?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The knowledge of demographic traits such as longevity, growth rates and age at sexual maturity is crucial for understanding the structure of a population in its natural environment and implementing appropriate strategies for its management and conservation. Based on counts of growth layer groups in sections of decalcified teeth using the paraffin technique, we estimated the age and growth of 25 individuals of striped dolphin (Stenella coeruleoalba) found dead stranded along the coast of Campania and Calabria (south Italy, central-western Mediterranean) from 2013 to 2018. Seven individuals, with TL of 100–110 cm, were calves under 1 year old. The oldest male and female individuals were 19 and 14 years old, respectively. Growth curve estimated using the Gompertz growth model (GGM) showed that in S. coeruleoalba male growth trajectories are partly in accordance with those reported in other studies on the same species from different Mediterranean areas. The high frequency (28%) of calves strongly suggests that females of this species use the marine area all around the south-western Italian coasts to give birth to their offspring. Furthermore, a comparison with the estimated age of striped dolphins from other Mediterranean marine areas shows that the longevity of the individuals examined in this study is much lower. Our study provides information toward understanding the demographic traits of S. coeruleoalba from Mediterranean Sea. The results reported here can be useful for future research aimed at understanding population structure, mortality patterns and the effects of anthropogenic activity on the survival of this species in this marine area.
Introduction
The striped dolphin, Stenella coeruleoalba (Meyen 1833) (Cetartiodactyla, Delphinidae), has a wide distribution, from tropical to warm-temperate waters of the Atlantic, Pacific and Indian oceans, as well as many adjacent seas, including the Mediterranean (Braulik Citation2019). Morphological and genetic evidence suggests that the Mediterranean and eastern North Atlantic striped dolphin populations are isolated from each other, with reduced or absent gene flow across the Strait of Gibraltar (Calzada & Aguilar Citation1995; Garcia-Martinez et al. Citation1995; Aguilar & Gaspari Citation2012). Within the Mediterranean, some clinal variations in body length among populations have been observed, suggesting restrictions in gene flow between areas (Calzada & Aguilar Citation1995) or even different demographic characteristics of distinct populations. In general, reduced gene flow may be a result of geographic isolation, genetic and chromosome differentiation (Mezzasalma et al. Citation2013, Citation2017a, Citation2017b). Evidence of genetic differentiation between the Adriatic and Tyrrhenian Seas has been also found (Gaspari et al. Citation2007), but currently there is no information available on different age structures in Mediterranean populations of S. coeruleoalba.
Based on the International Union for Conservation of Nature (IUCN) Red List criteria, S. coeruleoalba is listed as “Least concern” worldwide (Braulik Citation2019) but “Vulnerable” in the Mediterranean, owing to several threats to which this species is susceptible and the limited conservation actions currently taking place in this geographical area (Aguilar & Gaspari Citation2012). In the past, the Mediterranean population of S. coeruleoalba was strongly affected by accidental catches in pelagic nets for swordfish fishing (Magnaghi & Podestà Citation1987; Podestà & Magnaghi Citation1989; Notarbartolo Di Sciara Citation1990). From the end of the last century, despite a ban in the Mediterranean by all European Union countries, including Italy (EC Reg. 1239/98), S. coeruleoalba continued to suffer from illegal capture although to a lesser extent than in the past (Tudela et al. Citation2005; Fortuna et al. Citation2007). Furthermore, over the last 30 years, several morbillivirus epizootics were responsible for many die-offs of Mediterranean striped S. coeruleoalba (Aguilar & Raga Citation1993; Raga et al. Citation2008; Rubio-Guerri et al. Citation2013; Casalone et al. Citation2014; Profeta et al. Citation2015).
Despite its importance from a conservation point of view, information on the age structure of the Mediterranean population of this cetacean is scarce and limited to only a few studies (Calzada et al. Citation1994, Citation1997; Marsili et al. Citation1997, Citation2001). Data on age determination of Italian striped dolphin are also reported in Guglielmini et al. (Citation2002).
One of the most widely used methods to estimate the individual age of odontocetes is based on the interpretation of the growth incremental layers which may be recognized in tooth sections (Scheffer & Myrick Citation1980; Evans et al. Citation2002; Read et al. Citation2018). For many species of odontocetes it has been possible to define growth layer groups (GLGs), where each group represents the amount of mineralized tissue formed per year (e.g. Myrick Jr et al. Citation1984; Hohn et al. Citation1989; Myrick Jr & Cornell Citation1990). Counts of GLGs in the tooth dentine is the common practice for assessing the age of odontocetes because dentine is the most developed mineralized tissue of the tooth (Perrin & Myrick Citation1980; Luque et al. Citation2009; Murphy et al. Citation2014). However, some researchers have obtained more accurate estimates for older dolphins when cementum is used (Cockcroft & Ross Citation1990). The distinctness of dentinal GLGs may vary according to the species and is influenced by the growth pattern of the dentine (Klevezal & Myrick Citation1984; Evans et al. Citation2007). In addition, the identification of GLGs may be complicated by the presence of accessory layers within the dentine, i.e. adjacent layers discernible in a GLG (sensu Scheffer & Myrick Citation1980), and other tooth-tissue alterations, such as pulp stones in the pulp cavity and dentinal resorption (Luque et al. Citation2009, Citation2013; Della Bianca et al. Citation2012; Read et al. Citation2018). The reading efficiency of GLGs and, consequently, the reliability of the age estimate can be also influenced by the tooth preparation technique (Hohn & Fernandez Citation1999; Evans et al. Citation2002). Luque et al. (Citation2009) showed that the paraffin technique is a viable method and represents a cost-effective alternative to other techniques when preparing teeth of small odontocete species for age determination.
In this study, we aimed to estimate the individual age and growth of striped dolphins (Stenella coeruleoalba) stranded along the coast of Campania (Maio et al. Citation2019) and Calabria (south Italy, western Mediterranean) from 2013 to 2018 using the counts of GLGs on stained sections of decalcified teeth using the paraffin technique.
Materials and methods
Sampling
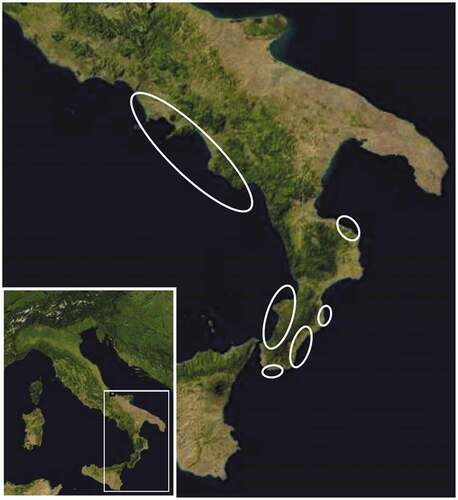
We analyzed the teeth of 25 individuals (17 males, 8 females) stranded along the coasts of Campania and Calabria (Southern Italy) from 2013 to 2018 (; Supplementary data, Table S1). Sex was determined by visual inspection of the gonads. For each animal, total body length (TL) was measured from the tip of the rostrum to the notch of the tail (American Society of Mammalogists Citation1961). During the necropsy, a portion with at least 3 teeth was collected from the middle of the lower jaw of each dolphin and fixed in 75% ethanol, exchanging the ethanol solution once a day for 5 days. Teeth were successively removed from the jaw bone using a scalpel blade, cleaned of soft tissue, and then stored in 75% ethanol until laboratory analysis.
Histological technique for age determination
Tooth histological sections were prepared according to Luque et al. (Citation2009), with slight modifications specified below. After gently rinsing in tap water, teeth were decalcified. Since the decalcification can affect the quality of the tooth histological sections and therefore their interpretation, for each animal, two decalcification solutions were used: RDO (Apex Engineering Products Corporation, Illinois, USA), a commercial rapid decalcifying agent of which hydrochloric acid is the principal active ingredient, and 5% nitric acid. The decalcification end point was defined as when teeth appeared translucent and pliable (Murphy et al. Citation2014; Read et al. Citation2018). The duration required for optimal decalcification varied between samples in relation to the decalcifier (type and concentration), tooth size and age, with longer times required for larger and older individuals. Using RDO, decalcification time ranged from 8 to 12 h for smaller individuals (TL < 150 cm) and up to 24 h for larger individuals. Using 5% nitric acid, decalcification time ranged from 12 to 20 h for smaller individuals (TL < 150 cm) and up to 48 h for larger individuals. After decalcification, the teeth were washed in running tap water for 6 h, dehydrated through a series of graded ethanol baths, cleared with Bioclear (Bio Optica, Milano, Italy) and then infiltrated with molten paraffin (60°C). Longitudinal serial paraffin sections (15 µm thick) were obtained using a standard rotative microtome (Reichert-Jung/Leica 2045, Germany), stained with Mayer’s Hematoxylin (30 min) and mounted with Bio Mount HM resin (Bio-Optica, Milano, Italy).
Tooth sections were examined using both a Leica EZ4 stereo microscope and a Motic BA340 compound light microscope, equipped with a digital camera. The former microscope was used to check for the best-stained sections closest to the midline of the tooth and to obtain a complete histological view (low magnification) of the sections; the latter allowed us to observe tooth sections at higher magnification which were successively photographed with the digital camera and stitched together into a single image. The acquired images of the tooth sections were optimized with respect to contrast and intensity using Adobe Photoshop 6.0 in order to enhance the distinctiveness of the GLGs. The count of GLGs was performed independently by three researchers (FMG, NM, CHL) and without prior knowledge of the TL or sex of the specimens. In the case of discrepancies in the GLG count, the sections were read again until a final consensus was reached.
Growth models
The growth of S. coeruleoalba was described using the GGM fitted to the age–length data according to other studies on odontocetes (Calzada et al. Citation1997; Venuto et al. Citation2020).
The GGM equation is as follows:
where lt is the total body length at age t; L∞ is the asymptotic length at which growth is zero; e is the base of the natural logarithm; k is the growth coefficient that defines the shape of the curve; and I is the age at the inflection point.
L∞, k, and their asymptotic confidence intervals (CIs) were estimated using a non-linear regression procedure by means of the Growth II software (Henderson & Seaby Citation2006).
Results
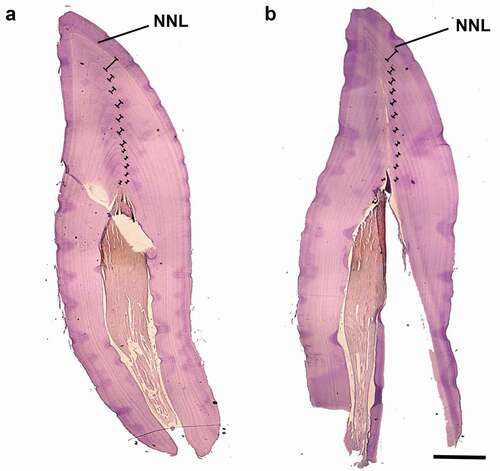
Dentinal GLGs were visible on all tooth sections, and their count was the same in teeth for which the two decalcification methods produced a similar result (). Accessory lines, which are subannual incremental layers (sensu Read et al., Citation2018), were often observed, especially within the first 3–4 GLGs following the neonatal line (NNL).
Figure 2. Representative tooth sections of Stenella coeruleoalba (ID Sc166277) obtained using different decalcifiers. The sections were obtained from two different teeth of the same individual. (a) Tooth decalcified using RDO; (b) tooth decalcified using 5% acid nitric. The neonatal line (NNL) and GLGs (Ⱶ˧) in dentine are indicated. In (b) one less GLG (n = 12) compared to (a) (n = 13) is visible, probably due to the greater erosion of the pulp cavity. Scale bar = 1600 μm for both figures
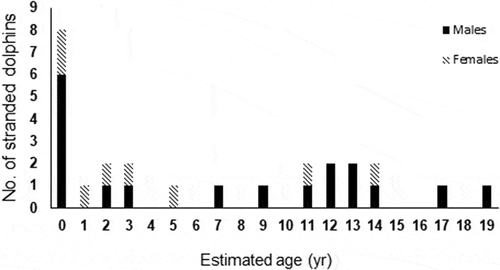
Estimated ages ranged from 0 to 19 years old (). Seven individuals (5 males, 2 females), with TL 100–110 cm, were calves under 1 year of age (); 7 individuals (3 males, 4 females), with TL 134–156 cm, were 1–7 years old; 11 individuals (9 males, 2 females), with TL 162–210 cm, were 9–19 years old. The oldest male and female were 19 (TL = 210 cm) and 14 (TL = 194 cm) years old, respectively.
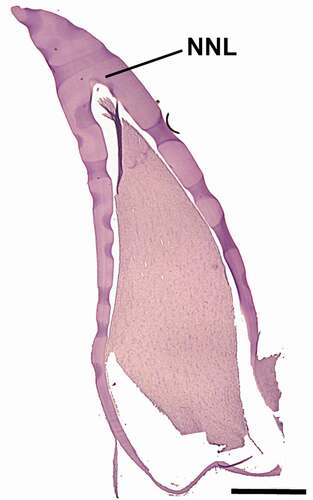
Figure 3. Tooth section of Stenella coeruleoalba calf under 1 year old (ID Sc9793). NNL: neonatal line. Scale bar = 1100 μm
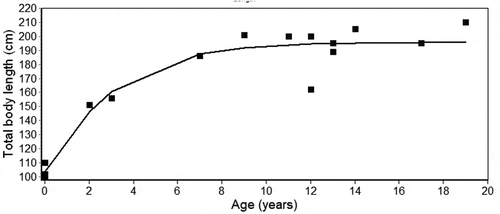
Since the female sample was too small, the growth curve was estimated using GGM only for males (). The estimated asymptotic total body length in males (L∞ ± CI: 195.7 ± 0.4 mm, k ± CI, 0.39 ± .1.32) was lower than the maximum total body length observed for this sex (TL: 210 cm).
Discussion
This study shows that the longevity of striped dolphins stranded along the coasts of south-western Italy (central-western Mediterranean) between 2013 and 2018 is much lower than that recorded in previous studies on striped dolphins stranded along Mediterranean coasts, and highlights the variability in the age structure of S. coeruleoalba populations in this marine area. In fact, in this study the estimated ages of the oldest male and female were 19 and 14 years, respectively, whereas other studies yielded estimates of 28 and 32 years for males and females, respectively, stranded along the Spanish coasts (Calzada & Aguilar Citation1995; Calzada et al. Citation1997), and 29 years (for the two sexes combined) for animals stranded along a wide coastal area all around the Italian peninsula (Marsili et al. Citation2001).
There are several possible explanations for the age structure of our sample, such as greater susceptibility to diseases in adults due to the effects of pollution or allopatric geographical distribution of adults and juveniles, as suggested in other studies (Calzada et al. Citation1994). In particular, it might be hypothesized that the age structure of our sample was affected by die-offs, mainly due to morbillivirus, that have frequently occurred in the Mediterranean striped dolphin population in the last few decades (Pertoldi et al. Citation2000; Valsecchi et al. Citation2004; Casalone et al. Citation2014). Furthermore, it has been suggested that animals dying from morbillivirus may venture more frequently toward the shore than those dying of old age (Valsecchi et al. Citation2004). Our study sample also had a very high percentage (about 90%) of animals infected with morbillivirus (data not shown). However, further targeted studies are needed on a larger sample to clarify the link between morbillivirus and age structure of Mediterranean striped dolphins.
Another interesting finding of this work is the high frequency (n = 7; 28% of our sample) of calves under 1 year old. We hypothesize that they very likely died in marine areas next to the stranding localities, for the following reasons. Most of the calf carcasses (n = 4) were in good condition (decomposition stage code 2, according to Geraci & Lounsbury Citation2005), while the remaining calves (n = 3) were in the initial decomposition state (decomposition stage code 2–3). In addition, the weather conditions and the average wind speed were stable and moderate, respectively, during the days of the stranding and those immediately preceding our research (see https://www.ilmeteo.it/portale/archivio-meteo). Finally, the trend of the speed and direction of surface currents, reported monthly for different stranding areas, was parallel to the coastlines (Istituto Idrografico della Marina Citation1982). Therefore, our results for the calves strongly suggest that the marine area along the Tyrrenian coasts of Campania and Calabria and Jonian coasts of Calabria (southern Italy) is used by the species to give birth to the offspring and as a nursery. This conclusion is in accordance with previous studies relating to the Salerno coast, where at least 15 calves under 1 year old were recorded between 1986 and 2019 (Maio et al. Citation2012, Banca Dati Spiaggiamenti: http://mammiferimarini.unipv.it). Furthermore, we collected calves both during May–early October (n = 4) and January–March (n = 3) (see Supplementary Table S1). Therefore, it is likely that in the study area S. coeruleoalba has two reproduction periods, roughly corresponding to the summer–early autumn and the late winter period, respectively, as previously suggested by Marini et al. (Citation1992), Mussi et al. (Citation1997) and Mussi and Miragliuolo (Citation2003).
The GGM showed that in S. coeruleoalba male growth trajectories are partly in accordance with those reported in other studies on the same species from different Mediterranean areas (Di Meglio et al. Citation1996; Calzada et al. Citation1997; Marsili et al. Citation1997, Citation2004). Indeed, we also observed initially high growth rates in males, until reaching an asymptote that likely corresponds to their attainment of sexual maturity. In our study, the asymptote begins at 12 years of age and a TL of 195 cm. In the study by Calzada et al. (Citation1997) the asymptote was approached at 18 years of age and a TL of 200 cm in males. In the papers of Marsili et al. (Citation1997, Citation2001, Citation2004), where the data for the two sexes were combined, the age of sexual maturity was estimated at 9 years, at a TL of about 190 cm. Similarly, Di Meglio et al. (Citation1996) reported that males of Mediterranean striped dolphins reach their asymptotic length, and then sexual maturity, at 8–9 years. Therefore, our findings are consistent with the observation that the males of S. coeruleoalba from central-western Mediterranean can reach sexual maturity at a much lower age than that reported by Calzada et al. (Citation1997). However, confirmation of the age at sexual maturity of striped dolphin males predicted on the basis of the growth curve can only be obtained via inspection of the gonads, which unfortunately were not available for this study.
To conclude, although this study was limited by the number of animals examined, it provides interesting information toward understanding the demographic traits of S. coeruleoalba from the Mediterranean Sea. The results reported here can useful for future research aimed at understanding mortality patterns and the effects of anthropogenic activity on the survival of this species in this marine area, as performed also for other cetacean species (Maio et al., Citation2016) and other marine vertebrates (see Guarino et al. Citation2020).
Supplemental Material
Download MS Word (26.2 KB)Acknowledgements
This research is part of the activities under a broad Memorandum of Understanding including the University of Naples Federico II and the Istituto Zooprofilattico del Mezzogiorno, Portici (Naples), ratified by decree No. 98 of 8 November 2009 of the Regional Council of Campania (Italy) and updated with decree No. 231 of 15 July 2015. We thank Dr. G. Lucifora, Istituto Zooprofilattico Sperimentale del Mezzogiorno (Vibo Valentia), for assistance with sample collection and associated biological data. We thank the National Park Authority of the Cilento, Vallo di Diano and Alburni for collaboration and stranded specimen permits. We thank the referees for their useful comments.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Aguilar A, Gaspari S. 2012. Stenella coeruleoalba (Mediterranean subpopulation). The IUCN Red List of Threatened Species 2012: e.T20731A2773889. Accessed January 2021 3. DOI:10.2305/IUCN.UK.2019-1.RLTS.T20731A50374282.en.
- Aguilar A, Raga JA. 1993. The striped dolphin epizootic in the Mediterranean Sea. Ambio 22:524–528.
- American Society of Mammalogists. 1961. Standardized methods for measuring and recording data on the smaller cetaceans. Journal of Mammalogy 42(4):471–476. DOI:10.2307/1377364.
- Braulik G. 2019. Stenella coeruleoalba. The IUCN Red List of Threatened Species 2019: e.T20731A50374282. Accessed September 2020 3. DOI:10.2305/IUCN.UK.2019-1.RLTS.T20731A50374282.en.
- Calzada N, Aguilar A. 1995. Geographical variation in body size in western Mediterranean striped dolphins (Stenella coeruleolba). Zeitschrift für Säugetierkunde 60:257–264.
- Calzada N, Aguilar A, Grau E, Lockyer C. 1997. Patterns of growth and physical maturity in the western Mediterranean striped dolphin, Stenella coeruleoalba (Cetacea: Odontoceti). Canadian Journal of Zoology 75(4):632–637. DOI:10.1139/z97-078.
- Calzada N, Lockyer CH, Aguilar A. 1994. Age and sex composition of the striped dolphin die-off in the western Mediterranean. Marine Mammal Science 10(3):210–399. DOI:10.1111/j.1748-7692.1994.tb00484.x.
- Casalone C, Mazzariol S, Pautasso A, Di Guardo G, Di Nocera F, Lucifora G, Ligios C, Franco A, Fichi G, Cocumelli C, Cersini A, Guercio A, Puleio R, Goria M, Podestà M, Marsili L, Pavan G, Pintore A, De Carlo E, Eleni C, Caracappa S. 2014. Cetacean strandings in Italy: An unusual mortality event along the Tyrrhenian Sea coast in 2013. Diseases of Aquatic Organisms 109(1):81–86. DOI:10.3354/dao02726.
- Cockcroft VG, Ross GJB. 1990. Age, growth and reproduction in bottlenose dolphins from the east coast of southern Africa. Fishery Bulletin Washington 88:289–302.
- Della Bianca NA, Hohn A, Goodal RNP. 2012. Age estimation and growth layer patterns in teeth of Commerson’s dolphins (Cephalorhynchus c. commersonii) in subantarctic waters. Marine Mammal Science 28(2):378–388. DOI:10.1111/j.1748-7692.2011.00475.x.
- Di Meglio N, Romero Alvarez R, Collet A. 1996. Growth comparison in striped dolphins, Stenella coeruleoalba, from the Atlantic and Mediterranean coasts of France. Aquatic Mammals 22:11–21.
- Evans K, Kemper C, McKenzie J, McIntosh R. 2007. Workshop on age determination of marine mammals using tooth structure. The South Australian Museum Commonwealth Department of the Environment, Water, Heritage and the Arts, Adelaide, South Australia: South Australian Museum. pp. 1–74.
- Evans KA, Hindell MA, Robertson KE, Lockyer CH, Rice DA. 2002. Factors affecting the precision of age determination of sperm whales (Physeter macrocephalus). Journal of Cetacean Research Management 4:193–202.
- Fortuna C, Canese S, Giusti M, Revelli E, Consoli P, Florio G, Greco S, Romeo T, Andaloro F, Fossi MC, Lauriano G. 2007. An insight into the status of the striped dolphins, Stenella coeruleoalba, of the southern Tyrrenian Sea. Journal of the Marine Biological Association of the United Kingdom 87(5):1321–1326. DOI:10.1017/S002531540705669X.
- Garcia-Martinez J, Barrio E, Raga JA, Latorre A. 1995. Mitochondrial DNA variability of striped dolphins (Stenella coeruleoalba) in the Spanish Mediterranean waters. Marine Mammal Science 11(2):183–199. DOI:10.1111/j.1748-7692.1995.tb00517.x.
- Gaspari S, Azzellino A, Airoldi S, Hoelzel AR. 2007. Social kin associations and genetic structuring of striped dolphin populations (Stenella coeruleoalba) in the Mediterranean Sea. Molecular Ecology 16(14):2922–2933. DOI:10.1111/j.1365-294X.2007.03295.x.
- Geraci JR, Lounsbury VJ. 2005. Marine Mammals Ashore: A Field Guide for Strandings. 2nd. Baltimore: National Aquarium. 0-9774609-0-8 https://www.semanticscholar.org/paper/Marine-Mammals-Ashore%3A-A-Field-Guide-for-Second-Geraci-Lounsbury/b7c42ae5e7c8579e50dad74329abe2bb0130eea8
- Guarino FM, Di Nocera F, Pollaro F, Galiero G, Iaccarino D, Iovino D, Mezzasalma M, Petraccioli A, Odierna G, Maio N. 2020. Skeletochronology, age at maturity and cause of mortality of loggerhead sea turtles Caretta caretta stranded along the beaches of Campania (south-western Italy, western Mediterranean Sea). Herpetozoa 33:39–51. DOI: 10.3897/herpetozoa.33.e47543.
- Guglielmini C, Zotti A, Bernardini D, Pietra M, Podestà M, Cozzi B. 2002. Bone density of the arm and forearm as an age indicator in specimens of stranded striped Dolphins (Stenella coeruleaolba). The Anatomical Record 267:225–230. DOI: 10.1002/ar.10107.
- Henderson PA, Seaby RM. 2006. Growth II. Pisces. Lymington, England: Conservation Ltd.
- Hohn AA, Fernandez S. 1999. Biases in dolphin age structure due to age estimation technique. Marine Mammal Science 15(4):1124–1132. DOI:10.1111/j.1748-7692.1999.tb00881.x.
- Hohn AA, Scott MD, Wells RS, Sweeney JC, Irvine AB. 1989. Growth layers in teeth from known-age, free-ranging bottlenose dolphins. Marine Mammal Science 5(4):315–342. DOI:10.1111/j.1748-7692.1989.tb00346.x.
- Istituto Idrografico della Marina. 1982. Atlante delle correnti superficiali dei mari italiani. Genova.
- Klevezal GA, Myrick AC. 1984. Marks in tooth dentine of female dolphins (genus Stenella) as indicators of parturition. Journal of Mammalogy 65(1):103–110. DOI:10.2307/1381206.
- Luque PL, Learmont JA, Begona Santos M, Ieno E, Pierce GJ. 2009. Comparison of two histological techniques for age determination in small cetaceans. Marine Mammal Science 25(4):902–919. DOI:10.1111/j.1748-7692.2009.00304.x.
- Luque PL, Pierce GJ, Learmonth JA, Ieno E, Santos B, López A, Reid RJ, Rogan E, Boon J, Lockyer CH. 2013. Are mineralization anomalies in common dolphin teeth associated with life-history events and/or the exposure to anthropogenic pollutants? Journal of Zoology 291(3):194–204. DOI:10.1111/jzo.12062.
- Magnaghi L, Podestà M. 1987. An accidental catch of 8 striped dolphins, Stenella coeruleoalba (Meyen, 1983) in the Ligurian Sea. (Cetacea Delphinidae). Atti Della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano 128(3–4):235–239.
- Maio N, Giovannotti M, Caputo Barucchi V, Petraccioli A, Pollaro F, Guarino FM, Splendiani A, De Stasio R, Odierna G. 2016. Haplotype characterization of a young stranded Common Minke Whale (Balaenoptera acutorostrata Lacépède, 1804): is the Mediterranean Sea a potential calving or nursery ground for the species? Hystrix It. J. Mamm 27(2):205–208. https://doi.org/10.4404/hystrix-27.2-11661
- Maio N, Petraccioli A, Guarino FM, Viglietti S, Loreto A, Pollaro F. 2019. La cetofauna dei mari della Campania: Particolarità e minacce. Rapporto Ambiente - SNPA. Edizione 2018. Doc. n. 07/2019, SNPA, Roma. 281–286. [ ISBN 978-88-448-0943-0].
- Maio N, Pollaro F, Di Nocera F, De Carlo E, Galiero G. 2012. Cetacei spiaggiati lungo le coste della Campania dal 2006 al 2011 (Mammalia: Cetacea). Atti Della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale di Milano 153(2):241–255.
- Marini L, Consiglio C, Angradi AM, Finoia MG, Sanna A. 1992. Cetacei nel mar Tirreno Centrale: Risultati della campagna di avvistamento 1989–1991. Rome: Università degli Studi di Roma, Dipartimento di Biologia Animale e dell'uomo. Tip. artistica di L. Nardini. pp. 1–107.
- Marsili L, Casini C, Marini L, Regoli A, Focardi S. 1997. Age, growth and organochlorines (HCB, DDTs and PCBs) in Mediterranean striped dolphins Stenella coeruleoalba stranded in 1988–1994 on the coasts of Italy. Marine Ecology Progress Series 5:273–282. DOI:10.3354/meps151273.
- Marsili L, D’Agostino A, Bucalossi D, Malatesta T, Fossi MC. 2004. Theoretical models to evaluate hazard due to organochlorine compounds (OCs) in Mediterranean striped dolphin (Stenella coeruleoalba). Chemosphere 56(8):791–801. DOI:10.1016/j.chemosphere.2004.03.014.
- Marsili L, Marini L, Casini C, Focardi S. 2001. Stenella striata (Stenella coeruleoalba) del Mar Mediterraneo: Studi morfologici e tossicologici. Natura – Società Italiana Scienze Naturali e Museo Civico di Storia Naturale di Milano 90:109–120.
- Mezzasalma M, Andreone F, Aprea G, Glaw F, Odierna G, Guarino FM. 2017a. When can chromosomes drive speciation? The peculiar case of the Malagasy tomato frogs (genus Dyscophus). Zoologischer Anzeiger 268:41–46.
- Mezzasalma M, Andreone F, Aprea G, Glaw F, Odierna G, Guarino FM. 2017b. Molecular phylogeny, biogeography and chromosome evolution of Malagasy dwarf geckos of the genus Lygodactylus (Squamata, Gekkonidae). Zoologica Scripta 46:42–54.
- Mezzasalma M, Guarino FM, Aprea G, Petraccioli A, Crottini A, Odierna G. 2013. Karyological evidence for diversification of Italian slow worm populations. Comparative Cytogenetics 7:217–227.
- Murphy S, Perrott M, McVee J, Read FL, Stokin KA. 2014. Deposition of growth layer groups in dentine tissue of captive common dolphins Delphinus delphis. Vol. 10. Tromsø, Norway: NAMMCO Scientific Publications. https://doi.org/10.7557/3.3017
- Mussi B, Miragliuolo A. 2003. I Cetacei della costa nord occidentale dell’Isola d’Ischia (Canyon di Cuma). In: Ambiente marino e costiero e territorio delle isole Flegree (Ischia, Procida e Vivara - Golfo di Napoli). Risultati di uno studio multidisciplinare. Gambi MC, De Lauro M, Jannuzzi F, editors. Memorie dell’Accademia di Scienze Fisiche e Matematiche, Società Italiana di Scienze, Lettere e Arti in Napoli. Liguori Editore. Napoli. pp. 213–232.
- Mussi B, Miragliuolo A, Battaglia M. 1997. Cetacei nell’arcipelago delle isole pontine e campane. In: Gaeta N, Italia U, Valerio L, editors. Atti del 5° Seminario Internazionale di Studi sull’Ecosistema marino. Gaeta: Oasi Blu del WWF Italia. pp. 157–167.
- Myrick Jr AC, Cornell LH. 1990. Calibrating dental layers in captive bottlenose dolphins from serial tetracycline labels and tooth extractions. In: Leatherwood S, Reeves RR, editors. The Bottlenose Dolphin. San Diego, CA: Academic Press. pp. 587–608.
- Myrick Jr AC, Shallenberger EW, Kang I, MacKay DB. 1984. Calibration of dental layers in seven captive Hawaiian spinner dolphins, Stenella longirostris, based on tetracycline labeling. Fisheries Bulletin (US) 82:207–225.
- Notarbartolo Di Sciara G. 1990. A note on the incidental catch in the Italian driftnet swordfish fishery, 1986-1988. Report of the International Whaling Commission 40:459–460.
- Perrin WF, Myrick JAC. 1980. Age determination of toothed whales and sirenians. Report of the International Whaling Commission (Special Issue 3): 1–50.
- Pertoldi C, Podestà M, Loescheke V, Schandorff S, Marsili L, Mancusi C, Nicolosi P, Randi E. 2000. Effect of the 1990 die-off in the northern Italian seas on the developmental stability of the striped dolphin Stenella coeruleoalba (Meyen, 1833). Biological Journal of the Linnean Society 71:61–70. DOI: 10.1111/j.1095-8312.2000.tb01242.x.
- Podestà M, Magnaghi L. 1989. Unusual number of cetacean bycatches in the Ligurian Sea. European Research on Cetaceans 3:67–70.
- Profeta F, Di Francesco CE, Marsilio F, Mignone W, Di Nocera F, De Carlo E, Lucifora G, Pietroluongo G, Baffoni M, Cocumelli C, Eleni C, Terracciano G, Ferri N, Di Francesco G, Casalone C, Pautasso A, Mazzariol S, Centelleghe C, Di Guardo G. 2015. Retrospective seroepidemiological investigations against Morbillivirus, Toxoplasma gondii and Brucella spp. in cetaceans stranded along the Italian coastline (1998–2014). Research in Veterinary Science 101:89–92. DOI: 10.1016/j.rvsc.2015.06.008.
- Raga J-A, Banyard A, Domingo M, Corteyn M, Van Bressem M-F, Fernandez M, Aznar F-J, Barrett T. 2008. Dolphin morbillivirus epizootic resurgence, Mediterranean Sea. Emerging Infectious Diseases 14(3):471–473. DOI:10.3201/eid1403.071230.
- Read FL, Hohn A, Lockyer CH. 2018. A review of age estimation methods in marine mammals with special reference to monodontids. Age estimation methods in marine mammals: With special reference to monodontids. NAMMCO Scientific Publications 10. DOI: 10.7557/3.4474.
- Rubio-Guerri C, Melero M, Esperón F, Bellière EN, Arbelo M, Crespo JL, Sierra E, García-Párraga D, Sánchez-Vizcaíno JM. 2013. Unusual striped dolphin mass mortality episode related to cetacean morbillivirus in the Spanish Mediterranean sea. BMC Veterinary Research 9(1):106. DOI:10.1186/1746-6148-9-106.
- Scheffer VB, Myrick JAC. 1980. A review of studies to1970 of growth layers in the teeth of marine mammals. In: Perrin WF, Myrick Jr AC, editors. Age determination of toothed whales and sirenians. Reports of the International Whaling Commission (Special Issue 3). Cambridge. pp. 51–61.
- Tudela S, Kai AK, Maynou F, El Andalossi M, Guglielmi P. 2005. Driftnet fishing and biodiversity conservation: The case study of the large-scale Moroccan driftnet fleet operating in the Alboran Sea (SW Mediterranean). Biological Conservation 121(1):65–78. DOI:10.1016/j.biocon.2004.04.010.
- Valsecchi E, Amos W, Raga JA, Podestà M, Sherwin W. 2004. The effects of inbreeding on mortality during a morbillivirus outbreak in the Mediterranean striped dolphin (Stenella coeruleoalba). Animal Conservation 7(2):139–146. DOI:10.1017/S1367943004001325.
- Venuto R, Botta S, Barreto AS, Secchi ER, Fruet PF. 2020. Age structure of strandings and growth of Lahille’s bottlenose dolphin (Tursiops truncatus gephyreus). Marine Mammal Science 1:15. DOI: 10.1111/mms.12683.