Abstract
Changes in the management and productivity of farmland affect foraging behaviour in migrating birds. Crops are increasingly being damaged (and farmers are sustaining ever greater economic losses) as a result of large flocks of waterfowl feeding on them. To investigate the differences in the time budget shared between natural grasslands and arable lands, migrating Greater White-fronted Geese (Anser albifrons) were filmed using digiscoping equipment at a spring stopover site. Four types of activity were noted in connection with habitat types, bird age, position in the flock and flock size. Foraging was the most common activity in both habitats, but was more common in the grasslands than on arable land. The mean times spent on vigilance, resting and other activities were also significantly different between the two habitat types. GLM analysis showed that young birds spent more time foraging than adults but revealed no differences in foraging times between the age categories in grasslands and arable land. In the latter, geese were more vigilant at the edge of a flock and rested more frequently in its centre. No such differences were found in the grasslands. Only resting time was adversely affected by flock size. These findings, which demonstrate that White-fronted Geese are flexible in their use of food resources, could be useful in agricultural management planning in the light of increasing conflicts with farmers.
Introduction
As with other forms of behavioural plasticity, flexibility in food intake has probably evolved in response to environmental uncertainty, which most land birds encounter in terms of food resource availability during migration (Parrish Citation2000). Changes in the management and productivity of farmland in the last few decades have elicited modified ways of utilizing foraging habitat in most agricultural areas (Atkinson et al. Citation2005). The destruction of natural wetlands and the population growth of some waterfowl have led to the increased use of crop fields by these birds (Owen Citation1990; MacMillan et al. Citation2004; Leito et al. Citation2008; Hake et al. Citation2010; Radtke & Dieter Citation2011; Rosin et al. Citation2012; Rees et al. Citation2019). Dense, single-species crops such as early-growth cereals in agricultural landscapes offer elevated energetic and nutritional intake rates of higher-quality food that is attractive to feeding birds, leading in effect to conflicts with farmers (Bischof et al. Citation2012; Eythórsson et al. Citation2017; Fox et al. Citation2017).
Very large, non-breeding populations of geese occur mostly on farmland foraging grounds, thus increasing their impact on agriculture (Fox et al. Citation2010; Madsen et al. Citation2014). Large flocks grazing over wide areas of farmland can damage spring-sown cereals and other crops (Owen, Citation1990; Van Eerden Citation1990; MacMillan et al. Citation2004; Hake et al. Citation2010), resulting in substantial economic losses and conflicts with farmers. Moreover, the availability and quality of abundant food on farmland, in conjunction with legal protection and management actions by bird conservation groups in some areas, have aggravated this situation (Abraham et al. Citation2005; Fox et al. Citation2005; Van Eerden et al. Citation2005; Wuczyński et al. Citation2012). The most common migrating goose species in Europe is the Greater White-fronted Goose (Anser albifrons) (hereafter White-fronted Goose/Geese). Birds belonging to the “Baltic-North Sea” group of this species move eastwards in the spring from the western Europe to the Russian Arctic (Madsen et al. Citation1999). Along the way, they stop over in various areas, where the large majority feed on farmland (e.g. Fox et al. Citation2005; Jankowiak et al. Citation2015; Grishchenko et al. Citation2019). Therefore, the appearance of enormous flocks of foraging geese in a relatively short period at stopover sites may cause very considerable crop damage.
One of the most important spring staging sites of White-fronted Geese in central Europe is the Biebrza Basin in north-eastern Poland (Polakowski et al. Citation2011). Higher numbers of these birds were recorded here in grasslands than on farmland, this preference being most conspicuous during the latter half of the stopover (Polakowski & Kasprzykowski Citation2016). Grasslands and meadows are natural stopover habitats for geese, offering appropriate conditions for foraging owing to the nutrient balance of natural food resources (Prop et al. Citation1998; Vickery & Gill Citation1999; Van Eerden et al. Citation2005; Jensen et al. Citation2008). An additional attraction of meadows for geese is their closer location to roosting sites compared with more distant arable lands, which entails extra energy expenditure on flying (Gill Citation1996). In turn, the use of grasslands by geese after rapid snowmelt is limited because these areas are then usually flooded. Arable land situated on somewhat higher ground is more readily accessible to geese in early spring and could therefore be an attractive foraging area for them (Polakowski & Kasprzykowski Citation2016). However, little is known about the behaviour of geese on both meadows and arable land, their activities there and their impact on farmland, which in the context of their currently very numerous flocks must be significant.
In the light of ubiquitous anthropogenic environmental change, understanding the influence of intraspecific factors on the use of space by geese has important consequences for the management and conservation of migratory waterfowl and the phenomenon of migration itself. Knowledge about how geese behave during spring migration in natural grasslands and crop fields may provide greater insight into their impact on agriculture and could be helpful for farmland management in places where geese congregate in large numbers. We therefore aimed to examine the time budget of White-fronted Geese on one of their most important spring stopover sites in Poland. Our hypothesis was that the time spent on particular activities was determined by the type of foraging grounds. We also anticipated that the time budget would depend not only on the type of feeding habitat, but also on the age of the birds, their physical position within the flock and the size of the flock.
Materials and methods
Study area
The study was conducted in the basin of the River Biebrza in north-eastern Poland. This area was described in detail and illustrated Polakowski et al. (Citation2018, Citation2019) and Polakowski & Kasprzykowski (Citation2016). The Biebrza Basin (area = ca 260 000 ha) is formed by the Biebrza valley (100 km long and up to 20 km wide) and part of the Narew valley (65 km long and up to 15 km wide). It is one of the best-preserved wetlands in Europe (Grygoruk et al. Citation2013) and for the most part is protected in the form of the Biebrza National Park (e.g. Grygoruk et al. Citation2013), two Natura 2000 SPAs (“Ostoja Biebrzańska” (PLB200006) and “Bagno Wizna” (PLB200005)) and an International Bird Area. Both rivers extensively inundate the meadows and pastures in their valleys, which cover 52.0% of the basin’s area, while the arable land lies mostly around its edge, occupying ca 25.2% of the study area (Polakowski & Kasprzykowski Citation2016). The Biebrza Basin is one of the most important staging sites for White-fronted Geese in central Europe, hosting up to 10% of the overall numbers wintering in Europe (Polakowski et al. Citation2011). Total numbers here have been estimated at more than 100 000, and single flocks can consists of 10 000–40 000 birds, almost exclusively White-fronted Geese (Polakowski et al. Citation2011).
Data collection
A total of 271 White-fronted Geese (181 adults and 90 immatures) in 66 flocks were filmed using the digiscoping method (see Leary Citation2004): 113 birds in 2016, 26 in 2017, 53 in 2018 and 79 in 2019. 173 of the geese were filmed in meadows or pastures (hereafter grasslands) and 98 on arable land. In the fields, situated on somewhat higher ground than the areas flooded by the rivers, winter cereals were usually grown, and there was no extraneous vegetation. The grasslands, situated at the same levels as the rivers and frequently flooded, consisted of all types of meadows and pastures, most of which were mown but some grazed. Flocks consisted of a mean number of 1015,0 birds. Filming took place at an average distance of 150–250 m using a Nikon Coolpix A10 camera combined with a Swarovski ATS 80HD scope fitted with a 25–50x zoom lens. All the films were 180 seconds long and all were recorded by the same person (MP). To prevent any selectivity bias, the birds were recorded at random in different habitats, in different places in the flock, at different periods and different sites in the Biebrza Basin. This material was digitally analysed by one person (MB). To calculate the time budget, all the data were entered into an Excel spreadsheet. Four types of activity were analysed: foraging, vigilance, resting, and others (i.e. all other activities). The geese were regarded as foraging when they were searching for food on the ground with their heads down. Raising the head to inspect the surroundings or keeping the head raised to look around was classified as vigilance. Also included in this category were responses to acute danger: jumping to one side, rapidly walking away from the danger area or even taking off from it. Birds sleeping, standing up or lying down were considered to be resting.
Statistical procedure
All the data were analysed in the R environment (R Core Team Citation2019). The differences in the mean time of activities between two habitat types were evaluated using Student’s t-test following logarithmic transformation of the data in order to approximate a normal distribution. The influence of habitat types, bird age, position in the flock and flock size on time spent on foraging, vigilance and resting was analysed using generalised linear mixed models (GLMM) with a Poisson distribution and logit link function (McCullagh & Nelder Citation1989). The models were constructed using the glmer function in the lme4 package for R (Bates et al. Citation2015). All three models were constructed in the same way. Time spent on a particular category of activity was treated as a dependent variable. Fixed effects, i.e. habitat type, age and position, were used as categorical factors, and flock size was treated as a numerical factor (after logarithmic transformation). Random effects included year nested within flock identity. The values reported are the means and 95% confidence limits. Only those results with a probability of α ≤ 0.05 were assumed to be statistically significant.
Results
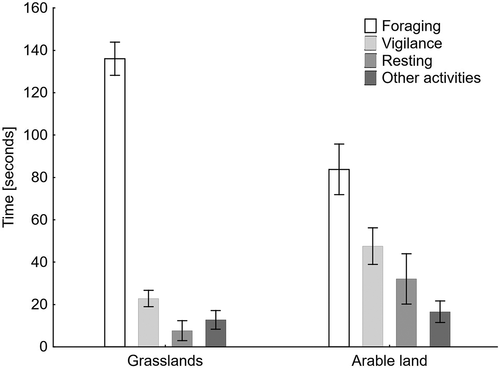
White-fronted Geese spent a lot of their time foraging in both habitat types, but in grasslands this activity took up 76.1% of the total time budget whereas on arable land it was 46.6%. The differences in the mean foraging times between the habitat types were significant (t = 5.36, df = 269, p < 0.001, ). On arable land, vigilance was the next most common form of activity (26.4% of time) compared with resting (17.8%) and other activities (9.2%). In contrast, the time spent on vigilance in the grasslands took up 12.7% of the time budget, whereas other activities there took up 7.1% and resting 4.1% of the time. The differences in the mean time spent on these three activity categories between habitat types were also significant (t = 4.34, df = 269, p < 0.001 for vigilance, t = 5.32, df = 269, p < 0.001 for resting and t = 2.09, df = 269, p = 0.037 for other activities).
Figure 1. Time budget of White-fronted Geese in two habitat types in the Biebrza Basin. Means (columns) and 95% confidence limits (whiskers) are shown
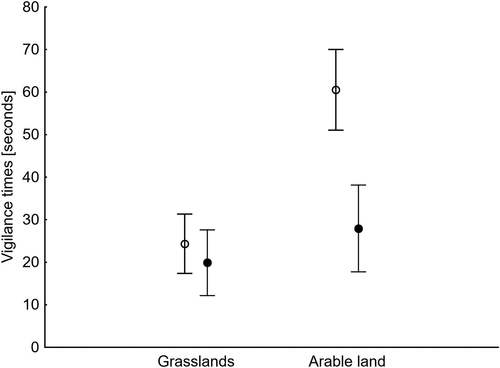
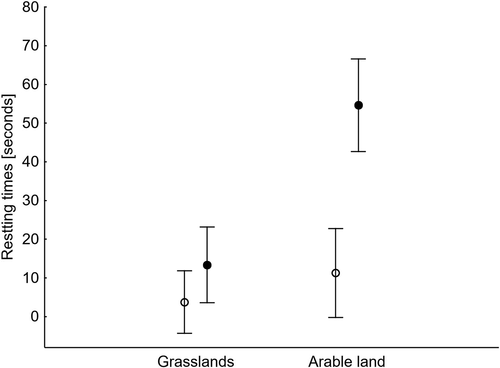
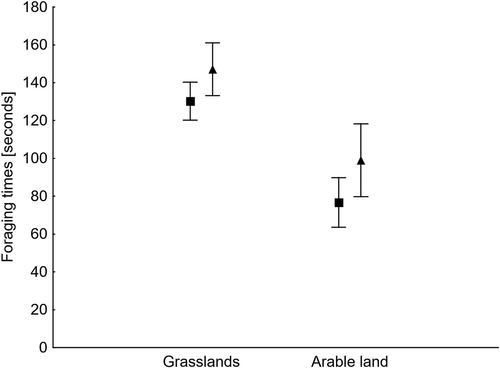
The first GLM analysis showed that the foraging time depended on the habitat type and the age of birds, but not on their position in the flock or flock size (). Young birds spent more time foraging than adults, but the differences in foraging time between the age categories in grasslands and arable land were not significant (t = 1.67, df = 171, p = 0.097 and t = 1.74, df = 96, p = 0.084; ). The second model showed that the vigilance time depended on the habitat and a bird’s position in a flock. Geese were less vigilant in grasslands than in arable fields, and they were statistically more vigilant at the edge of the flock than in its centre on arable land (t = 3.68, df = 96, p < 0.001, ). However, we found no differences in time spent on vigilance in grasslands between these two positions (t = 1.24, df = 171, p = 0.214). The other two predictors, i.e. bird age and flock size, did not affect the vigilance time. The third model showed that resting time was affected by habitat type, bird position and flock size, but not by bird age (). On arable land, geese rested significantly more often in the centre than at the edge of the flock (t = 3.36, df = 96, p = 0.001, ). At the same time, no differences in resting time between the two positions were found in grasslands (t = 1.69, df = 171, p = 0.093): resting time was negatively affected by flock size.
Table I. Results of the three mixed models analysed the influence of habitat types, bird age, position in the flock and flock size on time spent on foraging, vigilance and resting
Figure 2. Means (dots) and 95% confidence limits (whiskers) of the foraging times of adult (squares) and young (triangles) White-fronted Geese in two habitat types
Discussion
Our results indicate that the type of habitat determined the activity of White-fronted Geese at the stopover sites – foraging took up most of their time in both habitat types. This tallies with other studies showing that during stopovers, on their long way between western European wintering grounds and their breeding areas in Siberia (Madsen et al. Citation1999), Arctic geese spend most of the time foraging (Kear Citation2005). This is because they need to acquire energy to be deposited in the fat reserves which they then use for the next stages of the flight (e.g. Berthold Citation1993). Time spent foraging is especially important for adult female geese, which accumulate fat reserves on the way to the Arctic, where they incubate their eggs without leaving the nest (Drent et al. Citation2007; Hübner et al. Citation2010; Polakowski et al. Citation2019). Our analyses have also shown that younger individuals generally spend more time foraging than adults. On the one hand, this could be a way of increasing feeding efficiency by less experienced young birds, which seem to need more time to achieve the same effects as adults (see Polakowski et al. Citation2019). On the other hand, adults are under pressure of the approaching breeding season and are more time-limited on stopovers, so it seems that they need to feed more effectively during a shorter foraging time (Polakowski et al. Citation2019).
Suitable foraging grounds are apparently crucial for energy intake, both for further travel and, in some cases, for the upcoming breeding season. On the one hand, grasslands appear to provide more natural food resources and appropriate foraging conditions (e.g. Vickery & Gill Citation1999; Van Eerden et al. Citation2005; Polakowski & Kasprzykowski Citation2016), and some studies have shown that even short feeding bouts in such a habitat can ensure a sufficient intake rate (see Kristiansen & Jarrett Citation2002). On the other hand, fields with winter cereals are becoming increasingly important and attractive in many feeding areas situated along migration routes (Prop et al. Citation1998; Nilsson & Persson Citation2000; Fox et al. Citation2005). We found that geese spent less time feeding in fields. One reason for this could be that the crops in the fields of the Biebrza Basin are often of poorer quality, whereas maize and other nutrient-rich crops are not as abundant on the goose stopovers in western Europe (Rosin et al. Citation2012; Polakowski & Kasprzykowski Citation2016; own unpublished data). A more likely explanation, however, is that monoculture cereal fields can offer the birds higher intake rates (Fox et al. Citation2005; Fox & Abraham Citation2017): there, geese can feed faster and more effectively, without having to be selective, and in the expectation that each cereal leaf will be of high quality (Therkildsen & Madsen Citation2000). At the same time, grasslands are without doubt quite nutritious, but less so than cereals. In this latter habitat, geese need to spend more time selecting between grasses for the best leaves because the swards are of mixed species composition. The upshot is that geese can maintain higher intake rates in crop fields than in meadows and pastures, and foraging in the former may take up less time than in the latter. Because this applies to both age classes of geese, it will also explain the lack of differences in feeding times in crop fields and grasslands between them.
The next most common form of activity that we found during stopovers in both habitat types was vigilance. In fields, however, geese spent more time looking out for potential threats than on the grasslands. This is because most of the arable land in the Biebrza Basin is situated at the edge of the valleys and is exposed to frequent disturbance by farmers, photographers, bird-watchers, other people, dogs and foxes (own unpubl. data). Such disturbances may elicit higher levels of vigilance in the birds because this is known to be an important factor affecting their functioning (e.g. Jankowiak et al. Citation2015). We also found that vigilance was associated with a bird’s position in the flock, an observation corroborated by other studies (Inglis & Lazarus Citation1981; Black et al. Citation1992). The edge of a flock is not as safe place as its centre, so geese in that position spend more time being vigilant. This relationship is very obvious in crop fields, but not at all in grasslands. This could be because most of the grasslands in the Biebrza Basin are situated within protected and less accessible areas, where geese are not exposed to such intense human pressure.
Resting took up the least amount of time in the time budget of White-fronted Geese, especially in the grasslands. In the Biebrza Basin, these were their preferred feeding habitat since they were usually partly flooded (Polakowski & Kasprzykowski Citation2016). The birds thus spent much more time feeding than on other activities, including resting. Paradoxically, even though the crop fields seem to be a less secure habitat, geese spent much more time resting there than in grasslands. This was shown by the differences in the resting times in the various positions that the birds took up in the flock, noted only in the crop fields. In this habitat, geese at the centre of a flock spent more time resting, obviously for reasons of safety. Flock size negatively affected resting time: potential danger would be detected much more easily in a larger aggregation. In addition, the geese could have treated the arable fields in the Biebrza Basin as an alternative site used mainly for purposes other than foraging, e.g. resting, because at times no crops were being grown in them or they were lying fallow (our own data), as opposed to fields of cereals, rapeseed or beetroot, in which they often fed in other regions (e.g., Cramp & Simmons Citation1977; Rosin et al. Citation2012; Fox et al. Citation2017). However, the opposite explanation is also possible: geese foraged for a shorter time in the fields around the edge of the Biebrza Basin because they did so with greater efficiency, as described above.
In conclusion, we found that there were differences in the time budget of White-fronted Geese between grasslands and arable fields. Feeding and the choice of foraging habitat seem to be the most important activities, and birds spent much more time on these, regardless of age and position in the flock, than on any others. However, the time spent on vigilance and resting in arable fields did depend on a particular goose’s position in the flock. These findings, which demonstrate that White-fronted Geese are flexible in their use of food resources, could be useful in agricultural management planning in the light of increasing conflicts with farmers. With increasing numbers of geese, it seems impossible to manage the arable lands to obtain good crop yields and protect the geese at the same time. In case of Biebrza Basin, one solution might be replacing the winter cereals with spring cereals, which are sown when the most of geese departed. However, if this is not economically viable, it could be worth considering the introduction of subsidies for farmers who suffer damage to their crops, although such a system has yet to be implemented in Poland.
Acknowledgements
We are grateful to Anthony David Fox, Łukasz Jankowiak, Barbara Kapuśniak and Arkadiusz Kiszka for their help during the writing of the first draft of this manuscript. We also thank Joanna Przybylska, Peter Senn and Krzysztof Stępniewski for the language corrections.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Abraham KF, Jefferies RL, Alisauskas RT. 2005. The dynamics of landscape change and snow geese in mid-continent North America. Global Change Biology 11:841–855. DOI:10.1111/j.1365-2486.2005.00943.x.
- Atkinson PW, Fuller RJ, Vickery JA, Conway GJ, Tallowin JR, Smith REN, Haysom KA, Ings TC, Asteraki EJ, Brown VK. 2005. Influence of agricultural management, sward structure and food resources on grassland field use by birds in lowland England. Journal of Applied Ecology 42:932–942. DOI:10.1111/j.1365-2664.2005.01070.x.
- Bates D, Mächler M, Bolker B, Walker S. 2015. Fitting Linear Mixed-Effects Models Using lme4. Journal of Statistical Software 67:1–48. DOI:10.18637/jss.v067.i01.
- Berthold P. 1993. Bird migration. A general survey. Oxford: Oxford University Press. pp. 239.
- Bischof R, Loe LE, Meisingset EL, Zimmermann B, Moorter BV, Mysterud A. 2012. A migratory northern ungulate in the pursuit of spring: jumping or surfing the green wave? The American Naturalist 180:407–424. DOI:10.1086/667590
- Black JM, Carbone C, Wells RL, Owen M. 1992. Foraging dynamics in goose flocks: The cost of living on the edge. Animal Behaviour 44(1):41–50. DOI:10.1016/S0003-3472(05)80752-3.
- Cramp S, Simmons KEL. (eds.). 1977. The birds of the Western Palearctic. I. Oxford University Press.
- Drent RH, Eichhorn G, Flagstad A, Van Der Graaf AJ, Litvin KE, Stahl J. 2007. Migratory connectivity in Arctic geese: Spring stopovers are the weak links in meeting targets for breeding. Journal of Ornithology 148:501–514. DOI:10.1007/s10336-007-0223-4.
- Eythórsson E, Tombre IM, Madsen J. 2017. Goose management schemes to resolve conflicts with agriculture: Theory, practice and effects. Ambio 46(2):231–240. DOI:10.1007/s13280-016-0884-4.
- Fox AD, Abraham KF. 2017. Why geese benefit from the transition from natural vegetation to agriculture. Ambio 46(2):188–197. DOI:10.1007/s13280-016-0879-1.
- Fox AD, Ebbinge BS, Mitchell C, Heinicke T, Aarvak T, Colhoun K, Clausen P, Dereliev S, Faragó S, Koffijberg K, Kruckenberg H, Loonen MJJE, Madsen J, Mooij J, Musil P, Nilsson L, Pihl S, Van Der Jeugd H. 2010. Current estimates of goose population sizes in western Europe, a gap analysis and an assessment of trends. Ornis Svecica 20(3–4):115–127. DOI:10.34080/os.v20.19922.
- Fox AD, Elmberg J, Tombre IM, Hessel R. 2017. Agriculture and herbivorous waterfowl: A review of the scientific basis for improved management. Biological Reviews 92:854–877. DOI:10.1111/brv.12258.
- Fox AD, Madsen J, Boyd H, Kuijken E, Norriss DW, Tombre IM, Stroud DA. 2005. Effects of agricultural change on abundance, fitness components and distribution of two arctic-nesting goose populations. Global Change Biology 11(6):881–893. DOI:10.1111/j.1365-2486.2005.00941.x.
- Gill JA. 1996. Habitat choice in pink-footed geese: Quantifying the constraints determining winter site use. Journal of Applied Ecology 33(4):884–892. DOI:10.2307/2404959.
- Grishchenko M, Prins HH, Ydenberg RC, Schaepman ME, de Boer WF, de Knegt HJ. 2019. Land use change and the migration geography of Greater White‐fronted geese in European Russia. Ecosphere 10(8):e02754. DOI:10.1002/ecs2.2754.
- Grygoruk M, Mirosław-Świątek D, Chrzanowska W, Ignar S. 2013. How much for water? Economic assessment and mapping of floodplain water storage as a catchment-scale ecosystem service of wetlands. Water 5(4):1760–1779. DOI:10.3390/w5041760.
- Hake M, Månsson J, Wiberg A. 2010. A working model for preventing crop damage caused by increasing goose populations in Sweden. Ornis Svecica 20:225–233.
- Hübner CE, Tombre IM, Griffin LR, Loonen MJJE, Shimmings P, Jónsdóttir IS. 2010. The connectivity of spring stopover sites for geese heading to arctic breeding grounds. Ardea 98: 145–154. DOI:10.5253/078.098.0203.
- Inglis IR, Lazarus J. 1981. Vigilance and flock size in brent geese: the edge effect. Zeitschrift für Tierpsychologie 57(3‐4):193–200. DOI:10.1111/j.1439-0310.1981.tb01921.x.
- Jankowiak Ł, Skórka P, Ławicki Ł, Wylegała P, Polakowski M, Wuczyński A, Tryjanowski P. 2015. Patterns of occurrence and abundance of roosting geese: the role of spatial scale for site selection and consequences for conservation. Ecological Research 30(5):833–842. DOI:10.1007/s11284-015-1282-2.
- Jensen RA, Wisz MS, Madsen J. 2008. Prioritizing refuge sites for migratory geese to alleviate conflicts with agriculture. Biological Conservation 141:1806–1818.
- Kear J. (ed.) 2005. Ducks, geese and swans. Oxford: Oxford University Press.
- Kristiansen JN, Jarrett NS. 2002. Inter-specific competition between Greenland White-fronted Geese Anser albifrons flavirostris and Canada Geese Branta canadensis interior moulting in West Greenland: mechanism an d consequences. Ardea 90(1):1–13.
- Leary PR. 2004. Digiscope applications for shorebird studies. Bulletin-Wader Study Group 104:34–38.
- Leito A, Truu J, Õunsaar M, Sepp K, Kaasik A, Ojaste I, Mägi E. 2008. The impact of agriculture on autumn staging Eurasian Cranes (Grus grus) in Estonia. Agricultural and Food Science 17:53–62. DOI:10.2137/145960608784182281.
- MacMillan D, Hanley N, Daw M. 2004. Costs and benefits of wild goose conservation in Scotland. Biological Conservation 119(4):475–485. DOI:10.1016/j.biocon.2004.01.008.
- Madsen J, Bjerrum M, Tombre IM. 2014. Regional Management of Farmland Feeding, Geese Using an Ecological Prioritization Tool. Ambio 43:801–809. DOI:10.1007/s13280-014-0515-x.
- Madsen J, Cracknell G, Fox AD. (eds.). 1999. Goose populations of the western Palearctic. Wetlands International Pub. No. 48., Denmark: National Environmental Research Institute.
- McCullagh, P, Nelder, JA. 1989. Generalized linear models. London, United Kingdom: Chapman and Hall.
- Nilsson L, Persson H. 2000. Changes in field choice among staging and wintering geese in southwestern Scania, South Sweden. Ornis Svecica 10:161–169.
- Owen M. 1990. The damage‐conservation interface illustrated by geese. Ibis 132(2):238–252. DOI:10.1111/j.1474-919X.1990.tb01042.x.
- Parrish, JD. 2000. Behavioral, energetic, and conservation implications of foraging plasticity during migration. Studies in Avian Biology 20:53–70.
- Polakowski M, Broniszewska M, Jankowiak Ł, Ławicki Ł, Siuchno M. 2011. Numbers and dynamics of spring migration of geese in the Biebrza Basin. Ornis Polonica 52:159–180. [in Polish with English summary]
- Polakowski M, Jankowiak Ł, Cichocka A, Stępniewski K, Stępniewska K, Kruckenberg H, Ebbinge BS, Broniszewska M, Cichocka A. 2019. Timing and duration of spring staging of the Eurasian Greater White-fronted Goose (Anser albifrons albifrons) in relation to sex, age and progress of migration season. Ornis Fennica 96(1):24–32.
- Polakowski M, Kasprzykowski Z. 2016. Differences in the use of foraging grounds by Greylag Goose Anser anser and White-fronted Goose Anser albifrons at a spring stopover site. Avian Biology Research 9(4):265–272. DOI:10.3184/175815516X14739467542441.
- Polakowski M, Kasprzykowski Z, Golawski A. 2018. Influence of temperature on the timing of spring arrival and duration of migration in Arctic goose species at a central European stopover site.Ornis Fennica95(1):32–41.
- Prop J, Black JM, Shimmings P, Owen M. 1998. The spring range of barnacle geese Branta leucopsis in relation to changes in land management and climate. Biological Conservation 86(3):339–346. DOI:10.1016/S0006-3207(98)00029-9.
- R, Core Team. 2019. R: A Language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- Radtke TM, Dieter CD. 2011. Canada goose crop damage abatement in South Dakota. Human–Wildlife Interactions 5:315–320. DOI:10.26077/jwg9-4179.
- Rees EC, Cao L, Clausen P, Coleman, JT, Cornely, J, Einarsson, O, Ely CR, Kingsford RT, Ma M, Mitchell CD, Nagy S, Shimada T, Snyder J, Solovyeva DV, Tijsen W, Vilina YA, Włodarczyk R, Brides K. 2019. Conservation status of the world’s swan populations, Cygnus sp. and Coscoroba sp.: a review of current trends and gaps in knowledge. Wildfowl 5:35–72.
- Rosin ZM, Skórka P, Wylegała P, Krąkowski B, Tobolka M, Myczko Ł, Sparks TH, Tryjanowski P. 2012. Landscape structure, human disturbance and crop management affect foraging ground selection by migrating geese. Journal of Ornithology 153(3):747–759. DOI:10.1007/s10336-011-0791-1.
- Therkildsen OR, Madsen J. 2000. Energetics of feeding on winter wheat versus pasture grasses: A window of opportunity for winter range expansion in the pink-footed goose Anser brachyrhynchus. Wildlife Biology 6(1):65–74. DOI:10.2981/wlb.2000.002.
- Van Eerden, MR. 1990. The solution of goose damage in the Netherlands, with special reference to compensation schemes. Ibis 132:253–261. DOI:10.1111/j.1474-919X.1990.tb01043.x.
- Van Eerden MR, Drent RH, Stahl J, Bakke JP. 2005. Connecting seas: Western Palaearctic continental flyway for water birds in the perspective of changing land use and climate. Global Change Biology 11(6):894–908. DOI:10.1111/j.1365-2486.2005.00940.x.
- Vickery J, Gill J. 1999. Managing grassland for wild geese in Britain: A review. Biological Conservation 89(1):93–106. DOI:10.1016/S0006-3207(98)00134-7.
- Wuczyński A, Smyk B, Kołodziejczyk P, Lenkiewicz W, Orłowski G, Pola A. 2012. Long-term changes in numbers of geese stopping over and wintering in south-western Poland. Central European Journal of Biology 7(3):495–506. DOI:10.2478/s11535-012-0031-6.