?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The fecundity and sexual structure of weatherfish (Misgurnus fossilis) population, an inperilled and protected freshwater fish with a poorly known life history, was studied in three waterbodies: the River Ner, the Południowy canal and the Nowy Rów canal (Poland) differing in hydrological character. We compared reproductive traits; i.e. sex ratio, absolute and relative fecundity, oocyte size, gonado-somatic index and body condition. In all study sites, the sex ratio of weatherfish did not differ from parity (1: 1), though in the River Ner, the highest GSI values were recorded for females whilst male GSI did not differ among sites. The absolute (FA = 2860 ± 2065) and relative fecundity (FR = 120 ± 55 eggs per g of female body weight) in the River Ner were significantly lower than in the other two sites. In the River Ner the frequency distribution of oocyte diameter, decomposed using the Bhattacharya method, showed two distinct groups in equal numbers. Oocytes that were ready to spawn were larger in the River Ner than in the Południowy and Nowy Rów canals. Fish in the River Ner were also characterized by the lowest Fulton condition index (mean K = 0.36 ± 0.1). The trade-off between impaired fecundity and increased egg diameter may result from the different nature of the studied watercourses or levels of endocrine-disrupting chemicals (EDCs), such as steroid compounds.
Introduction
The weatherfish Misgurnus fossilis is a small, freshwater, benthic cobitid, naturally distributed through Central and Eastern Europe, from the northern border of the Alps (North France) to western Russia (Kottelat & Freyhof Citation2007). Weatherfish are demersal and have the ability to burrow into soft mud during dry periods or frosts (Boroń et al. Citation2002; Kottelat & Freyhof Citation2007). Because they are able to use atmospheric oxygen (intestinal breathing) the species can tolerate low dissolved oxygen levels (Jakubowski Citation1958). This fish species can tolerate unfavourable environmental conditions and a relatively high level of pollution (Jakubowski Citation1958; Drozd et al. Citation2009; Pyrzanowski et al. Citation2019). It prefers shallow waterbodies with sandy substrates covered with a thick layer of organic deposits, often densely overgrown with macrophytes. Typical habitats for weatherfish are slow-flowing rivers, canals and drainage ditches, oxbow and unmanaged lakes and fish ponds (Meyer & Hinrichs Citation2000; Pekarik et al. Citation2008; Mazurkiewicz Citation2012). Basic information on the life-history of M. fossilis, which is essential for conservation, is scarce (Kottelat & Freyhof Citation2007). Apart from this, M. fossilis is considered endangered due to habitat loss and was listed in the European Fauna-Flora-Habitat and Natura 2000 directives (Annex II of the Council Directive 92/43/EEC), representing species of European Community interest (E.U. Citation1992) and is included in many national red lists of endangered and protected fish species (Drozd et al. Citation2009; Hartvich et al. Citation2010). In Europe, the weatherfish has been classified as species of low concern (LC) (Freyhof & Brooks Citation2011), but observed genetic diversity is the lowest reported for any European freshwater fish, and it has been proposed that its threat level should be raised (Bohlen et al. Citation2007). In the light of a widespread decline in weatherfish populations, it is necessary to obtain baseline data on their life history traits, which are a prerequisite for rational conservation planning (Wootton & Elvira Citation2000). The aim of this study was to provide detailed information on the reproductive biology of M. fossilis populations inhabiting three waterbodies in west-central Poland.
Materials and methods
Study area and sampling site
Study sites were in three watercourses: the River Ner (52°08ʹ83.76ʹ’ N; 18°87ʹ70.17ʹ’ E), the Południowy drainage canal (52°13ʹ14.86ʹ’ N; 19°48ʹ03.62ʹ’ E) and the Nowy Rów drainage canal (51°12ʹ38.29ʹ’ N; 16°43ʹ17.34ʹ’E). All sites were located in the Nature 2000 areas, i.e. the River Ner and Południowy canal in Pradolina Bzury-Neru (PLH100006) and the Nowy Rów canal in Łęgi Odrzańskie (PLB020008). The River Ner is lowland, right-hand tributary of the River Warta, that receives treated wastewater from Łódź, the third largest city in Poland, and is characterized by a high level of water pollution (Kostrzewa Citation1999; Mosiej et al. Citation2007; Penczak et al. Citation2010; Pyrzanowski et al. Citation2015). The main source of pollution is municipal and industrial wastewater (Mosiej et al. Citation2007). The main characteristic of examined sited are given in . Along its entire length, the River Ner flows mainly among farmlands, wastelands and meadows. Both drainage canals, along their entire length, are located in Natura 2000 areas. Their catchment areas are typically agricultural, mainly dominated by meadows, pastures and peat bogs. The Nowy Rów canal is part of the drainage system that discharges water to the River Odra, whereas the Południowy canal drains water directly to the River Bzura. In the River Ner M. fossilis co-exists with bream (Blicca bjoerkna), roach (Rutilus rutilus), ruffe (Gymnocephalus cernua), perch (Perca fluviatilis), gudgeon (Gobio gobio) and bleak (Alburnus alburnus). In both drainage canals the weatherfish is the dominant fish species. In case of the Południowy canal it co-exists with pike (Esox lucius), crucian carp (Carassius carassius), roach and tench (Tinca tinca), while in the Nowy Rów canal it occurs together with tench (Pyrzanowski et al. Citation2015, Citation2020).
Table I. Characteristics of weatherfish M. fossilis sampling sites: Południowy canal, the River Ner and the Nowy Rów canal
Sampling
The fish samples were performed at the peak of reproductive season (April) 2014 and 2015 at three sampling sites, and to maintain the uniformity of gonad developmental conditions, sampling dates were adjusted to the pattern of prior local temperature. Fish were caught by electrofishing (EFGI 650; BSE Specialelektronik Bretschneider, Germany), immediately euthanized with an overdose of clove oil (Javahery et al. Citation2012), chilled and subsequently frozen in the laboratory.
Analysis
In the laboratory, all specimens were measured for total length (TL) and standard length (SL) to the nearest 1 mm and weighed (W) to the nearest 10 mg before dissection. The relationship between SL and TL was described by the equation: SL = 0.889 × TL – 1.728; r2 = 0.99; p < 0.001. Fish sex (female, male or juvenile) was determined by visual examination of secondary sexual characteristics and confirmed by dissection of the gonads. The sex-ratio of all populations was calculated as the number of males divided by the total number of individuals (males plus females) in the population. For each sampling date and site, the observed proportion of males to females was tested for deviations from a 1:1 sex ratio using a binomial test (Wilson & Hardy Citation2002). Fish gonads were removed, weighed (WG) (nearest 10 mg) and preserved in glycerine. To determine reproductive allocation, the gonado-somatic index (GSI) (Wootton Citation1998) was calculated using: GSI = 100WG/W. From each ovary, three subsamples (anterior, central and posterior part of left gonad) were collected and weighed (nearest 0.1 mg). Oocytes were photographed under a stereomicroscope (Nikon SMZ1000), counted and their diameters measured to the nearest 0.001 mm using LUCIA 5 image analysis software. The gravimetric method was used to evaluate absolute fecundity (FA) – oocytes number per female and relative fecundity (FR); i.e. number of oocytes per 1 g of female body weight was calculated (Bagenal Citation1978). For each site, oocyte diameters data were pooled and used to obtain their size-frequency histograms. If the frequency of oocyte size distribution was polynomial the Bhattacharya method followed by modal class progression analysis was used to decompose observed distributions into Gaussian components (Bhattacharya Citation1967). This procedure allows separation of discrete normal distributions in the data based on a separation index (SI) in the case that the difference between successive Gaussian means divided by the difference between their standard deviations exceeded 2 (Gayanilo et al. Citation2005). For each fish the Fulton condition factor (K) was calculated using (Le Cren Citation1951; Ricker Citation1975):
where:
W– fish weight (g)
TL – total length (cm)
One-way analysis of variance (ANOVA II) was used to test for differences among sites for the FA, FR and oocytes size and two-factor ANOVA (site × sex as factors) was performed for GSI and K. When significant differences were observed, multiple comparison tests (post-hoc HDS Tukey test) were used (Zar Citation2010). All values were reported as mean ± standard error. Before running ANOVA, the data were examined for normality (Shapiro-Wilk test) and homogeneity of variance (Levene’s test).
The relationships between body weight (W), gonad weight (WG), absolute fecundity (FA) and total length (TL) were determined by linear regression (log-transformed data). The weight-length relationship was tested against isometry; i.e. slopes b = 3, or allometry (positive; b > 3 or negative; b < 3) with Bailey’s t-test for sex and sites. To identify differences among sites, analysis of covariance (ANCOVA) was used. Firstly, differences in slopes (b) were tested and if the null hypothesis was rejected, the common slope (bc) was calculated and differences in intercepts (a coefficient) were tested (Zar Citation2010). Tukey’s HSD post-hoc test was also used to identify which sites were responsible for differences in linear regressions (Zar Citation2010).
Results
Sex ratio
A total of 242 individual weatherfish (117 females, 97 males and 28 juveniles) were collected, of which 76 individuals were from the Południowy canal (collected in 2014), while 84 individuals were from the River Ner and 82 from the Nowy Rów canal (both collected in 2015) (). In all sites the sex ratio did not differ from parity; the Południowy canal: fM = 0.426 (p = 0.047); the River Ner: fM = 0.471 (p = 0.086); the Nowy Rów canal: fM = 0.450 (p = 0.060).
Table II. Characteristics of weatherfish M. fossilis from the Południowy canal, the River Ner and the Nowy Rów canal study sites. Mean values with standard deviation and range for total length (TL), body weight (W) and gonad weight (WG). Dates in brackets indicate sampling year
Fish condition
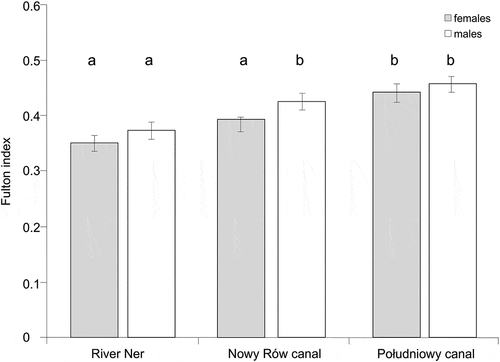
The Fulton condition index (K) () varied significantly among sites (F2, 208 = 16.80, p < 0.001) but not between males and females (F1, 208 = 1.89, p = 0.171). Moreover, there is no significant interaction between sex and site (F2, 208 = 1.93, p = 0.148). A post-hoc HDS Tukey test revealed significantly lower values for condition for both sexes in the River Ner and for females in the Nowy Rów canal. In contrast, the highest condition values were found in the Południowy (both sexes) and for males in the Nowy Rów canal ().
GSI conditions
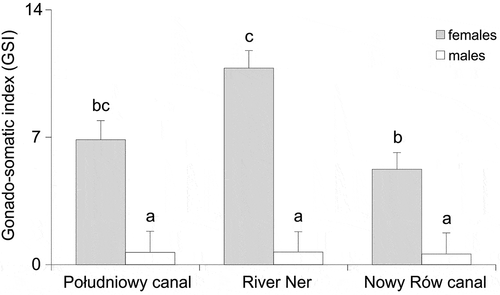
The gonado-somatic index varied significantly with sex (F1, 208 = 70.643, p < 0.001) and site (F2, 208 = 4.154, p = 0.017). In general, females GSI was over ten times higher than males GSI () and post-hoc HDS Tukey test revealed a lack of differences in males GSI among sites. For females there were differences between the Południowy canal and the River Ner (p = 0.039) and the Nowy Rów canal and the River Ner (p = 0.003) (). In all study sites, females and males GSI increased significantly with length (). Analysis of covariance (ANCOVA) showed a lack of difference in slopes among sites (F2;113 = 0.114, p = 0.891) and a common slope was bc = 5.053, (s.e.bc = 0.277). Nevertheless, differences between estimates of the coefficient a (intercept) were detected (F2;115 = 14.022, p < 0.001) and multiple comparisons (post-hoc HDS Tukey test) showed differences among all the study sites. For males, a regression of GSI on TL also showed a lack of difference in b among sites (F2,93 = 0.140, p = 0.867, bc = 2.382 (0.349)), though there were differences in intercepts (a coefficients) (F2,95 = 3.149, p = 0.044). However, multiple comparison (post-hoc HDS Tukey test) failed to indicate which sites differed from each other. In this case, the null hypothesis was not rejected and the lack of a difference in the regression of GSI on TL was accepted.
Table III. The gonadosomatic - total length regression parameters and their standard error (SE) (log-transformed data) for weatherfish M. fossilis males (M) and females (F) collected in the Południowy canal (A), the River Ner (B) and the Nowy Rów canal (C)
W-TL relationship
The relationship between body weight and total length () was isometric for females in the River Ner (t33 = 0.095, p = 0.925), but in both canals the relationship was positively allometric (t37 = 7.177, p < 0.001 in the Południowy and t41 = 2.675, p < 0.011 in the Nowy Rów). For males this relationship was positively allometric only in the Południowy canal (t27 = 6.214, p < 0.001), but in the River Ner and the Nowy Rów canal males showed isometric growth (t30 = 1.179, p = 0.248 and t34 = 1.303, p = 0.201, respectively). Weight-length relationships differed among all sampling sites, for both females (F2;111 = 6.727, p = 0.002) and males (F2;91 = 5.532, p < 0.001) (). Multiple comparisons (post-hoc HDS Tukey test) showed differences among all the study sites for both sexes.
Table IV. Weight-length regression parameters and their standard error (SE) (log-transformed data) of weatherfish M. fossilis males and females collected in the Południowy canal (A), the River Ner (B) and the Nowy Rów canal (C)
WG was significantly associated with TL (). For females, the slope of the relationship (coefficients b) between TL and WG did not differ among sites (F2;109 = 0.283, p = 0.754, bc = 8.182 (0.287)) but among sites differences were found for the coefficient a (F2;115 = 14.565, p < 0.001). A post-hoc HDS Tukey test showed differences among all study sites. A similar situation was observed in the case of males, with no among sites differences in coefficient b (F2,91 = 0.0714, p < 0.930, bc = 8.182 (0.287)), but significant differences for coefficient a (F2,91 = 49.349, p < 0.001). Post-hoc comparisons (HDS Tukey test) showed differences only between the drainage canals; i.e. the Południowy canal (A) and the Nowy Rów canal (C). Differences between the River Ner (B) and both drainage canals were not found.
Table V. Regression of gonad weight on total length (log-transformed data) of weatherfish M. fossilis males and females collected in the Południowy canal (A), the River Ner (B) and the Nowy Rów canal (C)
Fecundity
Fecundity was estimated for 48 mature females from all sites (17 from the Południowy canal, 21 from the River Ner and 10 from the Nowy Rów canal). In females from the Południowy canal absolute fecundity (FA) ranged from 2828 to 12,336 eggs (mean ± SD: 6153 ± 2812.8), relative fecundity (FR) varied from 43 to 222 eggs/g of body weight (1184 ± 51.77). In females from the River Ner, FA ranged from 355 to 5734 eggs (2859 ± 2065.0), FR from 75 to 320 eggs (124 ± 54.68), while in females from the Nowy Rów canal FA ranged from 459 to 23,776 eggs (8438 ± 10,116.2), FR from 39 to 423 eggs (148 ± 148.07).
Weatherfish absolute fecundity varied among sites (F2,45 = 4.557, p = 0.016), and a post-hoc HDS Tukey test showed differences between the Południowy canal and the River Ner (p = 0.012). Similarly, there were differences among sites in relative fecundity (F2,45 = 4.869, p = 0.012). A post-hoc Tukey’s test showed differences between the Południowy canal and the River Ner (p = 0.0415), the Nowy Rów canal and the River Ner (p = 0.030). However, relative fecundity (FR) did not differ between canals (p = 0.834).
Across all sites FA increased significantly with fish length, though coefficients b and a did not differ among sites. The relationship between TL and FA for all sites took the form: FA = 5.494 (0.633) × log TL - 8.816 (1.423), r2 = 0.621, p < 0.001.
Oocyte size
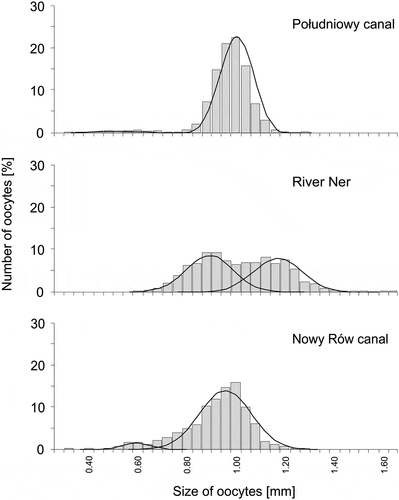
Oocyte diameter of females from the Południowy canal ranged from 0.30 to 1.30 mm (0.99 mm ± 0.63), while their mean (±SD) wet weight was 0.78 mg (± 0.331). For females from the River Ner oocyte diameter ranged from 0.54 to 1.64 mm (mean 1.16 mm ± 0.97) and mean weight was 1.51 mg (± 0.893). For females from the Nowy Rów canal oocyte diameter ranged from 0.29 to 1.25 mm (mean 0.91 mm ± 0.97) and mean weight was 1.11 mg (± 0.671). In all study sites, egg diameter histograms were polymodal and Bhattacharya’s method revealed two distinctive size groups in each population. In the Południowy and the Nowy Rów canals a small fraction of small oocytes were observed (). These small oocytes reached a size (mean ± SD) of 0.58 mm (± 0.081) and 0.59 mm (± 0.059) in Południowy and Nowy Rów canal and constituted 4.0 and 5.8% of the oocyte population, respectively. In the two canals, larger oocytes dominated in the gonads and measured: 1.00 mm (± 0.064) and 0.91 mm (± 0.098), respectively. Meanwhile, in the River Ner the number of small oocytes was similar to the number of larger oocytes, and differences in their size were less distinct (). In the River Ner, the diameter of small oocytes average 0.89 mm (± 0.094) and the average size of the larger class of oocytes was 1.17 mm (± 0.097). In the River Ner, oocytes ready to spawn were larger than those at the corresponding stage from the canal sites (F2,726 = 39.69, p < 0.0001). A post-hoc Tukey’s test revealed differences between the Południowy canal and the River Ner (p < 0.001), the Nowy Rów canal and the River Ner (p < 0.001) and with no difference between canals (p = 0.998).
Discussion
For the weatherfish populations the sex ratio did not differ from the 1: 1 ratio, typical for this species (Meyer & Hinrichs Citation2000; Pyrzanowski et al. Citation2020), though in wild populations different levels of ploidy may occur (Raicu & Taisescu Citation1972; Drozd et al. Citation2010). In the case of other cobitids, especially the genera Cobitis and Sabanejewia, the sex ratio is usually biased towards females due to polyploidy (Bohlen & Ritterbusch Citation2000). The mechanisms of sex determination and sex reversal in fish not only vary among species, but is also complex and still not well understood (Leet et al. Citation2011; Wootton & Smith Citation2015). Many factors in the aquatic environment may affect the proportion of the sexes in wild fish populations because of the sensitivity of gonad tissue to hormonal treatments, especially in the early life stages (Smith & Wootton Citation2016).
In the case of weatherfish there are no data on the condition of individuals of this species. In our study, Fulton’s condition coefficient varied significantly among sites but surprisingly not between sex. Fulton’s and other coefficient are an attempt to realize from relation of body weight and length (Le Cren Citation1951; Bolger & Connolly Citation1989; Blackwell et al. Citation2000) but if the weight-length relationship is allometric (b ≠ 3) Fulton’s coefficient index is not applicable. As our result showed, only males in the River Ner and the Nowy Rów canal display isometric growth pattern. Similar like condition, the weight-length regression data are also scarce. As we know such regression was published only for Belgium and also proved positive allometric weight-length relation (Verreycken et al. Citation2011). Also relative body weight (Wr) suggested as the index of condition (Blackwell et al. Citation2000), can not be calculated due to small dataset. In such a situation slope of weight-length regression (Bolger & Connolly Citation1989) or residual analysis (Fechhelm et al. Citation1995) could be a measure of the condition for population. Being aware of these problems Fulton’s factor was calculated because of its popularity in fish biology studies.
Gonadosomatic index (GSI), which relates gonad weight to body weight, is commonly used in fish ecology to quantify reproductive development in fish (Wootton Citation1998). Variation in the GSI fluctuation over time reflects not only gonad development but it is also a proxy to assess the energy allocation between reproduction and growth; i.e. as a surrogate of reproductive effort (Stearns Citation1992; Wootton Citation1998). Like other loaches, weatherfish showed common differences in GSI between the sexes, with female GSI over 10 times larger than that of males. Among cobitid loaches that are multiple spawners, female GSI usually varies between 18% and 26% immediately prior to reproduction (Mills & Eloranta Citation1985; Saat et al. Citation2003; Mousavi Sabet et al. Citation2011). The gonad weight of both sexes is usually significantly associated with fish size (). Thus, the GSI suffers from the disadvantage of all such indices (e.g. conditions factors) and the index reflects the appropriate regression of gonad size on body length (Bolger & Connolly Citation1989; Wootton Citation1998). However, in our study variation in weatherfish GSI among sites showed a similar pattern to that seen for the regression between gonad weight and TL.
In both drainage canals, weatherfish had relatively higher absolute fecundity (on average of 6100 oocytes in the Południowy canal and 8400 in the Nowy Rów canal), compared with the River Ner with an absolute fecundity of 2800 eggs per female. A study by Drozd et al. (Citation2009) for weatherfish from a floodplain area in the Czech Republic, showed the total number of eggs per female ranged between 5800 and 7900, a figure comparable to the Południowy and Nowy Rów canals. Under natural conditions, the reproductive potential of weatherfish can be double this figure, as reported by Podubski and Štedronský (Citation1954) for a population from southern Bohemia, Opalatenko (Citation1974), Kouril et al. (Citation1996) and Adamkova-Stibranyiova et al. (Citation1999).
The absolute maximum fecundity might be determined in the process that controls spawning. In German fish farms, fecundity was artificially increased from 15,900 to 25,800 eggs (Geldhauser Citation1992). In case of related species, such as the oriental weatherfish (Misgurnus anguillicaudatus), females can produce up to 1800–15,500 eggs per batch (Berg Citation1949; Suzuki Citation1983). Clearly, the absolute fecundity of females may vary depending on size. A similar pattern was observed in case of relative fecundity. The highest value was recorded in the Południowy canal (189 eggs per g b. m.), second in the Nowy Rów canal (148 eggs per g b. m.) and lowest in the River Ner (124 eggs per g b. m.). Our results are comparable to those obtained by Drozd et al. (Citation2009), where the stripped fecundity (number of eggs/g b. m.) averaged 121.6 eggs per female, as well as for Mazurkiewicz (Citation2012), who reported a figure of 250 eggs per g b. m. In contrast, Podubski and Štedronský (Citation1954), Opalatenko (Citation1974), Geldhauser (Citation1992) Kouril et al. (Citation1996) and Adamkova-Stibranyiova et al. (Citation1999) obtained values two or three times higher.
The diameter (without envelopes) of mature weatherfish eggs (in metaphase of the second meiotic division) ranges between 1.17 and 1.30 mm (Kostromarova Citation1991). After laying, the eggs swell to the diameter of 1.7–1.9 mm (Mazurkiewicz Citation2012). Oocyte diameter for female weatherfish from a floodplain area in the Czech Republic measured on average 1.42 mm with an average weight of 0.88 mg (Drozd et al. Citation2009). Slightly greater mass of wet eggs was reported by Kouril et al. (Citation1996) with an average weight of one egg obtained during artificial propagation of about 1.07 mg and by Adamkova-Stibranyiova et al. (Citation1999) of 1.00 mg. In the case of the related but smaller loaches, the oocyte diameter is slightly similar, i.e., for M. anguillicaudatus it ranges from 0.72 to 0.85 mm (Suzuki Citation1983; Zheng Citation1985), for Misgurnus mizolepis it measures on average 1.10 mm (Kim et al. Citation1987), for stone loach: 0.85 to 0.96 mm (Mills et al. Citation1983; Mills & Eloranta Citation1985; Saat et al. Citation2003) and for Cobitis taenia on average 1.14 mm (Bohlen Citation1998). In the present study, the observed average size of unspawned oocytes from females from the Południowy canal (0.99 mm) and Nowy Rów canal (0.91 mm) were smaller than in the River Ner (1.16 mm). Consequently, the average weight of eggs in the River Ner (1.51 mg) was almost twice as high as in the Południowy and Nowy Rów canals (0.78 and 1.1 mg, respectively). The average egg diameter and weight may vary during one individual season and between populations (Drozd et al. Citation2009). Two groups of oocyte sizes were observed in the ovaries of females from both canals and river. In the canals large, mature oocytes were typical. The dominance of mature oocytes in the gonads may indicate that all eggs were to be released once during the spawning season, suggesting a lack of batch spawning. In contrast, in the River Ner both size classes of oocytes were equally numerous. This difference may indicate another physiological response (at the mating system level) as an investment in iteroparity (Warner Citation1998), potentially leading to higher lifetime reproductive success.
Differences in reproductive traits of the weatherfish inhabiting the three watercourses presented in this study may have many reasons, but most probably is high level of pollution in aquatic ecosystems. The relatively large number of chemicals polluting the environment is the cause of numerous problems arising from their physiological side effects. The main elements are industrial chemicals, pharmaceuticals and endocrine-disrupting chemicals (EDCs), such as steroid compounds, including natural products, as well as their synthetic forms, which are widely used in the form of oral birth-control pills and in human disease therapy (Pojana et al. Citation2004; Wang et al. Citation2018). Despite their usual low concentrations in open waterbodies they have been classified as a potential environmental factor affecting the metabolic processes of living organisms (Vallejo-Rodríguez et al. Citation2018). EDCs increase the risk of disruption to the reproductive biology of vertebrates (Lange et al. Citation2012), directly impacting the fertility of fish and contributing to the decline of some fish species (Kim et al. Citation1997; Jobling et al. Citation2003, Citation2006; Mills & Chichester Citation2005; Lü et al. Citation2012). Common effects of oestrogen exposure include increased oestrogen and vitellogenin concentrations in plasma, reduced gonad development and disruption of gonad function. Steroid oestrogens also contribute to changes in the ratio of reproductive cell types, pathological changes in gonads, decreased sperm counts and, consequently, to a reduction in fertility. They might also cause changes to male secondary sex characteristics and potentially leading to the complete feminization of males (Leet et al. Citation2011; Huang et al. Citation2016; Hassell et al. Citation2016; Czarny et al. Citation2017), changes in sexual behaviour (Dammann et al. Citation2011; Reyhanian et al. Citation2011) and ultimately contributing to local extinctions (Czarny et al. Citation2017). The study conducted by Kim et al. (Citation1997) shows that in related species, such as the M. mizolepis, higher concentrations and/or longer exposure to oestrogen 17β-oestradiol (E2) can alter the sex ratio of young individuals. In addition to significant classical feminizing effects, morphological changes within the ovaries and size and morphology of the pectoral fins, being the expression of sexual dimorphism in weatherfish, were also observed by Kim et al. (Citation1997), which also contradicts our observations. The presence of oestrogens in the water, which as Mills et al. (Citation2003) reported, not only affects gamete quality, but are also responsible for decreases in the amount of eggs and sperm produced by fish. Reduced fertility after exposure to oestrogens, particularly to 17α-ethynyloestradiol (EE2) has also been observed by Voisin et al. (Citation2016). Considering that the River Ner is associated with a large urban agglomeration, the level of pollution, including EDCs, is higher than in the case of the other two watercourses, which are located in rural, non-urbanized areas with a lower potential to generate pollution (, Pyrzanowski, unpublished data). This assumption however, requires detailed study on the possible impact of endocrine-disrupting chemicals, especially oestrogenic steroids, on endangered weatherfish populations and contributing therefore to the better understanding of the impact of pollutant on fish life history traits in general.
Additional information
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Special thanks are extended to C. Smith from Nottingham Trent University (UK) for English correction and the useful suggestions on an earlier version of this manuscript. We are greatly indebted to S. Szklarek from European Regional Centre for Ecohydrology of the Polish Academy of Sciences for water sample analysis. We also thank two anonymous reviewers for valuable improvements to the manuscript. The weatherfish is protected in Poland, therefore all procedures were carried out under permission from the Local Ethics Committee (66/ŁB729/2014) and the Regional Directorate of Environmental Protection (WPN-II.6401.268.2014.KW2).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adamkova-Stibranyiova I, Adamek Z, Sutovsky I. 1999. A comparative study on the induced spawning in female loach (Misgurnus fossilis) by means of single and double pituitary injection technique. Czech Journal of Animal Science 44:403–407.
- Bagenal TB. 1978. Aspect of fish fecundity. In: Gerking SD, editor. Ecology of freshwater fish production. Oxford, UK: Blackwell Scientific Publications. pp. 75–101.
- Berg LS. 1949. Freshwater fishes of the USSR and neighbouring countries. Moskva-Leningrad: Izdatel’stvo Akademii Nauk SSSR.
- Bhattacharya CG. 1967. A simple method of resolution of a distribution into Gaussian components. Biometrics 23:115–135. DOI: 10.2307/2528285.
- Blackwell BG, Brown ML, Willis DW. 2000. Relative weight (Wr) status and current use. Fisheries Assessment and Management Reviews in Fisheries Science 8:1–44. DOI: 10.1080/10641260091129161.
- Bohlen J. 1998. Differences in clutch size, egg size and larval pigmentation between Cobitis taenia and C. bilineata (Cobitidae). Italian Journal of Zoology 65(Suppl):219–221. DOI: 10.1080/11250009809386817.
- Bohlen J, Ritterbusch D. 2000. Which factors affect sex ratio of spined loach (genus Cobitis) in Lake Müggelsee? Environmental Biology of Fishes 59:347–352. DOI: 10.1023/A:1007695703991.
- Bohlen J, Šlechtová V, Doadrioo I, Ráb P. 2007. Low mitochondrial divergence indicates a rapid expansion across Europe in the weather loach, Misgurnus fossilis (L.). Journal of Fish Biology 71:186–194. DOI: 10.1111/j.1095-8649.2007.01547.x.
- Bolger T, Connolly PP. 1989. The selection of suitable indices for the measurement and analysis of fish condition. Journal of Fish Biology 34:171–182. DOI: 10.1111/j.1095-8649.1989.tb03300.x.
- Boroń A, Kotusz J, Przybylski M. 2002. Spined loach, golden spined loach, weatherfish, stone loach. Olsztyn: Wydawnictwo IRŚ.
- Czarny K, Szczukocki D, Krawczyk B, Zieliński M, Miękoś E, Gadzała-Kopciuch R. 2017. The impact of estrogens on aquatic organisms and methods for their determination. Critical Reviews in Environmental Science and Technology 47(11):909–963. DOI: 10.1080/10643389.2017.1334458.
- Dammann AA, Shappell NW, Bartell SE, Schoenfuss HL. 2011. Comparing biological effects and potencies of estrone and 17β-estradiol in mature fathead minnows, Pimephales promelas. Aquatic Toxicology 105:559–568. DOI: 10.1016/j.aquatox.2011.08.011.
- Drozd B, Flajšhans M, Ráb P. 2010. Sympatric occurrence of triploid, aneuploid and tetraploid weatherfish Misgurnus fossilis (Cypriniformes, Cobitidae). Journal of Fish Biology 77(9):2163–2170. DOI: 10.1111/j.1095-8649.2010.02794.x.
- Drozd B, Kouril J, Blaha M, Hamackova J. 2009. Effect of temperature on early life history in weatherfish, Misgurnus fossilis (L. 1758). Knowledge and Management of Aquatic Ecosystems 04. DOI: 10.1051/kmae/2009010.
- E.U. 1992. Council directive 92/43/EEC on the conservation of natural habitats and wild fauna and flora. Official Journal of the European Union L206. Strasbourg, Germany. pp. 1–66. Available: http://eurlex.europa.eu/LexUriServ/LexUriServ.douri=CONSLEG:1992L0043:20070101:EN:PDF
- Fechhelm RG, Griffiths WB, Wilson WJ, Gallaway BJ, Bryan JD. 1995. Intra- and interseasonal changes in the relative condition and proximate body composition of broad whitefish from the Prudhoe Bay Region of Alaska. Transactions of the American Fisheries Society 124:508–519. DOI: 10.1577/1548-8659(1995)124<0508:IAICIT>2.3.CO;2.
- Freyhof J, Brooks E. 2011. European red list of freshwater fishes. Luxembourg: Publications Office of the European Union.
- Gayanilo FC Jr., Sparre P, Pauly D. 2005. FAO-ICLARM stock assessment tools II (FiSAT II). Revised version. User’s guide. FAO Computerized Information Series (Fisheries). No. 8. Rome: FAO.
- Geldhauser F. 1992. The controlled multiplication of the weatherfish (Misgurnus fossilis L.). Fischer &Teichwirt 43(1):2–6.
- Hartvich P, Lusk S, Rutkayová J. 2010. Threatened fishes of the world: Misgurnus fossilis (Linnaeus, 1758) (Cobitidae). Environmental Biology of Fishes 87:39–40. DOI: 10.1007/s10641-009-9564-6.
- Hassell K, Pettigrove V, Beresford N, Jobling S, Kumar A. 2016. No evidence of exposure to environmental estrogens in two feral fish species sampled from the Yarra River, Australia: A comparison with Northern Hemisphere studies. Ecotoxicology and Environmental Safety 131:104–117. DOI: 10.1016/j.ecoenv.2016.05.004.
- Huang GY, Liu YS, Chen XW, Liang YQ, Liu SS, Yang YY, Hu LX, Shi WJ, Tian F, Zhao JL, Chen J, Ying GG. 2016. Feminization and masculinization of western mosquitofish (Gambusia affinis) observed in rivers impacted by municipal wastewaters. Scientific Reports. DOI: 10.1038/srep20884.
- Jakubowski M. 1958. The structure and vascularization of the skin of the pond-loach (Misgurnus fossilis L.). Acta Biologica Cracoviensia 1:113–127.
- Javahery S, Nekoubim H, Moradlu AH. 2012. Effect of anaesthesia with clove oil in fish (review). Fish Physiology and Biochemistry 38:1545–1552. DOI: 10.1007/s10695-012-9682-5.
- Jobling S, Casey D, Rodgers-Gray T, Oehlmann J, Schulte-Oehlmann U, Pawlowski S, Baunbeck T, Turner AP, Tyler CR. 2003. Comparative responses of molluscs and fish to environmental estrogens and an estrogenic effluent. Aquatic Toxicology 65:205–220. DOI: 10.1016/S0166-445X(03)00134-6.
- Jobling S, Williams R, Johnson A, Taylor A, Gross-Sorokin M, Nolan M, Tyler CR, Van Aerle R, Santos E, Brighty G. 2006. Predicted exposures to steroid estrogens in U.K. rivers correlate with widespread sexual disruption in wild fish populations. Environmental Health Perspectives 114(1):32–39. DOI: 10.1289/ehp.8050.
- Kim DS, Nam YK, Jo J-Y. 1997. Effect of oestradiol-17β immersion treatments on sex reversal of mud loach, Misgurnus mizolepis (Günther). Aquaculture Research 28:941–946. DOI: 10.1111/j.1365-2109.1997.tb01019.x.
- Kim YU, Park YS, Kim DS. 1987. Development of eggs, larvae and juveniles of loaches, Misgurnus mizolepis Günther. Bulletin of the Korean Fisheries Society 20(1):16–23.
- Kostromarova AA. 1991. The loach – Misgurnus fossilis. In: Dettlaff TA, Vassetzky SG, editors. Animal species for developmental studies. New York and London: Consultants Bureau. pp. 125–144.
- Kostrzewa J. 1999. Chance for the River Ner. Aura 12:16–17.
- Kottelat M, Freyhof J. 2007. Handbook of European freshwater fishes. Switzerland and Berlin, Germany: Cornol.
- Kouril J, Hamackova J, Adamek Z, Sukop I, Vachta R. 1996. The artificial propagation and culture of young weatherfish (Misgurnus fossilis L.). In: Kirchhofer A, Hefti D, editors. Conservation of endangered freshwater fish in Europe. Basel: Birkhäuser Verlag. pp. 305–310.
- Lange A, Katsu Y, Miyagawa S, Ogino Y, Urushitani H, Kobayashi T, Hirai T, Shears JA, Nagae M, Yamamoto J, Ohnishi Y, Oka T, Tatarazako N, Ohta Y, Tyler CR, Iguchi T. 2012. Comparative responsiveness to natural and synthetic estrogens of fish species commonly used in the laboratory and field monitoring. Aquatic Toxicology 109:250–258. DOI: 10.1016/j.aquatox.2011.09.004.
- Le Cren ED. 1951. The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). The Journal of Animal Ecology 20:201–219. DOI: 10.2307/1540.
- Leet JK, Gall HE, Sepúlveda MS. 2011. A review of studies on androgen and estrogen exposure in fish early life stages: Effects on gene and hormonal control of sexual differentiation. Journal of Applied Toxicology 31(5):379–398. DOI: 10.1002/jat.1682.
- Lü XF, Liu FY, Zhou XP, Zhou QF, Deng YL. 2012. Effects of cadmium, 17β-estradiol and their interaction in the male Chinese loach (Misgurnus anguillicaudatus). Chinese Science Bulletin 57:858–863. DOI: 10.1007/s11434-011-4708-4.
- Mazurkiewicz J. 2012. Weatherfish Misgurnus fossilis Linnaeus, 1758. In: Makomaska-Juchiewicz M, Baran P, editors. Monitoring of animal species. Methodological guide. Vol. 3. Warszawa: GIOŚ. pp. 264–275.
- Meyer L, Hinrichs D. 2000. Microhabitat preferences and movements of the weatherfish, Misgurnus fossilis, in a drainage channel. Environmental Biology of Fishes 58(3):297–306. DOI: 10.1023/A:1007681313916.
- Mills CA, Eloranta A. 1985. Reproductive strategies in the stone loach Noemacheilus barbatulus. Oikos 44(2):341–349. DOI:10.2307/3544709.
- Mills CA, Welton JS, Rendle EL. 1983. The age growth and reproduction of the stone loach Noemacheilus barbatulus in a Dorset, England, UK chalk stream. Freshwater Biology 13:283–292. DOI: 10.1111/j.1365-2427.1983.tb00678.x.
- Mills LJ, Chichester C. 2005. Review of evidence: Are endocrine-disrupting chemicals in the aquatic environment impacting fish populations? Science of the Total Environment 343:1–34. DOI: 10.1016/j.scitotenv.2004.12.070.
- Mills LJ, Gutjahr-Gobell RE, Horowitz DB, Denslow ND, Chow MC, Zaroogian GE. 2003. Relationship between reproductive success and male plasma vitellogenin concentrations in cunner, Tautogolabrus adspersus. Environmental Health Perspectives 111(1):93–100. DOI:10.1289/ehp.5531.
- Mosiej J, Komorowski H, Karczmarczyk A, Suska A. 2007. Effect of pollutants discharged from Łódź conurbation on quality of water in Ner and Warta rivers. Acta Scientiarum Polonorum Formatio Circumiectus 6(2):19–30.
- Mousavi Sabet H, Kamali A, Soltani M, Bani A, Esmaeili HR, Rostami H, Vatandoust S, Moradkhani Z. 2011. Age, reproduction, and fecundity of a population of Cobitis sp. (Actinopterygii: Cypriniformes: Cobitidae) from the Babolrud River in the southern Caspian Sea basin. Acta Ichthyologica Et Piscatoria 41(2):117–122. DOI: 10.3750/AIP2011.41.2.07.
- Opalatenko LK. 1974. On the morphological characteristics (Cobitidae) Verchnevo Dniestr. VestZool 6:56–62.
- Pekarik L, Kosco J, Kosuthova L, Kosuth P. 2008. Coenological and habitat affinities of Cobitis elongatoides, Sabanejewia balcanica and Misgurnus fossilis in Slovakia. Folia Zoologica 57(1–2):172–180.
- Penczak T, Kruk A, Grabowska J, Śliwińska A, Koszaliński H, Zięba G, Tybulczuk S, Galicka W, Marszał L. 2010. Influence of gradual improvement in water quality on the regeneration of fish fauna in the Ner river. Roczniki Naukowe PZW. 23:97–117.
- Podubski V, Štedronský E. 1954. Contribution to the biology of the weatherfish (Misgurnus fossilis L.). SB Csazv 27:333–336.
- Pojana G, Bonfa A, Busetti F, Collarin A, Marcomini A. 2004. Estrogenic potential of the Venice, Italy, lagoon waters. Environmental Toxicology and Chemistry 23(8):1874–1880. DOI: 10.1897/03-222.
- Pyrzanowski K, Zięba G, Dukowska M, Smith C, Przybylski M. 2019. The role of detritivory as a feeding tactic in a harsh environment – A case study of weatherfish (Misgurnus fossilis). Scientific Reports 9(1). DOI: 10.1038/s41598-019-44911-y.
- Pyrzanowski K, Zięba G, Przybylski M. 2015. Artificial drainage ditches as undervalued habitats of threatened fish species – A case of weatherfish Misgurnus fossilis in the Natura 2000 site ‘Pradolina Bzury-Neru PLH100006ʹ. Chronmy Przyrode Ojczysta 71:266–272.
- Pyrzanowski K, Zięba G, Przybylski M. 2020. Endangered weatherfish (Misgurnus fossilis) age and growth is affected by the size of the watercourses. Journal of Vertebrate Biology (Folia Zoologica) 69(1). DOI: 10.25225/jvb.19041.
- Raicu P, Taisescu E. 1972. Misgurnus fossilis, a tetraploid fish species. Journal of Heredity 63:92–94. DOI: 10.1093/oxfordjournals.jhered.a108240.
- Reyhanian N, Volkova K, Hallgren S, Bollner T, Olsson P-E, Olsén H, Hällström IP. 2011. 17α-Ethinyl estradiol affects anxiety and shoaling behavior in adult male zebra fish (Danio rerio). Aquatic Toxicology 105(1–2):41–48. DOI: 10.1016/j.aquatox.2011.05.009.
- Ricker WE. 1975. Computation and interpretation of biological statistics of fish populations. Journal of the Fisheries Research Board of Canada 191:1–382.
- Saat T, Lauringson G, Lees J. 2003. Reproduction of the stone loach, Barbatula barbatula (L.) in Estonia. Folia Biologica 51:193–197.
- Smith C, Wootton JR. 2016. The remarkable reproductive diversity of teleost fishes. Fish and Fisheries 17:1208–1215. DOI: 10.1111/faf.12116.
- Stearns SC. 1992. The evolution of life histories. Oxford: Oxford University Press.
- Suzuki R. 1983. Multiple spawning of the cyprinid loach, Misgurnus anguillicaudatus. Aquaculture 31:233–243. DOI: 10.1016/0044-8486(83)90315-0.
- Vallejo-Rodríguez R, Sánchez-Torres PB, López-López A, Leoń-Becerril E, Murillo-Tovar M. 2018. Detection of steroids in tap and drinking water using an optimized analytical method by gas chromatography – Mass spectrometry. Exposure and Health 10:189–199. DOI: 10.1007/s12403-017-0254-x.
- Verreycken H, Van Thuyne G, Belpaire C. 2011. Length-weight relationships of 40 freshwater fish species from two decades of monitoring in Flanders (Belgium). Journal of Applied Ichthyology 27:1416–1421. DOI: 10.1111/j.1439-0426.2011.01815.x.
- Voisin A-S, Fellous A, Earley RL, Silvestre F. 2016. Delayed impacts of developmental exposure to 17-α-ethinylestradiol in the self-fertilizing fish Kryptolebias marmoratus. Aquatic Toxicology 180:247–257. DOI: 10.1016/j.aquatox.2016.10.003.
- Wang S, Zhu Z, He J, Yue X, Pan J, Wang Z. 2018. Steroidal and phenolic endocrine disrupting chemicals (EDCs) in surface water of Bahe River, China: Distribution, bioaccumulation, risk assessment and estrogenic effect on Hemiculterleucisculus. Environmental Pollution 243:103–114. DOI: 10.1016/j.envpol.2018.08.063.
- Warner RR. 1998. The role of extreme iteroparity and risk avoidance in the evolution of mating systems. Journal of Fish Biology 53(sA):82–93. DOI: 10.1111/j.1095-8649.1998.tb01019.x.
- Wilson K, Hardy ICW. 2002. Statistical analysis of sex ratios: An introduction. In: Hardy ICW, editor. Sex ratios: Concepts and research methods. Cambridge: Cambridge University Press. pp. 48–92.
- Wootton RJ. 1998. Ecology of teleost fishes. London: Chapman and Hall.
- Wootton RJ, Elvira BB. 2000. Life-history evolution biology and conservation of fish: Introductory note. Ecology of Freshwater Fish 9:90–91. DOI: 10.1034/j.1600-0633.2000.90110.x.
- Wootton RJ, Smith C. 2015. Reproductive biology of teleost fishes. Oxford: Walley-Blackwell.
- Zar JH. 2010. Biostatistical analysis. Prentice Hall, NJ: Englewood Cliffs.
- Zheng WB. 1985. Observations on the embryonic and larval development of Misgurnus anguillicaudatus (Cantor). Journal of Fisheries of China Shui Chan Xue Bao 9(1):37–47.