?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Mycoses of wild birds are rarely addressed in scientific research. We tested the hypothesis that urban populations of House Sparrow Passer domesticus, which in many cities showed downward trends in their number and are characterized by lower body mass and poorer body condition, would be more frequently colonized by fungi. To evaluate the degree of bird colonization by fungi, swabs were taken from the beak cavity and cloaca of birds from urban and rural populations in the breeding season. A high degree of bird colonization by fungi was determined in both types of habitats (urban: 86% of population; rural: 92% of population). In total, 26 species of fungi were isolated (urban: 15 species; rural: 22 species). No significant differences were determined between the number of fungi species identified in birds from urban and rural habitats and between the degree of colonization of the two ontocenoses (beak cavity and cloaca). In both environments, Candida krusei turned out to be the prevalent fungus, with a clear predominance in the urban population. The extent of ontocenoses colonization was higher in adult than in juvenile birds, which indicated that the colonization of ontocenoses progresses with age. A significant correlation was found between the presence of fungi, lower values body condition parameters from the urban population (F – degree of fat score, logBM – body mass logarithm, logT – tarsus length logarithm, BM/WL –body mass/wing length, logBM/logWL, BM/TL – body mass/tail length), physiological condition, and age of birds, which may suggest that adults birds with worse condition, and higher haemoglobin level were more often colonized by fungi. No important differences in the fungal colonization were detected between urban and rural areas, thus, the hypothesis that fungal infection may be the cause of decline of sparrows in urban habitats is still to be demonstrated.
Introduction
Birds are significantly more frequently colonized by fungi than other vertebrates, and thus become their carriers, natural reservoirs or active or passive vectors (Hubálek Citation2004; Cafarchia et al. Citation2006; Tsiodras et al. Citation2008; Biedunkiewicz et al. Citation2012; Dynowska et al. Citation2013, Citation2015; Meissner et al. Citation2015). Birds are particularly susceptible to colonization by fungi owing to anatomical and physiological determinants that promote their growth and development, including poorly vascularized air sacs, thin skin without sweat and sebaceous glands, very poor or even absent antimycotic activity in blood (Dahlhausen Citation2006). Fungi may colonize body coats of birds and its products (feathers and calloused parts) as well as internal organs by entering through the alimentary, respiratory or reproductive routes as well as damaged skin, mucosa, and cornea (Howard Citation2003; Reding Citation2003). The body temperature of birds, that ranges from 40 to 44°C, constitutes a physiological barrier that inhibits the growth and proliferation of most of the pathogenic fungi (Robert & Casadevall Citation2009). After infecting a macroorganism, the fungi – if not eliminated by non-specific and specific defense mechanisms – colonize the body of a host, thereby making it a carrier and entering into the commensal-type relation with it (Casadevall & Pirofski Citation2000). Colonization may proceed asymptomatically or transform into an infection, when biological homeostasis is disturbed between the fungi and the macroorganism (Casadevall & Pirofski Citation2000). Each disruption of this balance and dysfunctions of the immunological system predispose to the development of mycosis which is often fatal (Dynowska & Dynowski Citation1996; Casadevall & Pirofski Citation2000; Howard Citation2003). Disease symptoms were observed in captive (Wolff et al. Citation1992) and wild birds (Rippon et al. Citation2012) as well as in commercially-produced poultry (Khosravi et al. Citation2008). The incidence of mycoses is better recognized in commercially-produced birds, whereas knowledge about the prevalence of fungi and their effect on the general health status of wild bird populations is scarce. Only a few studies focused on fungi isolated from wild species, e.g. Black Stork Ciconia nigra (Dynowska & Dynowski Citation1996), Tree Swallow Tachycineta bicolor (Mills et al. Citation1999) or the genus Passer sp. (Pinowski et al. Citation1994; Kruszewicz et al. Citation1995; Stewart Citation2000). Authors of different works emphasize that microorganisms (viruses, bacteria, and fungi) and parasites can have a significant effect on the host’s condition, thereby becoming important selective forces in the evolution of many aspects of bird biology (Mills et al. Citation1999; Dolnik & Hoi Citation2010; González-Braojos et al. Citation2012; Dugas & Doumas Citation2014).
Until recently, House Sparrow was the dominant species in the urban avifauna (Dulisz & Nowakowski Citation1996; Kelcey & Rheinwald Citation2005), however, significant decreases were noted in its population since the 1970s in many European cities (Robinson et al. Citation2005; Kelcey & Rheinwald Citation2005; Węgrzynowicz Citation2013). Declines in House Sparrow population have also been observed in rural areas, but they were not as rapid as in city centers (De Laet & Summers-Smith Citation2007). The main causes for the downward trend in House Sparrow population size include factors related to urbanization pressure (Shaw et al. Citation2008), however, aspects of the ecological functioning of House Sparrow are still poorly recognized.
Investigations conducted so far on the condition of House Sparrow have demonstrated that these birds in heavily urbanized areas have lower body mass, shorter tarsus length and poorer body condition than those from rural areas (Liker et al. Citation2008; Seress et al. Citation2011; Dulisz et al. Citation2016). Smaller size or lower body condition of birds may be an adaptation to the urban environment (Witter & Cuthill Citation1993; Salleh Hudin et al. Citation2016). According to the Shochat credit card hypothesis (Shochat Citation2004), some species reach a high number in urban areas at the expense of body condition, because the city provides a constant supply of food, but does not provide good quality food (limited natural food resources). On the other hand, an inferior body condition may be the result of a compromise between the risk of predation and the risk of starvation (Lima Citation1986; McNamara Citation1987; Cresswell Citation2008). Avoiding predator at the cost of feeding time as an adaptation to the increased pressure of predation, in poor feeding habitats such as urban areas, may consequently lead to a decrease in the physiological condition of the organism and reduced adaptation to this environment, and ultimately to population size decrease (MacLeod et al. Citation2006, Citation2007). Andersson et al. (Citation2015) indicated that the availability of diet affected a number of physiological aspects, including oxidative stress, resistance to inflammation and cell membrane efficiency. A lower condition may be a risk factor, and according to the rule of necessary trade-offs, individuals displaying such a strategy may be more exposed to other factors that result from the multidimensional space of all features of the life history.
Considering the lower body condition of House Sparrow urban populations and the disturbed status of its population size, the following hypothesis was adopted in this study: birds with a poorer body condition will be more intensively colonized by fungi in urbanized areas, constituting qualitatively poorer habitats (Robinson et al. Citation2005; Donnelly & Marzluff Citation2006; Vangestel et al. Citation2010; Seress et al. Citation2012) with greater predatory pressure (Kübler et al. Citation2005; Nowakowski & Dulisz Citation2005; Rutz Citation2006; Chamberlain et al. Citation2009; Bell et al. Citation2010). This study was aimed at determining the degree of colonization of the beak cavity and cloaca of urban and rural House Sparrows by fungi in the breeding season, depending on the body and physiological condition of birds.
Materials and methods
Study area
Urbanized areas – the city of Olsztyn (88.33 km2, ca. 175,000 inhabitants) situated in the North Eastern Poland (53.46°N, 20.28°E) in the temperate climate with Atlantic and Baltic influences as well as lakeland climate characteristics (average annual temperature: +7.2°C; average for July: +17°C; average for January: –3.5°C; average annual precipitation: 642 mm). The birds were caught at three sites in residential areas with 15–45 years old apartment buildings of. The main abiotic elements of these residential areas were 5-store multi-family buildings and typical communication infrastructure (local roads, pavements and parking lots). Shopping and service centers and public facilities were sparse and isolated. Arranged green areas covered from 25 to 30% of the residential area. These high-rise residential areas were characterized by a relatively high population density (8,967.2 person/km2) and by the presence of many disposal sites of household wastes. Apart from the places where birds were fed by inhabitants of these residential areas, the latter were the main anthropogenic sources of feed for House Sparrows.
Rural areas – five villages (Szostaki, Wizna, Lisno, Bronowo, Kossaki-Falki) located in the rural landscape of the valleys of Biebrza and Narew rivers in north-eastern Poland (53.10°N, 22.36°E – 53.28°N, 22.45°E). The villages were situated 10 to 20 km from one another, and ca. 200 km away from the city of Olsztyn. The rural areas were also in the temperate climate with the influence of continental climate (average annual temperature: +6.5°C, average for July: +17°C, average for January: –4.2°C; average annual precipitation: 550 mm). The population size in these villages ranged from ca. 60 to ca. 4,150 inhabitants. The main source of income of village inhabitants was farming, hence the land development – apart from family houses – also included farm buildings with many cases of ancient wooden architecture. Open landscape areas were dominant around villages. The supply of anthropogenic resources of feed was lower compared to the urban area.
Methods of field experiments and study material
The birds were caught using MIST-NET ornithological nets (Japanese nylon nets) in the breeding season (May-July) of 2014. Fifty birds were caught from urban areas (Males: 20, Females: 5; juv.: 25) and another 50 from rural areas (Males: 21, Females: 17; juv.: 12). The study was conducted on populations nesting in buildings. No nestboxes for birds were present in both areas. The birds were individually ringed, their sex and age were determined, and their body fat composition was ranked on a scale from 0 to 5 according to the Operation Baltic methods (Busse Citation1990). Measurements of body mass, wing, alula, tail, tarsus length, Kipp’s distance and wing formula were taken. All measurements were conducted in accordance with the Operation Baltic methods (Busse Citation1990). Linear measurements of primaries, wings and tails were conducted using a ruler accurate to 1 mm. The length of the tarsus was measured with a MEASY 2000 caliper, accurate to 0.1 mm. Body mass was weighed on an electronic TANITA scale, accurate to 0.1 g. Glucose and blood haemoglobin level in all individuals was determined using a HemoCue Glucose 201+ and HemoCue Hb 201+ portable photometers. For this purpose, blood samples were collected into special cuvettes of 10 μl by wing vein venipuncture.
The studies were carried out in accordance with the approved guidelines and Polish national law. The experiments were carried out with the consent of the Local Ethical Committee for Animal Experiments in Olsztyn (Resolution No. 111/2010 of 29.09.2010).
Methods of mycological analyses
The biological material was sampled ex vivo from the beak cavity and cloaca of birds using a sterile cotton swabs, earlier immersed in a physiological salt. Cotton swabs with the collected materials were placed in a liquid Sabouraud and Czapek-Dox culture medium with chloramphenicol (0.1%) and gentamycin (0.025%). The samples were incubated at 25°C for 7 days, and then passaged onto the aforementioned solid culture media and incubated at 25, 37, and 40°C. Once fungal growth was observed, microcultures were established on Nickerson’s agar (yeast and yeast-like fungi), and microscopic imprint specimens were prepared (mold fungi). Taxonomic identification was performed with bioMérieux API-tests (API 20°C, API20°C AUX, APIZYM) and CHROM agar Candida media, applying the following keys: Barnett et al. (Citation2000), De Hoog et al. (Citation2000) and Kurtzman et al. (Citation2011). Works by Howard (Citation2003) and Reding (Citation2003) were used to characterize the fungi.
Statistical analyses
Characterization of birds’ body condition was based on several morphometric indices, as suggested by Labocha and Hayes (Citation2012). The following indices were used in order to create a bird’s body condition profile: F – degree of fat score; logBM – body mass logarithm; logT – tarsus length logarithm, BM/WL – body mass/wing length; logBM/logWL – body mass logarithm/wing length logarithm; BM/TL – body mass/tail length and BM/T – body mass/tarsus length.
The principal component analysis (PCA) was used to reduce the number of variables describing the condition of birds and detect regularities between variables. The results of the factor analysis were validated by Bartlett’s sphericity test (Bartlett Citation1954). The condition indices have been reduced to three principal component factors. The number of distinguished factors was based on the combined criteria of Kaiser (Citation1958) and Cattell (Citation1966). PC1 was mainly loaded by: logBM – body mass logarithm (factor coordinate = 0.953), BM/WL – body mass/wing length (0.984); logBM/logWL – body mass logarithm/wing length logarithm (0.989), BM/T – body mass/tarsus length (0.843) and BM/TL – body mass/tail length (0.824). PC2 was mainly loaded by logT – tarsus length logarithm (0.951) and partially by BM/T – body mass/tarsus length (−0.434). PC3 was loaded by F – Fat score (−0.903). The factor loadings of principal components were used to describe the condition of individual birds, because the result of the principal components analysis was the classification of objects in new spaces defined by the created factors.
Differences in the probabilities of colonization by fungi (binomial dependent variable: 1 – Yes, 0 – No) depending on the nominal variables (age, population – urban and rural), metric variables – factor loadings of PC1, PC2 and PC3 characterizing bird’s body condition and physiological features (glucose and haemoglobin level) were analyzed by the General Linear Models (GLMs) using Logit link functions (McCullagh & Nelder Citation1989), and searching for the most-fitting model using Akaike’s information criterion (AIC) (IBM Citation2017).
The relative risk (RR) and 95% confidence intervals of the likelihood of event occurrence (colonization by fungi) were estimated in the group of birds from urban areas, compared to those from rural areas, in the group of males vs. females, and in the group of adult vs. juvenile birds. This method is typical for the epidemiological studies and assesses the risk of infection in relation to the exposed population (Riffenburgh Citation2006).
Relative risk (RR – relative risk) is the ratio of the probability of a given effect in two groups
where: Nyes – number of birds with a positive test for fungal availability, Ntotal – total number of individuals in the studied population, indices a and b – compared populations.
The odds ratio (OR) coefficient commonly used in epidemiological studies (Riffenburgh Citation2006) was calculated as a chance of an event occurring in one of the group of birds versus the chance of an event occurring in the second group.
where:
P(a); P(b) – probability of infected birds in the group (a) and (b).
The study assessed the risk of fungal infection in the compared groups of birds, e.g. urban vs. rural, males vs. females, adults vs. juveniles.
Correlations between the prevalence of fungi in the cloaca and beak were tested with McNemar’s test; rγ – gamma coefficient of correlation, and ϕ coefficient were computed considering many correlations of the same ranks.
Differences in bird condition indices (PC1-3), glucose levels between the birds with positive and negative tests for fungi availability and types of population (urban/rural) were also tested in factorial ANOVA or main effect ANOVA models. Differences in glucose and blood haemoglobin levels between birds from two populations (urban/rural) and age of birds (juv./adults) were tested in the factorial ANOVA model.
Test functions were calculated accordingly to the assumptions of statistical analyses, based on algorithms developed in SPSS 25 (IBM Citation2017) and STATISTICA 13.0 software (TIBCO Software Inc Citation2017); the RR and OR coefficients and their 95% confidence intervals were calculated in accordance with the assumptions of Hackshaw (Citation2009), based on the algorithms implemented in the additional medical examination package (4.0) working in the STATISTICA 13.0 environment (StatSoft Polska Sp. z o.o. Citation2018). The level of significance was set at P = 0.05.
Results
Degree of urban and rural population colonization by fungi
Of the 100 analyzed House Sparrow birds, fungi were detected in 89 of them. The identified fungi represented 26 species (). In the urban population (U), fungi belonging to 15 species were isolated from 43 birds (86%), and the total number of isolates of all identified fungal species obtained from both ontocenoses reached 106 (beak cavity: 54, cloaca: 52). In the rural population (R), fungi representing 22 species were isolated from 46 House Sparrows (92%), and the total number of isolates of all identified fungal species acquired from both ontocenoses reached 115 (beak cavity: 62; cloaca: 53). No significant differences were determined between the number of fungi species identified in birds from urban and rural habitats (Mann-Whitney test: beak cavity: Z = 0.782, P = 0.434; cloaca: Z = 0.090, P = 0.929). In turn, a strong positive correlation was found between the prevalence of fungi in the beak cavity and cloaca (McNemar test: chi2 = 48.011, P < 0.000001; gamma correlation rγ = 0.938, P < 0.0001; ϕ = 0.591). In both types of habitats, Candida krusei was the most common fungus (), with a strong dominance in the urban population (46 isolates in 27 individuals: beak cavity – 25 isolates, cloaca – 21 isolates). Another very frequently recorded species was Candida albicans (), especially in the rural population (21 isolates in 12 individuals; beak cavity – 11 isolates, cloaca – 10 isolates).
Table I. Fungi isolated from ontocenoses of the beak cavity and cloaca of House Sparrows Passer domesticus occurring in two types of habitat
In both populations of birds, the extent of the fungal colonization of the two analyzed ontocenoses was high: Urban – 82% and Rural – 90% for the beak cavity and Urban – 80% and Rural – 80% for the cloaca (). No significant difference was determined in the degree of colonization between the two populations (beak cavity: Fisher’s exact test, P = 0.388; cloaca: Fisher’s exact test, P = 1.000). In addition, the relative risk (RR) and odds ratio (OR) calculated for the likelihood of colonization by fungi in urban population comparing to rural population did not diverge from random estimates (beak cavity: RR = 0.742, OR = 0.506; cloaca: RR = 1.000, OR = 1.000). No significant differences and overlapping of confidence limits were found in the odds ratio values calculated for the likelihood of beak and cloaca ontocenoses colonization by fungi ().
Table II. Number and percentage of birds with a positive test result for the presence of fungi in ontocenoses of the beak cavity and cloaca and scores of the relative risk coefficient of colonization by fungi in the compared groups of birds
The degree of colonization of the analyzed ontocenoses was similar in males and females (beak cavity – males: 87.8%, females: 95.5%, Fisher’s exact test, P = 0.656; cloaca – males: 90.2%, females: 86.4%, Fisher’s exact test, P = 0.687) (). There were also no differences between the degree of colonization of male and female ontocenoses in both analyzed populations (Fisher’s exact test – Urban: beak cavity, P = 1.000; cloaca, P = 1.000; Rural: beak cavity, P = 1.000; cloaca, P = 0.640).
The extent of ontocenoses colonization was higher in adult birds than in juveniles (beak cavity: Ad. – 90.5%, Juv. – 78.4%, RR = 1.547; cloaca: Ad. – 88.9%, Juv. – 67.6%, RR = 1.877) (), however, this difference was statistically significant only in the case of cloaca colonization (beak cavity: Fisher’s exact test, P = 0.134; cloaca: Fisher’s exact test, P = 0.016).
Colonization by fungi and body condition of birds
We found significant relationships between the probability of fungal colonization of birds and variables characterizing birds’ body condition (PC1 and PC2), physiological condition (blood haemoglobin level), and age of birds (). The best fitted models reduced the number of explanatory variables to the factor (PC1) characterizing birds’ body condition and to birds age (). Birds with lower body condition and also older (adults) were more often infected by fungi.
Table III. General Linear Models (A) and the best fitted model (B) of relation between probability of fungi occurrence in birds ontocenoses, body and physiological birds condition and age of birds (selection on AIC)
Body condition indices (F, logBM, logT, BM/WL, logBM/logWL, BM/TL, BM/T) were lower in urban habitats (Lambda Wilks = 0.841, F(7, 92) = 2.483, P = 0.022). Although no significant interactions between the probability of fungal infection, condition coefficients and the type of environment were found in the GLM model, body mass and condition coefficients loaded the PC1 factor (except BM/T) were higher in urban birds with a negative result of fungal presence in the beak cavity and cloaca than in infected birds (). In the rural population, differences in body condition coefficients were statistically insignificant between birds with positive and negative results of mycological tests ().
Table IV. Body condition coefficients (mean ± SD) and results of the Mann-Whitney test applied to compare the body condition of birds with positive (Yes) and negative (No) results of the test for fungal presence in the beak cavity ontocenosis
Table V. Body condition coefficients (mean ± SD) and results of the Mann-Whitney test applied to compare the body condition of birds with positive (Yes) and negative (No) results of the test for fungal presence in the cloaca ontocenosis
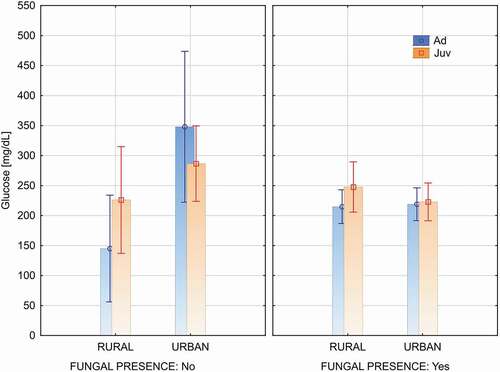
Glucose level depends on population type (urban/rural) and fungi presence and showed significant differences in the interaction between the variables: population x fungal presence (Factorial ANOVA: F(1, 71) = 6.805, P = 0.011). Glucose level was significantly lower in rural population compared to urban birds (Factorial ANOVA: F(1, 71) = 5.834, P = 0.018). Birds without fungal colonization in the beak and cloaca in the urban population had significantly higher glucose levels comparing to rural birds (Urban: mean = 298.8, Rural: mean = 185.5; Newman-Keuls test, P = 0.004). Such differences were not observed between birds from urban and rural areas colonized by fungi (Urban: mean = 220.5, Rural: mean = 224.9; Newman-Keuls test, P = 0.892) (). Glucose level doesn’t depends also on age of birds (F(1, 71) = 1.956, P = 0.166).
Figure 1. Glucose levels in birds with positive (Yes) and negative (No) results of the test for fungal presence in the urban (Urban) and rural (Rural) population. (Figure show means and 95% c.l.)
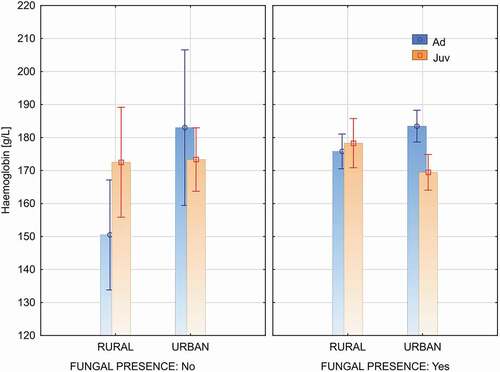
Birds infected by fungi had a significantly higher blood haemoglobin level (mean = 177.0) comparing to birds non infected by fungi (mean = 168.1) (F(1, 80) = 4.184, P = 0.044).
The level of blood haemoglobin in birds was similar in the both type of environment (F(1, 80) = 2.566, P = 0.113). The blood haemoglobin level depends also on occurrence of fungi in birds ontocenoses and birds age (Factorial ANOVA: F(1, 80) = 4.601, P = 0.035) – . Adults birds infected by fungi had higher blood haemoglobin level than non-infected birds (infected: mean = 180.0, non-infected: mean = 161.3; Newman-Keuls test, P = 0.022). This difference in the group of juvenile birds was statistically insignificant (infected: mean = 172.5, non-infected: mean = 173.1; Newman-Keuls test, P = 0.924).
Discussion
Prevalence of fungi in ontocenoses of birds
Only few studies provide data on the occurrence of fungi in wild bird organisms (e.g. Dynowska & Dynowski Citation1996; Cafarchia et al. Citation2006; Biedunkiewicz et al. Citation2012; Dynowska et al. Citation2013, Citation2015; Meissner et al. Citation2015). Pinowski et al. (Citation1994) pointed out the role of microorganisms as a significant causative agent of egg and chick mortality in sparrows nesting in hollows. The intersystemic prevalence of fungi in House Sparrow, analyzed with the use of the biological material originating from internal organs, was confirmed by Kruszewicz et al. (Citation1995), who analyzed chicks of House Sparrow and Tree Sparrow that were euthanized or died in the nest. The degree of colonization by fungi determined in the studied House Sparrow populations from the city of Olsztyn and rural areas of north-eastern Poland was high and reached 86% and 92%, respectively. The number of species isolated from House Sparrows relative to the sample size was also high (26 species) compared to the results obtained by Cafarchia et al. (Citation2006), where 15 species of fungi were isolated from 421 birds belonging to 7 migratory species, and by Dynowska et al. (Citation2015), where 23 species of fungi were isolated from 490 birds representing 9 species of Charadriiformes.
It seems that the species structure of fungi and the frequency of their occurrence in a given population of birds are related to aspects of bird ecology, their diet and habitat (Cafarchia et al. Citation2006). Lower fungal species diversity in urban sparrows may be associated with lower taxonomic diversity of fungi in this environment. Investigations concerning ecological determinants of mycotic infections in humans and animals explicitly show that each increase in the number of reported cases of fungus occurrence in their ontosphere is strictly correlated with the presence of the same species in the external environment (De Hoog et al. Citation2000; Tsiodras et al. Citation2008). Although the urban environment and human pressure create good conditions for the development of potentially-pathogenic mycoses from various ecophysiological groups (Meissner et al. Citation2015), higher temperatures and lower relative humidity of cities, compared to extra-urban areas, may exert inhibitory effects on fungal development. These climatic determinants and, in a consequence, higher temperatures of buildings that are the nesting sites of House Sparrow, may ensure a stronger barrier against infection in the urban populations, especially considering the physiological decrease of body temperature of sparrow at night even by 3°C from the average daily temperature of 41–43°C (Binkley et al. Citation1971).
A clear relation between the similar prevalence of fungi in the beak and cloaca may be indicative of the presence of the same species in feed and external environment (Dynowska et al. Citation2013), passage of fungi from the beak cavity to the gastrointestinal tract (Biedunkiewicz et al. Citation2012; Dynowska et al. Citation2015), and a similar physiological condition of the analyzed adult and juvenile birds in the compared populations. Transmission of fungi during copulation should not be excluded (Stewart Citation2000; Cafarchia et al. Citation2006; Tsiodras et al. Citation2008), and it also indicates a similar degree of infection of males and females.
In turn, a higher degree of ontocenosis colonization by fungi in adult than in juvenile birds, with a statistically significant difference in the case of cloaca infection, indicates that the colonization of ontocenoses progresses with bird age, mainly through digestive, respiratory and sexual routes, which is confirmed by the significantly higher degree of cloaca colonization in adult birds. Research conducted by Mills et al. (Citation1999) confirmed that cloaca colonization in Tree Swallow chicks by microorganisms, including fungi, began shortly after hatching, and the number of infected chicks was significantly increasing with age. A similar course of gastrointestinal tract colonization by fungi (e.g. Candida spp.) and bacteria (e.g. E. coli) was noted in chicks of House Sparrow and Tree Sparrow (Małyszko et al. Citation1991). An increasing number of chicks with mycotic infections with age was also determined by Kozłowski et al. (Citation1991a).
In both House Sparrow populations analyzed in the present study, the predominant species of fungi was Candida krusei, which was previously confirmed as the most abundant only in Great Cormorant (Biedunkiewicz et al. Citation2012). It was subdominant among the fungi isolated from Mallard Duck, as it constituted over 5% of all isolates (Meissner et al. Citation2015). In contrast, it was rarely found or absent in the group of the analyzed migratory birds (Cafarchia et al. Citation2006; Dynowska et al. Citation2015). Candida krusei is classified as a strongly expansive fungus, capable of infecting all tissues, organs and systems of most vertebrates (Kurnatowska & Kurnatowski Citation2006). Candida albicans was the second most abundant fungus, occurring mainly in the rural population of House Sparrow. It was previously reported as the predominant one in ontocenoses of Mallard Duck, especially in birds from extra-urban areas (Meissner et al. Citation2015), as well as in the analyzed species of Charadriiformes (Dynowska et al. Citation2015). An earlier study (Kozłowski et al. Citation1991a) concerning mortality rates of eggs and chicks of House Sparrow and Tree Sparrow in the urban population confirmed only one isolate of Candida albicans from eggs and dead embryos in the case of sparrow. Analyses at the successive stage of ontogenetic development of both species demonstrated colonization of ontocenoses with yeast-like fungi at a rate of 23.2% in House Sparrow chicks and a significantly lower one of 8.7% in Tree Sparrow chicks (Kozłowski et al. Citation1991b). Probably, the increase in fungal prevalence from the genus Candida in birds, demonstrated in successive years and different research projects, indicated a strong expansion of these fungi in the external environment and perhaps a higher susceptibility of House Sparrow to colonization.
Colonization of ontocenoses with fungi vs. body conditions
The weaker body conditions of House Sparrow in urban habitats demonstrated in many works (Liker et al. Citation2008; Seress et al. Citation2011), particularly the condition of males (Dulisz et al. Citation2016), on the background of a declining trend in the population number of the species, may accordingly to Labocha and Hayes (Citation2012) be the reason of the lower survivability rate of birds (Blums et al. Citation2005), a poorer reproductive success (Blums et al. Citation2002), and modification of behaviors (Bachman & Widemo Citation1999). Results obtained in this work demonstrated that House Sparrows with lower values of body condition coefficients and lower body mass were significantly more frequently colonized by fungi, and this pertained to their urban population. It may, therefore, be concluded that infections with fungi are directly linked with the general condition of the macroorganism. Most of the fungi capable of colonizing the ontosphere of animals and humans are opportunistic forms, incapable of inducing infections in organisms with not-impaired immunity and maintained systemic homeostasis. The attack of fungi has to be preceded by the appearance of immunosuppressive factors (Casadevall & Pirofski Citation2000) and they may be autogenic or allogenic. The former result from physiology, such as age, reproduction or pathology, e.g. viral, bacterial or parasitic diseases that debilitate the organisms and lead to the disruption of natural defensive barriers (Tsiodras et al. Citation2008); the latter ones are environmental factors that result mainly from the advancing human pressure, which facilitates the accumulation of organic matter of various origins in natural reservoirs of potentially-pathogenic fungi and broadly understood chemization (Dynowska & Dynowski Citation1996; Meissner et al. Citation2015). They indirectly or directly affect metabolic mechanisms and, consequently, lead to poorer body condition of a potential fungal host. Stress is a very important environmental factor that negatively affects body homeostasis and reduces immunity to multiple infections, including mycoses (Dynowska et al. Citation2015). It is very well reflected by most body condition coefficients, whose values are higher in non-infected birds. In turn, House Sparrows infected in the urban environment had lower body condition. Such a correlation was not observed in the rural environment, which would confirm the stress-inducing effect of the urban habitat.
Alas, the decrease in glucose levels in the infected birds is directly linked with the presence of fungi, for which glucose is a universal and the most readily available carbon source (De Hoog et al. Citation2000; Kurtzman et al. Citation2011).
The data obtained by the authors do not indicate significant differences in the concentration of blood haemoglobin in birds from urban and rural environments. Only adult birds colonized by fungi had significantly higher haemoglobin levels compared to adults non-colonized by fungi. Since numerous studies confirm positive relationships of blood haemoglobin concentrations with different measures of nutritional state and condition in birds (Minias Citation2015), lower concentrations of blood haemoglobin should be expected in urban environments, in which birds with lower body condition indices will are more frequently colonized by fungi. It seems that the increase in oxygen capacity can be a physiological regulation in response to adverse environmental factors, e.g. stress or intrasystemic factors, e.g. parasites. Just as bird preparation for migration is associated with fat load and increased oxygen capacity for long-distance flights (Minias et al. Citation2013), so higher blood haemoglobin levels may be the answer to the recorded in recent years increase in predation in urban areas to optimize the ability to avoid predators. Dulisz et al. (Citation2016) suggested that lower indicators of body condition, especially male sparrows during the breeding period, could be the result of balancing between the risk of predation and the risk of hunger, thanks to which they reach a compromise between optimal body size and preserving life. The authors of this work also showed that adult urban birds had different biometric indices than rural populations, and these differences seemed to be adaptation to a more maneuverable flight and a quick start and braking as a result of predation pressure. Other comparative studies in urban and rural areas confirmed a higher level of the H:L indicator (heterophile to lymphocyte ratio) in urban birds, suggesting that they may have experienced higher levels of chronic stress (Powell et al. Citation2013; Herrera-Dueñas et al. Citation2014). Clearly lower levels of glucose and haemoglobin of adult birds from the rural environment in relation to birds from the urban environment in the group of birds not infected with fungi, may indicate their higher survival rate at lower physiological parameters. Higher physiological condition in the urban environment may be a response to the long-term stress or predator pressure. However, a relatively high blood haemoglobin level in the group of birds infected with fungi, may be a condition for their higher survival. Birds with low physiological condition do not survive and are not recorded.
The results of this study provide much novel and important information related to the aspect of House Sparrow ecology in the urban and rural environments, its physical condition and associations with microfungi commonly occurring in its environment and feed. Considering that all birds from which the swabs were collected were healthy, it should be concluded that this relation has the character of a carrier state. Taking into account the downward trends in House Sparrow population, especially urban populations exposed to various factors of urbanization pressure, they may be additionally weakened by fungi colonizing them in the situation of disturbed body homeostasis. But no important differences in the fungal colonization detected between urban and rural areas do not allow to confirm the hypothesis that fungal infection may be the cause of decline of sparrows in urban habitats, and the problem requires further research.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Andersson MN, Wang H-L, Nord A, Salmón P, Isaksson C. 2015. Composition of physiologically important fatty acids in great tits differs between urban and rural populations on a seasonal basis. Frontiers in Ecology and Evolution 3:93. DOI: 10.3389/fevo.2015.00093.
- Bachman G, Widemo F. 1999. Relationships between body composition, body size and alternative reproductive tactics in a lekking sandpiper, the Ruff (Philomachus pugnax). Functional Ecology 13:411–416. DOI: 10.1046/j.1365-2435.1999.00323.x.
- Barnett JA, Payne RW, Yarrow D. 2000. Yeasts: Characteristics and Identification. 3rd ed. Cambridge, UK: Cambridge University Press.
- Bartlett MS. 1954. A note on the multiplying factors for various chi square approximations. Journal of the Royal Statistical Society 16:296–298.
- Bell CP, Sam WB, Parkes NG, Brooke MDL, Chamberlain DE. 2010. The role of the Eurasian Sparrowhawk (Accipiter nisus) in the decline of the House Sparrow (Passer domesticus) in Britain. The Auk 127:411–420. DOI: 10.1525/auk.2009.09108.
- Biedunkiewicz A, Dziekońska-Rynko J, Rokicki J. 2012. Black cormorant Phalacrocorax carbo (L., 1758) as a vector of fungi and parasites occurring in the gastrointestinal tract. Biologia 67:417–424. DOI: 10.2478/s11756-012-0012-2.
- Binkley S, Kluth E, Menaker M. 1971. Pineal function in Sparrows: Circadian rhythms and body temperature. Science 174:311–314. DOI: 10.1126/science.174.4006.311.
- Blums P, Clark RG, Mednis A. 2002. Patterns of reproductive effort and success in birds: Path analyses of long-term data from European ducks. Journal of Animal Ecology 71:280–295. DOI: 10.1046/j.1365-2656.2002.00598.x.
- Blums P, Nichols JD, Hines JE, Lindberg MS, Mednis A. 2005. Individual quality, survival variation and patterns of phenotypic selection on body condition and timing of nesting in birds. Oecologia 143:365–376. DOI: 10.1007/s00442-004-1794-x.
- Busse P. 1990. The key to determining the age and gender of European passerine birds. Notatki Ornitologiczne 31:5–368.
- Cafarchia C, Camarda A, Romito D, Campolo M, Quaglia NC, Tullio D, Otranto D. 2006. Occurrence of yeasts in cloacae of migratory birds. Mycopathologia 161:229–234. DOI: 10.1007/s11046-005-0194-z.
- Casadevall A, Pirofski LA. 2000. Minireview: Host e pathogen interactions; basic concepts of microbial commensalisms, colonization, infection and disease. Infection and Immunity 68:6511–6518. DOI: 10.1128/IAI.68.12.6511-6518.2000.
- Cattell RB. 1966. The scree test for the number of factors. Multivariate Behavioral Research 1:245–276. DOI: 10.1207/s15327906mbr0102_10.
- Chamberlain DE, Glue DE, Tomps MP. 2009. Sparrowhawk Accipiter nisus presence and winter bird abundance. Journal of Ornithology 150:247–254. DOI: 10.1007/s10336-008-0344-4.
- Cresswell W. 2008. Non-lethal effects of predation in birds. Ibis 150:3–17. DOI: 10.1111/j.1474-919X.2007.00793.x.
- Dahlhausen R. 2006. Implications of mycoses in clinical disorders. In: Harrison GJ, Lightfoot TL, editors. Clinical avian medicine. Palm Beach, FL, USA: Spix Publishing Inc. pp. 691–704.
- De Hoog GS, Guarro J, Gene J, Figuerras MJ. 2000. Atlas of clinical fungi. 2nd ed. Utrecht, Netherlands: Centraalbureau voor Schimmelcultures.
- De Laet J, Summers-Smith JD. 2007. The status of the urban house sparrow Passer domesticus in north-western Europe: A review. Journal of Ornithology 148(Suppl 2):S275–S278. DOI: 10.1007/s10336-007-0154-0.
- Dolnik OV, Hoi H. 2010. Honest signalling, dominance hierarchies and body condition in House Sparrows Passer domesticus (Aves: Passeriformes) during acute coccidiosis. Biological Journal of Linnean Society 99:718–726. DOI: 10.1111/j.1095-8312.2010.01370.x.
- Donnelly R, Marzluff JM. 2006. Relative importance of habitat quantity, structure and spatial pattern to birds in urbanizing environments. Urban Ecosystems 9:99–117. DOI: 10.1007/s11252-006-7904-2.
- Dugas MB, Doumas LT. 2014. Ectoparasite density is associated with mouth colour and size in nestling House Sparrows Passer domesticus. Ibis 156:682–686. DOI: 10.1111/ibi.12154.
- Dulisz B, Nowakowski JJ. 1996. The species diversity of the avifauna in built-up areas in the city of Olsztyn (NE Poland). Acta Ornithologica 31:33–38.
- Dulisz B, Nowakowski JJ, Górnik J. 2016. Differences in biometry and body condition of the House Sparrow (Passer domesticus) in urban and rural population during breeding season. Urban Ecosystems 19:1307–1324. DOI: 10.1007/s11252-016-0546-0.
- Dynowska M, Biedunkiewicz A, Kisicka I, Ejdys E, Kubiak D, Sucharzewska E. 2015. Epidemiological importance of yeasts isolated from the beak and cloaca of healthy Charadriiformes. Bulletin of the Veterinary Institute in Pulawy 59:65–69. DOI: 10.1515/bvip-2015-0010.
- Dynowska M, Dynowski J. 1996. A case of aspergillosis and candidosis of a black stork: Ciconia nigra (Linnaeus, 1758). Medycyna Weterynaryjna 52:127–128.
- Dynowska M, Wojczulanis-Jakubas K, Pacyńska JA, Jakubas D, Ejdys E. 2013. Potentially pathogenic yeast isolated from the throat and cloaca of an Arctic colonial seabird: The little auk (Alle alle). Polar Biology 36:343–348. DOI: 10.1007/s00300-012-1263-7.
- González-Braojos S, Vela AI, Ruiz-de-Castañeda R, Briones V, Moreno J. 2012. Age-related changes in abundance of enterococci and Enterobacteriaceae in Pied Flycatcher (Ficedula hypoleuca) nestlings and their association with growth. Journal of Ornithology 153:181–188. DOI: 10.1007/s10336-011-0725-y.
- Hackshaw A. 2009. A concise guide to clinical trials. New York, NJ, USA: Wiley-Blackwell, BMJ Books Hoboken.
- Herrera-Dueñas A, Pineda J, Antonio MT, Aguirre JI. 2014. Oxidative stress of House Sparrow as bioindicator of urban pollution. Ecological Indicators 42:6–9. DOI: 10.1016/j.ecolind.2013.08.014.
- Howard DH. 2003. Pathogenic Fungi in Humans and Animals. 2nd ed. New York, NY, USA: Marcel Dekker Inc.
- Hubálek Z. 2004. An annotated checklist of pathogenic microorganisms associated with migratory birds. Journal of Wildlife Diseases 40:639–659. DOI: 10.7589/0090-3558-40.4.639.
- IBM. 2017. IBM SPSS statistics 25 documentation. Available: www-01.ibm.com.
- Kaiser HF. 1958. The varimax criterion for analytic rotation in factor analysis. Psychometrica 23:158–163. DOI: 10.1007/BF02289233.
- Kelcey JG, Rheinwald G, editors. 2005. Birds in European Cities. Katharinen, Germany: Ginster Verlag.
- Khosravi AR, Shokri H, Ziglari T, Naeini AR, Mousavi Z, Hashemi H. 2008. Outbreak of severe disseminated aspergillosis in a flock of ostrich (Struthio camelus). Mycoses 51:557–559. DOI: 10.1111/j.1439-0507.2008.01504.x.
- Kozłowski S, Małyszko E, Pinowski J, Bernacka B, Pepiński W, Kruszewicz A. 1991a. Pathogenic microorganisms isolated from Passer domesticus and Passer montanus eggs and nestlings. In: Pinowski J, Kavanagh BP, Pinowska B, editors. Nestling mortality of granivorous birds due to microorganisms and toxic substances. Warszawa, Poland: PWN – Polish Scientific Publishers. pp. 153–165.
- Kozłowski S, Małyszko E, Pinowski J, Kruszewicz A. 1991b. The influence of pathogenic fungi on the mortality of sparrow (Passer spp.) eggs and nestlings. Preliminary report. In: Pinowski J, Kavanagh BP, Pinowska B, editors. Nestling mortality of granivorous birds due to microorganisms and toxic substances. Warszawa, Poland: PWN – Polish Scientific Publishers. pp. 167–169.
- Kruszewicz AG, Pinowski J, Kruszewicz AH, Mazurkiewicz M, Pawiak R, Małyszko E. 1995. Occurrence of fungi in House Sparrow (Passer domesticus) and Tree Sparrow (Passer montanus) nestlings. In: Pinowski J, Kavanagh BP, Pinowska B, editors. Nestling mortality of granivorous birds due to microorganisms and toxic substances: Synthesis. Warszawa, Poland: PWN – Polish Scientific Publishers. pp. 283–290.
- Kübler S, Kupko S, Zeller U. 2005. The kestrel (Falco tinnunculus L.) in Berlin: Investigation of breeding biology and feeding ecology. Journal of Ornithology 146:271–278. DOI: 10.1007/s10336-005-0089-2.
- Kurnatowska A, Kurnatowski P. 2006. Medical mycology. Łódź, Poland: Promedi.
- Kurtzman CP, Fell JW, Boekhout T, editors. 2011. The Yeasts: A taxonomic study. 5th ed. Amsterdam, Netherlands: Elsevier.
- Labocha MK, Hayes JP. 2012. Morphometric indices of body condition in birds: A review. Journal of Ornithology 153:1–22. DOI: 10.1007/s10336-011-0706-1.
- Liker A, Papp Z, Bókony V, Lendvai ÁZ. 2008. Lean birds in the city: Body size and condition of house sparrows along the urbanization gradient. Journal of Animal Ecology 77:789–795. DOI: 10.1111/j.1365-2656.2008.01402.x.
- Lima SL. 1986. Predation risk and unpredictable feeding conditions – Determinants of body-mass in birds. Ecology 67:377–385. DOI: 10.2307/1938580.
- MacLeod R, Barnett P, Clark J, Cresswell W. 2006. Mass-dependent predation risk as a mechanism for house sparrow declines? Biology Letters 2:43–46. DOI: 10.1098/rsbl.2005.0421.
- MacLeod R, Lind J, Clark J, Cresswell W. 2007. Mass regulation in response to predation risk can indicate population declines. Ecology Letters 10:945–955. DOI: 10.1111/j.1461-0248.2007.01088.x.
- Małyszko E, Pinowski J, Kozłowski S, Bernacka B, Pepiński W, Kruszewicz A. 1991. Auto- and allochtonous flora and fauna on the intestinal tract of Passer domesticus and Passer montanus nestlings. In: Pinowski J, Kavanagh BP, Pinowska B, editors. Nestling mortality of granivorous birds due to microorganisms and toxic substances. Warszawa, Poland: PWN – Polish Scientific Publishers. pp. 129–137.
- McCullagh P, Nelder JA. 1989. Generalized Linear Models. 2nd ed. London, UK: Chapman and Hall.
- McNamara JM. 1987. Starvation and predation as factors limiting population size. Ecology 68:1515–1519. DOI: 10.2307/1939235.
- Meissner W, Dynowska M, Góralska K, Rzyska H. 2015. Mallards (Anas platyrhynchos) staying in urban environments have higher levels of microfungi biota diversity than do birds from nonurban areas. Fungal Ecology 17:164–169. DOI: 10.1016/j.funeco.2015.07.004.
- Mills TK, Lombardo MP, Thorpe PA. 1999. Microbial colonization of the cloacae of nestling Tree Swallows. The Auk 116:947–956. DOI: 10.2307/4089674.
- Minias P. 2015. The use of haemoglobin concentrations to assess physiological condition in birds: A review. Conservation Physiology 3(1):cov007. DOI: 10.1093/conphys/cov007.
- Minias P, Kaczmarek K, Włodarczyk R, Janiszewski T. 2013. Hemoglobin concentrations in waders vary with their strategies of migration: A comparative analysis. Comparative Biochemistry and Physiology A 165:7–12. DOI: 10.1016/j.cbpa.2013.02.008.
- Nowakowski JJ, Dulisz B. 2005. Population densities and synurbization of corvids in Olszyn city (NE Poland). In: Jerzak L, Kavanagh BP, Tryjanowski P, editors. Corvids of Poland. Poznań, Poland: Bogucki Wydawnictwo Naukowe. pp. 481–500.
- Pinowski J, Barkowska M, Kruszewicz AH, Kruszewicz AG. 1994. The causes of the mortality of eggs and nestlings of Passer spp. Journal of Biosciences 19:441–451. DOI: 10.1007/BF02703180.
- Powell C, Lill A, Johnstone CP. 2013. Body condition and chronic stress in urban and rural noisy miners. The Open Ornithology Journal 6:25–31. DOI: 10.2174/1874453201306010025.
- Reding P. 2003. Fungal diseases. In: Samour J, editor. Avian medicine. Edinburgh, UK: Elsevier Science. pp. 275–291.
- Riffenburgh RH. 2006. Statistics in medicine. 2nd ed. Burlington, MA, USA: Elsevier Academic Press.
- Rippon RJ, Alley MR, Castro I. 2012. Candida albicans infection in free-living populations of hihi (stitchbird; Notiomystis cincta). New Zealand Veterinary Journal 58:299–306. DOI: 10.1080/00480169.2010.69760.
- Robert VA, Casadevall A. 2009. Vertebrate endothermy restricts most fungi as potential pathogens. Journal of Infectious Diseases 200:1623–1626. DOI: 10.1086/644642.
- Robinson RA, Siriwardena GM, Crick HQP. 2005. Size and trends of the House Sparrow Passer domesticus population in Great Britain. Ibis 147:552–562. DOI: 10.1111/j.1474-919x.2005.00427.x.
- Rutz C. 2006. Home range size, habitat use, activity patterns and hunting behaviour of urban-breeding northern goshawks Accipiter gentilis. Ardea 94:185–202.
- Salleh Hudin N, Strubbe D, Teyssier A, De Neve L, White J, Janssens G, Lens L. 2016. Predictable food supplies induce plastic shifts in avian scaled body mass. Behavioral Ecology 27:1833–1840. DOI: 10.1093/beheco/arw108.
- Seress G, Bókony V, Heszberger J, Liker A. 2011. Response to predation risk in urban and rural House Sparrows. Ethology 117:896–907. DOI: 10.1111/j.1439-0310.2011.01944.x.
- Seress G, Bókony V, Pipoly I, Szép T, Nagy K, Liker A. 2012. Urbanization, nestling growth and reproductive success in a moderately declining house sparrow population. Journal of Avian Biology 43:403–414. DOI: 10.1111/j.1600-048X.2012.05527.x.
- Shaw LM, Chamberlain DE, Evans M. 2008. The House Sparrow Passer domesticus in urban areas: Reviewing a possible link between post-decline distribution and human socioeconomic status. Journal of Ornithology 149:293–299. DOI: 10.1007/s10336-008-0285-y.
- Shochat E. 2004. Credit or debit? Resource input changes population dynamics of city-slicker birds. Oikos 106:622–626. DOI: 10.1111/j.0030-1299.2004.13159.x.
- StatSoft Polska Sp. z o.o. 2018. Zestaw medyczny wersja 4.0. Available: www.statsoft.pl.
- Stewart R. 2000. Cloacal microbes in House Sparrows. The Condor 102:679–684. DOI: 10.1650/0010-5422(2000)102.
- TIBCO Software Inc. 2017. Statistica (data analysis software system), version 13. Available: http://statistica.io.
- Tsiodras S, Kelesidis T, Kelesidis I, Bauchinger U, Falagas ME. 2008. Human infections associated with wild birds. Journal of Infection 56:83–98. DOI: 10.1016/j.jinf.2007.11.001.
- Vangestel C, Braeckman BP, Matheve H, Lens L. 2010. Constraints on home range behaviour affect nutritional condition in urban house sparrows (Passer domesticus). Biological Journal of the Linnean Society 101:41–50. DOI: 10.1111/j.1095-8312.2010.01493.x.
- Węgrzynowicz A. 2013. Changes in the House Sparrow Passer domesticus population in cities and towns of Poland in 1960–2010. Ornis Polonica 54:225–236.
- Witter MS, Cuthill IC. 1993. The ecological costs of avian fat storage. Philosophical Transactions of the Royal Society B Biological Sciences 340(1291):73–92. DOI: 10.1098/rstb.1993.0050.
- Wolff PL, Petrini KR, Kolmstetter C. 1992. An Outbreak of Aspergillosis in Crested Wood Partridges (Rollulus rouloul). Journal of Zoo and Wildlife Medicine 23:108–112.