Abstract
Pesticides are frequently applied to agricultural activities to improve harvest, in terms of yield and product quality. Useful tools for ecotoxicological studies of marine ecosystems are based on biomarker application on bioindicator key fish species. The main aim of the present study was to detect the potential presence of pesticides in a polluted coastal marine environment, namely Milazzo Gulf, situated in the north eastern coast of Sicily (Italy), by measuring the enzymatic activities of the ecotoxicological biomarkers acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) in brain and blood samples of Chelon labrosus. Also, Marinello Reserve was selected as a reference site. The data showed a significant inhibition in AChE (81%) and BChE (71%) activities in fish from Milazzo Gulf in respect to those from the reference site. The esterase inhibition is primarily due to the presence of organophosphorus insecticides and carbamates that resulted, in Milazzo Gulf, higher in concentration in respect to the reference quality standard decree (D.M. 260, 2010). The results obtained in this study confirm the suspected presence of insecticides in waters and fish from Milazzo Gulf, which may lead to a considerable hazard to humans. This study confirms the relevant advantages of the biomarker approach on fish species in the ecotoxicological evaluation of marine environments.
1. Introduction
Pesticides are frequently applied to agricultural commodities to enhance the quality and quantity of food. The unrestricted, heavy use of synthetic chemical pesticides results in deleterious until lethal effects on various non-target organisms in aquatic environments, including fish, and direct or indirect effects to users (Kalavathy et al. Citation2001; Kumaresan et al. Citation2018; Ranjbar Jafarabadi et al. Citation2019; Sathyamoorthi et al. Citation2019; Vignet et al. Citation2019; Al-Ghanim et al. Citation2020). Extensive application of pesticides in the agricultural field implies that fish accumulate various fold higher concentrations of chemical residues than the surrounding water in the aquatic environment because of the bioaccumulation phenomenon. Organophosphates (OPs) are one of the groups of insecticides more widely used in agriculture. These compounds provoke high neurotoxicity on non-target organisms, such as fish (Castillo et al. Citation2006; Almeida et al. Citation2010; Diepens et al. Citation2014; Maisano et al. Citation2016; Sandoval-Herrera et al. Citation2019) that may absorb them by epidermis, gill epithelium, and digestive system. Each of these chemical substances passes several biological membranes until they are assimilated into the body (Castillo et al. Citation2006). The structures of these biological membranes and those of toxic compounds are the most important factors controlling the access of pollutants into the organism. For example, small molecules and fat-soluble substances are taken by passive diffusion that does not require energy use from the organism (Topal & Onac Citation2020). Organophosphorus pesticides have a strong insecticidal activity and do not accumulate in the environment. In addition, pesticides are rapidly metabolized into the organism and subsequently transformed into highly toxic metabolites that can also damage the human body to varying degrees (Yancheva et al. Citation2020). Therefore, chemical pesticides that persist in water represent not only a threat to fish but also to the human population as a potential consumer of affected fish (Licata et al. Citation2012; Mahboob et al. Citation2014; Cappello et al. Citation2018; Hamed et al. Citation2019; Sayed et al. Citation2019; Soliman et al. Citation2019; Parrino et al. Citation2020).
Useful tools for ecotoxicological studies of marine ecosystems are based on biomarker application on key bioindicator species (Napierska & Podolska Citation2008; Licata et al. Citation2012; Mahboob et al. Citation2014; Maisano et al. Citation2015; Giannetto et al. Citation2017; Cappello et al. Citation2018, Citation2021; Caliani et al. Citation2019; Fazio et al. Citation2019; Hamed et al. Citation2019; Pereira et al. Citation2019; Sayed et al. Citation2019; Parrino et al. Citation2020; Missawi et al. Citation2021). Fish are ubiquitous in aquatic environments and have a key role in the food chain, thus representing an energy vector from lower to higher levels (Payne et al. Citation1996; Caliani et al. Citation2019; Parrino et al. Citation2020). The uptake mechanisms of pollutants and the behaviour and responses of fish are of high ecological relevance. Although many fish species are highly mobile or migratory, fish represent optimal bioindicators of pollution of aquatic ecosystems (Van der Oost et al. Citation2003; Guerriero et al. Citation2018; Abdel-Gawad et al. Citation2020). Among the various biomarkers of exposure to pesticides, for this study, the enzymatic activities of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) were selected, having both been widely used to specifically evaluate the toxic effects of pesticides, such as carbamates and organophosphates (De Domenico et al. Citation2013; Caliani et al. Citation2019; Sayed et al. Citation2019; Parrino et al. Citation2020). These ecotoxicological biomarkers are specific for these xenobiotic compounds and provide information about the real effect induced on target species. Exposure to pesticides at concentrations that might not be lethal to fish can still affect their physiology and behaviour, with potential for reduced growth, survival and reproduction, and metabolic disturbances (Murty Citation1986; Kegley et al. Citation1999; Mahboob et al. Citation2014; Vignet et al. Citation2019).
The Milazzo Gulf (north eastern coastline of Sicily, Italy) represents a natural laboratory for investigating the relationship between pollution due to human activities and enrichments and distribution of trace elements in marine ecosystems (Pepe et al. Citation2010). In 2005, the industrial area of Milazzo was included in the list of Contaminated Sites of National Interest (Directive 23 December 2005 n. 266, art. 1 com. 561, Italian national law). The Milazzo Gulf is also affected by small river spills with irregular flows (D’Alessandro et al. Citation2016). The study area is characterized by low water circulation, mainly driven by NW winds. The surface water circulation is defined by a branch of Modified Atlantic Water which, flowing eastward, forms a near-coast anticyclonic gyre and generates an accumulation area in the northern part of the Gulf (Sitran et al. Citation2009; D’Alessandro et al. Citation2016). Conversely, the Marinello Reserve was chosen as a reference site. It extends over 400 hectares at the foot of the Tindari promontory (north eastern coast of Sicily, Italy). The area of the reserve, from Capo Tindari to Marinello, is characterized by tongues of sand that enclose brackish lakes called Marinello, Mergolo della Tonnara, Verde, Fondo Porto and Porto Vecchio. It is a lagoon area that was formed about 200 years ago and is very unstable and subjected to constant changes along the coastline.
Chelon labrosus is the most common marine species of the Mugilidae family, belonging to the order Mugiliformes. The adults live in small shoals near the rocky or sandy coasts, especially where there are artifacts and stream mouths. The juvenile specimens gather in much larger groups. Its appearance is that one of Mugilidae, with a slender and fusiform body shape with an almost circular cross-section, and thus its silver livery with numerous dark longitudinal lines. However, it may be well distinguished by the other species especially for the mouth, which has a robust and fleshy upper lip without central indentation but with some series of tubercles. In addition, the pectoral fins are often dull gray. This species can reach 70 cm in length and 2 kg of weight. Its meat is particularly appreciated in April–May when the migration into inland waters begins and in December–February, the period preceding the spawning, when the fish is fattest.
The aim of the present study was to evaluate the presence of pesticides in a coastal marine environment, represented herein by the industrial area of Milazzo Gulf (Messina, Italy) on the north eastern coast of Sicily. This was achieved through investigation on the ecotoxicological biomarkers AChE and BChE, as applied to the key fish species Chelon labrosus collected from the study area. Furthermore, chemical analysis of water samples was also carried out to analyze and eventually confirm the presence of carbamate and organophosphorus insecticides in the study area.
2. Materials and methods
2.1. Sampling design and sample collection
The study area was the Milazzo Gulf (Messina, Italy; 38°13′21.36″N and 15°14′43.64″E), a natural bay located on the north eastern Tyrrhenian coast of Sicily (). The coastal area of the Gulf, since the 1960s, is characterized by several industrial activities (i.e., crude oil refinery and thermal power plants), besides being a marine and commercial harbor. Due to all these activities, as well as municipal and urban sewages, it is reasonable to consider this area as a potential source of anthropogenic trace element contamination. Moreover, in this study area, the natural reserve of Marinello (Messina, South Italy) was selected as a reference site. The reference sampling site (38°08′24.93″N and 15°03′32.66″E) was located within a protected area established in 1998 and entrusted in management to the Metropolitan City of Messina.
A total of 80 C. labrosus specimens with similar length were sampled in May 2017 from the study site (Milazzo Gulf; 40 specimens) and the reference site (Marinello Reserve; 40 specimens), using pots. All of the experimental procedures and sample collection were carried out in accordance with the ethical considerations of the European legislation concerning the protection of animals used for scientific purposes (European Directive 2010/63). During fish collection, the water temperature, pH, salinity and dissolved oxygen were measured using a multiparametric probe (WTW 340i/Set; Wissenschaftlich, Weilheim, Germany). Immediately after collection, fish were transported live to the laboratory in large tanks with aeration, and at a constant temperature.
Water samples were taken from the two sampling sites (Milazzo Gulf and Marinello Reserve) using Niskin bottles, and transported to the laboratory at a constant temperature, for pesticides analysis by atomic absorption spectroscopy (Analyst 400; Perkin Elmer). The data for each analysis are reported as means ± standard deviations of three replicates (Microsoft Excel 2016).
2.2. Biomarker analysis
All the forty specimens collected from Milazzo Gulf and Marinello Reserve were firstly identified sexually, measured and weighed. Brain and blood were collected for evaluation of the AChE and BChE enzymatic activities, respectively. Blood was taken from the caudal vein in specimens anesthetized by MS222 (ethyl-ester-3-amino-benzoic acid, Sigma, Saint Louis, Missouri, US), at the concentration of 0.6 g L−1, with a 2.5 ml syringe (Maisano et al. Citation2013). Blood samples were centrifuged (10 min at 3000 g x 4°C), and immediately stored at –80°C.
AChE activity was measured in accordance with the protocol of Westlake et al. (Citation1981) and expressed as μmol substrate minute-1 g brain-1. The kinetic reaction was registered for 3 minutes at 410 nm wavelength.
BChE analysis was carried out in accordance with the protocol of Ellmann et al. (Citation1961) and the kinetic reaction was registered for 3 minutes at 410 nm wavelength. The final activity was expressed as μmol substrate minute-1 ml plasma-1.
Spectrophotometric assays were carried out using a spectrophotometer Shimatzu 1260 UV, with cell regulated at 30°C.
2.3. Water sample analysis
For the analysis of seawater samples, a volume of 100 mL was firstly placed in flasks with NaCl, and then the solution was stirred at 900 rpm using a PDMS stir bar (Erkuden Pérez-Carrera et al. Citation2007). Therefore, extraction and a pre-treatment based on a thermodesorption and cryofocusing procedure were carried out according to León et al. (Citation2003). For GC-MS analysis, the cryofocused compounds were transferred in an HP-5 5% Phenyl Methyl Siloxan (30 m x 250 µm x 0.25 µm) with the following conditions and the following temperature program: 60°C for 1.2 min, then 600°C/min to 325°C for 5 min. The acquisition mode was SIM.
Temperature Transfer Line (°C)280
Temperature of the source (°C)230
Temperature of the quadrupole (°C)150
Injection volume (µl)4
Carrier Gas Flow (psi)25
Run Time (min)50.071
2.4. Statistical analysis
All the data obtained from the enzymatic measurements of AChE and BChE in fish from the study and reference areas were expressed as mean ± standard deviation (S.D.), statistically processed and analyzed with a one-way variance analysis program (ANOVA), with the application of the Student-Newman-Keuls post-hoc test. Statistical analysis was carried out by using the GraphPad software (Prism 5.0, San Diego CA, USA). Data were considered statistically significant at p < 0.05.
3. Results
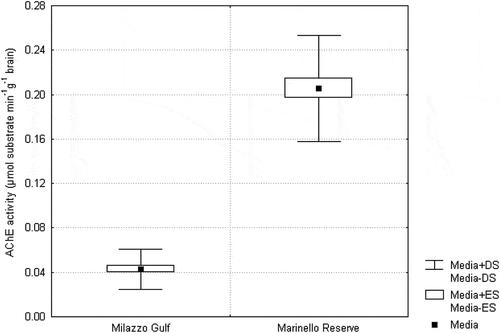
The AChE and BChE activities in the brain and blood samples from the C. labrosus collected in the study and reference site are shown in , respectively. The mean AChE activity in the brains of the fish from Milazzo Gulf was lower than that of the reference site (0.04 vs. 0.21 μmol substrate min−1 g−1 brain).
Figure 2. Mean AChE activity (µmol substrate minute−1 g brain−1) for Chelon labrosus, collected from the study and reference sites
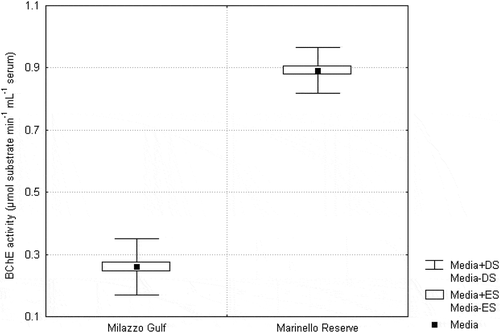
Similarly, the mean BChE activity was lower in fish from the industrial area in respect to that measured in fish from the reference site (0.26 vs. 0.89 μmol substrate min−1 mL−1 plasma).
Figure 3. Mean BChE activity (µmol substrate minute−1 mL plasma−1) for Chelon labrosus, collected from the study and reference sites
shows the physico-chemical parameters of the water samples collected from the two sites (Milazzo Gulf and Marinello Reserve).
Table I. Physico-chemical parameters of water from the study and reference sites
provides the concentrations of the carbamate and organophosphorus pesticides detected in the water samples from the two sites (Milazzo Gulf, Marinello Reserve), together with the reference water quality standard according to the Italian Law (Decree D.M. 260, 2010). All the carbamate and organophosphorus insecticides showed higher concentrations in the water samples from Milazzo Gulf (mean: 0.26 μg L−1 and 1.72 μg L−1, respectively) with respect to the reference quality standards. For the reference site of Marinello Reserve, all the pesticide concentrations were below the limit of detection, and hence below the reference quality standards.
Table II. Carbamate and organophosphorus concentrations (μg L−1) detected in the two different sites, with the reference water quality standard
4. Discussion
This study provides information on the ecotoxicological status of Chelon labrosus in the Milazzo Gulf, through the use of the esterase activities of AChE and BChE biomarkers, which have been validated as useful environmental diagnostic tools to recognize the presence of xenobiotic pollutants, such as insecticides in aquatic environments (Fossi et al. Citation2002; Minutoli et al. Citation2008, Citation2013; Fasulo et al. Citation2010; Mohammed Citation2014; Parrino et al. Citation2020). The neurotoxic effects of carbamates and organophosphates can be, in fact, evaluated by measuring the inhibition of the cholinesterase activity, even if many studies have also linked this inhibition to the toxic effects of heavy metal or hydrocarbon exposure (Payne et al. Citation1996; Guilhermino et al. Citation1998; Dethloff et al. Citation1999; Sturm et al. Citation1999; Parrino et al. Citation2020). AChE plays its role in catalysing the hydrolytic cleavage of acyl group in various esters of choline at the synaptic cleft, while BChE is able to hydrolyse larger molecules such as butyrylcholine. It serves as a co-regulator of cholinergic neurotransmission, and it is also involved in the detoxification processes of several compounds like cocaine, heroin, anaesthetics and pesticides (Taylor Citation1991; Çokugras Citation2003; Nicolet et al. Citation2003; Geula & Darvesh Citation2004; Gonzalez et al. Citation2012; Bajda et al. Citation2013; Nadorp & Soreq Citation2014; Cappello et al. Citation2015; Ahmad et al. Citation2016).
Through phosphorylation, xenobiotic agents can form a complex with AChE to make it unavailable for the degradation of acetylcholine. In the synaptic cleft, this produces an accumulation of endogenous acetylcholine and the consequent hyperstimulation at the level of the peripheral cholinergic terminals of neuromuscular, sympathetic ganglia, and the central nervous system. In the cholinergic endings of smooth muscle, high concentrations of acetylcholine can result in muscle contractions, which will be accompanied by increased secretion due to hyperstimulation of glandular cholinergic terminals. In neuromuscular plaques, the accumulation of acetylcholine can lead to hyperstimulation, with muscle contractions, or vice-versa to paralysis due to depolarisation. In the synapses of the central nervous system, increased acetylcholine can result in sensory and behavioural changes, loss of motor coordination, and respiratory depression (Parrino et al. Citation2020). In humans, inhibition of AChE results in biological complications, such as diarrhoea, muscle weakness, headache associated with the development of Alzheimer's disease and paralysis (Onor et al. Citation2007; Leibson & Lifshitz Citation2008; Ahmad et al. Citation2016).
Regarding the location of the study area herein selected, it must be considered that it is very close to an industrial centre, the Milazzo Refinery, covering 524 acres. It worked from 1961 to 1979 when, after the Iranian oil crisis, it was stopped. In March 1982, a branch of ENI Group purchased the Refinery and started up the production again. An agreement was signed in December 1996 between ENI and Kuwait Petroleum Italia, based on a 50/50 shareholding. The refinery of Milazzo is one of the most complex and efficient refineries in Europe, with a capacity of 10 million tonnes per year. Besides these intense industrial activities, the Milazzo area is interested in the large cultivation of vegetables and many greenhouses, being characterized by very large available flat soils. There are three NS-oriented streams that flow from the Peloritani Mountains into the Milazzo Gulf: the Muto and Corriolo, which are in proximity to its mouth flows and thus very close to the crude oil refinery, and Mela streams (Pepe et al. Citation2010). During the periods of high stream discharge, continental waters carry out a significant amount of sediments, sewage and agricultural land run-off waters. It is well known that pesticide usage is a critical concern in coastal areas, where inputs from agriculture and urbanization may impact the coastal marine environments (Fulton & Key Citation2001). Because of their relatively non-persistent characteristics in the environment, OPs have become one of the most widely used classes of insecticides worldwide. Although these compounds offer the advantage of rapid degradation in the environment, they generally lack target specificity and have high acute toxicity towards many non-target invertebrate and vertebrate species, like fish (Rodrigues et al. Citation2012).
The use of biomarkers in fish to evaluate the effects of pesticides, including insecticides, has already been demonstrated to be advantageous (Caliani et al. Citation2019; Fazio et al. Citation2019; Hamed et al. Citation2019; Sayed et al. Citation2019; Soliman et al. Citation2019; Parrino et al. Citation2020). In this study, it is possible to observe an enzymatic activity inhibition of 81% (AChE) and 71% (BChE) in samples of C labrosus from Milazzo Gulf compared to those from the reference site (Marinello). Therefore, these results highlight a pesticide contamination in the study area under examination. Indeed, this esterase inhibition can be primarily due to the presence of the carbamate and organophosphorus insecticides, which can act directly on these enzymes, although hydrocarbons and/or heavy metals can also contribute (Payne et al. Citation1996; Guilhermino et al. Citation1998; Dethloff et al. Citation1999; Sturm et al. Citation1999; Parrino et al. Citation2020). Considering the presence of the refinery, and therefore of the intense industrial activities, and furthermore considering that Milazzo hosts an important harbor with high maritime traffic, a contamination by hydrocarbons and/or heavy metals must be also expected. The analyses of water samples here confirmed the presence of the carbamate and organosphorous pesticides in the waters of Milazzo Gulf, while for the reference site, these compounds were not detectable, as they were below the threshold values. It is therefore likely that these compounds were responsible for the AChE and BChE inhibition in the C. labrosus specimens from the study site. This type of pollution in Milazzo Gulf can be justified with the runoff waters and to the streams that outflow into the sea, crossing the cultivated fields often with greenhouses for cultivation and intensive production of plants, flowers and vegetables. This can thus bring an important pollutant load in the ecosystems of the Milazzo Gulf that may induce stress in non-target species, like fish. The coastal environment of Milazzo Gulf because of its conformation does not allow efficient water exchange, so the sampling site is particularly interested in the accumulation of various substances, including chemical compounds. In contrast, the reference site of Marinello represents a natural Reserve, with a very low level of contamination degree, and therefore a better environmental quality status, as also supported by the high levels of esterase activities recorded in fish collected from this site. Further studies will be carried out testing the same biomarkers selected for this study, but applied in several different fish species, in order to elucidate their responses. Furthermore, it will be interesting to test other biomarkers in the same target species C. labrosus in order to gain a more clear picture of the quality status of the marine coastal area under examination.
5. Conclusion
Data presented in this study demonstrate the presence of pesticides in the ecosystem of the Milazzo Gulf that may result in a considerable hazard to humans due to the bathing and recreational fishing activities in the surrounding waters. This study also confirms the advantages that may be obtained from the application of a biomarker-based approach on fish species for the evaluation of the ecotoxicological status of marine ecosystems.
Ethics approval
All sampling, animal maintenance and experimental procedures of this study involving fishes were performed in accordance with the ethical guidelines of the European Union Council, namely the Guide for the Care and Use of Laboratory Animals, Directive 2010/63/EU.
Disclosure statement
The authors declare no conflicts of interest in this work.
Additional information
Funding
References
- Abdel-Gawad FK, Khalil WKB, Bassem SM, Kumar V, Parisi C, Inglese S, Temraz TA, Nassar HF, Guerriero G. 2020. The duckweed, Lemna minor modulates heavy metal-induced oxidative stress in the Nile tilapia, Oreochromis niloticus. Water 12:2983. DOI: 10.3390/w12112983.
- Ahmad SA, Sabullah MK, Basirun AA, Khalid A, Yasid NA, Iqbal IM, Shamaan NA, Syed MA, Shukor MY. 2016. Evaluation of cholinesterase from the muscle and blood of Anabas testudineus as detection of metal ions. Fresenius Environmental Bulletin 25:4253–4260.
- Al-Ghanim KA, Mahboob S, Vijayaraghavan P, Al-Misned FA, Kim YO, Kim HJ. 2020. Sub-lethal effect of synthetic pyrethroid pesticide on metabolic enzymes and protein profile of non-target Zebra fish, Danio rerio. Saudi Journal of Biological Sciences 27:441–447. DOI: 10.1016/j.sjbs.2019.11.005.
- Almeida JR, Oliveira C, Gravato C, Guilhermino L. 2010. Linking behavioural alterations with biomarkers responses in the European seabass Dicentrarchus labrax exposed to the organophosphate pesticide fenitrothion. Ecotoxicology 19:1369–1381. DOI: 10.1007/s10646-010-0523-y.
- Bajda M, Lċfnrzvnd A, Hebda M, Guzior N, Sotriffer CA, Malawska B. 2013. Structure-based search for new inhibitors of cholinesterases. International Journal of Molecular Sciences 14:5608–5632. DOI: 10.3390/ijms14035608.
- Caliani I, Rodrígue LP, Casini S, Granata A, Zagami G, Pansera M, Querci G, Minutoli R. 2019. Biochemical and genotoxic biomarkers in Atherina boyeri to evaluate the status of aquatic ecosystems. Regional Studies in Marine Science 28:100566. DOI: 10.1016/j.rsma.2019.100566.
- Cappello T, De Marco G, Oliveri Conti G, Giannetto A, Ferrante M, Mauceri A, Maisano M. 2021. Time-dependent metabolic disorders induced by short-term exposure to polystyrene microplastics in the Mediterranean mussel Mytilus galloprovincialis. Ecotoxicology and Environmental Safety 209:111780. DOI: 10.1016/j.ecoenv.2020.111780.
- Cappello T, Giannetto A, Parrino V, De Marco G, Mauceri A, Maisano M. 2018. Food safety using NMR-based metabolomics: Assessment of the Atlantic bluefin tuna, Thunnus thynnus, from the Mediterranean Sea. Food and Chemical Toxicology 115:391–397. DOI: 10.1016/j.fct.2018.03.038.
- Cappello T, Maisano M, Giannetto A, Parrino V, Mauceri A, Fasulo S. 2015. Neurotoxicological effects on marine mussel Mytilus galloprovincialis caged at petrochemical contaminated areas (eastern Sicily, Italy): 1H NMR and immunohistochemical assays. Comparative Biochemistry and Physiology: Toxicology & Pharmacology 169:7–15. DOI: 10.1016/j.cbpc.2014.12.006.
- Castillo LE, Martinez E, Ruepert C. 2006. Water quality and macroinvertebrate community response following pesticide applications in a banana plantation, Limon, Costa Rica. Science of the Total Environment 367:418–432. DOI: 10.1016/j.scitotenv.2006.02.052.
- Çokugras AN. 2003. Butyrylcholinesterase: Structure and physiological importance. Turkish Journal of Biochemistry 28:54–61.
- D’Alessandro M, Esposito V, Giacobbe S, Renzi M, Mangano MC, Vivona P, Consoli P, Scotti G, Andaloro F, Romeo T. 2016. Ecological assessment of a heavily human-stressed area in the Gulf Ryditerranean Sea: An integrated study of biological, physical and chemical indicators. Marine Pollution Bulletin 106:260–273. DOI: 10.1016/j.marpolbul.2016.01.021.
- De Domenico E, Mauceri A, Giordano D, Maisano M, Giannetto A, Parrino V, Natalotto A, D’Agata A, Cappello T, Fasulo S. 2013. Biological responses of juvenile European sea bass (Dicentrarchus labrax) exposed to contaminated sediments. Ecotoxicology and Environmental Safety 97:114–123. DOI: 10.1016/j.ecoenv.2013.07.015.
- Dethloff GM, Schlenk D, Hamm JT, Bailey HC. 1999. Alterations in physiological parameters of rainbow trout (Onchorhynchus mykiss) with exposure to copper and copper/zinc mixtures. Ecotoxicology and Environmental Safety 42:253–264. DOI: 10.1006/eesa.1998.1757.
- Diepens NJ, Pfennig S, Van den Brink PJ, Gunnarsson JS, Ruepert C, Castillo LE. 2014. Effect of pesticides used in banana and pineapple plantations on aquatic ecosystems in Costa Rica. Journal of Environmental Biology 35:73–84.
- Ellmann GL, Courtney KD, Andres Jr V, Featherstone RM. 1961. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochemical Pharmacology 7:88–95. DOI: 10.1016/0006-2952(61)90145-9.
- Erkuden Pérez-Carrera E, León León VM, Gómez Parra A, González-Mazo E. 2007. Simultaneous determination of pesticides, polycyclic aromatic hydrocarbons and polychlorinated biphenyls in seawater and interstitial marine water samples, using stir bar sorptive extraction–thermal desorption–gas chromatography–mass spectrometry. Journal of Chromatography A 1170:82–90. DOI: 10.1016/j.chroma.2007.09.013.
- Fasulo S, Marino S, Mauceri A, Maisano M, Giannetto A, D’Agata A, Parrino V, Minutoli R, De Domenico E. 2010. A multibiomarker approach in Coris julis living in a natural environment. Ecotoxicology and Environmental Safety 73:1565–1573. DOI: 10.1016/j.ecoenv.2010.01.008.
- Fazio F, Savoca C, Ferrantelli V, Cammilleri G, Capillo G, Piccione G. 2019. Relationship between arsenic accumulation in tissues and hematological parameters in mullet caught in Faro Lake: A preliminary study. Environmental Science and Pollution Research 26:8821–8827. DOI: 10.1007/s11356-019-04343-7.
- Fossi MC, Borsani JF, Di Mento R, Marsili L, Casini S, Neri G, Mori G, Ancora S, Leonzio C, Minutoli R, Notarbartolo D, Sciara G. 2002. Multi-trial biomarker approach in Meganycthiphanes norvegica as an early indicator of health status of the Mediterranean “whale sanctuary”. Marine Environmental Research 54:761–767. DOI: 10.1016/S0141-1136(02)00148-4.
- Fulton MH, Key PB. 2001. Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environmental Toxicology and Chemistry 20:37–45. DOI: 10.1897/1551-5028(2001)020<0037:aiiefa>2.0.co;2.
- Geula C, Darvesh S. 2004. Butyrylcholinesterase, cholinergic neurotransmission and the pathology of Alzheimer’s disease. Drugs Today 40:711–721. DOI: 10.1358/dot.2004.40.8.850473.
- Giannetto A, Maisano M, Cappello T, Oliva S, Parrino V, Natalotto A, De Marco G, Fasulo S. 2017. Effects of oxygen availability on oxidative stress biomarkers in the Mediterranean mussel Mytilus galloprovincialis. Marine Biotechnology 19:614–626. DOI: 10.1007/s10126-017-9780-6.
- Gonzalez V, Huen K, Venkat S, Pratt K, Xiang P, Harley KG, Kogut K, Trujillo CM, Bradman A, Eskenzi B, Holland NT. 2012. Cholinesterase and paraoxonase (PON1) enzyme activities in Mexican-American mothers and children from an agricultural community. Journal of Exposure Science & Environmental Epidemiology 22:641–648. DOI: 10.1038/jes.2012.61.
- Guerriero G, Bassem SM, Abdel-Gawad FK. 2018. Biological responses of white sea bream (Diplodus sargus, Linnaeus 1758) and sardine (Sardine pilchardus, Walbaum 1792) exposed to heavy metal contaminated water. Emirates Journal of Food & Agriculture 30:688–694. DOI: 10.9755/ejfa.2018.v30.i8.1762.
- Guilhermino L, Soares AMVM, Carvalho AP, Lopes MC. 1998. Effects of cadmium and parathion exposure on hematology and blood biochemistry of adult male rats. Bulletin of Environmental Contamination and Toxicology 60:52–59. DOI: 10.1007/s001289900590.
- Hamed M, Soliman HAM, Sayed AEH. 2019. Ameliorative effect of Spirulina platensis against lead nitrate–induced cytotoxicity and genotoxicity in catfish Clarias gariepinus. Environmental Science and Pollution Research 26:20610–20618. DOI: 10.1007/s11356-019-05319-3.
- Kalavathy KA, Sirvakumar A, Rashmi C. 2001. Toxic effect of pesticide dimethoate on the fish Sarotherodon mossambicus. Journal of Ecological Research and Bioconservation 2:27–32.
- Kegley S, Neumeister L, Martin T. 1999. Ecological impacts of pesticides in California. California: Pesticide Action Network.
- Kumaresan V, Pasupuleti M, Arasu MV, Al-Dhabi NA, Arshad A, Nurul Amin SM, Md Yusoff F, Arockiaraj J. 2018. A comparative transcriptome approach for identification of molecular changes in Aphanomyces invadans infected Channa striatus. Molecular Biology Reports 45:2511–2523. DOI: 10.1007/s11033-018-4418-y.
- Leibson T, Lifshitz M. 2008. Organophosphate and carbamate poisoning: Review of the current literature and summary of clinical and laboratory experience in Southern. The Israel Medical Association Journal 10:7704–7727.
- León VM, Álvarez B, Cobollo MA, Muñoz S, Valor I. 2003. Analysis of 35 priority semivolatile compounds in water by stir bar sorptive extraction–thermal desorption–gas chromatography–mass spectrometry: I. Method optimization. Journal of Chromatography A 999:91–101. DOI: 10.1016/S0021-9673(03)00600-9.
- Licata P, Naccari F, Dugo G, Fotia V, Turco VL, Potorti AG, Di Bella G. 2012. Organochlorine pesticides and polychlorinated biphenyls in common buzzard (Buteo buteo) from Sicily (Italy). Environmental Monitoring and Assessment 184:2881–2892. DOI: 10.1007/s10661-011-2157-9.
- Mahboob S, Ghazala Al-Ghanim KA, Sultana S, Al-Misned F, Ahme Z. 2014. Fish Cholinesterases as Biomarkers of sublethal effects of organophosphorus and carbamates in tissues of Labeo rohita. Pakistan Journal of Zoology 46:121–127.
- Maisano M, Cappello T, Catanese E, Vitale V, Natalotto A, Giannetto A, Barreca D, Brunelli E, Mauceri A, Fasulo S. 2015. Developmental abnormalities and neurotoxicological effects of CuO NPs on the black sea urchin Arbacia lixula by embryotoxicity assay. Marine Environmental Research 111:121–127. DOI: 10.1016/j.marenvres.2015.05.010.
- Maisano M, Cappello T, Oliva S, Natalotto A, Giannetto A, Parrino V, Battaglia P, Romeo T, Salvo A, Spanò N, Mauceri A. 2016. PCB and OCP accumulation and evidence of hepatic alteration in the Atlantic bluefin tuna, Thunnus thynnus, from the Mediterranean Sea. Marine Environmental Research 121:40–48. DOI: 10.1016/j.marenvres.2016.03.003.
- Maisano M, Trapani MR, Parrino V, Parisi MG, Cappello T, D’Agata A, Benenati G, Natalotto A, Mauceri A, Cammarata M. 2013. Haemolytic activity and characterization of nematocyst venom from Pelagia noctiluca (Cnidaria, Scyphozoa). Italian Journal of Zoology 80:168–176. DOI: 10.1080/11250003.2012.758782.
- Minutoli R, Fossi MC, Zagami G, Granata A, Guglielmo L. 2008. First application of biomarkers approach in the zooplanktonic copepod Acartia latisetosa for the early management and conservation of transitional waters ecosystems. Transitional Waters Bulletin 1:45–52. DOI: 10.1285/i1825229Xv2n1p45.
- Minutoli R, Granata A, Guglielmo L. 2013. Potential use of ecotoxicological biomarkers in Serratella ignita (Ephemeroptera) larvae for Alcantara river (Sicily, Italy) water quality assessment. Journal of Limnology 72:394–399. DOI: 10.4081/jlimnol.2013.e32.
- Missawi O, Bousserrhine N, Zitouni N, Maisano M, Boughattas I, De Marco G, Cappello T, Belbekhouche S, Guerrouache M, Alphonse V, Banni M. 2021. Uptake, accumulation and associated cellular alterations of environmental samples of microplastics in the seaworm Hediste diversicolor. Journal of Hazardous Materials 406:124287. DOI: 10.1016/j.jhazmat.2020.124287.
- Mohammed EH. 2014. Biochemical response of the cyclopoida copepod Apocyclops borneoensis exposed to nickel. Jordan Journal of Biological Sciences 7(1):41–47. DOI: 10.12816/0008212.
- Murty AS. 1986. Toxicity of pesticides to fish. Vol. 1. Boca Raton, Florida: CRC Press Inc. pp. 143.
- Nadorp B, Soreq H. 2014. Predicted overlapping microRNA regulators of acetylcholine packaging and degradation in neuroinflammation-related disorders. Frontiers in Molecular Neuroscience 7:1–9. DOI: 10.3389/fnmol.2014.00009.
- Napierska D, Podolska M. 2008. Relationship between biomarker responses and contaminant concentration in selected tissues of flounder (Platichthys flesus) from the Polish coastal area of the Baltic Sea. Oceanologia 50:421–442. DOI: 10.1007/s10646-009-0328-z.
- Nicolet Y, Lockridge O, Masson P, Fontecilla-Camps JC, Nachon F. 2003. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. The Journal of Biological Chemistry 278:41141–41147. DOI: 10.1074/jbc.M210241200.
- Onor ML, Trevisiol M, Aguglia E. 2007. Rivastigmine in the treatment of Alzheimer’s disease: An update. Clinical Interventions in Aging 2:17–32. DOI: 10.2147/ciia.2007.2.1.17.
- Parrino V, Minutoli R, Lo Paro G, Sulfaro D, Fazio F. 2020. Environmental assessment of the pesticides in Parablennius sanguinolentus along the Western Calabrian coast (Italy). Regional Studies in Marine Science 36:101297. DOI: 10.1016/j.rsma.2020.101297.
- Payne JF, Mathieu A, Melvin W, Fancey LL. 1996. Acetylcholinesterase, an old biomarker with a new future? Field trials in association with two urban rivers and a paper mill in Newfoundland. Marine Pollution Bulletin 32:225–231. DOI: 10.1016/0025-326X(95)00112-Z.
- Pepe F, Scopelliti G, Di Leonardo R, Ferruzza G. 2010. Granulometry, mineralogy and trace elements of marine sediments from the Gulf of Milazzo (NE Sicily): Evaluation of anthropogenic impact. Italian Journal of Geosciences 129:385–394. DOI: 10.3301/IJG.2010.23.
- Pereira P, Korbas M, Pereira V, Cappello T, Maisano M, Canario J, Almeida A, Pacheco M. 2019. A multidimensional concept for mercury neuronal and sensory toxicity in fish – From toxicokinetics and biochemistry to morphometry and behavior. Biochimica et Biophysica Acta. General Subjects 1863:129298. DOI: 10.1016/j.bbagen.2019.01.020.
- Ranjbar Jafarabadi A, Riyahi Bakhtiari A, Mitra S, Maisano M, Cappello T, Jadot C. 2019. First polychlorinated biphenyls (PCBs) monitoring in seawater, surface sediments and marine fish communities of the Persian Gulf: Distribution, levels, congener profile and potential health risk assessment. Environmental Pollution 253:78–88. DOI: 10.1016/j.envpol.2019.07.023.
- Rodrigues S, Antunes SC, Brandão FP, Castro BB, Gonçalvesab F, Nunes B. 2012. Effects of anticholinesterase drugs on biomarkers and behavior of pumpkinseed, Lepomis gibbosus (Linnaeus, 1758). Journal of Environmental Monitoring 14:1638–1644. DOI: 10.1039/c2em30033h.
- Sandoval-Herrera N, Mena F, Espinoza M, Romero A. 2019. Neurotoxicity of organophosphate pesticides could reduce the ability of fsh to escape predation under low doses of exposure. Scientific Reports 9:10530. DOI: 10.1038/s41598-019-46804-6.
- Sathyamoorthi A, Kumaresan V, Palanisamy R, Pasupuleti M, Arasu MV, Al-Dhabi NA, Marimuthu K, Nurul Amin SM, Arshad A, Md Yusoff F, Arockiaraj J. 2019. Therapeutic cationic antimicrobial peptide (CAP) derived from fish aspartic proteinase Cathepsin D and its antimicrobial mechanism. International Journal of Peptide Research and Therapeutics 25:93–105. DOI: 10.1007/s10989-017-9652-y.
- Sayed AH, Abd-Elkareem M, Khalil NS. 2019. Immunotoxic effects of 4-nonylphenol on Clarias gariepinus: Cytopathological changes in hepatic melanomacrophages. Aquatic Toxicology 207:83–90. DOI: 10.1016/j.aquatox.2018.12.002.
- Sitran R, Bergamasco A, Decembrini F, Guglielmo L. 2009. Microzooplankton (tintinnid ciliates) diversity: Coastal community structure and driving mechanisms in the southern Tyrrhenian Sea (Western Mediterranean). Journal of Plankton Research 31:153–170. DOI: 10.1093/plankt/fbn111.
- Soliman HAM, Hamed M, Leec J, Sayed AEH. 2019. The novel protective role of sensitized derivate pyrazole carboxamide in the lead nitrate-exposed African catfish Clarias gariepinus. Environmental Pollution 247:678–684. DOI: 10.1016/j.envpol.2019.01.074.
- Sturm A, da silva de Assis HC, Hansen PD. 1999. Cholinesterases of marine teleost fish: Enzymological characterisation and potential use in the monitoring of neurotoxic contamination. Marine Environmental Research 47:389–398. DOI: 10.1016/S0141-1136(98)00127-5.
- Taylor P. 1991. The cholinesterases. The Journal of Biological Chemistry 266:4025–4028. DOI: 10.1016/S0021-9258(20)64277-6.
- Topal T, Onac C. 2020. Determination of heavy metals and pesticides in different types of fish samples collected from four different locations of Aegean and Marmara Sea. Hindawi. Journal Food Quality Article ID 8101532, 12. DOI: 10.1155/2020/8101532.
- Van der Oost R, Beyer J, Vermeulen PEN. 2003. Fish bioaccumulation and biomarkers in environmental risk assessment: A review. Environmental Toxicology and Pharmacology 13:57–149. DOI: 10.1016/s1382-6689(02)00126-6.
- Vignet C, Cappello T, Fu Q, Lajoie K, De Marco G, Clerandeau C, Mottaz H, Maisano M, Hollender J, Schirmer K, Cachot J. 2019. Imidacloprid induces adverse effects on fish early life stages that are more severe in Japanese medaka (Oryzias latipes) than in zebrafish (Danio rerio). Chemosphere 225:470–478. DOI: 10.1016/j.chemosphere.2019.03.002.
- Westlake GE, Bunyan PJ, Martin AD, Stanley PL, Steel LC. 1981. Organophosphorus poisoning: Effects of selected organophosphorus pesticides on plasma enzymes and brain esterases of Japanese quail (Coturnix cortunix japonica). Journal of Agricultural and Food Chemistry 29:772–777. DOI: 10.1021/jf00106a021.
- Yancheva V, Stoyanova S, Velcheva I, Georgieva E. 2020. Fish as indicators for environmental monitoring and health risk assessment regarding aquatic contamination with pesticides. International Journal of Zoology and Animal Biology 3:000210. DOI: 10.23880/izab-16000210.