Abstract
New data about the Mediterranean pycnogonids are given. For this contribution, we analysed 315 samples that until now had remained unidentified, collected in 1979–1980 in the Tyrrhenian Sea close to Torrevaldaliga, Italy. We found six species: Ammothella appendiculata, Anoplodactylus californicus, Endeis biseriata, Endeis spinosa, Nymphon gracile and Tanystylum conirostre. Two of them are the subject of taxonomical and faunal remarks: morphological features of Anoplodactylus californicus specimens are reported and discussed, and the first record of Endeis biseriata for the Mediterranean Sea is reported.
Introduction
Pycnogonids are exclusive marine Arthropoda: Chelicerata whose morphology earned them the common name of “sea spiders”. In the basin of Mediterranean Sea about 56 species are known to date (Lehmann et al. Citation2014), 44 of which have been recorded in the Italian seas (Bartolino & Chimenz Citation2010). Very few studies about Pycnogonida have been performed in the Tyrrhenian Sea. Among these, Chimenz et al. (Citation1979) studied the fauna of Civitavecchia harbour, finding 15 species, and used those data to outline some faunal and zoogeographical considerations (Chimenz Gusso & Lattanzi Citation2003). In addition, Arnaud studied samples from the same area in 1978 and confirmed the presence of some species cited by Chimenz (Arnaud Citation1987). In this paper we examine an old and ignored collection from the nearby locality of Torrevaldaliga with the aim to enrich our knowledge of the pycnogonid fauna in the North Tyrrhenian Sea, updating the species list for this area.
Materials and methods
Study area
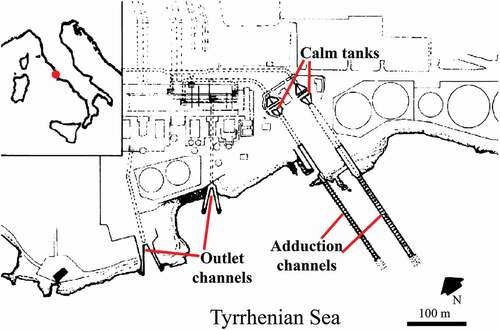
The samples analysed come from the Torrevaldaliga Power Station (one group of 200 MW and three of 320 MW). This is situated in the homonymous town along the coast (near Civitavecchia, 80 km North to Rome) in the Tyrrhenian Sea; a map of the area is provided in . Here, four main conduits pump the water from the sea, and two outfall zones discharge the water into the sea 10°C warmer than that entering the intakes (Relini et al. Citation1980). Between 1978 and 1979 a study of the fouling communities in different areas of this station was carried out (Relini & Bianchi Citation1979a; Morri Citation1980; Relini et al. Citation1980). In particular, it appears (from the available data) that pycnogonids were collected in adduction channels, with low brightness and considerable water current, and in calm tanks, characterised by exposure to light and low water speed. Only four samples were collected in the outlet channel, characterised by high water turbulence, the presence of light and warm water (Relini & Bianchi Citation1979b; Relini et al. Citation1980).
Figure 1. Localisation (red spot on the top left map) and plan of Torrevaldaliga Power Station (from Relini et al. Citation1980, mod.)
Collection and identification of specimens
Asbestos cement plates (200 × 300 × 3 mm) immersed in the different areas of the power station at a depth of 2 m were used to collect fouling. These panels were replaced at varying frequencies (every month, every 3 months and then after 6, 9 and 12 months) (Relini et al. Citation1980). Fouled panels were fixed in 10% buffered formalin in sea water. The fixing means was then inspected under a stereomicroscope and specimens were collected and preserved in pure ethanol.
The original study was focused on the presence and biodiversity of major fouling fauna, to determine dominant communities and their development (Relini et al. Citation1980). Pycnogonids were considered secondary organisms (Relini & Bianchi Citation1979a), and this is probably the reason why some samples (for a total of 40 individuals), although from the same collection, lack environmental data.
Identification of specimens was made under a stereomicroscope, based mainly on the monograph of the Iberian fauna by Munilla and Soler-Membrives (Citation2014); the analysis of some details and diagnostic measurements were performed by means of an interference contrast microscope (Leica DM LB2), a Leica DFC 295 camera and Leica Application Suite (v. 3.8). Three specimens of Anoplodactylus californicus (two males and one female) and three specimens (two males and one female) of Endeis biseriata were dehydrated in a graded ethanol series and then were transferred onto stubs, sputter coated with gold and observed under a scanning electron microscope (SEM: Vega3_TESCAN Microscope type LMU).
All the studied material is preserved in the museum collection (Zoology section) of the Department of Earth, Environment and Life Sciences (DISTAV) of Genoa University (MU-DISTAV-UGE-Zool–Pycno: 958–1245; 1496–1510; 1514–1515; 1517; 1565–1573). The nomenclature and the systematic order proposed by World Register of Marine Species (WoRMS) (WoRMS Editorial Board, Citation2020) is adopted.
Results
List of pycnogonid species
A total of 315 pycnogonids were collected in 1979–1980: only three specimens were identified at the genus level only (one juvenile of Achelia sp. and two juveniles of Ammothella sp.); the others were classified to the species level and assigned to the following six species: Ammothella appendiculata (Dohrn, Citation1881), Anoplodactylus californicus Hall, Citation1912, Endeis biseriata Stock, Citation1968, Endeis spinosa (Montagu, 1808), Nymphon gracile Leach, 1814 and Tanystylum conirostre (Dohrn, Citation1881). Anoplodactylus californicus and Tanystylum conirostre were the dominant species, with 178 and 101 individuals, respectively. Integrating our data with those available from Bartolino and Chimenz (Citation2010) and Lehmann et al. (Citation2014), an updated checklist of Central Tyrrhenian pycnogonids is given (), considering the biogeographical area from the coastline of Sardinia and from Piombino to the entire Gulf of Gaeta, belonging to the northern section of the central-western area of the Mediterranean Sea (Area 2, based on Bianchi Citation2004). In addition, when possible, some information about historical records of species in the entire Tyrrhenian area are reported.
Table I. Checklist of pycnogonid species in the Tyrrhenian Sea, with some indications of their distribution. Legend: CT, Central Tyrrhenian Sea; GN, Gulf of Naples; NT, North Tyrrhenian Sea; SAR, Sardinia; ST, South Tyrrhenian Sea. Bibliographic references to the distribution are reported; geographical indications for which no bibliographic reference is given are reported by Bartolino and Chimenz (Citation2010) without further references in the literature
Taxonomic accounts
Class Pycnogonida Latreille, 1810
Order Pantopoda Gerstäcker, 1863
Family Ammotheidae Dohrn, Citation1881
Genus Ammothella Verill, 1900
Ammothella appendiculata (Dohrn, Citation1881)
Syn: Ammothea appendiculata Dohrn, Citation1881
Material examined
2 ovigerous ♂♂, 1♀, August 1979, calm tank; 1♂, October 1980, adduction channel.
Remarks
The only female specimen collected displays small tubercles on each lateral process; because of these details it can be confused with A. longioculata (Faraggiana, 1940) or A. longipes (Hodge, 1864), but the abdomen’s morphology and the complete segmentation of the body allowed us to identify this individual as A. appendiculata (Munilla & Soler-Membrives Citation2014). Although in this collection A. appendiculata is represented by only four specimens, it is well known that this species is common in fouling communities, especially in the Mediterranean area (Arnaud & Bamber Citation1987).
Distribution
Species distributed worldwide but absent in polar environment (Munilla & Soler-Membrives Citation2014).
Depth range
Infralittoral species, registered at depths between 0 and 65 m (Munilla & Soler-Membrives Citation2014).
Family Endeidae Norman, 1908
Genus Endeis Philippi, 1843
Endeis biseriata Stock, Citation1968()
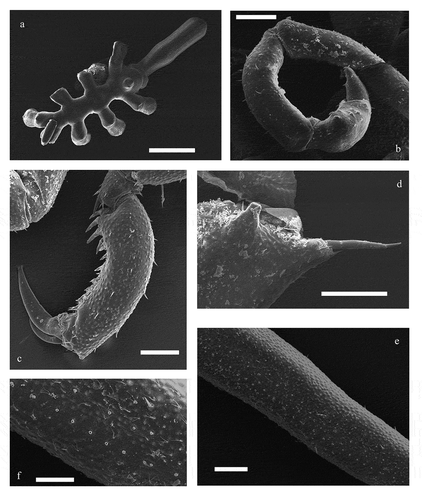
Figure 2. Endeis biseriata Stock, Citation1968 (scanning electron microscope images). (a) dorsal view of an ovigerous male; (b) detail of the oviger where it is visible a bulge on the 6th article; (c) detail of propodus and claws; (d) detail of the femur’s distal spur with a long seta (the insertion of the lateral one is also visible); (e) detail of femur on which two lines of cement gland pores are visible; (f) detail of cement gland pores. Scale bars: a = 500 μm, b–e = 200 μm, f = 100 μm
Material examined
1 ovigerous ♂, April 1979; 2 ovigerous ♂♂, 1♂, 2♀♀, August 1979, calm tank; 1♀, 2 juvenile ♂♂, November 1979; 1♂, September–November 1980; 1♂, 1♀, August 1980; 1 ovigerous ♂, 3♂♂, 4♀♀, October 1980, adduction channel.
Diagnosis
Trunk slender, with complete segmentation; lateral processes separated by a distance equal to or greater than their own diameters, with a variable number of small dorso-distal tubercles. Four eyes evident at the base of a conical ocular tubercle. Proboscis a little narrower at the base (with collar), with an enlargement at about 1/3rd of its length and parallel to the tip. Abdomen erect, much longer than the 4th lateral processes. The 7–segmented ovigers show a bulge on the 6th article with recurved spines and no denticulate ones. Very long legs, femora with a long distal spur, two smaller lateral spurs and a number of variable cement gland pores, from 32 to 48. Propodus is curved, with four heel spines followed by fewer than 10 smaller spines; auxiliary claws are at least 1/3rd the size of the main claw.
Remarks
The genus Endeis is very homogeneous, and single species are recognisable using characters that usually do not have a main role in the diagnosis of most other pycnogonid genera (Fry & Hedgpeth Citation1969).
Endeis biseriata shows a massive body ()) compared to other species, but the main diagnostic character for its identification consists in the different number of cement gland pores and their arrangement on the male femora (Stock Citation1968). With the use of SEM, we verified the presence of two sinuous lines of pores on male femora, variable in number from 40 to 48 ()). This character combined with all morphological features cited previously (some reported in ) made it possible to distinguish this species from others having two rows of cement glands. Endeis flaccida Calman, Citation1923 shows a large number of pores on the femora (about 57; Calman Citation1923), and Endeis nodosa Hilton, Citation1942 can be identified from its ventral femoral projection (Stock Citation1968; Müller Citation1992). Endeis holthuisi Stock, 1961 differs from the others by its very robust legs and straight propoda (Stock Citation1968). Finally, there are very slight differences between E. straughani (Clark, Citation1970) and E. biseriata (Staples Citation1982) – for example, the length of the auxiliary claws (half the length of the main claw in E. straughani and shorter in E. biseriata) and the distance between lateral processes (wider in E. biseriata) (Clark Citation1970; Staples Citation1982). In spite of this, the main diagnostic feature to distinguish these species is the number and configuration of cement gland pores: E. straughami possesses 25–30 cement gland openings in a main row and eight openings in a second irregular row (Staples Citation1982), while in E. biseriata the two rows are comparable.
Chimenz et al. (Citation1979) and Arnaud (Citation1987) did not find Endeis biseriata in their studies of pycnodonids from the nearby locality of Civitavecchia. This species was not even mentioned by Arnaud in his list of Torrevaldaliga’s fauna (see Final remarks). Despite this, based on the presence of ovigerous males and juveniles, the population of Endeis biseriata can be considered steady and reproductive in the studied site. For this reason, and due to the long amount of time that has passed since the collection of the specimens we studied, we can suppose the current presence of this species also in other areas of the Tyrrhenian Sea.
This is the first record of the species for the Mediterranean Sea. Although it has not been reported elsewhere, we cannot exclude the invasiveness of this species. The presence of E. biseriata in the Indo-Pacific waters and the Red Sea (Lucena et al. Citation2018) and its absence from the Eastern Atlantic coasts (Lucena et al. Citation2018) could suggest a Lessepsian migration, but an anthropochorous dispersal (fouling) seems more probable.
Distribution
Species widespread in Pacific, Indian and West Atlantic oceans; Arabian and Red seas (Stock Citation1970; Lucena et al. Citation2018). New record for the Mediterranean Sea.
Depth range
Endeis biseriata is a shallow-water species, found in a bathymetric range from 0 to 46 m (Stock Citation1992; Müller Citation1993; Lucena et al. Citation2018).
Endeis spinosa (Montagu, 1808)
Syn: Phallangium spinosum Montagu, 1808
Material examined
5♂♂, August 1979, calm tanks; 2♂♂, September–November 1980; 1 juvenile ♂, October 1980.
Remarks
In the Mediterranean Sea only two species of the genus Endeis Philippi, 1843 (Bartolino & Chimenz Citation2010) were recorded to date: E. charybdaea (Dohrn, Citation1881) and E. spinosa. The first is recognisable by its larger body than E. spinosa and by the higher number of cement gland pores (Stock Citation1968). There are some other characters which are easier to see but not as reliable, because of their frequent variability: in E. charybdaea the auxiliary claws are often longer than half the length of the main claw (Krapp Citation1975; King Citation1986; Munilla & Soler-Membrives Citation2014) and the mouth is often surrounded by numerous spines, more than in E. spinosa (King Citation1986). Another diagnostic feature is the distance between the lateral processes: in E. charybdaea they are separated by 3 times their diameter, while in E. spinosa they are separated by only 2 times their diameter (Munilla & Soler-Membrives Citation2014).
Endeis spinosa and E. biseriata differ in the general aspect of the body (more robust in E. biseriata), in the proboscis morphology (in E. spinosa the distal part is narrower than the proximal one) and in the length of auxiliary claws (about 1/3rd the length of the main claw in E. biseriata vs. half the length of the main claw in E. spinosa) (Munilla & Soler-Membrives Citation2014). Another character useful to distinguish these species is the number of cement gland pores: E. spinosa possesses up to 20 pores in a single row (Stock Citation1968).
Distribution
Species widespread in the Atlantic Ocean and the Mediterranean Sea (Munilla & Soler-Membrives Citation2014).
Depth range
0–537 m, on Posidonia and algae (Müller Citation1993; Munilla & Soler-Membrives Citation2014).
Family Nymphonidae Wilson, 1878
Genus Nymphon Fabricius, 1794
Nymphon gracile Leach, 1814
Syn: Nymphon gallicum Hoek, 1881
Material examined
1♂, 10 September 1979, 1 m depth.
Remarks
Three species belonging to the genus Nymphon Fabricius, 1794 are known from the Mediterranean waters (Bartolino & Chimenz Citation2010): N. puellula Krapp, 1974, N. parasiticum Merton, Citation1906 and N. gracile. The first two species lack auxiliary claws, although there are some doubts about N. parasiticum because the original description was based on a juvenile (Merton Citation1906); N. gracile, on the contrary, is characterised by the presence of auxiliary claws (Munilla & Soler-Membrives Citation2014).
Distribution
Species widespread in the North-East Atlantic and the Mediterranean Sea (Müller Citation1993; Munilla & Soler-Membrives Citation2014).
Depth range
0–52 m (Müller Citation1993; Munilla & Soler-Membrives Citation2014).
Family Phoxichilidiidae Sars, 1891
Genus Anoplodactylus Wilson, 1878
Anoplodactylus californicus Hall, Citation1912()
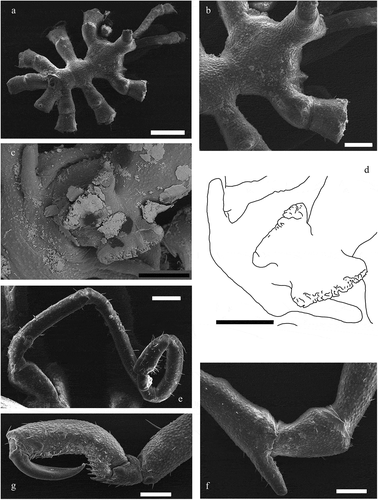
Figure 3. Anoplodactylus californicus Hall, Citation1912 (scanning electron microscope images). (a) dorsal view of a male; (b) detail of dorso-distal tubercles on lateral processes; (c) detail of the wing-shaped structures on the ventral side of female’s proboscis; (d) drawing made from (c), for clarification; (e) detail of the oviger (last two segments are particularly hairy); (f) detail of a ventral spur on coxa II; (g) detail of propodus and claws. Scale bars: a = 500 μm, b–g = 200 μm
Syn: Anoplodactylus carvalhoi Marcus, 1940; Anoplodactylus portus Calman, 1927; Anoplodactylus projectus Hilton, Citation1942
Material examined
2 ovigerous ♂♂, 1♂, April 1979; 3 ovigerous ♂♂, 23♂♂, 15♀♀, 13 juvenile ♂♂, 2 juvenile ♀♀, 12 juveniles, August 1979, calm tank; 1♂, 3 juvenile ♂♂, 3 juveniles, November 1979; 1 juvenile ♂, June–November 1980; 2 ovigerous ♂♂, 3♂♂, 3♀♀, 1 juvenile ♂, September–November 1980, adduction channel; 10 ovigerous ♂♂, 12♂♂, 23♀♀, 10 juvenile ♂♂, 27 juveniles, October 1980, adduction channel; 1 ovigerous ♂, 2♀♀, 5 juveniles, December 1980.
Diagnosis
Body slender with partially segmented trunk (suture between third and fourth segments almost never visible). Each lateral process with a dorso-distal tubercle; conical ocular tubercle; abdomen orthogonal to the trunk, erect and tall as the ocular tubercle. Cheliphore fingers without teeth. Ventral spurs on coxa II on legs III and IV, or II, III and IV in males and all legs in females. Females with wing-shaped processes on the ventral side of proboscis (), and rudimental ovigers. Oviger with the last two segments very hairy. Two small bumps on the side of the proboscis. Propodus heel with two strong spines followed by two parallel rows of 2 or 3 smaller spines; auxiliary claws present but reduced.
Remarks
Child (Citation1992a) pointed out that A. californicus is an easily recognisable species, even if the large number of synonyms (for example A. portus Calman, 1927) creates some difficulties in identification. This may be due to the variability of certain characters in different geographical populations (Lucena & Christoffersen Citation2018). Because of the plasticity of many morphological characters, the genus Anoplodactylus is characterised by some species complexes, such as the californicus-digitatus complex (Arango & Maxmen Citation2006).
Most papers do not mention the presence of tubercles on lateral processes (): these body structures are described without ornamentation, or with some setae or small buds (Hall Citation1912; Hilton Citation1942; Child Citation1987, Citation1992b; Arango & Maxmen Citation2006; Lucena Citation2013). Additionally, some individuals show a rounded ocular tubercle (Lucena Citation2013), not very evident eyes (Hall Citation1912; Hilton Citation1942), a completely segmented trunk (Arango & Maxmen Citation2006) and only one major spine on propodus heel (Child Citation1987).
The character of ventral spurs on coxae II ()) (present on each female leg, while in males on pairs of legs II, III and IV, or only on III and IV) it is not mentioned in the original description (Hall Citation1912). Some authors do not find differences between females and males (Lucena & Christoffersen Citation2018) and others point out this feature only on males (Hilton Citation1942; Child Citation1987, Citation1992a).
Examining the species of Anoplodactylus reported in Mediterranean Sea, the identification of females of A. californicus, because of the ventral wing-shaped proboscis protuberances (), is easy. This structure is typical of various species belonging to the californicus-digitatus complex (Arango & Maxmen Citation2006). On the contrary, the identification of the males is based on less evident characters of the ovigers and cement glands (Nakamura & Child Citation1991; Child Citation1992b).
Several specimens of A. californicus (under the synonym Anoplodactylus portus Calman, 1927) have previously been found in Civitavecchia harbour near Torrevaldaliga (Chimenz et al. Citation1979), and their presence in this area is confirmed by the samples collected around the power station (Arnaud Citation1987). Anoplodactylus californicus in the Italian seas was also recorded in northern Sicily (Palermo) (Chimenz Gusso Citation2000). It is considered an alien species in the Italian waters (Bartolino & Chimenz Citation2010), probably introduced by means of shipping fouling (Occhipinti-Ambrogi et al. Citation2010).
Distribution
Species with pantropical and pantemperate distribution, in the Atlantic and Pacific oceans and the Mediterranean Sea. It is found in a variety of substrates and often on algal vegetation in shallow waters (Child Citation1992a; Müller Citation1993; Lucena Citation2013; Lucena & Christoffersen Citation2018).
Depth range
0 to <100 m (Child Citation1992a; Müller Citation1993; Lucena Citation2013; Lucena & Christoffersen Citation2018).
Family Ammotheidae Dohrn Citation1881
Genus Tanystylum Miers, 1879
Tanystylum conirostre (Dohrn, Citation1881)
Syn: Clotenia conirostris Dohrn, Citation1881
Material examined
13♂♂, 56♀♀, 2 juveniles, 15 indet., August 1979, calm tank; 1♀, 10 September 1979, 1 m depth; 1 ovigerous ♂, November 1979; 1♀, 1 juvenile, June–November 1980; 2 ovigerous ♂♂, 2 juveniles, September–November 1980; 1 juvenile August 1980; 1 ovigerous ♂, 3♀♀, 1 juvenile, October 1980, adduction channel; 1♂, December 1980.
Remarks
Two species of Tanystylum are reported for the Mediterranean Sea: T. conirostre and T. orbiculare. The first has a sloped abdomen, a truncated conical proboscis and dorsal tubercles on the tibiae, bearing some setae. In T. orbiculare the abdomen is erect, the proboscis is cylindrical and there are no tubercles on the legs (Munilla & Soler-Membrives Citation2014).
Distribution
Species widespread in Mediterranean Sea, with amphi-Atlantic distribution (Müller Citation1993; Munilla & Soler-Membrives Citation2014).
Depth range
0–15 m (Munilla & Soler-Membrives Citation2014).
Final remarks
As already mentioned, pycnogonids were not the main target of the study on macrofouling due to their low abundance (Relini & Bianchi Citation1979a). Even in a recent technical report (Meloni & Moruzzi Citation2019), Pycnogonida amount to no more than 0.08% of the total arthropods of the macrobenthic community. Despite this, Relini and colleagues sent some of the specimens to the eminent specialist Françoise Arnaud, who compiled a list of the identified species attached to the technical report for the Torrevaldaliga Power Station managers, referring to some of these species in his work about Mediterranean pycnogonids (Arnaud Citation1987). He recorded five taxa: A. appendiculata, E. spinosa, N. gracile, T. conirostre and Anoplodactylus sp. The authors consider these results a partial validation of their analysis of this paper’s specimens, because of some recurrent species in this area (A. appendiculata, E. spinosa, N. gracile and T. conirostre).
Conclusions
Our contribution highlights the importance of the proper and accurate preservation of labelled samples obtained from faunal and/or ecological research. This is true also for those taxa not specifically studied in the original research, in order to always be able to obtain useful information from their study.
In particular, through the examination of unstudied samples from the Torrevaldaliga Power Station collected more than 30 years ago and preserved by the marine biologist Dr M. Mori, we were able to make a contribution to the knowledge of marine biodiversity of the Tyrrhenian Sea, bringing the number of known Mediterranean pycnogonid species to 37. Moreover, we outlined some remarks on the geographical distribution of Endeis biseriata, a new record for the Mediterranean Sea. The viable population of such an alien species, despite not yet being recorded from other localities in the Mediterranean Sea, should be carefully monitored in the future to prevent its further dispersion and any impact on native species and ecosystems.
Acknowledgements
We wish to thank Carlo Nike Bianchi, Mario Mori and Carla Morri for providing us with the samples and all the necessary information used to implement this work; special thanks to Laura Negretti for her valuable help in the acquisition of SEM images. Many thanks also to the anonymous reviewers whose suggestions helped us to improve our manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Arango CP, Maxmen A. 2006. Proboscis ornamentation as a diagnostic character for the Anoplodactylus californicus-digitatus complex (Arthropoda: Pycnogonida) with an example from the Anoplodactylus eroticus female. Zootaxa 64(1):51–64. DOI: 10.11646/zootaxa.1311.1.3.
- Arnaud F. 1987. Les pycnogonides (Chelicerata) de Méditerranée: distribution écologique, bathymetrique et biogéographie. Mesogee (Bulletin du Museum d’Histoire naturelle de Marseille) 47:37–58.
- Arnaud F, Bamber RN. 1987. The biology of Pycnogonida. Advances in Marine Biology 24:1–96.
- Bartolino V, Chimenz C. 2010. Pycnogonida. In: Relini G, editor. Checklist of the flora and fauna in Italian seas. Biologia Marina Mediterranea 17(suppl):394–397.
- Bianchi CN. 2004. Proposta di suddivisione dei mari italiani in settori biogeografici. Notiziario SIBM 46:57–59.
- Bouvier EL. 1923. Pycnogonides. Faune de France 7. Paris: Lechevalier. pp. 70.
- Calman WT. 1923. Pycnogonida of the Indian museum. Records of the Indian Museum 25(3):265–299.
- Child CA. 1987. The pycnogonida types of HVM Hall. Proceedings of the Biological Society of Washington 100:552–558.
- Child CA. 1992a. Pycnogonida of the Southeast Pacific Biological Oceanographic Project (SEPBOP). Smithsonian Contributions to Zoology (526):1–43. DOI: 10.5479/si.00810282.526.
- Child CA. 1992b. Shallow-water Pycnogonida of the Gulf of Mexico. Memoirs of the Hourglass Cruises IX:1–86.
- Chimenz C, Brignoli PM, Basciano G. 1979. Pantopodi del porto di Civitavecchia e dintorni (Italia Centrale). Cahiers de Biologie Marine X:471–497.
- Chimenz C, Cottarelli V. 1986. Soft bottom Pycnogonids from the gulf of Salerno (Italy). Oebalia 13:17–146.
- Chimenz C, Fresi E, Cinelli F, Mazzella I, Pansini M, Pronzato R. 1978. Evoluzione delle biocenosi bentoniche su substrato duro contro un gradiente di luce in una grotta marina superficiale. 2. Pantopodi. Memorie di Biologia Marina ed Oceanografia 8(4):91–103.
- Chimenz Gusso C. 2000. Picnogonidi delle coste italiane: quadro delle conoscenze. Memorie della Società Entomologica Italiana 78(2):541–574.
- Chimenz Gusso C, Lattanzi L. 2003. Mediterranean Pycnogonida: Faunistic, taxonomical and zoogeographical considerations. Biogeographia the Journal of Integrative Biogeography 24:251–262. DOI:10.21426/B6110015.
- Clark WC. 1970. New Pycnogonida from Queensland. Transactions of the Royal Society of New Zealand. Biological Sciences 12(3):13–17.
- Dohrn A. 1881. Die Pantopoden des Golfes von Neapel. Fauna u. Flora des Golfes von Neapel 3:1–252.
- Fry WG, Hedgpeth JW. 1969. Pycnogonida, 1: Colossendeidae, Pycnogonidae, Endeidae, Ammotheidae. In: New Zealand Oceanographic Institute Memoir 49. Fauna of the Ross Sea 7. Government Printer. pp. 1–139.
- Hall HVM. 1912. Studies on Pycnogonida. 1. Annual Report of the Laguna Marine Laboratory 1:91–99.
- Hilton WA. 1942. Pycnogonids from Allan Hancock expeditions. Reports of Allan Hancock Expedition 5:227–339.
- King PE. 1986. Sea spiders: A revised key to the adults of littoral Pycnogonida in the British Isles. Field Study 6:493–516.
- Krapp F. 1975. New records of Endeis charybdaea (Dohrn, 1881) (Pycnogonida) in the Atlantic and Mediterranean seas. Sarsia 59(1):85–94. DOI: 10.1080/00364827.1975.10411290.
- Lehmann T, Heß M, Melzer RR. 2014. Common littoral pycnogonids of the Mediterranean sea. Zoosystematics and Evolution 90(2):163–224. DOI: 10.3897/zse.90.7520.
- Lucena A 2013. Taxonomia de Pycnogonida do litoral do Estado da Paraíba - Monografia Taxonomia de Pycnogonida do litoral do Estado da Paraíba - Monografia. pp. 1–100.
- Lucena RA, Christoffersen ML. 2018. Anoplodactylus (Pycnogonida: Phoxichilidiidae)from Brazil, new records and two new species. Turkish Journal of Zoology 42(4):372–388. DOI: 10.3906/zoo-1712-1.
- Lucena RA, Fatemi Y, Christoffersen ML. 2018. Checklist of sea spiders (Arthropoda: Pycnogonida) from the Persian Gulf and the Gulf of Oman with new record of Endeis biseriata (Böhm, 1879) for the region. Turkish Journal of Zoology 42(6):709–714. DOI: 10.3906/zoo-1806-1.
- Meloni ML, Moruzzi L 2019. Monitoraggio relativo agli effetti dello scarico termico e della conversione a carbone della Centrale.
- Merton H. 1906. Eine auf Tethys leporina parasitisch lebende Pantopodenlarve (Nymphon parasiticum n. sp.). Mittheilungen aus der Zoologischen Station zu Neapel 18:136–141.
- Morri C. 1980. Osservazioni sugli idroidi raccolti nelle condotte della centrale termoelettrica di Torvaldaliga (Civitavecchia). Natura - Soc. ital. Sci. nat., Museo civ. Stor.nat. e Acquario civ., Milano 71:3–14.
- Müller H-G. 1992. Shallow-water Pycnogonida from Barbados, Lesser Antilles with description of Anoplodactylus justi n. sp. Studies on the Natural History of the Caribbean Region 71(1):42–52.
- Müller H-G. 1993. World catalogue and bibliography of the recent Pycnogonida. H.-G. Müller. Wetzlar: Laboratory of Tropical Ecosystems Research & Information Service, pp. 388.
- Munilla T, Soler-Membrives A. 2014. Pycnogonida. Madrid: Editorial CSIC Consejo Superior de Investigaciones Científicas. pp. 292.
- Nakamura K, Child CA. 1991. Pycnogonida of waters adjacent to Japan. Smithsonian Contributions to Zoology 512:74.
- Occhipinti-Ambrogi A, Marchini A, Cantone G, Castelli A, Chimenz C, Cormaci M, Froglia C, Furnari G, Gambi MC, Giaccone G, Giangrande A, Gravili C, Mastrototaro F, Mazziotti C, Orsi-Relini L, Piraino S. 2010. Alien species along the Italian coasts: An overview. Biological Invasions 13(1):215–237. DOI: 10.1007/s10530-010-9803-y.
- Relini G, Bianchi C. 1979a. Aspetti stagionali dell’insediamento di organismi nei canali di una centrale termoelettrica del medio Tirreno. Bollettino di zoologia 46(suppl):190–191.
- Relini G, Bianchi C, Pisano E. 1980. Macrofouling in the conduits of a Middle Thyrrenian power station. In: Proceedings of 5th International Congress on Marine Corrosion and Fouling. Madrid. pp. 279–292.
- Relini G, Bianchi CN. 1979b. Prime osservazioni sul fouling della centrale termoelettrica di Torvaldaliga (Civitavecchia). Atti della Società toscana di scienze naturali, residente in Pisa. Memorie 86:308–311.
- Staples DA. 1982. Pycnogonida of the Calliope River and Auckland Creek, Queensland. Memoirs of the Queensland Museum 20(3):455–471.
- Stock JH. 1968. Pycnogonida collected by the Galathea and Anton Bruun in the Indian and Pacific Oceans. Videnskabelige Meddelelser fra Dansk naturhistorisk Forening i Kjøbenhavn 131:7–65.
- Stock JH. 1970. A new species of Endeis and other pycnogonid records from the Gulf of Aqaba. Bulletin Zoologisch Museum 2:1–4.
- Stock JH. 1992. Pycnogonida from southern Brazil. Tijdschrift voor Entomologie 135:113–139.
- WoRMS Editorial Board. 2020. World register of marine species. Available: http://www.marinespecies.org Accessed Jun 2020 18. DOI: 10.14284/170.
