Abstract
Three species belonging to the genus Dolichopoda (Orthoptera; Rhaphidopohoridae) are known so far from the Peloponnese, all endemic to the area. In particular, D. matsakisi is known from two mountains in the North, while D. dalensi is present in the east region. The third species, D. unicolor, is distributed in the southern part of the Peloponnese, inhabiting caves on Mt Taygetos and Mani Peninsula. Recently, extensive sampling work in most of the Peloponnese has led to the discovery of new taxa, morphologically differentiated by the above three known species.
To investigate the delimitation of the Peloponnesian species of Dolichopoda, we performed both morphological and molecular analyses. Morphological analysis was carried out by considering diagnostic characters generally used to distinguish different taxa, as the shape of epiphallus in males and the subgenital plate in females. Molecular analysis was performed by sequencing three mitochondrial genes, 12S rRNA, 16S rRNA, and COI, and one nuclear gene, 28S rRNA.
Results from both morphological and molecular analyses were used to revise the taxonomic arrangement of the Peloponnesian species. On the whole, we were able to distinguish seven lineages of Peloponnesian Dolichopoda species, of which D. kofinasi n.sp., D.epidavrii n.sp., D. poseidonica n.sp., and D. propanti n.sp. are described as new species.
http://www.zoobank.org/urn:lsid:zoobank.org:pub:857875CB-22B9-4CFC-9B7F-3FDDFC47A4E6
Introduction
The Mediterranean basin is one of the world’s most geographically complex regions (Blondel et al. Citation2010) with remarkable paleogeographic evolution and tectonic history (Cavazza & Wexel Citation2003).
It is also one of the global hotspots of biodiversity, showing a high proportion of both plant and animal endemic species especially in the eastern part, including the Balkans and Anatolia (Blondel et al. Citation2010). The Hellenic area and the Aegean islands show an intricate geological history with a series of land connection events throughout the late Tertiary (Dermitzakis & Papanikolaou Citation1981; Dermitzakis Citation1990). The area is also considered a temperate refugium and genetic diversity may have been accumulated during the several ice ages of the late Pliocene and Pleistocene epochs, leading to great lineage diversity in both terrestrial invertebrates and vertebrates (Poulakakis et al. Citation2015; Legakis et al. Citation2018).
The complex palaeogeographical history of the Mediterranean region seems to have driven the colonization and speciation processes in Dolichopoda Bolivar, 1880, a cavernicolous genus of Orthoptera belonging to the family Rhaphidophoridae and distributed from the eastern Pyrenees to the Caucasus Mountains and eastwards to northern Iran (Alborz Mountains). Most species of this genus are strictly dependent upon caves. However, especially in the northern part of the range, Dolichopoda populations inhabit many different habitats, including soil crevices in the forest, catacombs, Etruscan tombs, and other man-made habitats, natural caves, and large hypogean karst systems, representing a range from quasi epigean to hypogean conditions. Depending on the exploited habitat, they present variation in their semivoltine life cycle due to the different environmental conditions that change from variable climate regimes to a more constant environment (Di Russo et al. Citation1994).
Based on both molecular phylogenetic reconstruction and biogeographic analysis carried out on the ninety percent of known species, an eastern origin of Dolichopoda species could be hypothesized (Allegrucci et al. Citation2005, Citation2009, Citation2011). In particular, the colonization of Greece by Dolichopoda species followed two different routes, probably originating in Anatolia. The northern lineage currently occurring in the western Mediterranean, Thasos island, northern Greece, Ionian islands, and north-eastern Peloponnese could be the result of dispersal from the north through the Balkan peninsula. The southern lineage, including species currently inhabiting Aegean islands, Crete, south-eastern Greece, and south Peloponnese, likely arose from trans-Aegean colonization during the Messinian salinity crisis (5.96–5.33 Mya). The opening of the Mid-Aegean trench would have promoted an initial diversification within the uplift of the Anatolian Plateau, while the Messinian marine regression offered the conditions for a rapid dispersal through the whole Aegean-Hellenic region. Rather, climatic events linked to the Plio-Pleistocene are responsible for the speciation within each of the two different lineages, mainly driven by vicariance events. Also, adaptation to cave life seems to have played an important role in this process. The ancestors of Dolichopoda might have used caves as refugia during the unfavorable climatic conditions, beginning their adaptation to subterranean habitat. Therefore, the current distribution of Dolichopoda has been explained by a combination of both vicariance and dispersal events, with many processes occurring in ancestral epigean populations before the invasion of the subterranean habitat (Allegrucci et al. Citation2009, Citation2011).
Three species belonging to the genus Dolichopoda are known so far from the Peloponnese (Rampini et al. Citation2008; Di Russo et al. Citation2019; ), all endemic to the area. Our knowledge on the actual distribution of those species within Peloponnese became clearer recently (Di Russo et al. Citation2019). In particular, D. matsakisi Boudou-Saltet, Citation1972 is known from two mountains at the northern parts of Peloponnese, namely Mt Chelmos and Mt Panachaiko, while D. dalensi Boudou-Saltet, Citation1972 is present on the east (Korinthia and Argolis). The third species, D. unicolor Chopard, 1964, described from specimens collected in the Katafygi cave (Selenitsa), is distributed in the southern part of Peloponnese, inhabiting caves on Mt Taygetos and Mani Peninsula.
Figure 1. Geographic distribution of the Peloponnesian Dolichopoda populations sampled and analyzed in this study. Codes are as in . Black arrow indicates the probable way of colonization of Dolichopoda ex-Petrochilosina from mainland Greece
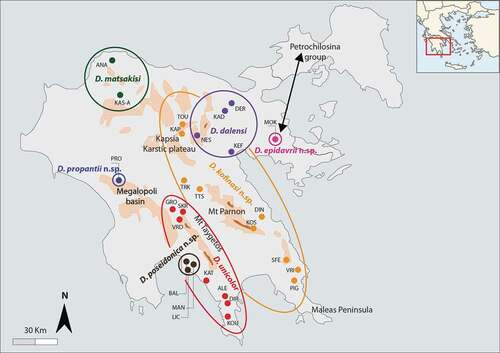
Since 2002, we have been collecting cave cricket specimens from caves of Peloponnese. The involvement of the members of Speleological Club Poseidon during recent years, proved very fruitful, allowing for investigation of a large number of caves in the area. The preliminary results were recently published, mainly with new distributional data (Di Russo et al. Citation2019). Based on the diagnostic morphological characters, cave crickets collected in 19 caves could not be assigned to any known species.
The main aims of this paper were to study these populations both at a morphological and molecular level to confirm the existence of new species in this region. The new taxa were compared with the known species from Peloponnese, as well as with those from nearby areas to evaluate the hypothesis of colonization as already drawn in the previous studies (Allegrucci et al. Citation2009, Citation2011).
Materials and methods
Taxon sampling and laboratory procedures
Twenty cave populations from Peloponnese in Greece were analyzed in this study for a total of 31 individuals (). Geographical locations of the present sampled caves are illustrated in , where are also reported the geographic locations of all known Peloponnesian species.
Table I. Dolichopoda species and outgroup taxa included in this study
Eight additional species from nearby areas previously analyzed (Allegrucci et al. Citation2009, Citation2011) were used for comparison. In particular, we considered D. lustriae (Rampini, di Russo, Pavesi & Cobolli, Citation2008) from central-western Greece, D. vandeli (Boudou-Saltet, 1970), D. insignis (Chopard, 1955), D. petrochilosi (Chopard, 1954), D. cassagnaui (Boudou-Saltet, Citation1980), and D. makrykapa (Boudou-Saltet, Citation1980) from central-eastern Greece, D. parakevi Boudou-Saltet, 1973 from Crete Island, D. naxia (Boudou-Saltet, Citation1972), D. giulianae Rampini & Di Russo, 2012 and D. calidnae Rampini & Di Russo, 2012 from the central and eastern Greek islands Naxos, Samos and Kalimnos. One species belonging to genus Troglophilus Krauss, 1879 within the same family was used as outgroup (T. cavicola (Kollar, 1833) ; Allegrucci et al. Citation2009, Citation2017).
DNA was isolated from the leg muscle of each individual, using a C-TAB protocol (Doyle & Doyle Citation1987) resuspended in 100 µl of sterile water and stored at −40°C.
The entire Cytochrome Oxidase I gene (COI, a total of 1500 bp), a 550-bp fragment of the 16S rRNA gene, and a 450-bp fragment of the 12S rRNA gene were amplified through the polymerase chain reaction (PCR) and sequenced for each individual. The large subunit of the nuclear ribosomal DNA (28S rRNA) was also sequenced. The primers used were: LCO1490, HCO2198 (Folmer et al. Citation1994), UEA1, UEA5, and UEA10 (Lunt et al. Citation1996) for the COI gene, 12Sai, 12Sbi (Kocher et al. Citation1989; Simon et al. Citation1994) for the 12S rRNA gene and 16Sar, 16Sbr (Simon et al. Citation1994) for the 16S gene. As regards 28S rRNA, it was partially amplified and sequenced for a fragment of 580 base pairs, belonging to domains 3–5, using primers from Friedrich and Tautz (Citation1997). Optimal cycling parameters varied for each primer pair used. PCR products were purified using the ExoSAP digestion (Amersham Pharmacia Biotech), directly sequenced in both directions using the BigDye terminator ready-reaction kit, and resolved on ABI 3100 Genetic Analyzer (PE Applied Biosystems), following the manufacturer’s protocols. Sequence data were edited and compiled using Codon-Code Aligner 9.0.1. All sequences were submitted to GenBank (Accession Numbers are reported in ).
Each gene fragment (12S, 16S, COI, and 28S) was considered separately for the alignment. Non protein coding sequences of 16S, 12S, and 28S were aligned using ClustalX 2.1 (Larkin et al. Citation2007) with opening gap = 10 and extending gap = 0.10. These sets of parameters appear to work at best in the alignment of Dolichopoda sequences and have been used in all previous studies (Allegrucci et al. Citation2005, Citation2009, Citation2011, Citation2019). No heterozygous haplotypes were observed in 28S sequences. Coding sequences of COI were assembled, aligned, and translated with Codon-Code Aligner.
Data analysis
Species delimitation and phylogenetic analysis
The Automatic Barcode Gap Discovery (ABGD, Puillandre et al. Citation2012) was employed to carry out species delimitation analysis. ABGD automatically finds the distance at which a barcode gap occurs and sorts the sequences into putative species based on this distance. The method statistically infers the barcode gap from the data and partitions the data accordingly. Populations belonging to the same species therefore should be grouped in the same partition. This procedure is then recursively applied to the previously obtained groups of sequences. This analysis was carried out on the present samples and all Dolichopoda species previously analyzed ( and Allegrucci et al. Citation2005, Citation2011, Martinsen et al., Citation2009), considering only the COI data set and only the common base pairs consisting of 964 bp. The resulting inferences were then recursively applied to yield finer partitions (recursive partitions) until no further partitioning was possible. Genetic distance matrix (p-distance) was uploaded at https://bioinfo.mnhn.fr/abi/public/abgd/abgdweb.html and ABGD was run with the default settings (Pmin = 0.001, Pmax = 0.1, Steps = 10, X (relative gap width) = 1.5, Nb bins = 20) and using p-distance.
Genetic distances between haplotypes were calculated using p-distance as implemented in mega 5.2 (Tamura et al. Citation2007). network 10.0.0.0 (Bandelt et al. Citation1999) was employed to calculate a median-joining network representing the genealogical relationships among mtDNA haplotypes. In this analysis, all the Peloponnesian species were considered with the addition of D. vandeli from Voiotia to point out the differences with D. epidavrii n.sp. Phylogenetic analyses were carried out on each gene fragment separately and then data were combined by constructing a concatenated matrix, partitioned by genes. All phylogenetic analyses were performed on the Peloponnesian taxa and those from nearby areas as detailed above, using Bayesian Inference (BI) analysis as implemented in the software MrBayes 3.2.7 (Ronquist et al. Citation2012). J model test 2.1.7 (Darriba et al. Citation2012) was used to perform a hierarchical likelihood ratio test and calculate approximate Akaike Information Criterion (AIC) values of the nucleotide substitution models for each gene fragment.
At least two simultaneous searches were conducted comprising four Markov chains (MCMC) started from a randomly chosen tree and run for 1,000,000 generations, with sampling every 100 generations. The following descriptors were assumed to indicate convergence on a common phylogenetic topology by separate Bayesian searches: similarity in log-likelihood scores at stationarity, the similarity in consensus tree topologies and PP values for supported nodes, and a final average standard deviation of split frequencies (ASDSF) for simultaneous searches approaching zero. Convergence to stationarity was indagated also using TRACER 1.7 (Rambaut et al. Citation2018), and the effective sample size (ESS) of all parameters showed values above 1000 (values much higher than the threshold of statistical significance) in both simultaneous searches, indicating that MCMC had converged. The first 1,000 trees were discarded as burn-in and posterior probabilities (PP) were calculated from post-burn-in trees.
To reconstruct a possible biogeographic scenario, a molecular clock analysis was performed, using beast 2.5.2 (Bouckaert et al. Citation2019) and substitution rates as previously obtained for each gene. In particular, we used rates equal to 1.6%, 1.1% and 0.7% per site/lineage/million years for COI, 12S and 16S rRNA, respectively. While a mean substitution rate of 0.06% was considered for 28S rRNA (Allegrucci et al. Citation2011)
In particular, we used a relaxed molecular clock, following an uncorrelated lognormal (UCLN) model of molecular evolutionary rate heterogeneity as implemented in beast. The UCLN model was used in BEAST to estimate the posterior density of divergence times. A Yule or “pure birth” prior process was used for the branching rate in the phylogeny. The time to the most recent common ancestor (MRCA) between each clade was estimated under the models highlighted in J model test 2.1.7 (Darriba et al. Citation2012) for each partition within each gene. We did three independent runs with BEAST, each for 20 million steps. Convergence to stationarity and effective sample size (ESS) of model parameters were assessed using Tracer 1.7 (Rambaut et al. Citation2018), with the species tree reconstructed after a 10% burn-in using TreeAnnotator 2.5.2 (Bouckaert et al. Citation2019).
Taxonomy and morphological analysis
A total of 64 adult individuals of Dolichopoda from most of the same localities as those for the molecular study were used for the taxonomy and the morphological investigation. All the studied specimens were collected by hand on the wall of the caves or by pitfall traps, during several field trips conducted in the years between 2013 and 2019. Specimens were preserved in 70% ethanol and deposited in the collection of the Museum of Zoology of the University “La Sapienza” of Rome, Italy (MZUR). Permissions for collection of samples were obtained by the Ephorate of Palaeoanthropology and Speleology of the Ministry of Culture, Education, and Religious Affairs, Athens. The specimens were studied using a Leica MZ12.5 stereomicroscope. Pictures were taken using a Samsung NX mini camera. For the morphological analysis, nine external body characters were utilized: lobes of the tenth tergum; median and basal processes of the epiphallus; plica dorsalis, amount of spinulation of the hind tibia; the shape of the female subgenital plate and ovipositor; the number of denticles on the inner valve of the ovipositor. Measures of the morphological parameters were taken using a digital caliper (0.1 mm).
Results
A total of 2970 base pairs corresponding to the entire COI gene, to 394 bp of 12S, to 547 bp of 16S, and 611 of 28S were successfully sequenced and aligned in 31 specimens of Dolichopoda populations from Peloponnese. Out of 2970 characters 636 sites are variable and 450 are parsimony informative.
Genetic distances and Species delimitation
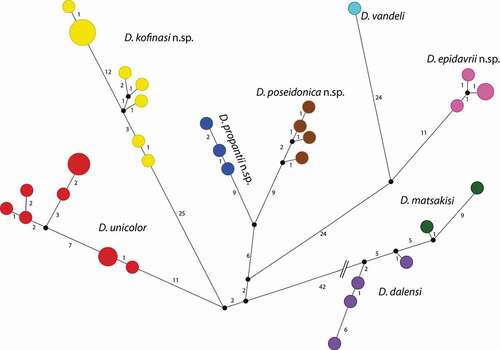
The genealogical relationships among mtDNA haplotypes are shown in , where Dolichopoda Peloponnesian haplotypes were organized in six main well-differentiated haplogroups. Five of them are well differentiated and correspond to the different species, D. unicolor, D. kofinasi n. sp., D. propantii, n.sp. D. poseidonica n.sp. and D. epidavrii n.sp. while D. matsakisi and D. vandeli, corresponding to the sixth haplogroup show a lower genetic differentiation.
Figure 2. Median-joining network analysis in the populations of Peloponnesian Dolichopoda considered in this study: circled areas are proportional to the number of individuals sharing the same haplotype; numbers, along connections, indicate the number of nucleotide substitutions
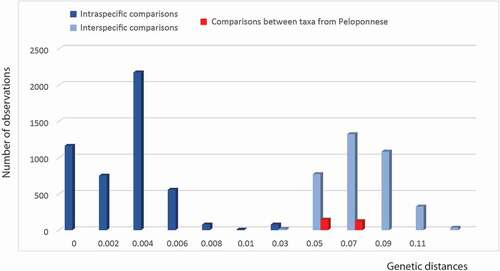
To investigate this further we carried out a genetic distance analysis using the COI gene as a barcode and p distance between all studied Dolichopoda species (Allegrucci et al. Citation2005, Citation2009, Citation2011, Citation2014; Martinsen et al. Citation2009). Genetic distance values at intra- and interspecific levels are compared in . In particular, shows the genetic distance values found in intra- and interspecific comparisons of all studied Dolichopoda species compared to the values found in comparisons between the populations from Peloponnese. Intraspecific values between all studied Dolichopoda species ranged from 0 to 0.025, with a mean of 0.012 (± 0.008SD), while interspecific values ranged from 0.016 to 0.126, with a mean of 0.073 (± 0.017SD). Intraspecific pairwise comparisons between the Peloponnesian populations ranged from 0.0018 to 0.0094 with a mean of 0.007 (± 0.003SD), while interspecific values ranged from 0.025 to 0.083 with a mean of 0.057 (± 0.013SD).
Figure 3. Distribution of genetic distance values (p-distances) at different taxonomic levels. Pairwise comparisons at the intra- and inter-specific level in all studied Dolichopoda species are reported. Comparisons between taxa from Peloponnesus are evidenced in red
ABGD analysis proposed several partitions that varied according to the different a priori thresholds. Apart from the two extreme a priori threshold values (P = 0.001 and P = 0.035), for which an aberrant number of species hypotheses were obtained (almost every haplotype was considered as a different species hypothesis for P = 0.001 and, conversely, all the haplotypes were combined in a single species hypothesis for P = 0.035), all the tested a priori thresholds lead to the same splitting with all the groups corresponding to the nominal species. The Peloponnesian taxa were subdivided into seven different groups: one group consisted of populations from eastern Messinia and south-western Lakonia and belonging to D. unicolor, the second group included populations from Central Arkadia up to eastern Lakonia and belonging to D. kofinasi, n. sp. The third group comprised populations from south-western Messinia limited to the Kambos and Kitries bay area and belonging to D. poseidonica, n. sp., the fourth group included taxa from a single cave (Propanti cave) in north-central Messinia and belonging to D. propantii, n. sp. A fifth group included taxa from a single cave in western Argolida, belonging to D. epidavrii n. sp. while a sixth group identified taxa from North Peloponnese, belonging to D. matsakisi and the seventh group included taxa belonging to D. dalensi and coming from the eastern Peloponnese ().
Phylogenetic analysis
J model test (Darriba et al. Citation2012) indicated GTR + I + G (Lanave et al. Citation1984; Gu et al. Citation1995) as the best model of DNA substitution for the COI and 16S genes, while the best models were GTR+G and GTR for 12S and 28S, respectively.
The phylogeny based on the combined data sets is highly supported () The studied Greek Dolichopoda species are separated into four main clusters with the Aegean species (D. calidnae, D. giulianae, D. naxia, and D. paraskevi) being sister to all the other clusters, as expected. Phylogenetic analysis also emphasized the presence of new species. In particular, D. epidavrii n. sp. is sister to D. vandeli while D. kofinasi n. sp., D. propantii n. sp., and D. poseidonica n. sp., are sisters to D. unicolor.
Figure 4. Bayesian Inference analysis for each gene separately and for the combined data set carried out on the Peloponnesian species of Dolichopoda. The geographically closest species were also considered. Values above branches indicate posterior probabilities derived from BI analysis. Scale bars: 0.00035–0.01 substitutions per site. Only posterior probability (PP) values ≥ 0.90 are shown
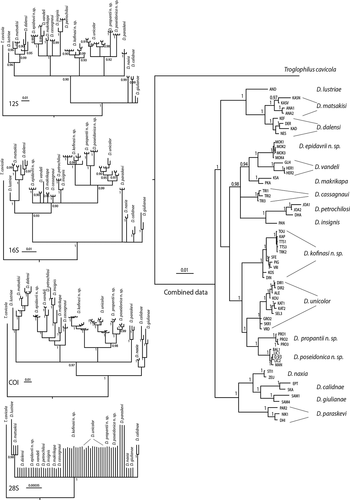
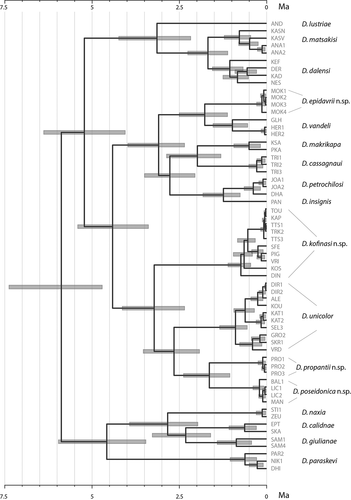
Although the deep nodes of the phylogeny are not resolved, the tree topology of each studied gene is rather similar to the topology obtained by combining all data sets (). Nuclear ribosomal gene (28S rRNA) produced a topology distinguishing only some clades, and most of the taxa relationships were unresolved. This is due to the low level of polymorphism revealed in this gene. However, its presence in the combined dataset increases the resolution of the deep nodes in phylogeny.The chronogram in shows that the four new species from Peloponnese have been separated during the Plio-Pleistocene era.
Figure 5. Divergence times among the analyzed Dolichopoda species inferred by Bayesian analysis using relaxed molecular clocks. Bars at the nodes represent the 95% highest posterior density (HPD) credibility interval
Systematics and morphological analysis
In this section, we report the description of the new four species identified for the Peloponnese.
Family RHAPHIDOPHORIDAE Walker, 1871Genus Dolichopoda Bolivar,1880
Dolichopoda epidavriiDi Russo & Rampini n. sp.()
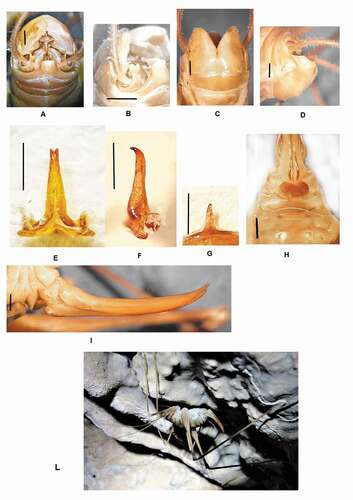
Figure 6. Dolichopoda epidavrii n. sp: (a) male tenth tergum; (b) tenth tergum lateral view; (c) male subgenital plate (ventral view); (d) male subgenital plate (lateral view); (e) median process of epiphallus (dorsal view); (f) median process of epiphallus (lateral view); (g) plica dorsalis; (h) female subgenital plate; (i) ovipositor (lateral view); (L) Female habitus D.epidavrii. Scale bars: 1 mm
Type material
– Holotype ♂: Greece, Peloponnese, Argolida, Epidavros, Spilaio Monis Kalamiou, 697 m, 08.12.2019, Di Russo & Kofinas leg. (MZUR)
Other material examined
– 1♂, 3♀, 2 nymphs, same locality, date, and collector as the holotype;
Type locality
The Spilaio (cave) Monis Kalamiou is located on a small mountain called Psili Rachi. The entrance is facing south and is located close to the monastery (Moni-μονή) of Kalamiou.The presence of cave crickets in the cave was communicated to us by Giannis Farsarakis.
Etymology
The name of the new taxon refers to the archaeological site of Epidavros, not far from the cave locality.
Diagnosis
This taxon presents a unique characteristic for the cave crickets of Peloponnese: the median process of the epiphallus is bifurcate at the apex, contrary to the pointed median process present in all the other species of Peloponnese. The male shows affinities to male D. vandeli, differing mainly in the trapezoidal ninth tergum, the more elongated lobes of the tenth tergum, and the finger-like plica dorsalis. The female has a subgenital plate similar to those of D. petrochilosi, being hearth-shaped.
Thus it belongs to the species complex widespread in the eastern central Greece (Attica and Voiotia) and W Aegean Islands (Evvoia, Skyros) (ex - Petrochilosina subgenus).
Description
Male
Relatively big in size; body-color not uniform, thorax and abdomen brownish dorsally while paler ventrally. Legs long, uniformly yellowish with the posterior edge darker. Femora unarmed. Fore tibia armed with 4–5 spines on sides of the lower edge, 3/3 spines on the upper edge. Mid tibia with 5/7 short spines on both sides of the upper edge, 3/4 spines on the lower edge. The hind tibia is longer, with 16/18 spines of varying length on both sides of the upper edge and 2 homogeneous spines on the lower external edge. ninth abdominal tergite prominent trapezoidal with the posterior edge slightly rounded ()); tenth tergum with two narrow and elongated lateral lobes, rectangular in shape, with the terminal edge strongly sinuous ()). The lobes are separated by a large median depression. Sub genital plate globular, deeply incised in the middle, lateral lobes are triangular with two very short styli ()). Median process of the epiphallus almost slender, triangular, bifurcate at the apex, in the lateral view slightly curved. Narrow basal process with the anterior lobes more developed than the posterior ones ()). Plica dorsalis sclerotized fingerlike in shape ()).
Length (mm): body16.1; pronotum 3.9; fore femur16.0; mid femur 15.5; hind femur 24.0; fore tibia18.0; mid tibia 18.0; hind tibia 32.5; hind tarsum11.0; 1st article of hind tarsus 6.0.
Female
Relatively bigger than male (mm 16.2–18.8). VII, VIII, and IX sternites are well developed showing posterior edge prominent. Subgenital plate, sclerotized, heart-shaped ()). Ovipositor uniformly curved (mm 12–14) with apex strongly curved upward. The inferior valves with the base strongly squared have 15–16 denticles ())
Dolichopoda kofinasiDi Russo & Rampini n. sp.()
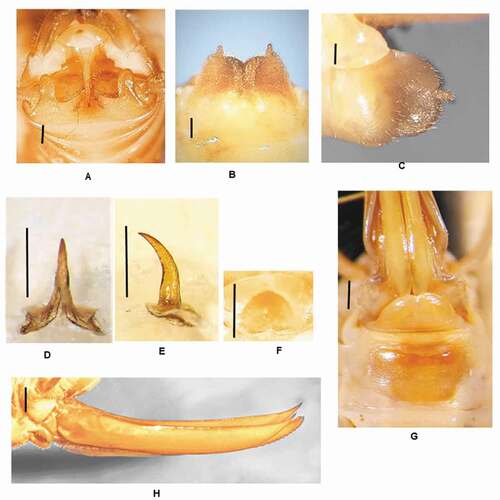
Figure 7. Dolichopoda kofinasi n. sp.: (a) male tenth tergum; (b) male subgenital plate (ventral view); (c) male subgenital plate (lateral view); (d) median process of epiphallus (dorsal view); (e) median process of epiphallus (lateral view); (f) plica dorsalis; (g) female subgenital plate; (h) ovipositor (lateral view). Scale bars: 1 mm
Type material
– Holotype ♂: Greece, Peloponnese, Lakonia, Mt Koulochera, Sfendami cave, 19.09.2015, Kofinas leg., 1025 m (MZUR).
Other material examined
– 8♂. 5♀, same locality, date, and collector as the holotype;
Lakonia: Monemvasia, Vri cave, 2♂, 2♀, 05.11.2014, Kofinas leg., 37 m; Demonia, cave Aghion Anargiron, 3♂, 2♀, 24.01.2015, Kofinas leg., 177 m; Vellies, cave Pigaza, 2♀, 1 nymph, 24.01.2015, Kofinas leg., 115 m; Arkadia, Kosmas, cave Kosma, 21.03.2013, F. Ballarin leg., 3♂, 2♀, 04.12.2016, Di Russo leg., 1144 m; Leonidio, cave Dionysou 04.12.2016, Di Russo leg., 542 m; Kapsia, cave Kapsia, 5♀, 5 nymphs 21.02.2016, Di Russo leg.; 2♀, 27.08.2019, Rousiotis leg., 630 m; Kapsia, Katavothra Tousi, 3♀, 4 nymphs 08.08.2016, Kofinas leg., 653 m; Kollines, Spilaio Tyrias, 7 nymphs, 27.07.2019, Kofinas leg., 592 m; Skortsinos, cave Troupitses, 11 nymphs, 27.07.2019, Kofinas leg., 474 m.
Type locality
Sfendami cave is a pit hole located close to the peak of Mt Koulochera. The entrance is facing west, towards the valley of Molai and Mt Taygetos. Next to the entrance is standing one of the few maple trees (Acer sp., sfendami) of the area. The pit hole was explored for the first time two decades ago by Giannis Kofinas-Kallergis, who observed the cave crickets.
Etymology
The new taxon is dedicated to our friend Giannis Kofinas-Kallergis, founder and active member of the “Poseidon” Speleological Club, who explored and collected for the first time Dolichopoda specimens from the type locality.
Diagnosis
The new taxon is similar to D. unicolor, differing mainly on leg spinulation, the sub-rectangular apex of the lobes of the tenth tergum and the wide triangular median process of the epiphallus. The inferior valves of the ovipositor have 12–14 denticles.
Description
Male
Size relatively short with legs slender and elongated; color brownish darker dorsally.
Legs long, slender, uniformly yellowish. Femora unarmed. Fore tibia armed with 4–5 spines on sides of the lower edge, 2/0 spines on the upper edge. Mid tibia with 3/4 short spines on both sides of the upper edge, 4/5 spines on the lower edge. Hind tibia longer, with 14/16 (3/3) spines of varying length on both sides of the upper edge and 2 homogeneous spines on the lower external edge.
X tergum with the lateral lobes subrectangular and rounded at the apex; the two lobes are separated by a large concave strongly haired margin ()). Sub genital plate globular, deeply incised in the middle. The wide lateral lobes are trapezoidal and haired, with the posterior edges almost rounded, showing two large cylindrical styli ()).
Median process of the epiphallus triangular in shape, uniformly curved enlarged at the base, and acute at the apex ()). The basal process is wide with the posterior edge curved, while the anterior one is bilobate and deeply incised. Plica dorsalis short, membranous, domed in shape ()).
Length (mm): body 12.3; pronotum 3.4; fore femur 13.9; fore tibia 16.0; mind femur 14.0; mind tibia 16.5; hind femur 20.5; hind tibia 27.3; hind tarsus 9.00; 1st article of hind tarsus 5.0.
Female
The length of the body ranges between 11–15 mm (ovipositor excluded) and the general form is similar to the male. VIII sternite transverse and sclerificate. Sub genital plate triangular, sclerified, slightly incised in the middle ()). Ovipositor relatively elongated (10–11 mm), curved distally; the inferior valves, slightly curved at the base have 12–14 denticles (Fig. H).
Dolichopoda propantiiDi Russo & Rampini n. sp.()
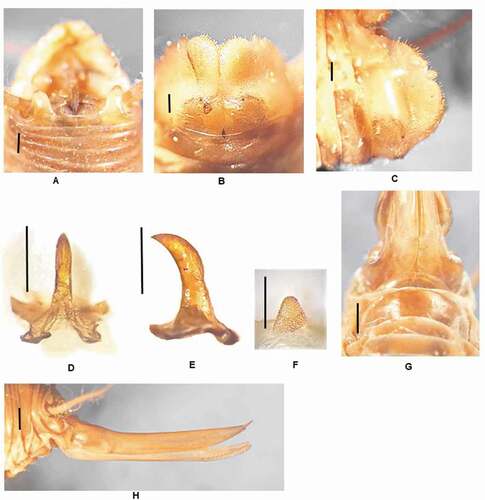
Figure 8. Dolichopoda propantii n. sp.: (a) male tenth tergum; (b) male subgenital plate (ventral view); (c) male subgenital plate (lateral view); (d) median process of epiphallus (dorsal view); (e) median process of epiphallus (lateral view); (f) plica dorsalis; (g) female subgenital plate; (h) ovipositor (lateral view). Scale bars: 1 mm
Type material
– Holotype ♂: Greece, Peloponnese, Ilia, Andritsena, Propanti cave, 966 m., 18.08.2019, C. Di Russo leg. (MZUR)
Other material examined
– 2♂, 2♀, 18.08.2019, same locality, date, and collector as the holotype; 1♀, 5 nymphs, 20.08.2017, Di Russo leg.; 2♂, 1♀, 3 nymphs, 21.05.2018, Di Russo leg.
Type locality
Propanti cave is located NW of the town of Andritsena. The cave was investigated for the first time by the pioneer speleologist Anna Petrochilou in 1966 (Petrochilou Citation1969). She was the first to report on the presence of cave crickets in the cave, as “D. petrochilosi”.
Etymology
– The new species name refers to the name of the type locality.
Diagnosis
– Closely resembling D. unicolor, differing mainly in the triangular, relatively short and almost entirely flattened epiphallus, the plica dorsalis having a median finger-like protuberance and the different number of the leg spinulation.
Description
Male
Size relatively small. Body-color is uniformly brownish. Legs very long, slender, and brownish with the femora unarmed. Fore tibia armed with 3–5 spines on sides of the inferior edge, 2/2 spines on the upper edge, a pair of spurs of equal length on the apex. Mid tibia with 5/7 short spines on both sides of the upper edge, 4/4 spines on the lower edge, and two apical spurs similar to those of the fore tibia. Hind tibia longer, with 21/24 spines of varying length on both sides of the upper edge and 1/2 homogeneous spines on the lower edge.
Posterior edge of the tenth tergum with two prominent rounded lobes ()). Epiproctum triangular Subgenital plate globular, deeply incised in the middle, with two enlarged lateral lobes, the styli are short (). Epiphallus sclerotized with relatively short median process almost entirely flattened and triangular in shape; from the side, the median process is thickened and strongly curved at apex; the posterior basal processes are quite well developed while the anterior ones are reduced ). Plica dorsalis little sclerotized at the base, with a cylindrical fingerlike protuberance, covered with bristles in the middle of the apical part ()).
Length (mm): body 15.00; pronotum 3.0; fore femur 15,00; fore tibia 18; mid femur 14.5; mid tibia 19; hind femur 23; hind tibia 29; hind tarsus 11.00; 1st article of hind tarsus 6.0.
Female
The length of the body ranges between 13,5 and 14.5 .mm (ovipositor excluded) and the general form is similar to the male. Sub genital plate transverse with a rounded posterior edge slightly incised in the middle ()). Ovipositor almost straight, with an average length of 10 mm, showing a pointed apex hooked; the inferior valves are curved at the base and have 15–16 denticles ()).
Dolichopoda poseidonicaDi Russo & Rampini n. sp.()
Figure 9. Dolichopoda poseidonica n. sp.: (a) male tenth tergum; (b) male subgenital plate (ventral view); (c) male subgenital plate (lateral view); (d) median process of epiphallus (dorsal view); (e) median process of epiphallus (lateral view); (f) plica dorsalis; (g) female subgenital plate; (h) ovipositor (lateral view). Scale bars: 1 mm
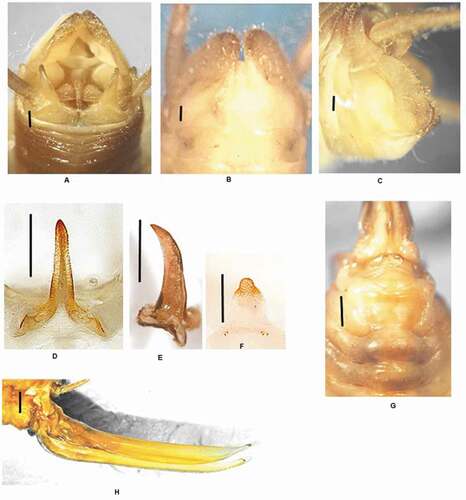
Type material
– Holotype ♂: Greece, Peloponnese, Messinia, western Mani, Kardamili: cave Katafygi Mantagari, 15.02.2015, G. Kofinas leg. (MZUR)
Other material examined
– 2♂, 3♀ same locality, date and collector as the holotype; Greece, Peloponnese, Messinia, western Mani, Kardamili: Spilaio (cave) Balli, 3♂, 3♀, 10 nymphs, 27.01.2018, G. Kofinas leg.; pit-hole Lykourgou, 3♂, 2♀, 4 nymphs, 27.01.2018, G. Kofinas leg.
Type locality
– cave Katafygi Mantagari is located NW of Kardamili. It is a horizontal cave, was investigated for the first time by members of “Poseidon” Speleological Club.
Etymology
– The name of the new taxon is dedicated to the “Poseidon” Speleological Club (PSC), Kalamata, members of which collected for the first time specimens from the type locality and more than that greatly improved our research on the cave crickets of Peloponnese. The specimens were collected during the 13th Christmas pie cutting event and a seminar held by PSC at the type locality.
Diagnosis
– Closely resembling D. unicolor, differing mainly in the tenth tergum which has two prominent elongations, laterally folded, the different number of the leg spinulation and the median process of the epiphallus being less elongated, more thickened and slightly curved.
Description
Male
Size relatively small. Body-color is uniformly yellow-brown. Legs very long, slender, and brownish with the femora unarmed. Fore tibia armed with 3–4 spines on sides of the inferior edge, 2/4 spines on the upper edge, a pair of spurs of equal length on the apex. Mid tibia with 5/5 short spines on both sides of the upper edge, 4/5 spines on the lower edge, and two apical spurs similar to those of the fore tibia. Hind tibia longer, with 17/19 spines of varying length on both sides of the upper edge and 0/2 homogeneous spines on the lower edge. Posterior edge of the tenth tergum with two prominent elongations, laterally folded ()).
Subgenital plate globular at the bottom, with two symmetrical lateral lobes and rounded posterior edges, the styli are cylindrical and short ()). Epiphallus sclerotized with a relatively short median process, triangular in shape; from the side, the median process is thickened at the base and slightly curved; the posterior basal processes are rather well developed while the anterior ones are reduced ()). Plica dorsalis little sclerotized, the basal lobes with 2–3 strong bristles and a cylindrical domelike protuberance covered with bristles occurs in the middle of the apical part. ()).
Length (mm): body 13.5; pronotum 3.5; fore femora 13.8; middle femora 13.0; hind femora 21.4; fore tibia 14.4; middle tibia 14.6; hind tibia 24.8; hind tarsus 9.7; 1st article of hind tarsus 5.7.
Female
The length of the body ranges between 12–13 mm (ovipositor excluded) and the general form is similar to the male. VII–IX sternites carinate, with a protuberance in the middle. Subgenital plate triangular ()) with the rounded distal part more thickened.
Ovipositor almost straight with an average length of 12.5 mm, and a pointed apex curved upwards. Inferior valves slightly curved at the base with 15–16 denticles ()).
Discussion
Barcode and morphological analyses confirm the existence of four new species in the Peloponnese. From the genetic point of view, the median-joining network analysis (), carried out on all Peloponnesian species, revealed six distinct haplogroups with five of them corresponding to the nominal species D. unicolor, D. kofinasi n. sp., D. propantii n. sp., D. poseidonica n. sp., and D. epidavrii n. sp. The sixth haplogroup corresponds to D. matsakisi and D. dalensi, that resulted closely related from a genetic point of view, but morphologically well differentiated (Di Russo et al. Citation2014), suggesting that they separated from each other recently and complete lineage sorting has not yet been achieved. All the haplogroups are geographically distributed with a resulting scenario rather heterogeneous. Pairwise comparisons between the Peloponnesian species range between 2.5% and 8.3%, falling in the range of interspecific comparisons observed in all the other studied Dolichopoda species (, Allegrucci et al., Citation2014) and consistent with divergence values found in other insect species (see for example, Hernández-Triana et al. Citation2019; Lencioni et al. Citation2021). ABGD analysis confirms these results, grouping the Peloponnesian taxa into seven different groups. Morphological analysis is in agreement with the molecular one, indicating that, at least two or three combinations of characters, as the shape of the tenth tergum, epiphallus, and plica dorsalis, discriminate these new four taxa in Peloponnese. In particular, the two western Peloponnesian species D. poseidonica n. sp. and D. propantii n. sp. are well-differentiated from D. unicolor, showing the tenth tergum’s lobes elongated and folded and the epiphallus short and almost flattened, respectively. D. kofinasi n. sp. can be distinguished from D. unicolor, mainly for the wide triangular median process of epiphallus and the domelike plica dorsalis. Finally, D. epidavrii n. sp., confined in the Northeast Peloponnese (Argolis) is strongly related to the “Petrochilosina” group of endemic species to Attica and Voiotia, Evvoia and Skyros island. In particular, it appears closely related to D. vandeli from Voiotia for the shape of the ninth tergum, and to D. petrochilosii from Attica, for the heart-shaped subgenital plate in the female. Moreover, D. epidavrii shares the synapomorphy, represented by the bifurcate apex of the epiphallus, with D. christosnifoni Di Russo & Rampini, 2018 from the western Cyclades, D. lycia (Galvagni, 2006) and D. fortuita Gorochov & Ünal, 2015 from south Turkey. On the contrary in all the other Peloponnesian species the pointed apex of the epiphallus may represent a plesiomorphic character, being shared by the majority of the Dolichopoda known both in the western and in the eastern part of the geographic distribution of the genus. Only in the Transcaucasian species, the epiphallus shows another different shape being truncated at the apex.
The Peloponnese is known for its remarkable and endemic fauna (Sfenthourakis & Legakis Citation2001) both within invertebrates and vertebrates, and new species are continuously discovered. For example, endemic new species of Coleoptera Cerambycidae (Vartanis & Borek Citation2019), Diptera Hybotidae (Grootaert & Alexiou Citation2020) have been recently described. The phylogeny illustrated in based on the combined data sets support the major phylogenetic relationships previously demonstrated (Allegrucci et al. Citation2009, Citation2011) highlighting that the Peloponnesian populations represent differentiated clades. In particular, D. epidavrii n. sp. links to D. vandeli as expected also from geographical and morphological points of view. D. kofinasi n. sp., D. propantii n. sp., and D. poseidonica n. sp. link to D. unicolor, being D. kofinasi n. sp. the most differentiated one. Interestingly, D. kofinasi n. sp. is one of the Greek species with the largest spatial range (). Greece is characterized by a dry and warm climate with the vegetation represented mainly by Mediterranean bush present with low shrubs. Forests can be found only at elevated altitudes. Mediterranean bush with low shrubs represents an environment fundamentally inhospitable to Dolichopoda; most species of this genus are strictly dependent upon caves, although individuals are often observed outside in moist or mesic woods, especially in the northern part of the genus’s range. Here, the favorable environmental conditions permit the gene flow between the different populations (Sbordoni et al. Citation1985; Allegrucci et al. Citation1997) making the range of each species rather large. The different bioclimatic and vegetational conditions found in the south-eastern part of the genus’s range make that the highest Dolichopoda species diversity is found in the Hellenic area, where we might expect that gene flow among cave populations could be more limited. Indeed, the spatial range of each species is restricted and often limited to only one cave (Boudou-Saltet Citation1980). Dolichopoda kofinasi n. sp. extends its range from the Arcadian plateau in the north, up to the northern foothills of Mt Taygetos and, through the Mt Parnon, it arrives at the extremities of the Maleas Peninsula. The inhabited area is very diverse, being wet and cold in the north, and very dry and hot in the south. This geographic diversity is also evident from the phylogeny in , where a phylogeographic pattern can be observed with the northern populations well differentiated from the southern ones.
D. kofinasi n. sp. is sister to D. propantii n. sp., D. poseidonica n. sp., and D. unicolor (), being the most differentiated one. Divergence times suggested that it originated 3.2 Ma (HPD 2.33–4.12 Ma; ) in the Pliocene. In that period and starting from the Upper Miocene, Mount Parnon has undergone a series of settlements due to the extensional faults causing its displacement and deformation (Skourtsos & Lekkas Citation2011). These settlements could have determined the formation of geographic barriers between the ancestor of D. kofinasi n. sp. and the other Peloponnesian species, causing its isolation and subsequent speciation.
D. propantii n. sp. and D. poseidonica n. sp. separated from each other about 1.7 Ma (HPD 1.03–2.36 Ma; ), in the Pleistocene. Their separation from the sister taxon D. unicolor happened 2.7 Ma (HPD 1.91–3.5 Ma; ), during the Pliocene. D. propantii n. sp. seems to be isolated on a small mountain, connected by a low ridge with the southern populations of D. unicolor and isolated from the eastern populations of D. kofinasi through the basin of Alfios river, the largest Peloponnesian river, originating from the northern foothills of Mt Taygetos. The presence of large paleo-lakes in the Megalopolis basin, since Late Pliocene (2.8 Mya), placed between the northern slope of Mt Taygetos and Arcadia plateau (Dermitzakis & Papanikolaou Citation1981; Fountoulis et al. Citation2014), may have affected dispersion, and allowed speciation on this small mountain. D. poseidonica n. sp. inhabits caves situated in a small area of land inside the Messinian Gulf. Only a shallow narrow basin isolates this area from the overhanging Mt Taygetos and the populations of D. unicolor. In particular, the Kambos and Kitries bay area is characterized by a depression that separates it from the rest of Mt Taygetos by at least 1.8–2 Mya (Fountoulis et al. Citation2014). This depression has been probably filled with shallow brackish water for a long time, constituting an efficient barrier to the gene flow with the close populations of D. unicolor.
Our data suggest that the present-day distribution of Peloponnesian cave crickets seems to be in alignment with the palaeogeographic history of the area. Several studies demonstrated that the genetic variation and differentiation of different species of both invertebrates and vertebrates has been influenced by the complex palaeogeographic and climate history of the Aegean region (Poulakakis et al. Citation2015 and references therein). In the present case, dating estimates () confirm the previous hypothesis that the dispersion toward Peloponnese occurred two times, independently. In particular, colonization of North Peloponnese hosting D. matsakisi and D. dalensi, proceeded from North-western Greece, while the central southern Peloponnese, hosting D. unicolor, D. kofinasi n. sp., D. propantii n. sp. and D. poseidonica n. sp. was colonized starting from eastern Greece (Allegrucci et al. Citation2009, Citation2011). This is also confirmed by the discovery of D. epidavrii n. sp. in the North Eastern Peloponnese (Argolis). This species belongs to a species group distributed in the Central – Eastern mainland Greece and Western Aegean. The group was once considered as a separate subgenus, Petrochilosina Boudou-Saltet Citation1980, but the taxonomic value and the use of the subgenus category in the genus Dolichopoda has been abandoned (Boudou-Saltet Citation1983; Alexiou et al. Citation2015). The finding of a member of this group in Argolis suggests a past land connection. In fact, during most of the Pleistocene, Peloponnese became reconnected with the north mainland, most probably causing the invasion and establishment of “ex-Petrochilosina” populations in the area of Argolis. It is clear that the shallow west Saronic Gulf, connecting Argolis Peninsula with east-central Greece, was land during most of the Pleistocene (Dermitzakis Citation1989), allowing colonization of new areas through dispersal. This hypothesis seems to agree with the separation of D. epidavrii n. sp. from the rest of the group, being the divergence time estimate equal to 1.8 Ma (HPD1.11, 2.5; ).
In conclusion, present results, in alignment with previous studies (Allegrucci et al. Citation2009, Citation2011), suggest that the climatic and tectonic events that occurred in the Plio-Pleistocene are important factors driving the isolation and consequently speciation in the Dolichopoda cave crickets. The silvicolous ancestors of Dolichopoda might have used caves as refugia during the unfavorable climatic conditions, beginning their adaptation to subterranean habitat. Therefore, the current distribution of Dolichopoda can be explained by a combination of both vicariance and dispersal events, with many processes occurring in ancestral epigean populations before the invasion of the subterranean environment.
Acknowledgements
We are indebted to our friend Giannis Kofinas-Kallergis and members of ‘Poseidon’ Speleological Club for greatly supported and helped our research on cave crickets of Peloponnese. Without them, this study would have been incomplete. Several friends and colleagues kindly provided us with cave cricket samples: S. Zoia, L. Dell ‘Anna, P.M. Giachino, D. Vailati, Grigoris Rousiotis, F. Ballarin, and Konstantinos Bakolitsas. They are greatly appreciated. We thank also our speleologist friends Kostas Laganas, Giannis Farsarakis, Valerio Cenni, Giorgio Pintus, and Vasilis Trizonis for their help in the field. Finally, we are particularly grateful to all the nice people of Kastorio, especially Giannis Arfanis, for their friendly hospitality in the Taygetos area. Ephorate of Palaeoanthropology and Speleology of the Ministry of Culture, Education and Religious Affairs, Athens, provided permissions to visit and collect cave cricket samples. A special thanks to Martin Husemann and to other three anonymous reviewers for their helpful comments on the original manuscript and to Matt Berry for English revision.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Alexiou S, Di Russo C, Rampini M. 2015. The cave crickets of the genus Dolichopoda from Evvia and Skyros islands: Formal description of D. octhoniai and D. saraolacosi (Orthoptera: Rhaphidophoridae). Fragmenta entomologica 47(2):133–137. DOI: 10.4081/fe.2015.141.
- Allegrucci G, Ketmaier V, Di Russo C, Rampini M, Sbordoni V, Cobolli M. 2017. Molecular phylogeography of Troglophilus cave crickets (Orthoptera, Rhaphidophoridae): A combination of vicariance and dispersal drove diversification in the East Mediterranean region. Journal of Zoological Systematics and Evolutionary Research 55:310–325. DOI: 10.1111/jzs.12172.
- Allegrucci G, Minasi MG, Sbordoni V. 1997. Patterns of gene flow and genetic structure in cave-dwelling crickets of the Tuscan endemic, Dolichopoda schiavazzii (Orthoptera, Rhaphidophoridae). Heredity 78:665–673. DOI: 10.1038/hdy.1997.106.
- Allegrucci G, Rampini M, Di Russo C, Lana E, Cocchi S, Sbordoni V. 2014. Phylogeography and systematics of the westernmost Italian Dolichopoda species (Orthoptera, Rhaphidophoridae). ZooKeys 437:1–23. DOI: 10.3897/zookeys.437.7917.
- Allegrucci G, Rampini M, Gratton P, Todisco V, Sbordoni V. 2009. Testing phylogenetic hypothesis for reconstructing the evolutionary history of Dolichopoda cave crickets in the eastern Mediterranean. Journal of Biogeography 36:1785–1797. DOI: 10.1111/j.1365-2699.02130.x.
- Allegrucci G, Sbordoni V. 2019. Insights into the molecular phylogeny of Raphidophoridae, an ancient, worldwide lineage of Orthoptera. Molecular Phylogenetics and Evolution 138:126–138. DOI: 10.1016/j.ympev.2019.05.032.
- Allegrucci G, Todisco V, Sbordoni V. 2005. Molecular phylogeography of Dolichopoda cave crickets (Orthoptera, Rhaphidophoridae): A. scenario suggested by mitochondrial DNA. Molecular Phylogenetics and Evolution 37:153–164. DOI: 10.1016/j.ympev.2005.04.022.
- Allegrucci G, Trucchi E, Sbordoni V. 2011. Tempo and mode of species diversification in Dolichopoda cave crickets (Orthoptera, Rhaphidophoridae). Molecular Phylogenetics and Evolution 60:108–121. DOI: 10.1016/j.ympev.2011.04.002.
- Bandelt HJ, Forster P, Rohl A. 1999. Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution 16:37–48. DOI: 10.1093/oxfordjournals.molbev.a026036.
- Blondel J, Aronson J, Bodiou JY, Boeuf G. 2010. The Mediterranean region: Biological diversity in space and time. vol. 4. Oxford, UK: Oxford University Press. pp. 1–401.
- Bouckaert R, Vaughan TG, Barido-Sottani J, Duchêne S, Fourment M, Gavryushkina A. 2019. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Computational Biology 15(4):e1006650. DOI: 10.1371/journal.pcbi.1006650.
- Boudou-Saltet P. 1972. Les Dolichopodes (Orth. Rhaph.) de Grèce. VII. Nouvelles espèces du Péloponnèse. Bulletin de la Société d’Histoire Naturelle de Toulouse 108:420–425.
- Boudou-Saltet P. 1980. Les Dolichopodes (Orth. Rhaph.) de Grèce. IX. Une espèce nouvelle en Eubee: D. makrykapa. Biologia gallo-hellenica 9:123–134.
- Boudou-Saltet P. 1983. Sur les Dolichopoda (Orth. Rhaph.) du sous-genre Petrochilosina. Mémoire de Biospéleologie 10:321–323.
- Cavazza W, Wexel FC. 2003. The Mediterranean region – A geological primer. Episodes 26:160–168. DOI: 10.18814/epiiugs/2003/v26i3/002.
- Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: More models, new heuristics and high-performance computing. Nature Methods 9:772. DOI: 10.1038/nmeth.2109.
- Dermitzakis MD. 1989. The colonisation of Aegean Islands in relation with the paleogeographic evolution. Biologia Gallo-hellenica 14(2):99–121.
- Dermitzakis MD. 1990. Paleogeography, geodynamic processes and event stratigraphy during the late Cenozoic of the Aegean area. Biogeographical aspects of insularity, Roma 1987. Accademia Nazionale dei Lincei 85:263–288.
- Dermitzakis MD, Papanikolaou DJ. 1981. Paleogeography and geodynamics of the Aegean Region during the Neogene. Annales Geologiques des Pays Helleniques, Hors Serie 4:245–289.
- Di Russo C, Carchini G, Sbordoni V. 1994. Life-history variation in Dolichopoda cave crickets. In: Danks HV, editor. Insect life-cycle polimorphism. Dor-drecht, Netherlands: Kluwer Academy Publisher. pp. 205–226.
- Di Russo C, Kofinas-Kallergis G, Alexiou S, Rampini M. 2019. New records of Dolichopoda (Orthoptera, Rhaphidophoridae) from Peloponnisos, Greece. Parnassiana Archives 7:55–63.
- Di Russo C, Rampini M, Cobolli M. 2014. The cave crickets of Greece: A contribution to the study of Southern Balkan Rhaphidophoridae diversity (Orthoptera), with the description of a new species of Troglophilus Krauss, 1879. Biodiversity Journal 5(3):397–420.
- Doyle JJ, Doyle JL. 1987. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochemical Bulletin 19:11–15.
- Folmer O, Black MB, Hoch W, Lutz RA, Vrijehock RC. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology Biotechnology 3:294–299.
- Fountoulis I, Mariolakos I, Ladas I. 2014. Quaternary basin sedimentation and geodynamics in SW Peloponnese (Greece) and late stage uplift of Taygetos Mt. Bollettino di Geofisica Teorica ed Applicata 55(2):303–324.
- Friedrich M, Tautz D. 1997. An episodic change of rDNA nucleotide substitution rate has occurred during the emergence of the insect order Diptera. Molecular Biology and Evolution 14:644–653. DOI: 10.1093/oxfordjournals.molbev.a025804.
- Grootaert P, Alexiou S. 2020. Description of a new species of Platypalpus of the candicans - cursitans subgroup from the Peloponnesus, Greece (Diptera: Hybotidae, Tachydromiinae). Entomologia Hellenica 29(1):17–26. DOI: 10.12681/eh.21760.
- Gu X, Fu YX, Li WH. 1995. Maximum likelihood estimation of heterogeneity of substitution rate among nucleotide sites. Molecular Biology and Evolution 12:546–557. DOI: 10.1093/oxfordjournals.molbev.a040235.
- Hernández-Triana ML, Brugman VA, Nikolova NI, Ruiz-Arrondo I, Barrero E, Thorne L, Fernández De Marco M, Krüger A, Lumley S, Johnson N, Fooks AR. 2019. DNA barcoding of British mosquitoes (Diptera, Culicidae) to support species identification, discovery of cryptic genetic diversity and monitoring invasive species. ZooKeys 832:57–76. DOI: 10.3897/zookeys.832.32257.
- Kocher TD, Thomas WK, Meyer A, Edwards SV, Paabo S, Villablanca FX, Wilson AC. 1989. Dynamics of mitochondrial DNA evolution in animals: Amplification and sequencing with conserved primers. Proceedings of the National Academy of Sciences USA 86:6196–6200. DOI: 10.1073/pnas.86.16.6196.
- Lanave C, Preparata C, Saccone C, Serio G. 1984. A new method for calculating evolutionary substitution rates. Journal of Molecular Evolution 20:86–93. DOI: 10.1007/BF02101990.
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and clustal X version 2.0. Bioinformatics 23:2947–2948. DOI: 10.1093/bioinformatics/btm404.
- Legakis A, Constantinidis T, Petrakis PV. 2018. Biodiversity in Greece. In: Pullaiah T, editor. Global biodiversity, vol.2: Selected countries in Europe. New York: Apple Academic Press. pp. 510. DOI: 10.1201/9780429487750.
- Lencioni V, Rodriguez‐Prieto A, Allegrucci G. 2021. Congruence between molecular and morphological systematics of Alpine non-biting midges (Chironomidae, Diamesinae). Zoologica Scripta. DOI: 10.1111/zsc.12480.
- Lunt DH, Zhang DX, Szymura JM, Hewitt GM. 1996. The insect cytochrome oxidase I gene: Evolutionary patterns and conserved primers for phylogenetic studies. Insect Molecular Biology 5:153–165. DOI: 10.1111/j.1365-2583.1996.tb00049.x.
- Martinsen L, Venanzetti F, Bachmann L. 2009. Phylogeography and mitochondrial DNA divergence in Dolichopoda cave crickets (Orthoptera, Rhaphidophoridae). Hereditas 146:33–45. DOI: 10.1111/j.1601-5223.2008.02068.x.
- Petrochilou A. 1969. To Spileo Propanti Andritsenis [In Greek with French summary]. Bulletin of Hellenic Speleological Society 10(3–4):72–77.
- Poulakakis N, Kapli P, Lymberakis P, Trichas A, Vardinoyiannis K, Sfenthourakis S, Mylonas M. 2015. A review of phylogeographic analyses of animal taxa from the Aegean and surrounding regions. Journal of Zoological Systematics and Evolutionary Research 53:18–32. DOI: 10.1111/jzs.12071.
- Puillandre N, Lambert A, Brouillet S, Achaz G. 2012. ABGD, automatic barcode gap discovery for primary species delimitation. Molecular Ecology 21:1864–1877. DOI: 10.1111/j.1365-294X.2011.05239.x.
- Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. 2018. Posterior summarization in Bayesian phylogenetics using tracer 1.7. Systematic Biology 67(5):901–904. DOI: 10.1093/sysbio/syy032.
- Rampini M, Di Russo C, Pavesi F, Cobolli M. 2008. The genus Dolichopoda in Greece. A. Description of new species from the Ionian regions and Peloponnisos (Orthoptera, Rhaphidophoridae). Zootaxa 1923 1–17.
- Ronquist F, Teslenko M, Van Der Mark P, Ayres D, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. 2012. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61:539–542. DOI: 10.1093/sysbio/sys029.
- Sbordoni V, Allegrucci G, Cesaroni D, Cobolli Sbordoni M, De Matthaeis E. 1985. Genetic structure of populations and species of Dolichopoda cave crickets: Evidence of peripatric divergence. Bollettino di zoologia 52(1–2):139–156. DOI: 10.1080/11250008509440347
- Sfenthourakis S, Legakis A. 2001. Hotspots of endemic terrestrial invertebrates in southern Greece. Biodiversity and Conservation 10:1387–1417. DOI: 10.1023/A:1016672415953.
- Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P. 1994. Evolution, weighting and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Annals of the Entomological Society of America 87:651–701. DOI: 10.1093/aesa/87.6.651.
- Skourtsos E, Lekkas S. 2011. Extensional tectonics in Mt Parnon (Peloponnesus, Greece. International Journal of Earth Science (Geol Rundsch) 100:1551–1567. DOI: 10.1007/s00531-010-0588-0.
- Tamura K, Dudley J, Nei M, Kumar S. 2007. MEGA 4: Molecular evolutionary genetics analysis. MEGA. software version 4.0. Molecular Biology and Evolution 24:1596–1599. DOI: 10.1093/molbev/msm092.
- Vartanis J, Borek R. 2019. Ropalopus carolini sp. nov. - Description of a new species from Greece-Peloponnese Peninsula (Coleoptera: Cerambycidae). Munis Entomology & Zoology 14:610–614.
