Abstract
In this study the morphology, the conventional glycohistochemistry and the ultrastructure of adult rainbow trout (Oncorhynchus mykiss) stomach is reported. For this purpose, stomach belonging to 10 adult female rainbow trout, weighting ~ 500 ± 20 gr and 32 ± 2.50 cm long, were collected and processed. Oncorhynchus mykiss has a siphonal stomach, with a J letter shape, showing a descending portion (cardiac region), an ascendant one (pyloric region), connected through the most represented middle portion (fundic region). Morphologically gastric wall shows an overlay of four typical vertebrate tunicae: mucosa, sub-mucosa, muscularis and serosa. Tunica mucosa, lacking of muscularis mucosae, is raised into longitudinal folds. The tunica propria submucosa is of considerable thickness and receives the bodies of many gastric glands whose lumen is lined with oxyntopeptic cells. Nervous cells, organized in ganglion, that can be compared to Mammals plexus submucosus, are noted. Glycohistochemistry was performed by staining with Periodic acid–Schiff (PAS), Alcian blue (AB) pH 2.5, AB pH 1 and AB-PAS. Histochemical analysis revealed a consistent presence of glycoconjugates produced by epithelial lining and gastric pit cells. Ultrastructural studies, performed at scanning electron microscopy, showed a mucosa that rises in folds with a geometrically well-defined shape and an abundance of gastric pits in the fundic region. Taken together our results integrate the anatomical description of rainbow trout stomach and will be helpful for future studies related to digestive physiology, development of appropriate feeding strategies and gastric disease control of this teleostean species.
Introduction
The digestive apparatus of fishes displays a remarkable diversity in its morphology and function among species. This is correlated to taxonomy, feeding habits, body size, shape and even sex (Kapoor et al. Citation1975; Smith Citation1989). Thereby different studies have been conducted to define the structure of the digestive apparatus in various fish species (Carrassón et al. Citation2006; Chatchavalvanich et al. Citation2006; Cao & Wang Citation2009; Faccioli et al. Citation2014; Kasozi et al. Citation2017; Moawad et al. Citation2017; de Oliveira et al. Citation2019). Although significant differences can be observed macroscopically, the basic histological structure of the digestive tract is similar among species (Wilson & Castro Citation2010). Several authors have described the presence of mucosubstances in digestive system of many Teleosts (Scocco et al. Citation1996, Citation1997, Citation1998; Pedini et al. Citation2004; Domeneghini et al. Citation1998; Domeneghini et al. Citation2005; Diaz et al. Citation2008; Dos Santos et al. Citation2015; Ghosh & Chakrabarti Citation2015). Glycoconjugates are correlated with different functions, such as lubrication, protection of the epithelium against proteolytic degradation, inhibition of microorganisms, buffering of intestinal fluid, link and transport of ions (Reifel & Travill Citation1979; Allen Citation1981; Reid et al. Citation1988).
The stomach is the organ of the digestive apparatus were the differences among fish species are clearly marked. In fact, the stomach is absent in some Teleosts or modified as intestinal bulb. When it is present as true organ, it generally consists of three regions (or portions), named cardiac, fundic and pyloric regions (Wilson & Castro Citation2010). The stomach is characterized by the presence of gastric glands, showing a single cell type secreting both hydrochloric acid and pepsinogen (Diaz et al. Citation2003; Gallagher et al. Citation2005). The efficiency of the gastric digestion depends, mainly, on the pH and the mucous secretions that provide a suitable environment to the activity of gastric enzyme (Garrido et al. Citation1998; Saadatfar et al. Citation2010; Namulawa et al. Citation2014). The function of mucous substances in the stomach is generally thought to be linked to processes that increase and improve the digestive efficiency, to lubricate the cavity of the organ and defend it from pathogens (Reifel & Travill Citation1977; Murray et al. Citation1994; Pedini et al. Citation2005).
The structure and the function of fish gut represent a complex system. That is why, in-depth characterization of fish’s alimentary canal tract is fundamental to better understand digestive physiology and delineate nutritional researches.
Rainbow trout (Oncorhynchus mykiss) is a carnivorous fish belonging to Salmonidae family. It represents the most-widely cultivated cold freshwater fish in the world and an important model species for many research areas, including basic biological disciplines like comparative physiology and toxicology (Mennigen & Zhang Citation2016). Although its intestinal morphology has been already investigated (Ezeasor & Stokoe Citation1981; Bielek Citation2002; Banan Khojasteh et al. Citation2009; Heidarieh et al. Citation2012; Ramos et al. Citation2015; Shabanzadeh et al. Citation2015; Berillis & Mente Citation2017; Nofouzi et al. Citation2019; Verdile et al. Citation2020), few study are dedicated to the histology of trout stomach (Weinreb & Bilstad Citation1955; Ezeasor Citation1981; Ostos Garrido et al. Citation1993; Marchetti et al. Citation2006; Mireşan et al. Citation2012). However, most recent publications analyze the morpho-histology of stomach in young trout reared in indoor standardized condition (Ostos Garrido et al. Citation1993); other authors analyze sexually immature subjects by conventional and lectin histochemistry (Marchetti et al. Citation2006) or adult subjects by means of morphological staining (Mireşan et al. Citation2012), but among them there isn’t a glycohistochemical study of stomach on adult trout reared in natural-like condition and none of them focused on scanning electron microscopy (SEM). SEM uses a focused beam of electrons to scan a surface of a sample to create a high-resolution image, that can show information on sample’s surface composition and topography (Choudhary & Choudhary Citation2017).
The purpose of present study is to integrate the description of the morphology of adult rainbow trout stomach and the characterization and the distribution of complex carbohydrates in this organ. These data could contribute to establish a complete morpho-histochemical picture to take into account as comparative base point for future studies aimed to understand the nutritional physiology of this species.
Materials and methods
Animals and sampling
A total number of ten adult female rainbow trout (Oncorhynchus mykiss), weighting ~ 500 ± 50 gr and 32 ± 2.5 cm long, were used for this study. The animals, intended for human consumption, were obtained by a local agro-fish company (Val Sele, Eboli, Sa). Fishes were bred in natural-like conditions, feed twice day with a commercial diet, according the instruction of manufacturer (Oregon OP-2). Samples were collected fresh at the morning after 24 hours from the last meal. The animal care procedures were in accordance with Legislative Decree No. 146, implementing Directive 98/58/EC of 20 July 1998 concerning the protection of animals kept for farming purposes. Fish were manipulated and sacrificed using the MS-222 (Coyle et al. Citation2004).
An incision was made at the ventral aspect of the body from the anal opening to the interbranchial membrane to take the stomach. Small pieces (0.5 cm × 0.5 cm) from descending, bottom and ascending parts of stomach (cardiac, fundic and pyloric regions; see for details) were obtained and immediately fixed by immersion in:
Bouin’s fluid fixative for 24 hours (h) to evaluate the morphology;
Carnoy’s fluid for 24 h followed by post-fixation in 2% calcium acetate–4% paraformaldehyde solution (1:1) for 3 h at room temperature for conventional histochemistry;
2.5% glutaraldehyde solution in phosphate buffer (PBS) pH 7.4 for 24 h at 4°C for scanning electron microscopy (SEM).
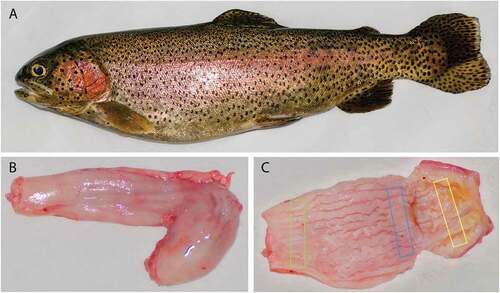
Figure 1. Rainbow trout. Adult female (a). Outer surface of stomach (b), note the J shape with the short descending segment and the longest ascending one. Inner surface of stomach (c), note the wall longitudinal folds. Rectangles (c) indicate the sampling sites: green rectangle for cardial region, blue one for fundic region and yellow one for pyloric region
Morphological and conventional glycohistochemical treatments
Specimens for optical analysis were dehydrated through a series of graded ethanol, cleared in xylene, embedded in paraffin wax and cut into 5-μm-thick serial sections at microtome Reichert-Jung 2050.
For morphological study at optic level, sections were stained with Harris’s hematoxylin-eosin (HE) and Toluidine Blue (TB) (Melis et al. Citation1992). Complex carbohydrate characterization was performed using the established screening staining procedures, carrying out the following histochemical reactions: Periodic acid-Schiff (PAS), Alcian blue (AB) pH 2.5, AB pH 1, AB/PAS (Pearse Citation1985; Bancroft & Gamble Citation2008; Mercati et al. Citation2020). PAS reaction was followed by counterstaining with hematoxylin. Staining solutions were purchased by Bio-Optica Milano SPA: AB pH 2.5 code 05-M26003; AB pH 1 code 05-M26005; PAS code 04–130802A; AB/PAS code 04–163802. All reactions were carried out according the manufacturer’s instructions.
Sugar moieties visualized by different histochemical treatments are shown in .
Table I. Sugar moieties visualized by the histochemical treatments carried out
All stained sections were observed and photographed under Leica DM 1000 light microscope. The digital raw images were optimized for image resolution, contrast, evenness of illumination and background by using Adobe Photoshop CS5 (Adobe Systems, San Jose, CA). For each animal, three independent observers, unaware of the treatments carried out, evaluated five microscopic fields of each intestinal tract and the intensity of the staining was graded in arbitrary units as follows: negative (-), weak (±), moderate (+), strong (++) and very strong (+++).
Scanning electron microscopy (SEM)
Specimens of 5 × 5 mm were postfixed with 1% osmium tetroxide in PBS for 6 h at 4°C in the dark. After three washes in PBS, the specimens were dehydrated first through graded series of ethanol and subsequently in CO2 atmosphere using a critical point-dried EMITECH K850 (Emitech, Asford, UK). They were attached to aluminum stubs facing upwards, covered with carbon tabs, and then the samples were sputtered with gold-palladium (AGARIB 7340, Agar Scientific Ltd, Stansed, UK). The sections were examined with a scanning electron microscopy ZEISS EVO40 (20.000KU EHT, 11.5 mm ˂ ωD ˂ 16.5 and 90x ˂ magnification ˂ 8k).
Statistical analyses
Arbitrary units of glycohistochemical reactivity sorted from 0 (negative) to 4 (very strong), were converted into ranks to run the statistical analysis (Scocco et al. Citation2017; Dall’Aglio et al. Citation2020a). To test the null hypothesis that the location shift between histochemical treatments was equal to zero, we performed paired t-test if the variables satisfied conditions for parametric tests or Wilcoxon signed rank test when they did not. Normality was tested using the Shapiro-Wilk test; homogeneity of variance using the Levene test. In both the analyses, we applied the Holm’s correction for multiple comparisons to avoid type I error.
Statistical elaborations were performed using the R version 3.5.3 (R Core Team Citation2019), the stats R-package, version 3.5.3 (shapiro.test, t.test, wilcox.test functions), and the car R-package, version 3.0–2 (leveneTest function).
Results
Oncorhynchus mykiss has a siphonal stomach, with a J letter shape, showing a descending portion whose early tract represents the cardiac region and an ascendant one whose last tract represents the pyloric region, connected through the main fundic portion. The inner wall is raised in several longitudinal folds () (Mireşan et al. Citation2012).
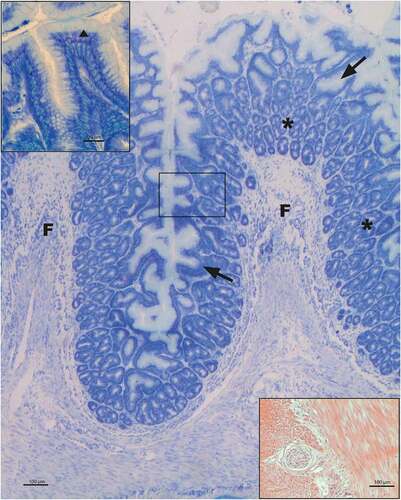
The wall of the organ shows the vertebrate typical overlapping of four tunicae, but the muscolaris mucosae is lacking. The epithelial lining is represented by a simple columnar epithelium that deepens to form mucous gastric pits on whose bottom the tubular gastric glands open. The epithelium lies on a thick tunica propria-submucosae of loose connective richly occupied by the gastric glands (). The epithelium, supported by the conspicuous underlying loose connective hosting the tubular gastric glands, everts out to form the above-described folds. Tubular gastric glands showed an epithelial wall constituted of a single cell type, as previously described in the literature (Ezeasor Citation1981). The tunica muscularis consists of smooth muscle tissue organized in two layers, with an inner consistent circular and an outer slender longitudinal fiber’s pattern. Between these two muscle layers, there is a consistent layer of connective tissue, in which blood vessels, nerve fibers and ganglia are observed (). The tunica serosa of peritoneal derivation is represented by a thin mesothelium supported by a thinner connective layer.
Figure 2. Morphology of rainbow trout fundic portion of stomach at optical level. Toluidine blue staining shows the simple columnar epithelium that deepens to form the gastric pits (↑); on the floor of these, tubular gastric glands open (*). Nor lining epithelium (▲) neither gastric pits show metachromatic behavior (see enlarged upper left corner). Between the two muscular tunica layers a nervous net with ganglia is present (down right corner; hematoxylin-eosin stained). F = folds
Stomach wall organization does not show any difference among the three analyzed segments; also as far as conventional histochemistry is concerned, no differences were observed among the three analyzed segments of trout stomach (see in Supplementary material).
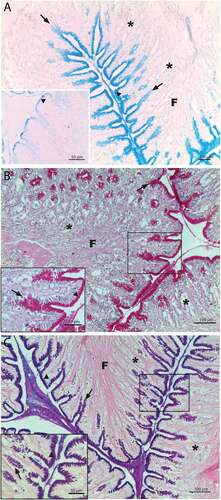
PAS showed very strong positivity in the supranuclear region and cell coat of the epithelial lining cells and in the gastric pits ()). AB pH 2.5 showed a similar binding pattern distribution at epithelial lining level, while showed a positivity ranging from moderate to strong in gastric pits. AB reactive sites decreased with pH 1 treatment, above all in gastric pits ()). AB/PAS were positive in epithelium and gastric pits, all reactive structures showed a prevalently PAS affinity particularly evident in gastric pits (). In gastric glands no binding patterns were present for any histochemical treatment.
Figure 3. Rainbow trout fundic portion of stomach. AB staining pH 2.5 (a) show reactive binding sites at epithelial lining, cell coat and gastric pit level. The same treatment at pH 1 strongly decrease the reaction response, above all as epithelial supranuclear region and gastric pits regards (down left corner). PAS staining (b) evidence very strong response by epithelium and gastric pits; down left corner is the enlargement of the box in the photo. AB/PAS staining (c) show that epithelial cells are prevalently AB positive at both cell coat and cytoplasm level, while gastric pits show a prevalently PAS positivity to the histochemical treatment; down left corner is the enlargement of the box in the photo. Epithelial lining (▲), gastric pit (↑), gastric glands (*), F = folds
The above results for trout stomach structures to conventional histochemistry are summarized in .
Table II. Histochemical reactivity of stomach structures
Comparison between AB pH 2.5 and AB pH 1 showed statistically significant differences in the response of the reactive structures, except for gastric glands, to the two sequential treatment (). The most significant differences were identified in the supranuclear region and gastric pits ().
Table III. Statistical significance of differences (p values) between histochemical treatments AB pH 2.5 and AB pH 1, as performed by Wilcoxon signed rank test and paired t-test. p values were adjusted for multiple testing using the Holm correction
To better understand the results, it should be considered that the specific complex carbohydrate kinds are mainly identified taking into account the reactivity difference by comparing sequential treatments (Dall’Aglio et al. Citation2020b). So, the difference between the AB pH 2.5 and pH 1 reactivity is ascribable to carboxylated complex carbohydrates, while the AB pH 1 residual reactivity is due to sulphated complex carbohydrates with the exception of highly sulphated-like material since the lack of metachromasia to toluidine blue staining. In addition, the prevalently PAS positive response to AB/PAS treatment of some structures indicates the presence of neutral glycoproteins.
On the basis of the above considerations, glycohistochemical evidences allow to hypothesize that in rainbow trout the stomach structures of produce the complex carbohydrate types listed in , in descending semi-quantitative order.
Table IV. Complex carbohydrate types produced by stomach structures
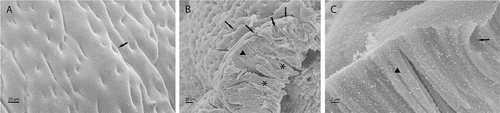
Scanning electron microscope studies showed that the mucosa lifts up to form longitudinal folds supported by a connective axis. The luminal surface is characterized by a large number of opening related to numerous gastric pits (). The lining epithelium of the mucosa is of a simple prismatic type, with high cells that at the apical membrane level show a thin cell coat ()) and which rest on a connective layer thickly occupied by tubular gastric glands ()).
Figure 4. SEM images of fundic portion of stomach inner surface (a) in which the outlets of the gastric pits are well visible (↑). SEM cross section of stomach mucosae (b) showing the epithelial columnar lining (▲), the gastric pits (↑) and the tubular gastric glands (*). In C it is showed an enlargement of epithelium (▲) with a gastric pit (↑)
Discussion
This study provided more in-depth information on the morphological and glycohistochemical aspects of rainbow trout stomach in adult subjects reared in natural-like conditions, completing the anatomical description by SEM technique.
Rainbow trout stomach showed a J-shaped, that is the configuration most commonly found in fish species (Wilson & Castro Citation2010). Presumably, this shape may help in extending the duration that food stays in the stomach, thus promoting better enzymatic digestion. Rainbow trout, as other salmonid species, is a carnivore fish: its diet is composed by insects, crustaceans and small fishes, and the presence of wall inner folds allows trout’s stomach to be easily extendable enabling the fish to swallow large prey whole and increases the surface area allowing efficient mixing of food with gastric juice.
Although the structure of the wall has the same stratification noted in the majority of other Teleosts, there are some variations that are mostly related to the species’ feeding habits. We observed four layers: mucosa in which the muscularis mucosae is not present, in contrast to what described by Weinreb and Bilstad (Citation1955), propria-submucosa, muscular and serosa. Like in other Teleosts, the lining epithelium of gastric mucosa is represented by a simple layer of prismatic cells showing the same morphology along the entire wall of the organ. The supranuclear portion of these epithelial cells evidenced the production of a variety of complex carbohydrates. The mucosal epithelium invaginates to form gastric pits at bottom of which gastric glands open. These are common characteristics of carnivorous fish species (Vieira-Lopes et al. Citation2013). The single cell type presents in the gastric glands is described as oxyntopeptic cell, because it is generally assumed that they synthesize both hydrocloridric acid and pepsinogen, that in Mammals are produced by the oxyntic and chief cells respectively (Diaz et al. Citation2003). The presence of a consistent submucosal connective layer might have a mechanical function mostly limiting the damage to tissues in any moments of extreme fullness organ. In the stomach wall localizes a nervous control system formed by two different patterns that intertwine giving rise to two plexuses and, therefore, the development of an intramural network with cells that are organized to form small ganglia. They can be compared to a real small autonomous system with action on the smooth muscle similar to mammalian myenteric plexus of Auerbach.
Based on conventional glycohistochemical treatments response from tunica mucosae pertaining structures, we provided also the histochemical profile of rainbow trout stomach. Findings allow to argue that epithelial lining cells produce both surface and secretion glycoconjugates represented by glycoproteins and glycosaminoglycan-like carbohydrates, while gastric pits produce secretion glycosaminoglycan-like material. In particular, at epithelial cell coat level, a consistent amount of surface glycoproteins was evidenced. The strong positivity to AB pH 1 and the prevailing PAS positivity to AB/PAS treatments allows us to hypothesize that in the cell coat, the glycoproteins are mainly acid, showing the oligosaccharidic chain in which many glucosidic residues linked sulphated groups, and in lesser quantity neutral glycoproteins. Indeed, previous research (Marchetti et al. Citation2006) evidenced on the epithelial cell coat of trout stomach, the presence of both acid sialylated and neutral fucosylated glycoproteins in addition to oligosaccharidic chain with terminal residues represented by β-galactose and α-Nacetyl-galactosamine, two sugar residues proved to be linked to sulphated groups (Parillo & Verini-Supplizi Citation2001).
The acid glycoconjugates could likely confer a permanent negative charge to the cell plasma membranes, so protecting the mucosae epithelium from the highly acid gastric juice action (Scocco et al. Citation1996; Marchetti et al. Citation2006). In this last function, surface glycoconjugates are largely supported by the similar action carried out by secretion glycoconjugates produced by both stomach epithelial lining, which also acts like a secreting lamina, and gastric pits. These structures prevalently secrete carboxylated glycosaminoglycans like to hyaluronic acid and/or chondroitin, in addition to lesser amount of both chondroitinsulphate A/B/C-like glycosaminoglycans and neutral glycoproteins. Heparin and/or heparansulphated-like glycosaminoglycan production can be excluded by the lack of metachromatic response of toluidin blue treated samples. The great amount of glycosaminoglycans draws large quantities of water to form a viscoelastic barrier able to guarantee a good mucosae hydration degree, in addition to protect the epithelial apical membrane from both HCl and peptidase action (Mercati et al. Citation2020; Dall’Aglio et al. Citation2020a). Finally, an important involvement of produced complex carbohydrates in the defense of rainbow trout stomach mucosae from pathogen agents have to be considered; in fact, the several kinds of glycoconjugates could hamper the attach of viruses and/or bacteria to the stomach mucosae, acting as hapten-like sites and enveloping the pathogen agents as hypothesized in fish and in different animal species (Scocco & Pedini Citation2006; Scocco et al. Citation2017; Dall’Aglio et al. Citation2020b), in addition to prevent some pathogen enzymatic strategies (Ohya & Kaneko Citation1970; Yamada & Hirano Citation1973; Hanaoka et al. Citation1989; Zimmer et al. Citation1992; Nieuw-Amerongen et al. Citation1995; Scocco et al. Citation1997, Citation1998).
In conclusion, our work integrates the morphology description of adult rainbow trout’s stomach and defines its glycohistochemical profile. Our findings offer the baseline to further researches aimed to monitor modifications linked to environmental changes and to assess the best feed strategies in aquaculture. However, more studies should be carried out for a deeper understanding of the digestion process and nutrient absorption of these fish.
Author contributions
Conceptualization, A.C. and P.S.; methodology and validation, E.D.F. and A.C.; formal analysis, F.M.T.; investigation, A.C. and C.M.A.B.; data curation, A.P., D.G. and C.M.A.B.; writing—original draft preparation, E.D.F. and P.S.; writing—review and editing, E.D.F.; supervision, A.C and P.S. All authors have read and agreed to the published version of the manuscript.
Supplemental Material
Download MS Word (385 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Allen A. 1981. Structure and function of gastrointestinal mucus. In: Johnson LR, editor. Physiology of the gastrointestinal tract. New York: Raven Press. pp. 617–639.
- Banan Khojasteh SM, Sheikhzadeh F, Mohammadnejad D, Azami A. 2009. Histological, histochemical and ultrastructural study of the intestine of rainbow trout Oncorhynchus mykiss). World Applied Sciences Journal 6(11):1525–1531.
- Bancroft JD, Gamble A. 2008. Theory and practice of histological techniques. 6th ed. London, UK: Churchill-Livingstone.
- Berillis P, Mente E. 2017. Histology of goblet cells in the intestine of the rainbow trout can lead to improvement of the feeding management. Journal of Fisheries Sciences 11(4):032–033. DOI: 10.21767/1307-234X.1000139.
- Bielek E. 2002. Rodlet cells in teleosts: New ultrastructural observations on the distribution of the cores in trout (Oncorhynchus mykiss, Salmo trutta L.). Journal of Submicroscopic Cytology and Pathology 34:271–278.
- Cao XJ, Wang WM. 2009. Histology and mucin histochemistry of the digestive tract of yellow catfish, Pelteobagrus fulvidraco. Anatomia, Histologia, Embryologia 38:254–261. DOI: 10.1111/j.1439-0264.2009.00932.x.
- Carrassón M, Grau A, Dopazo R, Crespo SA. 2006. Histological, histochemical and ultrastructural study of the digestive tract of Dentex dentex (Pisces, Sparidae). Histology and Histopathology 21:579–593. DOI: 10.14670/HH-21.579.
- Chatchavalvanich K, Marcos R, Poonpirom J, Thongpan A, Rocha E. 2006. Histology of the digestive tract of the freshwater stingray Himantura signifer Compagno and Roberts, 1982 (Elasmobranchii, Dasyatidae). Anatomy and Embryology 211:507–518. DOI: 10.1007/s00429-006-0103-3.
- Choudhary OP, Choudhary P. 2017. Scanning electron microscope: Advantages and disadvantages in imaging components. International Journal of Current Microbiology and Applied Sciences 6:1877–1882. DOI: 10.20546/ijcmas.2017.605.207.
- Coyle SD, Durborow RM, Tidwell JH. 2004. Anesthetics in aquaculture. USA: Southern Regional Aquaculture Center, Mississippi State University. SRAC Publication No. 3900.
- Dall’Aglio C, Mercati F, De Felice E, Tardella FM, Kamphues J, Cappai MG, Scocco P. 2020b. Influence of different feed physical forms on mandibular gland in growing pigs. Animals 10(5):910. DOI: 10.3390/ani10050910.
- Dall’Aglio C, Mercati F, Faeti V, Acuti G, Trabalza Marinucci M, De Felice E, Tardella FM, Franciosini MP, Casagrande Proietti P, Catorci D, Stacchini P, Pastorelli A, Scocco P. 2020a. Immuno- and glyco-histochemistry as a tool to evaluate the oregano supplemented feed effects in pig gut. European Journal of Histochemestry 64(1):3110. DOI: 10.4081/ejh.2020.3110.
- de Oliveira MIB, de Matos LV, da Silva LA, Chagas EC, da Silva GS, Gomes ALS. 2019. The digestive tube of Piaractus brachypomus: Gross morphology, histology/histochemistry of the mucosal layer and the effects of parasitism by Neoechinorhynchus sp. Journal of Fish Biology 94:648–659. DOI: 10.1111/jfb.13934.
- Diaz AO, Garcia AM, Devincenti CV, Goldemberg AL. 2003. Morphological and histochemical characterization of the mucosa of the digestive tract in Engraulis anchoita (Hubbs and Marini, 1935). Anatomia, Histologia, Embryologia 32:341–346. DOI: 10.1111/j.1439-0264.2003.00490.x.
- Diaz AO, Garcia AM, Gldemberg AL. 2008. Glicoconjugates in the mucosa of the digestive tract of Cynoscion guatucupa: A histochemical study. Acta Histochemica 110:76–85. DOI: 10.1016/j.acthis.2007.08.002.
- Domeneghini C, Arrighi S, Radaelli G, Bosi G, Veggetti A. 2005. Histochemical analysis of glycoconjugate secretion in the alimentary canal of Anguilla anguilla L. Acta Histochemica 106:477–487. DOI: 10.1016/j.acthis.2004.07.007.
- Domeneghini C, Pannelli Straini R, Veggetti A. 1998. Gut glycoconjugates in Sparus aurata L. (Pisce, Teleostei). A comparative histochemical study in larval and adult ages. Histology and Histopathology 13:359–372. DOI: 10.14670/HH-13.359.
- Dos Santos ML, Arantes FP, Santiago KB, Dos Santos JE. 2015. Morphological characteristics of the digestive tract of Schizodon knerii (Steindachner,1875), (Characiformes: Anostomidae): An anatomical, histological and histochemical study. Anais Da Academia Brasileira De Ciencias (Annals of the Brazilian Academy of Sciences) 87:867–878. DOI: 10.1590/0001-3765201520140230.
- Ezeasor DN. 1981. The fine structure of the gastric epithelium of the rainbow trout, Salmo gairdneri, Richardson. Journal of Fish Biology 19:611–627. DOI: 10.1111/j.1095-8649.1981.tb03828.x.
- Ezeasor DN, Stokoe WM. 1981. Light and electron microscopic studies on the absorptive cells of the intestine, caeca and rectum of the adult rainbow trout, Salmo gairdneri, Rich. Journal of Fish Biology 18:527–544. DOI: 10.1111/j.1095-8649.1981.tb03794.x.
- Faccioli CK, Chedid RA, Do Amaral AC, Bastos Franceschini Vicentini I, Vicentini CA. 2014. Morphology and histochemistry of the digestive tract in carnivorous freshwater Hemisorubim platyrhynchos (Siluriformes: Pimelodidae). Micron 64:10–19. DOI: 10.1016/j.micron.2014.03.011.
- Gallagher ML, Lczkovich JJ, Stellwag EJ. 2005. Characterization of the ultrastructure of the gastrointestinal tract mucosa, stomach contents and liver enzyme activity of the pinfish during development. Journal of Fish Biology 58:1704–1713. DOI: 10.1111/j.1095-8649.2001.tb02324.x.
- Garrido MV, Torres MI, Equisoain MA. 1998. Histological, histochemical and ultrastructural analysis of the gastric mucosa in Oncorhynchus mykiss. Aquaculture 115:121–132. DOI: 10.1016/0044-8486(93)90363-4.
- Ghosh SK, Chakrabarti P. 2015. Histological and histochemical characterization on stomach of Mystus cavasius (Hamilton), Oreochromis niloticus (Linnaeus) and Gudusia chapra (Hamilton): Comparative study. The Journal of Basic & Applied Zoology 70:16–24. DOI: 10.1016/j.jobaz.2015.04.002.
- Hanaoka K, Pritchett TJ, Tokasaki S, Kochibe N, Sabesan S, Paulson JC, Kobata A. 1989. 4-O-acetyl-N-acetylneuraminic acid in the N-linked carbohydrate structures of equine and guinea pig α2 – Macroglobulins, potent inhibitors of influenza virus infection. Journal of Biological Chemistry 264:9842–9849. DOI: 10.1016/S0021-9258(18)81735-5.
- Heidarieh M, Mirvaghefi AR, Akbari M, Farahmand H, Sheikhzadeh N, Shahbazfar AA, Behgar M. 2012. Effect of dietary Ergosan on growth performance, digestive enzymes, intestinal histology, hematological parameters and body composition of rainbow trout (Oncorhynchus mykiss). Fish Physiology and Biochemistry 38:1169–1174. DOI: 10.1007/s10695-012-9602-8.
- Kapoor BG, Smit H, Verighina A. 1975. The alimentary canal and digestion in teleosts. In: Russell FS, Yonge M, editors. Advances in marine biology 13. London, New York: Academic Press. pp. 109–239.
- Kasozi N, Degu GI, Mukalazi J, Drago Kato C, Kisekka M, Owori Wadunde A, Kityo G, Tibenda Namulawa V. 2017. Histomorphological description of the digestive system of Pebbly Fish, Alestes baremoze (Joannis, 1835). The Scientific World Journal 2017:8591249. DOI: 10.1155/2017/8591249.
- Marchetti L, Capacchietti M, Sabbieti G, Accili D, Materazzi G, Menghi G. 2006. Histology and carbohidrate histochemistry of the alimentary canal in the rainbow trout Oncorhynchus mykiss. Journal of Fish Biology 68:1808–1821. DOI: 10.1111/j.1095-8649.2006.01063.x.
- Melis M, Carpino F, Di Tondo U. 1992. Tecniche in anatomia patologica. Milan, Italy: Edi-Ermes. pp. 1–512.
- Mennigen JA, Zhang D. 2016. MicroTrout: A comprehensive, genome-wide miRNA target prediction framework for rainbow trout, Oncorhynchus Mykiss. Comparative Biochemistry and Physiology. Part D, Genomics & Proteomics 20:19–26. DOI: 10.1016/j.cbd.2016.07.002.
- Mercati F, Dall’Aglio C, Acuti G, Faeti V, Tardella FM, Pirino C, De Felice E, Scocco P. 2020. Oregano feed supplementation affects glycoconjugates production in swine gut. Animals 10(1):149. DOI: 10.3390/ani10010149.
- Mireşan V, Cocan D, Miclauş V, Cadar M. 2012. Morpho – Histological study of esophagus and stomach in rainbow trout (Onchorynchus mykiss) from fiad farm. Animal Science and Biotechnologies 69:297–298.
- Moawad UK, Awaad AS, Tawfiek MG. 2017. Histomorphological, histochemical, and ultrastructural studies on the stomach of the adult African catfish (Clarias gariepinus). Journal of Microscopy and Ultrastructure 5(3):155–166. DOI: 10.1016/j.jmau.2016.08.002.
- Murray HM, Wright GM, Goff GP. 1994. A comparative histological and histochemical study of the stomach from three species of pleuronectid, the Atlantic halibut, Hippoglossus hippoglossus, the yellowtail founder, Pleuronectes ferruginea, and the winter flounder, Pleuronectes americanus. Canadian Journal of Zoology 72(7):1199–1210. DOI: 10.1139/z94-161.
- Namulawa VT, Kato CD, Nyatia E, Kiseka M, Rutaisire J. 2014. Histochemistry and Ph characterization of the gastrointestinal tract of Nile perch Lates niloticus. World Journal of Fish and Marine Sciences 6(2):162–168. DOI: 10.5829/idosi.wjfms.2014.06.02.82216.
- Nieuw-Amerongen AV, Bolscher JGM, Veermar ECI. 1995. Salivary mucins: Protective functions in relation to their diversity. Glycobiology 5(8):733–740. DOI: 10.1093/glycob/5.8.733.
- Nofouzi K, Sheikhzadeh N, Varshoie H, Sharabyani SK, Jafarnezhad M, Shabanzadeh S, Ahmadifar E, Stanford J, Shahbazfar A. 2019. A beneficial effect of killed Tsukamurella inchonensis on rainbow trout (Oncorhynchus mykiss) growth, intestinal histology, immunological, and biochemical parameters. Fish Physiology and Biochemistry 45:209–217. DOI: 10.1007/s10695-018-0555-4.
- Ohya T, Kaneko Y. 1970. Novel hyaluronidase from streptomyces. Biochimica et Biophisica Acta – Enzimology 198:607–609. DOI: 10.1016/0005-2744(70)90139-7.
- Ostos Garrido MV, Nunez Torres MI, Abaurrea Equisoain MA. 1993. Histological, histochemical and ultrastructural analysis of the gastric mucosa in Oncorhynchus mykiss. Aquaculture 115:121–132. DOI: 10.1016/0044-8486(93)90363-4.
- Parillo F, Verini-Supplizi A. 2001. Glycohistochemistry of the zona pellucida of developing oocytes in the rabbit and hare. Research in Veterinary Science 70:257–264. DOI: 10.1053/rvse.2001.0472.
- Pearse AGE. 1985. Histochemistry, theoretical and applied. Edinburgh, UK: Churchill Livingstone.
- Pedini V, Dall’Aglio C, Parillo F, Scocco P. 2004. A lectin histochemical study of the oesophagus of shi drum. Journal of Fish Biology 64:625–631. DOI: 10.1111/j.1095-8649.2004.00326.x.
- Pedini V, Dall’Aglio C, Parillo F, Scocco P. 2005. Glycoconjugate distribution in gastric fundic mucosa of Umbrina cirrosa L. revealed by lectin histochemistry. Journal of Fish Biology 66:222–229. DOI: 10.1111/j.0022-1112.2005.00596.x.
- R Core Team. 2019. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available: http://www.R-project.org/.
- Ramos MA, Gonçalves JFM, Batista S, Costas B, Pires MA, Rema P, Ozorio ROA. 2015. Growth, immune responses and intestinal morphology of rainbow trout (Oncorhynchus mykiss) supplemented with commercial probiotics. Fish & Shellfish Immunology 45:19–26. DOI: 10.1016/j.fsi.2015.04.001.
- Reid PE, Volz D, Cho KY, Owen DA. 1988. A new method for the histochemical demonstration of O-acyl sugars in human colonic epithelial glycoproteins. The Histochemical Journal 20:510–518. DOI: 10.1007/BF01002649.
- Reifel CW, Travill AA. 1977. Structure and carbohydrate histochemistry of the oesophagus in ten teleostean species. Journal of Morphology 152:303–314. DOI: 10.1002/jmor.1051520303.
- Reifel CW, Travill AA. 1979. Structure and carbohydrate histochemistry of the intestine in ten teleostean species. Journal of Morphology 162:343–360. DOI: 10.1002/jmor.1051620305.
- Saadatfar Z, Shahsavani D, Khoshnegah S. 2010. Development of stomach in Sturgeon (Acipenser persicus). Journal of Applied Animal Research 37:153–156. DOI: 10.1080/09712119.2010.9707115.
- Scocco P, Accili D, Menghi G, Ceccarelli P. 1998. Unusual glycoconjugates in the oesophagus of a tilapine polyhybrid. Journal of Fish Biology 53:39–48. DOI: 10.1111/j.1095-8649.1998.tb00107.x.
- Scocco P, Ceccarelli P, Menghi G. 1996. Glycohistochemistry of the Tilapia spp. stomach. Journal of Fish Biology 49:584–593. DOI: 10.1111/j.1095-8649.1996.tb00056.x.
- Scocco P, Forte C, Franciosini MP, Mercati F, Casagrande-Proietti P, Dall’Aglio C, Acuti G, Tardella FM, Trabalza-Marinucci M. 2017. Gut complex carbohydrates and intestinal microflora in broiler chickens fed with oregano (Origanum vulgare L.) aqueous extract and Vitamin E. Journal of Animal Physiology and Animal Nutrition 101:676–684. DOI: 10.1111/jpn.12588.
- Scocco P, Menghi G, Ceccarelli P. 1997. Histochemical differentiation of glycoconjugates occurring in the tilapine intestine. Journal of Fish Biology 51:848–857. DOI: 10.1111/j.1095-8649.1997.tb02005.x.
- Scocco P, Pedini V. 2006. Equine mandibular gland: In situ characterisation of sialoderivatives. Equine Veterinary Journal 38:410–415. DOI: 10.2746/042516406778400637.
- Shabanzadeh S, Shapoori M, Sheikhzadeh N, Nofouzi K, Oushani AK, Enferadi MHN, Mardani K, Shahbazfar A. 2015. A growth performance, intestinal histology, and biochemical parameters of rainbow trout (Oncorhynchus mykiss) in response to dietary inclusion of heat-killed Gordonia bronchialis. Fish Physiology and Biochemistry 42:65–71. DOI: 10.1007/s10695-015-0117-y.
- Smith LS. 1989. Digestive functions in teleosts fishes. In: Halver JE, editor. Fish nutrition. 2nd ed. San Diego: Academic Press. pp. 331–421.
- Verdile N, Pasquariello R, Scolari M, Scirè G, Brevini TAL, Gandolfi FA. 2020. Detailed study of rainbow trout (Onchorhynchus mykiss) intestine revealed that digestive and absorptive functions are not linearly distributed along its length. Animals (Basel) 10(4):745. DOI: 10.3390/ani10040745.
- Vieira-Lopes DA, Pinheiro NL, Sales A, Ventura A, Araújo FG, Gomes ID, Nascimento AA. 2013. Immunohistochemical study of the digestive tract of Oligosarcus hepsetus. World Journal of Gastroenterology 19(12):1919–1929. DOI: 10.3748/wjg.v19.i12.1919.
- Weinreb EL, Bilstad N. 1955. Histology of the digestive tract and adjacent structures of the rainbow trout, Salmo gairdneri irideus. Copeia 3:94–204. DOI: 10.2307/1440460.
- Wilson JM, Castro LFC. 2010. Morphological diversity of the gastrointestinal tract in fishes. Fish Physiology 30:1–55. DOI: 10.1016/S1546-5098(10)03001-3.
- Yamada K, Hirano K. 1973. The histochemistry of hyaluronic acid-containing mucosubstances. Journal of Histochemistry and Cytochemistry 21:469–472. DOI: 10.1177/21.5.469.
- Zimmer G, Reuter G, Schauer R. 1992. Use of influenza c-virus for detection of 9-O-acetylated salic acids on immobilized conjugates by esterase activity. European Journal of Biochemistry 204:209–215. DOI: 10.1111/j.1432-1033.1992.tb16626.x.
