Abstract
During the studies on the invertebrate fauna of the subterranean part of the “Wieliczka” Salt Mine in Wieliczka, Poland, the presence of many specimens of the dipteran species Meoneura obscurella (Fallén 1823) was observed. Organic remains and faeces related to the presence of mice (Mus musculus) were indicated as a potential food source for the insects. M. obscurella displays lecithotrophic viviparity (ovoviviparity), which has developed due to extremely harsh abiotic conditions and a lack of food. This is the first documented case of lecithotrophic viviparity within the fly family Carnidae. Based on the ability of this species to inhabit and reproduce in the conditions of the subterranean part of the “Wieliczka” Salt Mine, it is proposed to classify the species as a troglophile. The occurrence of Niptus hololeucus (Faldermann 1835), a species of beetle representing the family Ptinidae, was also confirmed in the subterranean part of the “Wieliczka” Salt Mine.
Introduction
Even though salt deposits and salt mines are widespread throughout the world (Kozary et al. Citation1968; Lefond Citation1969), information about the fauna living in such habitats is rare and fragmented (Zejszner Citation1843; Nitzu et al. Citation1998–1999; Kłys et al. Citation2018). Consequently, there is a widespread belief that the high salinity of this specific underground environment is unsuitable to the fauna that inhabits it (Humphreys et al. Citation2009), which in turn makes studies of such fauna very rare.
The salt mine in Wieliczka has been in continuous operation since the mid-13th century. The mine consists of nine levels reaching up to 327 metres deep, with a total of 26 open-top shafts and 180 shafts connecting the different levels. Since the 13th century, salt has been mined from over 2000 chambers, and over 240 km of galleries have been dug, creating a labyrinth that stretches from Level I (57 metres below the ground) to Level IX (327 metres below the ground) (Dębkowski et al. Citation2005).
Geologically speaking, the mine is related to the Carpathian foreland plate, which was filled 15–20 million years ago (Late Miocene) with the waters of the Baden Sea. Crystallisation of the salt dissolved in the seawater created sediments that have accrued in an area called the Carpathian Foredeep. These sediments are primarily composed of rock salt, anhydrite and gypsum, all of which are present in Wieliczka (Gaweł Citation1962). Detailed information about the origin and geology of the Carpathian Foredeep can be found in many articles (e.g., Oszczypko Citation1996, Citation1997; Andreyeva-Grigorovich et al. Citation2003; Oszczypko et al. Citation2006).
The salt industry in the neighbourhood of Wieliczka began with the extraction of salt from natural, surface sources of brine (Jodłowski Citation1988). In 1964, the mining of rock salt in Wieliczka was discontinued, and on 30 June 1996, all mining ceased completely (Beiersdorf Citation2011). Currently, salt is produced from brine pumped out of the mine to the surface, while the mine itself and its picturesque surroundings have become a tourist attraction, with a museum and a health resort.
Because a study in 2018 confirmed the presence of beetles representing Niptus hololeucus (Faldermann 1835) (Coleoptera: Ptinidae) (Kłys et al. Citation2018), which had already been observed over 150 years ago (Zejszner Citation1843) in abandoned areas of the mine, the authors of this paper decided to conduct further research on the entomofauna of the subterranean environment in the “Wieliczka” Salt Mine.
Methods
Research in the subterranean part of the “Wieliczka” Salt Mine was conducted between April 2018 to October 2019, in areas where the specimens of Niptus hololeucus (Coleoptera: Ptinidae) had previously (Kłys et al. Citation2018) been found. The Barber traps baited with bovine liver were used to collect specimens from 14 sites (so-called “chambers”) located in the excavations on various mine levels. The traps were checked and replaced on average once per month. Between June 2019 and August 2019, individual traps were also set on Levels IV through VII.
The collected insects were placed in 70% ethyl alcohol, inappropriately labelled tubes, and identified based on specialised identification keys (Trojan Citation1957; Papp Citation1978; Borowski Citation1996; Brake Citation2011). Determination of embryonic stages and their morphological nomenclature follows Panagopoulos (Citation2012) and Martín-Vega and Hall (Citation2016).
Photographs of the specimens were taken using the following equipment: Olympus SZX-9 stereo microscope, CX-41 optical microscope, Olympus DP-12 cameras and Analysis software.
Results
Many specimens of the Diptera species Meoneura obscurella (Fallén 1823) representing the family Carnidae were observed during studies in the Kieratowa Mortiz Chamber (). These flies were not detected anywhere outside the chamber. They were also extremely annoying to the people who entered the chamber.
Over 60 adult specimens of Meoneura obscurella (Diptera: Carnidae) () were collected in the study area. An examination of their morphology revealed the presence of between one and several large eggs (in the abdomens of about a dozen females). The eggs were transferred onto slides and observed using the aforementioned optical microscope. The samples were immersed in paraffin oil.
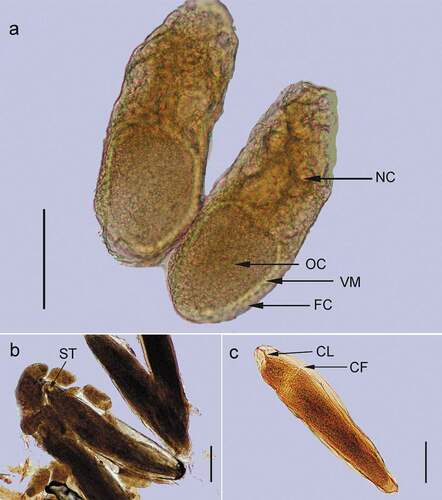
The eggs were found to contain embryos at different stages of development () or even fully-formed larvae ()). Older larvae and pupae ()) were also observed outside the flies bodies.
Figure 3. (a) A stage 10 follicle. FC – follicle cells. NC – nurse cells. OC – oocyte. VM – vitellin membrane. (b) An egg at the stage of about 40% of its total embryonic development. SI – stomodeal invagination. (c) An egg at the stage of 50% of its total embryonic development. CF – cephalic furrow. CL – clypeolabrum. Scale bar = 0.1 mm
Besides, the conducted study confirmed the presence of many specimens of the beetle Niptus hololeucus in the analysed excavations within the mine.
Discussion
The research to date has been unable to identify the anatomical, morphological and physiological traits that allow a species inhabiting such extreme environments as the “Wieliczka” Salt Mine to survive and reproduce. This subterranean habitat is characterised by a lack of light, a stable temperature (about 11.3°C throughout the year), high relative humidity (75%) and high salinity (Kłys et al. Citation2018). Furthermore, the mine’s only connection to the surface environment is through a series of corridors (Szlązak et al. Citation2006).
All sampled specimens of Meoneura obscurella (Diptera: Carnidae) inhabited only the Kieratowa Mortiz Chamber. To date, this species has been observed only in epigeal environments within its entire Holarctic range (Brake Citation2011; Stuke & Bächli Citation2015).
The presence of M. obscurella, a species rarely observed in Europe (Stuke & Bächli Citation2015), has only been recorded in Poland twice (Brake Citation2011), one of which is a mention in a catalogue of Polish fauna (Nowakowski Citation1991). Karl (Citation1936) is the only author to indicate a particular site inhabited by this species in Poland; namely, Słupsk (Stolp-Waldkatze, currently Zalesiczki).
Specimens of M. obscurella primarily inhabit the nests of birds, such as the blackbird, thrush, finch and sand martin; however, they have also been frequently observed on dead vertebrates and in vertebrate faeces (Smith Citation1989). Consequently, it can be speculated that thanks to such a specific diet, the species can easily make use of all, even the smallest, of the organic remains available in the mine.
Also, an interesting adaptation of M. obscurella never previously recorded in this species was observed during the study, which may aid in its survival in the harsh environment of a salt mine, namely, the development of eggs within the bodies of the females. The eggs collected from the abdomen of females contained embryos at different stages of growth or even fully-formed larvae ().
According to the subject literature, lecithotrophic viviparity in Diptera has evolved independently at least 61 times (Meier et al. Citation1999) due to a harsh environment and a lack of food. Using the small amounts of resources provided by a quickly-decaying food supply (faeces and dead animals) enforces a shortening of the development time for larvae outside the body of the mother and a decrease in the number of larvae. Lecithotrophic viviparity naturally reduces the feeding time of the larvae and decreases their number (Sulikowska-Drozd Citation2012).
It is worth mentioning that to date, M. obscurella has never been indicated as a species with lecithotrophic viviparity; however, Engel (Citation1931) suggested such a possibility. He observed some larvae in powdered tobacco (snuff) and decided to conduct an experiment in which he attempted to breed them in this environment. He described both the larvae and the pupae, and noted that no egg-laying took place, nor were any eggs present in the powdered tobacco. Instead, fully-formed larvae appeared in the tobacco.
The presence of a previously observed beetle, Niptus hololeucus (Zejszner Citation1843; Kłys et al. Citation2018) in the “Wieliczka” Salt Mine is unsurprising, as this species has inhabited the mine for many years and is a permanent part of its fauna. N. hololeucus originates from East Asia, and was introduced to North and South America and to Europe, where it inhabits human settlements, reaching in Poland even the northern-most range of the continent (Burakowski et al. Citation1986). The species has also been observed in European caves (Jeannel Citation1909) and Kan-Gohar Cave in the Fars Province of Iran (Dashan et al. Citation2014). Because N. hololeucus has also been observed in rodent burrows (Borowski Citation1996), it may be speculated that its habitat in the “Wieliczka” Salt Mine is in the nests of mice, which are abundant there, and that the organic remains and faeces of the mice are an excellent material for the cocoons to house the beetle larvae (Borowski Citation1996; Kłys et al. Citation2018).
Engel’s study was completely omitted in a monograph by Meier et al. (Citation1999) on viviparity and lecithotrophic viviparity in Diptera. The only Carnidae species mentioned in this monograph that may display lecithotrophic viviparity was Carnus hemapterus (Nitzsch, 1818). However, detailed research has not confirmed this possibility (Meier et al. Citation1999). Consequently, it should be concluded that M. obscurella (Fallén, 1923) is the first recorded representative of the family Carnidae to display lecithotrophic viviparity, which allows it to inhabit and reproduce in the extremely harsh conditions of a salt mine or even in powdered tobacco.
Both species observed in the “Wieliczka” Salt Mine, N. hololeucus and M. obscurella, seem to be more troglophilic than was previously thought. Their specimens were collected throughout the year, which means that they can complete their life cycles in both epigeal and subterranean environments. The original populations of these two species likely allowed for migration and gene flow between both environments. Currently, the populations present in the mine are most likely completely isolated. However, only genetic research can verify this hypothesis.
The small number of insect species inhabiting the “Wieliczka” Salt Mine is related to the specific abiotic conditions in the mine and limited contact with the surface (Halse et al. Citation2014).
Acknowledgements
The authors of this paper would like to thank the management of the ‘Wieliczka’ Salt Mine for allowing them to conduct field research in the mine, especially Chief Geologist Elżbieta Włodarczyk and Jerzy Przybyłło for their supervision and assistance in setting up the field research. The authors would also like to thank Professor Jerzy Lis (Institute of Biology, University of Opole) for his critical remarks on the first draw of the manuscript of this paper.
Disclosure statement
No potential conflict of interest is reported by the authors.
References
- Andreyeva-Grigorovich AS, Oszczypko N, Savitskaya NA, Ślączka A, Trofimovich NA. 2003. Correlation of Late Badenian salts of the Wieliczka, Bochnia and Kalush areas (Polish and Ukrainian Carpathian Foredeep. Annales Societatis Geologorum Poloniae 73:67–89.
- Beiersdorf Z. 2011. Wieliczka jako przykład polskiej ochrony zabytków, w szczególności wpisanych na Listę Światowego Dziedzictwa Kulturowego i Przyrodniczego UNESCO. In: Purchla J, editor. Zarządzanie miejscami wpisanymi na Listę Światowego Dziedzictwa UNESCO w Polsce i w Norwegii. Kraków: Międzynarodowe Centrum Kultury. pp. 280–305.
- Borowski J. 1996. Chrząszcze – Coleoptera. Pustoszowate – Ptinidae. Część XIX. Klucze do Oznaczania Owadów Polski. T. 149. z. 42.
- Brake I. 2011. World Catalog of the Family Carnidae (Diptera, Schizophora). MYIA 12:113–169.
- Burakowski B, Mroczkowski M, Stefańska J. 1986. Chrząszcze – Coleoptera. Dermestoidea, Bostrichoidea, Cleroidea i Lymexyloidea. Katalog Fauny Polski., XXIII., 11. Warszawa.
- Dashan M, Sadeghi S, Bakhshi Y, Malek-Hossein MJ. 2014. First record and redescription of Niptus hololeucus (Faldermann, 1835) from Kangohar Cave (Coleoptera: Ptinidae). Iranian Journal of Animal Biosystematics 10:81–85.
- Dębkowski R, Parchanowicz J, Trzósło A. 2005. Zabytkowe wyrobiska komorowe Kopalni Soli „Wieliczka” – Metody i kierunki ich zabezpieczenia. Prace Naukowe Instytutu Górnictwa Politechniki Wrocławskiej 111. Konferencje nr 43. Górnictwo, obudowa wyrobisk, zabezpieczenie wyrobisk zabytkowych, 21–23 April 2005, Lądek Zdrój. pp. 51–60.
- Engel EO. 1931. Fliegenmaden im Schnupftabak (Meoneura obscurella Fall.). Zetschrift für angewandte Entomologie 17:184–188. DOI: 10.1111/j.1439-0418.1931.tb00170.x.
- Gaweł A. 1962. Geology of the Wieliczka salt deposit. Prace Instytutu Geologicznego 30(3):305–327.
- Halse SA, Scanlon MD, Cocking JS, Barron HJ, Richardson JB, Eberhard SM. 2014. Pilbara stygofauna: Deep groundwater of an arid landscape contains globally significant radiation of biodiversity. Records of the West Australian Museum Supplement 78:443–483. DOI: 10.18195/.0313-122x.78(2).2014.443-483.
- Humphreys WF, Watts CHS, Cooper SJB, Leijs R. 2009. Groundwater estuaries of salt lakes: Bur-ied pools of endemic biodiversity on the western plateau, Australia. Hydrobiologia 626:79–95. DOI: 10.1007/s10750-009-9738-4.
- Jeannel R. 1909. Biospéléologica, X. Coléoptères (2e serie). Archives de zoologie expérimentale et générale (Paris) 1:447–532.
- Jodłowski A. 1988. Początki eksploatacji soli na terenie żup krakowskich do połowy XIII wieku. Wieliczka: Dzieje żup krakowskich. pp. s.71–101.
- Karl O. 1936. Die Fliegenfauna Pommerns. Diptera Brachycera. Stettiner Entomologische Zeitung 97:318–330.
- Kłys G, Ziarkiewicz A, Przybyło J, Włodarczyk-Żurek E. 2018. Chrząszcze w soli/Beetles in rock salt. Przeglad Solny 14:145–147.
- Kozary MT, Dunlap JC, Humphrey WE. 1968. Incidence of saline deposits in global time. Geological Society of America Special Paper 88:43–57.
- Lefond SJ. 1969. Handbook of world salt resources. Monographs in Geoscience XXIII. New York: Plenum Press.
- Martín-Vega D, Hall MJR. 2016. Estimating the age of Calliphora vicina eggs (Diptera, Calliphoridae): Determination of embryonic morphological landmarks and preservation of egg samples. International Journal of Legal Medicine 130(3):3–12. DOI: 10.1007/s00414-051-1308s.
- Meier R, Kotarba M, Ferrar P. 1999. Ovoviviparity and viviparity in the Diptera. Biological Reviews of the Cambridge Philosophical Society 74:199–258. DOI: 10.1017/S0006323199005320.
- Nitzu E, Giurginca A, Ilie V, Vănoaica L. 1998–1999. First note on the edaphic and subterranean fauna from the evaporitic karstic regions of Romania. Trav. Inst. Spéol. «Émile Racovitza», XXXVII – XXXVIII, Bucarest, pp. 143–157.
- Nowakowski JT. 1991. Carnidae. In: Razowski J, red. Wykaz zwierząt Polski. Tom II. Część XXXII/25-29, Insecta: Trichoptera – Siphonaptera. Część XXXIII-XLIII, Chaetognatha – Mammalia. Kraków: PAN, Instytut Systematyki i Ewolucji Zwierząt. pp. 342.
- Oszczypko N. 1996. The Miocene dynamics of the Carpathian Foredeep in Poland. Przeglad Geologiczny 44(10):1007–1018.
- Oszczypko N. 1997. The early-middle Miocene Carpathian peripheral foreland basin (Western Carpathians, Poland). Przeglad Geologiczny 45(10/2):1054–1063.
- Oszczypko N, Krzywiec P, Popadyuk I, Peryt T. 2006. Carpathian Foredeep Basin (Poland and Ukraine): Its sedimentary, structural, and geodynamic evolution. In: Golonka J, Picha FJ, editors. The Carpathians and their foreland: Geology and hydrocarbon resources. American Association of Petroleum Geologists, Memorie 84:293–350.
- Panagopoulos DJ. 2012. Gametogenesis, embryonic end post-embryonic development of Drosophila melanogaster, as a model system for the assessment of radiation and environmental genotoxicity. Chapter I. In: Spindler-Barth M, editor. Drosophila melanogaster: Life cycle, genetics, and development (insects and other terrestrial arthropods: Biology, chemistry and behavior). New York: Nova Science Publishers, Inc. (1879). pp. 1–38.
- Papp L. 1978. 72a család: Carnidae. Fauna Hungariae 133:202. Budapest, Akadémiai Kiadó.
- Smith KGV. 1989. An introduction to the immature stages of British flies. Diptera larvae, with notes on eggs, puparia and pupae. Handbooks for the Identification of British Insects 10(4):166.
- Stuke JH, Bächli G. 2015. Faunistical data of Carnidae (Diptera) from Switzerland and additional countries with the description of three new Meoneura species. Mitteilungen der Schweizerischen Entomologischen Gesellschaft 88:379–401.
- Sulikowska-Drozd A. 2012. Ewolucja strategii rozrodczych bezkręgowców – żyworodność i przetrzymywanie jaj. Kosmos. Problemy Nauk Przyrodniczych 61(4):563–572.
- Szlązak N, Obracaj D, Borowski M. 2006. Warunki mikroklimatu w podziemnych obiektach zabytkowych kopalni soli. Górnictwo i Geoinżynieria. Rok 30, Zeszyt 4.
- Trojan P. 1957. Muchówki – Diptera. Zeszyt 1 – Wstępny. Część XXVIII. Klucze Do Oznaczania Owadów Polski 20:145.
- Zejszner L. 1843. Krótki opis historyczny, geologiczny i górniczy Wieliczki. Berlin: B. Behr’s Buchhandlung Publisher. pp. 12.



