pieces in the Petri dishes). There were no differences in per capita productivity between the three groups (p = 0.33); per capita productivity was no affected by the initial number of workers (p = 0.28)
Colonies produced 0–70 (median 5.5) sexual individuals; 37 of 58 colonies produced at least one sexual individual. Most of the colonies (31 of 37) produced males only. From each of the three experimental groups, two colonies produced only or also queens. For the colonies which produced at least one sexual, per capita productivity of sexual individuals was similar for the three groups (GLM: F2,33 = 0.05, p = 0.95); however, it was affected by the initial number of workers (GLM: F1,33 = 5.30, p = 0.028) – per capita production of sexuals was greater for larger colonies.
During the experiment, 0–9 dead workers per colony were found: 0–6 (median: 1.5) for “the fresh wood group”; 0–6 (median: 1) for the “sterilised wood group”; and 0–9 (median: 4) for the “control group”. The number of found dead workers was larger for the control group (GLM: F2,52 = 4.50, p = 0.016), and for larger colonies more dead individuals were found (GLM: F1,52 = 20.48, p < 0.0001).
Discussion
The results of the study show that the acorn ant Temnothorax crassispinus colonies – an ant typically inhabiting cavities in dead wood and empty acorns – prefer artificial cavities containing dead wood to empty nest cavities (see: Preference test 1). It is interesting that colonies chose cavities with pieces of wood, but most of the colonies then removed all or a significant part of the pieces of wood. Similar behaviour for the same species was reported earlier (Mitrus Citation2019). The volume of cavity is important for the ant (cf. Mitrus Citation2015), but during this study colonies more frequently chose cavities with pieces of wood (= with smaller volume), and then enlarged the cavities (Preference test 1). Additionally, they presented no preference when one cavity was partially filled with sand (see: Preference test 2). Thus, the volume of the cavity available for the ants was enough for colonies. The results of these preference tests suggest that dead wood – or/and the microorganisms connected with dead wood – are important for the ant. However, the results fail to explain why the ant chooses cavities with pieces of wood. The choice could be, for example, an effect of the odour of the wood pieces, or the presence of microorganisms on the surface of the wood, but also could be the effect of the avoidance of “sterile” artificial nest sites made of glass, plastic and paper. For another preference test (see: Preference test 3), the colonies presented a trend towards choosing a cavity with “fresh” dead wood to cavity with dead wood previously “sterilised”. Again, although it suggests that dead wood is important for acorn ants, it is not possible based on the results to say whether – for example – they prefer wood with living microorganisms, or just avoid the wood with dead microorganism (= killed using 70°C). However, a direct factor affecting the choice could be another factor, e.g., smell, as the odour of the “sterilised” wood probably differs from the “fresh” wood.
The results of the three-month experiment showed no effect of wood availability on either colony size development (i.e., the percentage increase in colony size) or on per capita productivity. Although in many ant species per-capita productivity declines in larger colonies (see review in Kramer et al. Citation2014), no such relation was observed in this experiment; this result is in accordance with previous data for T. crassispinus (cf. Kramer et al. Citation2014; Mitrus Citation2015, Citation2016). Studies by Segers et al. (Citation2019) showed a relationship between a colony’s productivity and diversity of its gut fauna, and that the gut microbiota of insects in laboratory conditions differs to microbiota of insects under natural conditions.
During the experiment, the largest mortality of workers was observed for the control group (for colonies without access to dead wood), but for all groups mortality in the laboratory was very low. It is difficult to explain this difference. What is more, under natural conditions, mortality (e.g., caused by predation or difficult environmental conditions) may be much higher, but no data on the subject are available.
Wood is an important habitat for many animals including invertebrates, and dead wood is rich in microorganisms (e.g., Ulyshen Citation2016; Ulyshen & Sobotnik Citation2018; Lupala et al. Citation2019). Karlik et al. (Citation2016) demonstrated that T. curvispinosus colonies avoided the part of the nest (acorns) with microbes. During their experiment, they used cultures mostly of mouldy fungi (Karlik et al. Citation2016), but during this research, pieces of dead wood were changed every 3.5 weeks to avoid covering the wood by mould; however, mould could be present inside the wood pieces. Avoidance of areas rich in pathogenic microorganisms such as fungi could be favourable for ants. It is probable that for animals typically using dead wood for nesting, a lack of access to such material can affect gut microbiota, and consequently behaviour and fitness. However, there is a lack of data if such habitat (i.e., dwelling in dead wood or access to such wood) directly alters the composition of the microbiota of ants. What is more, it is possible that the gut microbiota of the ants from the three experimental groups in the experiment was similar, as, e.g., physical interaction between workers (e.g., trophallaxis – the exchange of food between two individuals) and brood care provides opportunities to transform bacteria and other microorganisms between individuals, also newly emerged (Engel & Moran Citation2013). The gut microbiota can affect the brood production, and so influence fitness (see: Segers et al. Citation2019). It is possible that lack of access to dead wood can be visible after longer periods (the long-term experiment in the study only lasts about 3 months). Nevertheless, for longer laboratory experiments, the diet in the artificial conditions can have larger effect on the microbiota.
The natural habitat of any animal species is quite different from laboratory conditions used during experiments. This difference could affect results and thus conclusions. For example, laboratory studies are often conducted using constant temperatures, while in nature, temperature typically shows daily fluctuations, and using constant temperature regimes significantly influences results (e.g., Fischer et al. Citation2011; Sprenger et al. Citation2018). Of course, the stress of collection, handling, and transport could also influence a behaviour (cf. Lichtenstein et al. Citation2016). Understanding factors which significantly affect biology of different species is essential in preparing appropriate laboratory conditions for experiments. The significance the dead wood for acorn ants is still unsatisfactory.
Conclusion
The differences between natural and artificial conditions, and their possible effects on results, should be taken into consideration, especially during long-term laboratory experiments. This study shows that the lack of access to dead wood during a multi-month laboratory experiment using colonies of the acorn ant Temnothorax crassispinus – whose colonies typically inhabit cavities in decaying wood and empty acorns – has no influence on life history parameters. However, the ant colonies showed a preference for nest cavities with pieces of dead wood, and dead wood looks attractive for workers. Thus, the significance of wood for the acorn ants should be explained.
Acknowledgements
I wish to thank anonymous reviewers for helpful comments on the previous version of the manuscript. The study was supported by an internal grant (No. 2018/18-13-108) from the Institute of Biology, University of Opole.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Białas B, Granieczny P, Pędzisz A, Mitrus S. 2011. Colony size, density and type of nesting sites of the ant Temnothorax crassispinus (Hymenoptera: Formicidae). Opole Scientific Society Nature Journal 44:185–191.
- Blüthgen N, Feldhaar H. 2010. Food and shelter: How resources influence ant ecology. In: Lach L, Parr CL, Abbott KL, editors. Ant ecology. Oxford, UK: Oxford University Press. pp. 115–136.
- Cremer S, Armitage SAO, Schmid-Hempel P. 2007. Social immunity. Current Biology 17(16):R693–R702. DOI: 10.1016/j.cub.2007.06.008.
- Currie CR. 2001. A community of ants, fungi, and bacteria: A multilateral approach to studying symbiosis. Annual Review of Microbiology 55(1):357–380. DOI: 10.1146/annurev.micro.55.1.357.
- Czechowski W, Radchenko A, Czechowska W, Vepsäläinen K. 2012. The ants of Poland with reference to the myrmecofauna of Europe. Warszawa: Museum and Institute of Zoology of the Polish Academy of Sciences, Natura optima dux Foundation.
- Dell Inc. 2016. Dell Statistica (data analysis software system), version 13. software.dell.com. Round Rock (TX): Dell Inc.
- Dillon RJ, Dillon VM. 2004. The gut bacteria of insects: Nonpathogenic interactions. Annual Review of Entomology 49(1):71–92. DOI: 10.1146/annurev.ento.49.061802.123416.
- Engel P, Moran NA. 2013. The gut microbiota of insects - diversity in structure and function. Fems Microbiology Reviews 37(5):699–735. DOI: 10.1111/1574-6976.12025.
- Ezenwa VO, Gerardo NM, Inouye DW, Medina M, Xavier JB. 2012. Animal behavior and the microbiome. Science 338(6104):198–199. DOI: 10.1126/science.1227412.
- Fernandes TT, Silva RR, Souza-Campana DR, Silva NS, Silva OGM, Morini MSC. 2020. Occurrence of Ants (Hymenoptera: Formicidae) in both Leaf Litter and Twigs in Atlantic Forest. Sociobiology 67(2):163–172. DOI: 10.13102/sociobiology.v67i2.4504.
- Fischer K, Kolzow N, Holtje H, Karl I. 2011. Assay conditions in laboratory experiments: Is the use of constant rather than fluctuating temperatures justified when investigating temperature-induced plasticity? Oecologia 166(1):23–33. DOI: 10.1007/s00442-011-1917-0.
- Foitzik S, Backus VL, Trindl A, Herbers JM. 2004. Ecology of Leptothorax ants: Impact of food, nest sites, and social parasites. Behavioral Ecology and Sociobiology 55(5):484–493. DOI: 10.1007/s00265-003-0718-9.
- Foitzik S, Heinze J. 2000. Intraspecific parasitism and split sex ratios in a monogynous and monandrous ant (Leptothorax nylanderi). Behavioral Ecology and Sociobiology 47(6):424–431. DOI: 10.1007/s002650050686.
- Foitzik S, Strätz M, Heinze J. 2003. Ecology, life history and resource allocation in the ant, Leptothorax nylanderi. Journal of Evolutionary Biology 16(4):670–680. DOI: 10.1046/j.1420-9101.2003.00562.x.
- Franks NR, Dornhaus A, Best CS, Jones EL. 2006. Decision making by small and large house-hunting ant colonies: One size fits all. Animal Behaviour 72(3):611–616. DOI: 10.1016/j.anbehav.2005.11.019.
- Franks NR, Mallon EB, Bray HE, Hamilton MJ, Mischler TC. 2003. Strategies for choosing between alternatives with different attributes: Exemplified by house-hunting ants. Animal Behaviour 65(1):215–223. DOI: 10.1006/anbe.2002.2032.
- Heinze J. 2006. Life in a nutshell - social evolution in formicoxenine ants. In: Kipyatkov VE, editor. Life cycles in social insects: Behaviour, ecology and evolution. St. Petersburg: St. Petersburg University Press. pp. 49–61.
- Hoggard SJ, Wilson PD, Beattie AJ, Stow AJ. 2011. Social complexity and nesting habits are factors in the evolution of antimicrobial defences in Wasps. PLoS One 6(7):7. DOI: 10.1371/journal.pone.0021763.
- Hölldobler B, Wilson EO. 1990. The ants. Cambridge, Massachusetts: Harvard University Press.
- Hunt ER, O’Shea-Wheller T, Albery GF, Bridger TH, Gumn M, Franks NR. 2014. Ants show a leftward turning bias when exploring unknown nest sites. Biology Letters 10(12):20140945. DOI:10.1098/rsbl.2014.0945.
- Ibarra-Juarez LA, Desgarennes D, Vazquez-Rosas-Landa M, Villafan E, Alonso-Sanchez A, Ferrera-Rodriguez O, Moya A, et al. 2018. Impact of rearing conditions on the Ambrosia Beetle’s microbiome. Life-Basel 8(4). DOI: 10.3390/life8040063.
- Johnston SR, Boddy L, Weightman AJ. 2016. Bacteria in decomposing wood and their interactions with wood-decay fungi. Fems Microbiology Ecology 92(11):fiw179. DOI:10.1093/femsec/fiw179.
- Karlik J, Epps MJ, Dunn RR, Penick CA. 2016. Life inside an acorn: How microclimate and microbes influence nest organization in Temnothorax Ants. Ethology 122(10):790–797. DOI: 10.1111/eth.12525.
- King JR, Warren RJ, Maynard DS, Bradford MA. 2018. Ants: Ecology and impacts in dead wood. In: Ulyshen MD, editor. Saproxylic insects: Diversity, ecology and conservation. Cham: Springer. pp. 237–262.
- Kramer BH, Scharf I, Foitzik S. 2014. The role of per-capita productivity in the evolution of small colony sizes in ants. Behavioral Ecology and Sociobiology 68(1):41–53. DOI: 10.1007/s00265-013-1620-8.
- Lichtenstein JLL, Pruitt JN, Modlmeier AP. 2016. Intraspecific variation in collective behaviors drives interspecific contests in acorn ants. Behavioral Ecology 27(2):553–559. DOI: 10.1093/beheco/arv188.
- Liu L, Zhao XY, Tang QB, Lei CL, Huang QY. 2019. The mechanisms of social immunity against fungal infections in eusocial insects. Toxins 11(5):244. DOI:10.3390/toxins11050244.
- Lupala AS, Oh SY, Park MS, Kim T, Yoo JS, Seelan JSS, Lim YW. 2019. Co-occurrence patterns of wood-decaying fungi and ants in dead pines of South Korea. Journal of Asia-Pacific Entomology 22(4):1154–1160. DOI: 10.1016/j.aspen.2019.10.009.
- McFall-Ngai M, Hadfield MG, Bosch TCG, Carey HV, Domazet-Loso T, Douglas AE, Dubilier N, et al. 2013. Animals in a bacterial world, a new imperative for the life sciences. Proceedings of the National Academy of Sciences of the United States of America 110(9):3229–3236. DOI: 10.1073/pnas.1218525110.
- Mehdiabadi NJ, Schultz TR. 2010. Natural history and phylogeny of the fungus-farming ants (Hymenoptera: Formicidae: Myrmicinae: Attini). Myrmecological News 13:37–55.
- Mitrus S. 2015. The cavity-nest ant Temnothorax crassispinus prefers larger nests. Insectes Sociaux 62(1):43–49. DOI: 10.1007/s00040-014-0372-4.
- Mitrus S. 2016. Emigration speed and the production of sexuals in colonies of the ant Temnothorax crassispinus under high and low levels of disturbance. Insectes Sociaux 63(1):127–134. DOI: 10.1007/s00040-015-0447-x.
- Mitrus S. 2019. Nest modifications by the acorn ant Temnothorax crassispinus (Hymenoptera: Formicidae). Myrmecological News 29:147–156. DOI: 10.25849/myrmecol.news_029:147.
- Modlmeier AP, Foitzik S, Scharf I. 2013. Starvation endurance in the ant Temnothorax nylanderi depends on group size, body size and access to larvae. Physiological Entomology 38(1):89–94. DOI: 10.1111/phen.12007.
- Ness J, Mooney K, Lach L. 2010. Ants as mutualists. In: Lach L, Parr CL, Abbott KL, editors. Ant ecology. Oxford, UK: Oxford University Press. pp. 97–114.
- Obadia B, Keebaugh ES, Yamada R, Ludington WB, Ja WW. 2018. Diet influences host-microbiota associations in Drosophila. Proceedings of the National Academy of Sciences of the United States of America 115(20):E4547–E8. DOI: 10.1073/pnas.1804948115.
- Pereira H, Detrain C. 2020. Pathogen avoidance and prey discrimination in ants. Royal Society Open Science 7(2):2. DOI: 10.1098/rsos.191705.
- Pratt SC. 2010. Nest Site Choice in Social Insects. In: Breed MD, Moore J, editors. Encyclopedia of animal behavior. Vol. 2. Oxford: Academic Press. pp. 534–540.
- Pratt SC, Pierce NE. 2001. The cavity-dwelling ant Leptothorax curvispinosus uses nest geometry to discriminate between potential homes. Animal Behaviour 62(2):281–287. DOI: 10.1006/anbe.2001.1777.
- Segers F, Kaltenpoth M, Foitzik S. 2019. Abdominal microbial communities in ants depend on colony membership rather than caste and are linked to colony productivity. Ecology and Evolution 9(23):13450–13467. DOI: 10.1002/ece3.5801.
- Seifert B. 2007. Die Ameisen Mittel- und Nordeuropas. Görlitz: Lutra.
- Sprenger PP, Burkert LH, Abou B, Federle W, Menzel F. 2018. Coping with the climate: Cuticular hydrocarbon acclimation of ants under constant and fluctuating conditions. Journal of Experimental Biology 221(9):9. DOI: 10.1242/jeb.171488.
- Stow A, Briscoe D, Gillings M, Holley M, Smith S, Leys R, Silberbauer T, Turnbull C, Beattie A. 2007. Antimicrobial defences increase with sociality in bees. Biology Letters 3(4):422–424. DOI: 10.1098/rsbl.2007.0178.
- Teseo S, van Zweden JS, Pontieri L, Kooij PW, Sorensen SJ, Wenseleers T, Poulsen M, Boomsma JJ, Sapountzis P. 2019. The scent of symbiosis: Gut bacteria may affect social interactions in leaf-cutting ants. Animal Behaviour 150:239–254. DOI: 10.1016/j.anbehav.2018.12.017.
- Tragust S. 2016. External immune defence in ant societies (Hymenoptera: Formicidae): The role of antimicrobial venom and metapleural gland secretion. Myrmecological News 23:119–128.
- Ulyshen MD. 2016. Wood decomposition as influenced by invertebrates. Biological Reviews 91(1):70–85. DOI: 10.1111/brv.12158.
- Ulyshen MD, Sobotnik J. 2018. An introduction to the diversity, ecology, and conservation of saproxylic insects. In: Ulyshen MD, editor. Saproxylic insects: Diversity, ecology and conservation. Cham: Springer. pp. 1–47.
