Abstract
Brachiopods exhibit a particular preference for cryptic habitats such as submarine caves. However, their assemblages have rarely been investigated quantitatively in this habitat. In this work, brachiopod assemblages were studied in detail for the first time in two Aegean submarine caves, Fara and Agios Vasilios, Lesvos Island, Eastern Mediterranean Sea. Six species of Recent brachiopods, i.e. Novocrania turbinata (Poli, 1795), Tethyrhynchia mediterranea Logan, 1994, Megathiris detruncata (Gmelin, 1791), Argyrotheca cuneata (Risso, 1826), A. cistellula (Searles–Wood, 1841), and Joania cordata (Risso, 1826), have been identified. The cave-exclusive species Tethyrhynchia mediterranea is reported for the first time from the Aegean Sea and Greek waters, increasing the regional brachiopod fauna to 13 species, and for the second time in the Eastern Mediterranean. Five species were present in both caves while T. mediterranea was found only in the internal dark ceilings and walls of Fara cave. In both caves the dominant species was Argyrotheca cuneata. Abundance and diversity increased towards the internal dark ceilings of both caves, which harboured a well-differentiated brachiopod assemblage compared to that of the outer cave zones.
Introduction
Brachiopods are sessile, marine invertebrates with a long geological history. Today, represented by approximately 400 species (Emig et al. Citation2013), brachiopods are considered a minor phylum, however, they are widely distributed geographically, living in all oceans. The shallow-water micromorphic species are commonly found in shaded, light-poor environments, such as cryptic and cave habitats where they can occur in high densities. Mediterranean marine caves constitute important hot-spots for brachiopod diversity, harbouring 10 (70%) out of the 14 species occurring in the Mediterranean Sea (Gerovasileiou & Bianchi Citation2021). Although brachiopods have been reported from many submarine caves (e.g. Logan Citation1979, Citation2003; Monteiro-Marques Citation1981; Logan & Noble Citation1983; Logan & Zibrowius Citation1994; Simon & Willems Citation1999; Boury-Esnault et al. Citation2001; Logan et al. Citation2002, Citation2004; Harmelin et al. Citation2003; Rosso et al. Citation2013; Gerovasileiou et al. Citation2015; Radolović et al. Citation2015; Sanfilippo et al. Citation2015; Guido et al. Citation2017; Simon et al. Citation2018; Jimenez et al. Citation2019), their assemblage structure has rarely been investigated in detail. Existing quantitative studies have focused on particular species, but never in the Eastern Mediterranean Sea. Taddei Ruggiero (Citation1994, Citation1996, Citation2001) carried out studies on the population of Novocrania anomala (Müller, 1776) from Isca cave, Tyrrhenian Sea, Italy. Logan (Citation2001) conducted a morphometric analysis of the populations of Megathiris detruncata (Gmelin, 1791) from caves in the Western Mediterranean and Northeastern Atlantic. Recently Bergamin et al. (Citation2020) studied the composition and distribution patterns of the dead brachiopod assemblage found in cave sediment of the CT12 cave in southern Spain.
Here we present the first quantitative study of brachiopod biodiversity, assemblage structure and spatial variability in two submarine caves of the Aegean Sea, Eastern Mediterranean (). The main goals of the study were to increase knowledge on the regional cave species diversity but also to provide a better understanding of the spatial variability of brachiopod assemblages in this peculiar habitat.
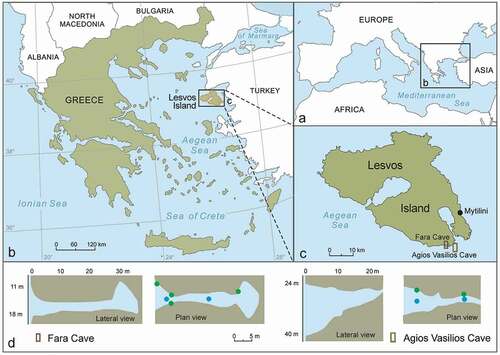
Figure 1. (a–c) Location of the studied caves in Lesvos Island, Aegean Sea, Eastern Mediterranean. (d) Lateral and plan views of the caves with indication of depth and location of collected samples in relation to distance from the entrances; blue circles: ceiling samples; green circles: wall samples
Material and methods
Study sites
Two marine caves were studied in Lesvos Island, Northeastern Aegean Sea (Eastern Mediterranean). The caves are located in the rock islets Fara and Agios Vasilios, off the southeastern coast of the island, hereafter indicated as F and AV, respectively (). The F cave (38.969° N, 26.477° E; 14–18 m depth) is 32-m-long, ending in a dark chamber, connected through narrow fissures to a second chamber on the other side of the islet. The AV cave (38.969°N, 26.541°E; 24–40 m depth) is a funnel-shaped blind cave with a large entrance (16 m high x 7 m wide). In this study only the first 25 m part of the cave was surveyed, because beyond this distance, an ascending narrow and dark tunnel makes underwater work practically impossible. Both caves have been thoroughly studied regarding their morphology (Gerovasileiou et al. Citation2013) and sessile benthic assemblages (Gerovasileiou et al. Citation2017; Guido et al. Citation2019a, Citation2019b), including detailed studies on sponges (Gerovasileiou & Voultsiadou Citation2016), serpulids (Sanfilippo et al. Citation2017) and bryozoans (Rosso et al. Citation2019), but this is the first study focusing on their brachiopod fauna.
Sampling process and sample analysis
Three replicate quadrats of 400 cm2 (20 × 20 cm) were scraped from 10 sampling stations (6 in F cave and 4 in AV cave), in the summer 2010, by SCUBA diving. Sampling stations represented different assemblages of the sidewalls and ceiling, at different distances from the entrance of the caves (). A total of 30 samples were collected (18 from F cave and 12 from AV cave) and preserved in 10% formalin. After the sorting process, all brachiopods were collected and identified. For the calculation of abundance, the number of complete individuals was added to the highest number of either ventral or dorsal valves (see Schrøder et al. Citation2019). The total number of specimens is 1186 (615 in F cave and 571 in AV cave).
Table I. Brachiopods recorded in the Fara and Agios Vasilios caves. The total number of individuals per sampling station is presented. For each sampling station the location inside the cave (L w, Left wall; R w, Right wall; C, Ceiling; and Entr, Entrance), distance from the entrance (m), biocoenosis (Cor, Coralligenous Biocoenosis; SD, Semidark Cave Biocoenosis; Trans, Transitional Zone; and Dark, Dark Cave Biocoenosis) and main sessile encrusters (Rh, Rhodophytes, Sc, Scleractinian corals; Sp, Sponges; Sr, Serpulid polychaetes; and B, Bryozoans) are indicated
To remove soft tissue, specimens were treated with hypochlorite bleach, followed by a water wash. For scanning electron microscope (SEM) examination, the selected specimens were dried, mounted on stubs, coated with platinum, and examined using a Philips XL-20 microscope at the SEM laboratory of the Institute of Paleobiology, Warszawa. The investigated specimens are housed at the Hellenic Centre for Marine Research, Heraklion, Crete and in the collection of the Institute of Paleobiology, Warszawa under the catalogue number ZPAL Bp.85.
Data analysis
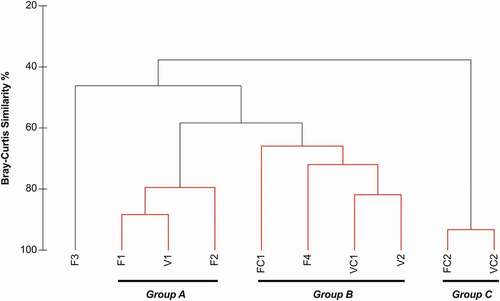
For each station, the brachiopod abundance (N), species richness (S), Shannon–Wiener diversity index (H’), Simpson’s diversity index (1-λ’) and species evenness (J’) were calculated. One-way permutational ANOVA (PERMANOVA) was used to examine the variability of the above measures across the stations of each cave (factor, Station; fixed with six levels for F cave and four levels for AV cave). The multivariate resemblance of sampling stations was investigated with cluster analysis, based on the Bray–Curtis similarity index (square root-transformed mean abundance) and using the SIMPROF (similarity profile) test to examine the null hypothesis of no meaningful structure within samples (significant differences at 5% level). The contribution of species to the Bray–Curtis similarity of samples within the resulting groups was estimated with SIMPER (similarity percentages). Statistical analysis was undertaken using the PRIMER-E v.6 software package (Clarke & Gorley Citation2006).
Results
Taxonomic composition
Six brachiopod species belonging to five genera have been identified in the material collected in the two Aegean submarine caves, Fara and Agios Vasilios (). Inarticulate and rhynchonellide brachiopods have one representative each, Novocrania turbinata (Poli, 1795) and Tethyrhynchia mediterranea Logan, 1994, respectively. The remaining four species, Megathiris detruncata (Gmelin, 1791), Argyrotheca cuneata (Risso, 1826), A. cistellula (Searles–Wood, 1841) and Joania cordata (Risso, 1826) are representatives of the family Megathyrididae. Two species, Argyrotheca cuneata and Joania cordata dominate in the studied material (52.8% and 22.8% of the total brachiopod specimens, respectively), followed by Megathiris detruncata (16.8%), Novocrania turbinata (6.1%), Argyrotheca cistellula (1.3%) and Tethyrhynchia mediterranea (0.2%) (). The latter was found only in the F cave while the other five species were present in both caves.
Novocrania turbinata (Poli, 1795) is a cementing inarticulate brachiopod (), commonly found attached to hard substrates in shallow-water cryptic habitats, including walls or ceilings of caves (Logan & Long Citation2001; Logan et al. Citation2004). In the studied material it is present in both caves, being relatively common (see ). Initially, those brachiopods were assigned to Novocrania anomala (O.F. Müller, 1776) by Gerovasileiou et al. (Citation2015, Citation2017) and Rosso et al. (Citation2019). However, the examination of all specimens collected in the two caves of Lesvos Island revealed a thick ventral valve, thus corroborating attribution to N. turbinata according to Robinson (Citation2017).
Figure 2. (a–d) Novocrania turbinata (Poli, 1795): (a) external outer view of dorsal valve, station FC2, ZPAL Bp.85/1; (b–c) inner view of dorsal valve and enlargement of posterior part to show details muscle scars, station FC2, ZPAL Bp.85/2; (d) lateral view of complete specimen, station VC2, ZPAL Bp.85/3. (e–i) Tethyrhynchia mediterranea Logan, in Logan & Zibrowius, Citation1994: (e–f) dorsal view of complete specimen and enlargement of umbonal part to show fine capillation anterior to the protegular node, station FC2, ZPAL Bp.85/4; (g–i) dorsal view of complete specimen, and enlargements of the umbonal part to show details of the beak and fine capillation anterior to the protegular node, station F4, ZPAL Bp.85/5. All SEM photos
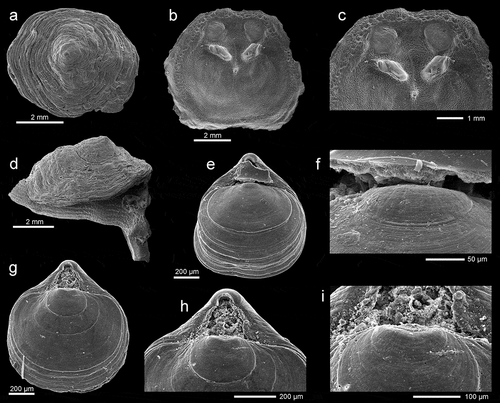
Tethyrhynchia mediterranea Logan in Logan & Zibrowius, Citation1994 is a diminutive rhynchonellide species, hardly exceeding 1 mm. It is characterized by the smooth surface with numerous growth lines, rectimarginate anterior commissure and high beak with a large, subtriangular, hypothyrid foramen (–i)). In the material under study, this species is very rare (three specimens), found only in F cave (). Tethyrhynchia mediterranea can be considered as a cave-exclusive species and is likely to be endemic to the Mediterranean Sea (Logan et al. Citation2004).
Megathiris detruncata (Gmelin, 1791), a representative of the family Megathyrididae, is one of the most common species in both caves. It is common throughout the Mediterranean and Northeastern Atlantic (Logan Citation1979, Citation1993, Citation2003; Harmelin et al. Citation2003; Gerovasileiou et al. Citation2015; Sanfilippo et al. Citation2015; Gerovasileiou & Bailly Citation2016; Bergamin et al. Citation2020). It is characterized by the broadly transverse shell ornamented by rounded ribs (–d)). The presence of three septa in the dorsal valve ()) makes this species easily distinguishable from other members of Megathyrididae.
Figure 3. (a–e) Megathiris detruncata (Gmelin, 1791): (a–b) dorsal views of complete specimens, station FC2, ZPAL Bp.85/6–7; (c) complete specimen in living position attached to a bryozoan, station VC2, ZPAL Bp.85/8; (d) ventral view of complete specimen with attached immature specimen, station FC2, ZPALBp.85/9; (e) interior view to show three dorsal septa and brachidium, station VC2, ZPAL Bp.85/10. (f–i) Argyrotheca cuneata (Risso, 1826): (f–g) dorsal views of complete specimens, station FC2, ZPAL Bp.85/11–12; (h) complete specimen in living position, station VC2, ZPAL Bp.85/13; (i) interior view to show dorsal medium septum, brachidium and two rudimentary ascending branches diverging from the septum, station V2, ZPAL Bp.85/14. All SEM photos
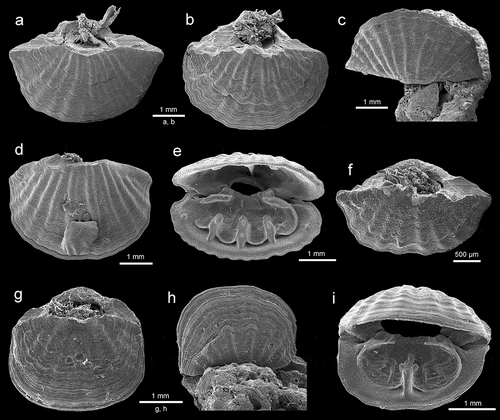
Argyrotheca cuneata (Risso, 1826) is the commonest species in the investigated material, present in both caves. This species is easily recognized by distinctive pink-red colouration between broad ribs. Its shell is variable from transversely elongate to subrectangular (–i)). It is widely distributed throughout the Mediterranean, being typical of the infralittoral-circalittoral zone (Logan Citation1979, Citation2003; Logan et al. Citation2004) but is also commonly found in caves (Taddei Ruggiero Citation1994, Citation1996; Grobe & Lüter Citation1999; Rosso et al. Citation2013; Gerovasileiou et al. Citation2015; Sanfilippo et al. Citation2015; Guido et al. Citation2017; Jimenez et al. Citation2019; Bergamin et al. Citation2020). Argyrotheca cuneata is also reported from the Northeastern Atlantic (Logan Citation1993).
Argyrotheca cistellula (Searles–Wood, 1841) has been found in both caves but it is very rare in the collected material (). Its shell is extremely small, not exceeding 1.4 mm in width, squarely transverse in outline, smooth except numerous growth lines, and strongly biconvex (–d)). This species is usually found in shallow water, commonly occurring in caves (Logan Citation1979; Logan Citation2001, Citation2003; Grobe & Lüter Citation1999; Simon & Willems Citation1999; Harmelin et al. Citation2003; Logan et al. Citation2004; Rosso et al. Citation2013; Gerovasileiou et al. Citation2015; Bergamin et al. Citation2020). It is also noted from the Northeastern Atlantic (Logan Citation1993).
Figure 4. (a–d) Argyrotheca cistellula (Searles–Wood, 1841): dorsal views of complete specimens and enlargement (d) of umbonal part, stations F4 (a-b) and FC2 (c-d), ZPAL Bp.85/14–16. (e–h) Joania cordata (Risso, 1826), station F2: (e–h) dorsal views of complete specimens, ZPAL Bp.85/16–17; (g–h) ventral views of complete specimens with attached immature individuals, ZPAL Bp.85/18–19. All SEM photos
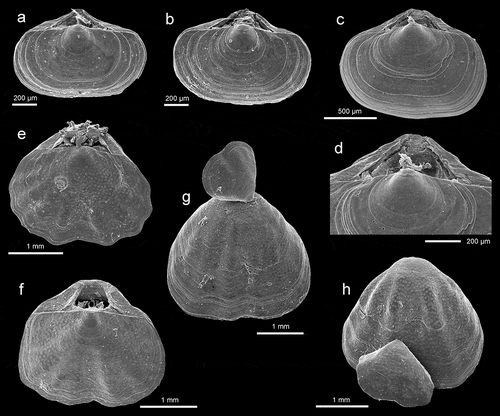
Joania cordata (Risso, 1826) is the second most common species in both caves. It is characterized by an elongate heart-shaped outline, semi-transparent shell with weakly defined ribs (–h)). Internally it is easily distinguishable from other megathyridid species described here by the presence of tubercles on the inner margins of both valves. This species is very common in the Mediterranean, occupying the infralittoral-circalittoral zone and often found attached to cave walls and ceilings (Logan Citation1979, Citation2003; Taddei Ruggiero Citation1996, Citation2001; Grobe & Lüter Citation1999; Logan et al. Citation2004; Rosso et al. Citation2013; Gerovasileiou et al. Citation2015; Sanfilippo et al. Citation2015; Bergamin et al. Citation2020). Joania cordata is also common in the Northeastern Atlantic (Logan Citation1993).
Spatial distribution and assemblage resemblance patterns
A total of six brachiopod species were found in the two studied Aegean caves (6 in F and 5 in AV cave). Two species, Argyrotheca cuneata and Joania cordata are reported from all stations while Megathiris detruncata was absent only from one station (F2). Only three individuals of Tethyrhynchia mediterranea were found on the dark wall and ceiling stations of F cave (F4 and FC2, respectively), which also exhibited the highest number of species and notably high abundance values (). Mean abundance differed significantly among sampling stations in both F (Pseudo-F = 14.231, P < 0.01, df = 5) and AV caves (Pseudo-F = 14.995, P < 0.05, df = 3). The highest numbers of brachiopods were recorded on the internal dark ceilings of both caves (89–144 individuals in FC2 and 85–127 individuals in VC2). On the other hand, the lowest abundance value was observed at station F3 (N = 2), which was characterized by high sediment cover. Variability was not statistically significant for the two diversity indices and species evenness in the F cave. However, in the deeper AV cave, values of Shannon–Wiener (Pseudo-F = 5.1466, P < 0.05, df = 3) and Simpson’s diversity index (Pseudo-F = 8.2958, P < 0.05, df = 3) differed significantly, generally being higher at the ceilings and internal wall stations ().
Table II. Total species richness (S) and mean abundance (N), Shannon–Wiener diversity (H’), Simpson’s diversity (1-λ’) and species evenness (J’) per sampling station
Cluster analysis and SIMPROF test () showed a homogeneous composition between: (a) the outer wall stations of the two caves (Group A: F1, V1, F2; similarity 79.51%), (b) the intermediate ceiling and internal wall stations of the two caves (Group B: FC1, F4, VC1, V2; similarity 65.95%), and (c) the two internal ceiling stations (Group C: FC2, VC2; similarity 93.29%). Station F3 was differentiated from the above-mentioned groups. The species that contributed the most to the similarity of stations within the above-mentioned groups were Joania cordata for Group A (contrib. 74.25%) and Argyrotheca cuneata for Groups B (contrib. 35.1%) and C (contrib. 48.79%).
Discussion
Taxonomic composition
All six species recognized in the studied material are typical of Mediterranean marine cave environments (e.g. Rosso et al. Citation2013; Gerovasileiou et al. Citation2015; Sanfilippo et al. Citation2015; Bergamin et al. Citation2020; Gerovasileiou & Bianchi Citation2021). However, in the western parts of the Mediterranean Novocrania turbinata is usually replaced by N. anomala (e.g. Taddei Ruggiero Citation1994, Citation1996, Citation2001; Rosso et al. Citation2013; Bergamin et al. Citation2020; Pino de la Torre et al. Citation2020). The latter is often present in considerable numbers, cemented on walls and ceilings of dark submarine caves where it can live in clusters (Logan et al. Citation2004; Radolović et al. Citation2015; Gerovasileiou & Bianchi Citation2021). A similar behaviour was also observed in the studied Aegean caves for N. turbinata where it contributed to the formation of nodules resulting mainly from the concretion of bryozoans and other encrusting invertebrates (Rosso et al. Citation2019 as N. anomala). The validity of the species N. turbinata has long been the subject of discussion (Logan Citation1979; Brunton Citation1988; Logan & Long Citation2001). Emig (Citation2014) regards N. turbinata as a synonym of N. anomala. According to him the differences observed in those two species fit within the intraspecific variability. However, in his revision of the genus Novocrania, Robinson (Citation2017) considers N. turbinata as a separate species, mostly based on a ventral valve that is strongly calcified in N. turbinata while in N. anomala the ventral valve is very thin, varying from organic to fully calcitic. The differences are also observed in the morphology of muscle scars in the interiors of dorsal valves. In N. turbinata anterior adductor muscle scars are elevated and its support structure scars (formerly brachial retractor scars; see Robinson Citation2014) are not separated from adductors (Logan & Long Citation2001; Kroh et al. Citation2008; Robinson Citation2017). Also the molecular phylogenic analysis of craniid brachiopods seems to support the separation of those two species (Cohen et al. Citation2014).
The rhynchonellide Tethyrhynchia mediterranea is reported here for the first time from the Aegean Sea and Greek waters, increasing the regional brachiopod fauna to 13 species (Logan et al. Citation2002; Gerovasileiou & Bailly Citation2016; this study), and for the second time in the Eastern Mediterranean Sea. It was first discovered in the dark zones of submarine caves along the Mediterranean coast of France and from the Gulf of Tunis (Logan & Zibrowius Citation1994). Later, it was also found in caves of the Adriatic Sea, Croatia (Simon & Willems Citation1999; Lüter Citation2001; Simon et al. Citation2018), Sicily (Di Geronimo et al. Citation2001; Rosso et al. Citation2013; Sanfilippo et al. Citation2015), Cyprus (Guido et al. Citation2017) and southern Spain (Bergamin et al. Citation2020; Pino de la Torre et al. Citation2020). Lüter (Citation2001) and Logan et al. (Citation2004) suggested that T. mediterranea might be a relict species of the Tethyan fauna. In this study, T. mediterranea was found exclusively in the most confined sectors of F cave, confirming its preference for “true” cave conditions. According to the ecological groups identified by Rosso et al. (Citation2013) for Mediterranean serpulids, bryozoans and brachiopods, T. mediterranea is the unique brachiopod belonging to the group of “cave species”. The remaining five species recorded in the two studied caves have been assigned to another ecological group, the “sciaphilic/coralligenous species” (Rosso et al. Citation2013). For instance, although Argyrotheca cuneata and Joania cordata are the most frequently reported brachiopods from Mediterranean submarine caves (Gerovasileiou & Bianchi Citation2021), they are also known from a wide variety of low-light habitats (Logan Citation1979; Gerovasileiou & Bailly Citation2016; Albano & Stockinger Citation2019).
Interestingly, the cave-dwelling brachiopods Gwynia capsula (Jeffreys, 1859) and Megerlia truncata (Linnaeus, 1767) which occur in the Greek Seas (Gerovasileiou & Bailly Citation2016) were not recorded in the studied caves despite the high number of samples collected.
Spatial variability and assemblage resemblance patterns
Spatial variability patterns regarding abundance and diversity were similar in both caves. Brachiopod abundance and diversity increased towards the internal confined dark zones of the studied caves, and especially on ceilings. However, the entrance zone of the deeper AV cave had higher abundance and diversity values when compared to the equivalent zones of the shallower F cave (). Contrastingly, in the internal cave zones, F cave had higher abundance and diversity values than in AV cave. A similar pattern was observed for other sessile invertebrates, such as sponges (Gerovasileiou & Voultsiadou Citation2016), serpulid polychaetes (Sanfilippo et al. Citation2017) and bryozoans (Rosso et al. Citation2019), and was attributed to the different depth and topography of the two caves, such as entrance dimensions and internal morphology (Gerovasileiou et al. Citation2013).
The increase of abundance and diversity towards the internal zones of the studied caves could be possibly linked to the lower competition for space with other sedentary fauna, which is sparser in the dark cave sectors, as well as the scarcity of predators (e.g. gastropods and crabs) and absence of grazing herbivores (Taddei Ruggiero Citation1994, Citation1996). In addition, brachiopods have a very low metabolic rate compared to other marine invertebrates (Peck Citation2001a). The long studies carried out on the brachiopod population from Isca cave showed that in the stable cave environment Novocrania anomala, a species closely related to N. turbinata, is marked by a very slow growth, possibly reaching the age of 40 years, while the lifespan of Argyrotheca cuneata can be deduced to be at least 4 years (Taddei Ruggiero Citation1996, Citation2001).
Brachiopods are able to survive long periods of low food availability. Although being considered poor competitors, in cases where food supply is limited or restricted, brachiopods become very strong competitors (Peck Citation2001a). As shown by Bergamin et al. (Citation2020), the abundance of brachiopods in cave sediments – where they are often found in high abundances having detached from the cave ceiling – is influenced by several factors, such as light, nutrients and water parameters. On the other hand, cave sectors with high sedimentation rates (e.g. station F3 in this study) support poorer brachiopod assemblages (Sempere-Valverde et al. Citation2019). This may be caused by the fact that brachiopods, as filter-feeding organisms, avoid the situation where their lophophore apparatus can be clogged by fine sediment particles.
Despite their preference for cryptic habitats, brachiopods have a minor cover in submarine caves when compared with other sessile invertebrates (Harmelin Citation1980; Harmelin et al. Citation2003; Martí et al. Citation2004). For instance, based on photoquadrat coverage data from a previous study in F and AV caves (Gerovasileiou et al. Citation2017), the mean brachiopod cover is only 0.167% ± 0.046 (SE: standard error) in F cave and 0.03% ± 0.02 (SE) in AV cave. In both caves the maximum brachiopod cover was observed in the internal dark ceilings and cave walls, 20–25 m away from the cave entrance (Gerovasileiou et al. Citation2017). Although brachiopods were not identified at lower taxonomic ranks (except for Novocrania which had the highest cover among all brachiopods), due to limitations involved in the photographic identification of small-sized taxa, these findings are in agreement with the current study, where the highest abundance and diversity was recorded in the same sampling locations ().
Resemblance patterns for brachiopod assemblages in the two studied Aegean caves are also in agreement with those described for other sessile invertebrate taxa in the same caves, showing a clear differentiation between the outer and internal cave sectors (Gerovasileiou & Voultsiadou Citation2016; Sanfilippo et al. Citation2017; Rosso et al. Citation2019). Intermediate ceiling stations, characterized by a transitional assemblage between the semidark and dark cave biocoenoses (, ) were grouped with the internal dark wall stations, due to the sharper decrease of light as a result of the substratum inclination (Gerovasileiou et al. Citation2017).
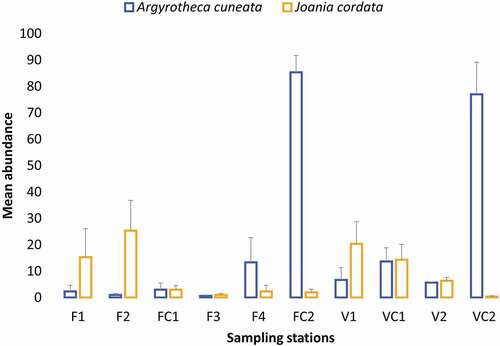
The species that contributed the most to the resemblance patterns were Joania cordata and Argyrotheca cuneata. Despite their presence in all sampling stations (), J. cordata was more abundant in the cave entrance and semidark zones, while A. cuneata was very abundant in the dark parts of both caves (, ). A recent study on brachiopods from sediments in a submarine cave in southern Spain also showed that J. cordata was present only at the cave entrance zone (Bergamin et al. Citation2020). This pattern might indicate the difference in environmental preference between those two species where J. cordata prefers dim-light conditions, and A. cuneata prefers dark internal zones of caves. However, differences in breeding behaviour of A. cuneata and J. cordata may influence such differences in distribution (Grobe & Lüter Citation1999). Under laboratory conditions larvae in both species were observed at the same time, during October to January (Atkins Citation1960), however, observations by Grobe & Lüter (Citation1999) have shown that in J. cordata there is no seasonality in the reproductive cycle; eggs or larvae have been observed throughout the year. On the contrary, in A. cuneata the larvae were found only in September and November (Grobe & Lüter Citation1999). As a result of endogenous development, the larvae of A. cuneata and J. cordata settle rapidly after having left the brood pouches thereby increasing the opportunities for success (Taddei Ruggiero Citation1994). In a fairly restricted environment like a cave, this growth pattern may not allow the larvae to travel far; this fact, coupled with a low degree of hydrodynamism, may cause gregariousness of A. cuneata and J. cordata in caves. In the examined material from the Aegean caves we have observed immature individuals of J. cordata attached to mature specimens (). Preferential settlement on conspecific shells is commonly observed among articulated brachiopods (see also )), especially in dense populations and when larval dispersal is limited (Peck Citation2001b).
Figure 6. Mean abundance of the species Argyrotheca cuneata and Joania cordata per sampling station in the two studied caves (error bars indicate standard error)
Seasonal variability of sessile benthos in submarine caves has been rarely investigated (e.g. Martí et al. Citation2004; Bussotti et al. Citation2006), revealing some differences in species number and cover. The dispersal of larvae in submarine caves is determined by various factors, such as water circulation – which is generally lower in confined dark sectors (Harmelin et al. Citation1985; Bianchi & Morri Citation1994) – larval behaviour and differential post-settlement survival against environmental conditions, among others (Gerovasileiou & Bianchi Citation2021 and references therein). Further research is required in order to investigate the reasons behind the observed distribution patterns of these two megathyridids.
Future research in other marine caves of the Eastern Mediterranean Sea, focusing also on cave sediment thanatocoenoses, is expected to increase knowledge on the regional diversity of brachiopods and will also provide a better understanding of the spatial and temporal variability of brachiopod assemblages in the marine cave habitat.
Acknowledgements
The authors would like to thank Maria Sini and Drosos Koutsoubas for their help during the fieldwork. Aleksandra Hołda-Michalska (Institute of Paleobiology, Warszawa) is thanked for her help in the preparation of . We wish to thank Jeffrey H. Robinson (University of Otago, Dunedin) and an anonymous reviewer for their constructive comments. J.H. Robinson kindly improved the language.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Albano PG, Stockinger M. 2019. The rhizome layer of Posidonia oceanica: An important habitat for Mediterranean brachiopods. Marine Biodiversity 49(5):2467–2472. DOI:10.1007/s12526-019-00968-6.
- Atkins D. 1960. The ciliary feeding mechanism of the Megathyridae (Brachiopoda), and growth stages of the lophophore. Journal of the Marine Biological Association of the United Kingdom 39(3):459–479. DOI:10.1017/S0025315400013485.
- Bergamin L, Taddei Ruggiero E, Pierfranceschi G, Andres B, Constantino R, Crovato C, d’Ambrosi A, Marassich A, Romano E. 2020. Benthic foraminifera and brachiopods from a marine cave in Spain: Environmental significance. Mediterranean Marine Science 21(3):506–518. DOI:10.12681/mms.23482.
- Bianchi CN, Morri C. 1994. Studio bionomico comparativo di alcune grotte marine sommerse; definizione di una scala di confinamento. Memorie dell’Istituto Italiano di Speleologia serie II 6:107–123.
- Boury-Esnault N, Harmelin J-G, Ledoyer M, Saldanha L, Zibrowius H. 2001. Peuplement benthique des grottes sous-marines de Sagres (Portugal, Atlantique Nord-Oriental). In: Biscoito M, Almeida AJ, Ré P, editors. A Tribute to Luiz Saldanha. Funchal, Madeira: Boletim do Museu Municipal do Funchal. Suplemento 6. pp. 15–38.
- Brunton CHC. 1988. Some brachiopods from the eastern Mediterranean Sea. Israel Journal of Zoology 35:151–169. DOI: 10.1080/00212210.1988.10688609.
- Bussotti S, Terlizzi A, Fraschetti S, Belmonte G, Boero F. 2006. Spatial and temporal variability of sessile benthos in shallow Mediterranean marine caves. Marine Ecology Progress Series 325:109–119. DOI:10.3354/meps325109.
- Clarke KR, Gorley RN. 2006. PRIMER v6: User manual/tutorial. Plymouth: PRIMER-E.
- Cohen B, Kaulfuss A, Lüter C. 2014. Craniid brachiopods: Aspects of clade structure and distribution reflect continental drift (Brachiopoda: Craniiformea). Zoological Journal of the Linnean Society 171(1):133–150. DOI:10.1111/zoj.12121.
- Di Geronimo I, La Perna R, Rosso A, Sanfilippo R, Taddei Ruggiero E. 2001. Associazioni bentoniche da sedimenti di grotto carsiche in Sicilia. Atti del 1° Seminario di Studi sul il Carsismo negli Iblei e nell’area sud Mediterranea, Ragusa, 9–11 aprile, 1999. Speleologia Iblea 8:97–102.
- Emig CC. 2014. Novocrania turbinata synonyme de N. anomala. Carnets de Géologie [Notebooks on Geology]. Brest 14:159–171.
- Emig CC, Bitner MA, Álvarez F. 2013. Phylum Brachiopoda. In: Zhang Z-Q, editor. Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness (Addenda 2013). Zootaxa 3703. Auckland, New Zealand: Magnolia Press. pp. 75–78. DOI:10.11646/zootaxa.3703.1.15
- Gerovasileiou V, Bailly N. 2016. Brachiopoda of Greece: An annotated checklist. Biodiversity Data Journal 4:8169. DOI: 10.3897/BDJ.4.e8169.
- Gerovasileiou V, Bianchi CN. 2021. Mediterranean marine caves: A synthesis of current knowledge. Oceanography and Marine Biology - an Annual Review 59. inpress.
- Gerovasileiou V, Chintiroglou CC, Vafidis D, Koutsoubas D, Sini M, Dailianis Y, Issaris T, Akritopoulou E, Dimarchopolou D, Voultsiadou E. 2015. Census of biodiversity in marine caves of the eastern Mediterranean Sea. Mediterranean Marine Science 16(1):245–265. DOI:10.12681/mms.1069.
- Gerovasileiou V, Dimitriadis C, Arvanitidis C, Voultsiadou E. 2017. Taxonomic and functional surrogates of sessile benthic diversity in Mediterranean marine caves. PLoS ONE 12(9):e0183707. DOI:10.1371/journal.pone.0183707.
- Gerovasileiou V, Trygonis V, Sini M, Koutsoubas D, Voultsiadou Ε. 2013. Three-dimensional mapping of marine caves using a handheld echosounder. Marine Ecology Progress Series 486:13–22. DOI:10.3354/meps10374.
- Gerovasileiou V, Voultsiadou Ε. 2016. Sponge diversity gradients in marine caves of the eastern Mediterranean. Journal of the Marine Biological Association of the United Kingdom 96(2):407–416. DOI:10.1017/S0025315415000697.
- Grobe P, Lüter C. 1999. Reproductive cycles and larval morphology of three Recent species of Argyrotheca (Terebratellacea: Brachiopoda) from Mediterranean submarine caves. Marine Biology 134(3):595–600. DOI:10.1007/s002270050574.
- Guido A, Gerovasileiou V, Russo F, Rosso A, Sanfilippo R, Voultsiadou E, Mastandrea A. 2019a. Dataset of biogenic crusts from submarine caves of the Aegean Sea: An example of sponges vs microbialites competition in cryptic environments. Data in Brief 27:104745. DOI: 10.1016/j.dib.2019.104745.
- Guido A, Gerovasileiou V, Russo F, Rosso A, Sanfilippo R, Voultsiadou E, Mastandrea A. 2019b. Composition and biostratinomy of sponge-rich biogenic crusts in submarine caves (Aegean Sea, Eastern Mediterranean). Palaeogeography, Palaeoclimatology, Palaeoecology 534:109–338. DOI: 10.1016/j.palaeo.2019.109338.
- Guido A, Jimenez C, Achilleos K, Rosso A, Sanfilippo R, Hadjioannou L, Petrou A, Russo F, Mastandrea A. 2017. Cryptic serpulid‑microbialite bioconstructions in the Kakoskali submarine cave (Cyprus, Eastern Mediterranean). Facies 63(3):21. DOI:10.1007/s10347-017-0502-3.
- Harmelin J-G. 1980. Etablissement des communautés de substrats durs en milieu obscur. Résultats préliminaires d’une expérience à long terme en Méditerranée. Memorie di Biologia Marina e di Oceanografia 10:29–52.
- Harmelin J-G, Boury-Esnault N, Fichez R, Vacelet J, Zibrowius H. 2003. Peuplement de la grotte sous-marine de Bagaud (Parc national de Port-Cros, France, Méditerranée). Rapport scientifique du Parc national de Port-Cros, France 19:117–134.
- Harmelin J-G, Vacelet J, Vasseur P. 1985. Les grottes sous-marines obscures: Un milieu extrême et un remarquable biotope refuge. Téthys 11:214–229.
- Jimenez C, Achilleos K, Petrou A, Hadjioannou L, Guido A, Rosso A, Gerovasileiou V, Albano PG, Di Franco D, Andreou V, Abu AR. 2019. A dream within a dream: Kakoskali Cave, a unique marine ecosystem in Cyprus (Levantine Sea). In: Öztürk B, editor. Marine Caves of the Eastern Mediterranean Sea. Biodiversity, threats and conservation. Vol. 53. Istanbul: Turkish Marine Research Foundation (TUDAV) Publication. pp. 91–110.
- Kroh A, Bitner MA, Ávila SP. 2008. Novocrania turbinata (Brachiopoda) from the Early Pliocene of the Azores (Portugal). Acta Geologica Polonica 58:473–478.
- Logan A. 1979. The recent Brachiopoda of the Mediterranean Sea. Bulletin de l’Institut Océanographique Monaco 72:1–112.
- Logan A. 1993. Recent brachiopods from the Canarian-Cape Verdean Region: Diversity, biogeographic affinities, bathymetric range and life habits. Courier Forschungsinstitut Senckenberg 159:229–233.
- Logan A. 2001. Recent cave-dwelling brachiopods from western Portugal and Madeira. In: Biscoito M, Almeida AJ, Ré P, editors. A Tribute to Luiz Saldanha. Funchal, Madeira: Boletim do Museu Municipal do Funchal. Suplemento 6. pp. 65–73.
- Logan A. 2003. Marine fauna of the Mljet National Park (Adriatic Sea, Croatia). 3. Brachiopoda. Natura Croatica 12:233–243.
- Logan A, Bianchi CN, Morri C, Zibrowius H. 2004. The present-day Mediterranean brachiopod fauna diversity, life habits, biogeography and paleobiogeography. Scientia Marina 68(S1):163–170. DOI:10.3989/scimar.2004.68s1163.
- Logan A, Bianchi CN, Morri C, Zibrowius H, Bitar G. 2002. New records of recent brachiopods from the eastern Mediterranean Sea. Annali del Museo Civico di Storia Naturale “G. Doria” XCIV:407–418.
- Logan A, Long SL. 2001. Shell morphology and geographical distribution of Neocrania (Brachiopoda, Recent) in the eastern North Atlantic and Mediterranean Sea. In: Brunton CHC, Cocks LRM, Long SL, editors. Brachiopods: Present and past. The Systematics Association Special Volume Series 63. London: Taylor & Francis. pp. 71–79.
- Logan A, Noble JPA. 1983. Recent brachiopods from Malta. The Central Mediterranean Naturalist 1(2):33–42.
- Logan A, Zibrowius H. 1994. A new genus and species of rhynchonellid (Brachiopoda, Recent) from submarine caves in the Mediterranean Sea. P.S.Z.N.I: Marine Ecology 15:77–88.
- Lüter C. 2001. Larval brooding and development of the micromorph rhynchonellid Tethyrhynchia mediterranea (Brachiopoda: Recent). Journal of the Marine Biological Association of the United Kingdom 81(6):939–942. DOI:10.1017/S0025315401004866.
- Martí R, Uriz MJ, Ballesteros E, Turón X. 2004. Benthic assemblages in two Mediterranean caves: Species diversity and coverage as a function of abiotic parameters and geographic distance. Journal of the Marine Biological Association of the United Kingdom 84(3):557–572. DOI:10.1017/S0025315404009567h.
- Monteiro-Marques V. 1981. Peuplements des planchers envasés de trois grottes sous-marines de la région de Marseille. Étude préliminaire. Téthys 10:89–96.
- Peck LS. 2001a. Physiology. In: Carlson SJ, Sandy MR, editors. Brachiopods ancient and modern: A tribute to G. Arthur Cooper. The Paleontological Society Papers 7. New Haven: Yale University. pp. 89–104.
- Peck LS. 2001b. Ecology of articulated brachiopods. In: Carlson SJ, Sandy MR, editors. Brachiopods ancient and modern: A tribute to G. Arthur Cooper. The Paleontological Society Papers 7. New Haven: Yale University. pp. 171–183.
- Pino de la Torre L, Navarro-Barranco C, Gofas S. 2020. Malacofauna from soft bottoms in the Cerro Gordo marine cave (Alboran Sea): Biodiversity and spatial distribution. Mediterranean Marine Science 21(3):684–704.
- Radolović M, Bakran-Petricioli T, Petricioli D, Surić M, Perica D. 2015. Biological response to geochemical and hydrological processes in a shallow submarine cave. Mediterranean Marine Science 16(2):305–324. DOI:10.12681/mms.1146.
- Robinson JH. 2014. The muscles, body wall and valve-opening mechanism of extant craniid (inarticulated) brachiopods. Journal of Natural History 48(21–22):1231–1252. DOI:10.1080/00222933.2013.840941.
- Robinson JH. 2017. A review of all Recent species in the genus Novocrania (Craniata, Brachiopoda). Zootaxa 4329(6):501–559. DOI:10.11646/zootaxa.4329.6.1.
- Rosso A, Gerovasileiou V, Sanfilippo R, Guido A. 2019. Bryozoan assemblages from two submarine caves in the Aegean Sea (Eastern Mediterranean). Marine Biodiversity 49(2):707–726. DOI:10.1007/s12526-018-0846-0.
- Rosso A, Sanfilippo R, Taddei Ruggiero E, Di Martino E. 2013. Faunas and ecological groups of Serpuloidea, Bryozoa and Brachiopoda from submarine caves in Sicily (Mediterranean Sea). Bollettino della Societa Paleontologica Italiana 2:1–10.
- Sanfilippo R, Rosso A, Guido A, Gerovasileiou V. 2017. Serpulid communities from two marine caves in the Aegean Sea, eastern Mediterranean. Journal of the Marine Biological Association of the United Kingdom 97(5):1059–1068. DOI:10.1017/S0025315417000297.
- Sanfilippo R, Rosso A, Guido A, Mastrandrea A, Russo F, Riding R, Taddei Ruggiero E. 2015. Metazoan/microbioal biostalactites from present-day submarine caves in the Mediterranean Sea. Marine Ecology 36(4):1277–1293. DOI:10.1111/maec.12229.
- Schrøder AE, Sørensen AM, Surlyk F. 2019. Morphological adaptations of the brachiopods from a Late Cretaceous rocky shore, Ivø Klack, southern Sweden. Palaeogeography, Palaeoclimatology, Palaeoecology 514:785–799. DOI: 10.1016/j.palaeo.2018.09.019.
- Sempere-Valverde J, Sabino Lorenzo Á, Espinosa F, Gerovasileiou V, Sánchez-Tocino L, Navarro-Barranco C. 2019. Taxonomic and morphological descriptors reveal high benthic temporal variability in a Mediterranean marine submerged cave over a decade. Hydrobiologia 839(1):177–194. DOI:10.1007/s10750-019-04005-2.
- Simon E, Motchurova-Dekova N, Mottequin B. 2018. A reappraisal of the genus Tethyrhynchia Logan, 1994 (Rhynchonellida, Brachiopoda): A conflict between phylogenies obtained from morphological characters and molecular data. Zootaxa 4471(3):535–555. DOI:10.11646/zootaxa.4471.3.6.
- Simon E, Willems G. 1999. Gwynia capsula (Jeffreys, 1859) and other Recent brachiopods from submarine caves in Croatia. Bulletin de l’Institut royal des Sciences naturelles de Belgique, Biologie 69:15–21.
- Taddei Ruggiero E. 1994. Brachiopods from bio- and thanatocoenoses of the Isca submarine cave (Sorrento Peninsula). In: Matteucci R, Carboni MG, Pignatti JS, editors. Studies on ecology and palaeoecology of benthic communities. Bollettino della Società Paleontologica Italiana Special volume 2. Modena. pp. 313–323.
- Taddei Ruggiero E. 1996. Notes on living brachiopod ecology in a submarine cave off the Campania coast, Italy. In: Copper O, Jin J, editors. Brachiopods. Rotterdam: A.A. Balkema Press. pp. 227–231.
- Taddei Ruggiero E. 2001. Brachiopods of the Isca submarine cave: Observations during ten years. In: Brunton CHC, Cocks RL, Long SL, editors. Brachiopods past and present. The Systematics Association Special Volume series 63. London: Taylor & Francis. pp. 261–267.