Abstract
In this work, a polymorphic variability of Muscovy duck histone H1.c’ is presented along with a link of its allelic variants with an extent of ADP (Adenosine Diphosphate)-ribosylation of histone H1 non-allelic subtypes. With the use of a two-dimensional electrophoretic system, histone H1.c’ was identified as differentiated into the variously charged isoforms H1.c’1 and H1.c’2. In the population studied, they occur in the form of homozygous phenotype c’1 (frequency 0.384) and c’2 (frequency 0.288) as well as the heterozygous phenotype c’1c’2 (frequency 0.326). Because fewer heterozygotes were observed (0.326) than expected (0.496), the duck population is under inbreeding (F = 0.342, HI = 0.325) and does not conform to Hardy–Weinberg assumptions (p < 0.05). The total population differentiation (δ = 0.5) and polymorphism information content (PIC = 0.496) show that about half the bird individuals are genetically different in an intermediate diverse population. Because an array of Muscovy duck H1 histones is enriched by the complex LHL (Linker Histone Like), which is a conjugate of histone H1 subtypes with ADP-ribose, its juxtaposition with histone H1.c’ allelic variants was examined. This was to uncover a correlation of the phenotype c’2 and c’1c’2 with low intensity of modification (mean LHL density = 316284.9 ± 47776.7) and the relation of the phenotype c’1 to high modification (LHL density = 1380419.6 ± 116471.3). A disparity of more than 4 times in the abundance of ADP–ribose linkage indicates a histone H1.c’ phenotype-specific association with the level of ADP-ribosylation. It seems that such a difference between histone H1.c’ allelic variants might be related to their individual participation in the determination of histone H1 subtype modification status. With respect to this, allelic variants of histone H1.c’ may variously influence the ADP-ribosylation reaction, presumably by differentiated stimulation of the enzymes catalysing the histone H1-ADP-ribose linkage.
Introduction
Histone H1 belongs to multifunctional proteins controlling processes running in both nuclear and nucleolar chromatin. The first of them are realised through the interactions with DNA to build chromatin structure and regulation of gene expression (Hergeth & Schneider Citation2015; Parseghian Citation2015), while the next are coupled to the interactions with partner proteins to organise RNA splicing and biogenesis of the ribosome (Szerlong et al. Citation2015; Kalashnikova et al. Citation2016). The multifaceted activity of histone H1 is determined by its structural disorder, dynamicity and posttranslational modifications attributable to its non-allelic variants, also called subtypes (Kowalski & Pałyga Citation2016). They possess characteristic amino acid sequences containing tandem repeat motifs localised in the peculiar tripartite-domain structure (Roque et al. Citation2016a) that confer on them specific functional features. So, histone H1 subtypes display an individual capability to fold and associate with chromatin fibre and to induce a higher-order structure of chromatin (Kowalski & Pałyga Citation2012a). Moreover, histone H1 subtypes are non-uniformly distributed in chromatin of cells of different origin, reflecting their imbalanced expression (Millãn-Ariño et al. Citation2016) and selective association with replication, stabilisation and repair of the DNA as well as epigenetic control of chromatin functioning (Fyodorov et al. Citation2017).
An equally important trait of histone H1 subtypes that modulates their activity is polymorphic variation. The reported data show that H1 allelic isoforms that differ in their amino acid sequence (Górnicka-Michalska et al. Citation2014) and chromatin-binding affinity (Flanagan et al. Citation2016) might perform a specific function. Other studies demonstrate that polymorphic H1 isoforms are useful as informative markers associated with physiological (Kowalski et al. Citation2015) and environmental features (Kowalski Citation2016) of organisms, critical for their adaptation and fitness. Besides a few cases of histone H1 polymorphic subtypes presented in mammals, such as H1.4 in rabbit (Pałyga Citation1990) and H1.2 in brown hare (Kowalski & Markowski Citation2018), their occurrence is best documented in examples of birds. Whereas eight out of nine avian H1 subtypes (H1.a, H1.a’, H1.b, H1.b’, H1.c, H1.d, H1.z and H5) were already detected as allelic proteins (for a review see Kowalski Citation2018), the results of recent work (Kowalski Citation2019) and the data presented herein show that the remaining subtype, H1.c’, is also a heterogeneous protein.
As mentioned above, histone H1 activity is also specified by posttranscriptional modifications. Histone H1 subtypes are extensively modified, mainly by acetylation, phosphorylation and methylation (Wood et al. Citation2009; Andrés et al. Citation2020), and the type and extent of modification evoke functional effects relating to the modulation of chromatin condensing capacity (Roque et al. Citation2016b) and regulation of gene expression (Izzo & Schneider Citation2016). Although the kinds and functions of H1 modifications are well known at the cellular level, rarely detected and, therefore, poorly explored are modified H1 histones observed in natural populations. Such an occurrence was, however, demonstrated on an example protein, dubbed X, occurring along with histone H1 subtypes in a population of Muscovy duck (Kowalski et al. Citation2004). Subsequently, this protein was identified as a complex, named LHL, consisting of histone H1 subtypes (H1.b and H5) linked to the ADP-ribose. In two-dimensional polyacrylamide gel, it is visible as a slowly migrating protein spot that forms a dimer in one-dimensional SDS (Sodium Dodecyl Sulfate)-polyacrylamide gel (Kowalski et al. Citation2009). Such protein doublets with retarded electrophoretic mobility were identified earlier as poly-ADP-ribosylated histone H1 forms (Poirier et al. Citation1982; Niedergang et al. Citation1985), so the presence of LHL conjugate in the Muscovy duck H1 histones may correspond to the effect of poly(ADP-ribosylation). This modification is generated by poly(ADP-ribose) synthesised by poly(ADP-ribose) polymerase from ADP-ribose in the presence of NAD (Nicotinamide Adenine Dinucleotide), releasing nicotinamide and protons. Through the binding of Asp, Glu and Lys residues it promotes modifications of nuclear proteins (Hottiger et al. Citation2010; Aredia & Scovassi Citation2014), including histones (Messner & Hottiger Citation2011). Because H1 histones are not only main acceptors of ADP-ribose (Huletsky et al. Citation1989; Hottiger Citation2011) but also efficient activators of ADP-ribosyltransferase, which catalyses modification (Moss & Stanley Citation1981; Huletsky et al. Citation1985), it seems possible that some Muscovy duck histone H1 subtypes may display such a stimulatory effects. Therefore, a similar functioning can be expected of their polymorphic variants. Hence, one purpose of the current study was to check whether histone H1.c’ might influence the extent of ADP-ribosylation in a way dependent on the activity of its polymorphic variants. If so, histone H1.c’ allelic variants could be considered specific in relation to the regulation of ADP-ribosylation-dependent processes and, thus, to play different functions in the erythrocyte chromatin of Muscovy duck.
Material and methods
Animal material
A total of 52 Muscovy duck individuals used in this study were raised for non-commercial purposes on a privately owned farm in a free-range backyard system. The bird individuals were about 2 years old. The sex structure of the analysed population is given in .
Table I. The sex structure of the Muscovy duck population
Extraction and electrophoresis of histones H1
About 1 mL of whole blood collected from the wing vein of each duck individual was placed in a tube containing a cooled solution of SSC (0.15 M NaCl with 0.015 M sodium citrate). Isolation of H1 histones and their electrophoretic analysis were conducted according to the procedure described by Neelin et al. (Citation1995) and Kowalski and Pałyga (Citation2012b), respectively. The erythrocytes obtained from the pelleted and leukocyte-depleted blood samples were a source of nuclei isolated with a solution of 3% saponin buffered with 0.1 M sodium phosphate (pH 7.0). After purification of the nuclear pellet by several washings with 0.9% NaCl, histones H1 were recovered by two-step extraction with decreasing concentrations of perchloric acid solution, i.e. 1 M and 0.5 M. To avoid a proteolytic degradation of proteins, 1 mM phenylmethylsulfonyl fluoride (PMSF) was added to all working solutions. Histones H1 were precipitated from the pooled supernatants with 20% trichloroacetic acid and washed with acetone, acidified with hydrochloric acid (HCl, 500:2 v/v) and alone.
Histone H1 preparations were prepared for electrophoresis by adding 1 mg of dry preparation to 100 µL of buffer containing 8 M urea, 0.9 M acetic acid and 10% 2-mercaptoethanol. Proteins were separated in a two-dimensional polyacrylamide gel system. It comprised acetic acid-urea (containing 15% acrylamide and 8 M urea) and SDS (containing 13.5% acrylamide and 0.1% SDS) electrophoresis in the first and second dimension, respectively. Both polyacrylamide gels were 24 cm long, which gives an effective migration distance of 21 cm. For electrophoresis conducted in the second dimension, the gel slices containing H1 histones resolved in the first dimension were cut out and equilibrated (2 times for 15 min each) in the buffer composed of 100 mM Tris-base pH 6.8, 10% glycerol, 2.1% SDS and 2% 2-mercaptoethanol. The gel strips with resolved histone H1.c’ isoforms were excised and electrophoresed again, in the acetic acid-urea polyacrylamide gel, by loading into a separate gel well about five protein spots corresponding to each isoform. Before electrophoresis, the gel pieces were immersed 2 times for 15 min each in the adaptation buffer containing 0.9% acetic acid, 2% Temed, 10% glycerol and 8 M urea.
After electrophoresis was stopped, the resolved H1 histones were visualised by a routine two-step staining with Coomassie Brillant Blue R-250 stain, at concentrations of 0.05% and 0.0035%, dissolved in the mixture of acetic acid and propanol-2, at concentrations of 10% and 25%. To improve the visibility of weakly stained proteins, especially low-intensity LHL complexes, some gels were additionally stained with a solution of 0.1% silver nitrate and developed by immersion in a solution of 3% potassium carbonate containing 0.05% formalin.
Quantitative estimation of LHL conjugates
The intensity of LHL complexes was analysed in the electrophoretic images processed with ImageJ 1.44c software. The area and integrated density were measured in square millimetres and as a sum of the values of the pixels, respectively, in the selected protein spots. The measurements were conducted on inverted gel images, giving transparent protein spots allowing right calculation of their area and density. An evaluation was performed for 20 LHL complexes (n = 20) in relation to a given phenotype of histone H1.c’. The coefficient of variation (CV) for the LHL complex protein spots was calculated by dividing the standard deviation by the mean of their area and density. It was assumed that CV values below 0.25 indicate a low relative variability. To check for significance of differences between obtained measurements, the Student’s t-test was used. Values corresponding to p < 0.05 were regarded as statistically significantly different.
Statistical assessment of Muscovy duck population
A conformity of the bird population to the Hardy–Weinberg expectation was estimated using the chi-square (χ2) test of goodness of fit, while the sex structure and difference in the distribution of histone H1.c’ phenotypes were checked by the chi-square (χ2) test of homogeneity. In both cases, p values of less than 0.05 were regarded as statistically significantly different. The coefficient of inbreeding (F), indicating a deficit of heterozygosity, was calculated from the equation F = 1 − (Hobs/Hexp) (Lowe et al. Citation2004). A reduction of heterozygosity under inbreeding, indicating a rate of inbreeding, was determined from the equation HI = 2pq(1−F) (Neal Citation2002). Total population differentiation, corresponding to the number of individuals by which the population differs, was estimated with the use of the equation δT = N/N − 1 × (1−ΣXi2) (Gregorius Citation1988). To designate a value for the polymorphism information content (PIC), showing the genetic variability in a population, the equation PIC = 1 − ΣPij2, proposed by Anderson et al. (Citation1993), was applied.
Results and discussion
Identification of Muscovy duck histone H1.c’ polymorphism
In two-dimensional polyacrylamide gels, Muscovy duck histone H1 was resolved into six subtypes (H1.a, H1.b, H1.c, H1.c’, H1.d and H1.z.), of which subtypes H1.c’ and H1.z were shown to be polymorphic (). While polymorphic variants of histone H1.z have already been characterised in the Muscovy duck (Kowalski et al. Citation2004), in this work they are mentioned only to point out their lack of association with the extent of LHL complex intensity (see the subsection Histone H1.c’ allelic variants and extent of histone H1 ADP-ribosylation). What is not documented so far is the allelic diversity of Muscovy duck histone H1.c’, and this is described below in detail.
Figure 1. Two-dimensional electrophoretic pattern of Muscovy duck histone H1 complement (subtypes H1.a, H1.b, H1.c, H1.c’ and H1.z) supplemented with LHL conjugates. The allelic heterogeneity of histones H1 is reflected by variously localised isoforms of histone H1.c’, i.e. H1.c’1 and H1.c’2 that form homozygous phenotypes (c’1 and c’2) and heterozygous phenotype (c’1c’2), and differently migrating isoforms of histone H1.z, i.e. H1.z1 and H1.z2 that create homozygous phenotypes (z1 and z2) and heterozygous phenotype (z1z2)
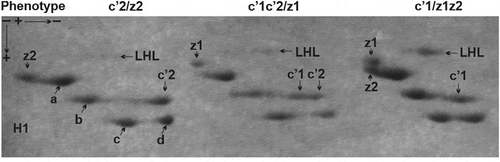
So, a difference in the localisation of histone H1.c’ detected in the electrophoretic gels allowed us to distinguish two isoforms (H1.c’1 and H1.c’2) observed in the form of three phenotypes. A homozygous phenotype c’1 and c’2 is created by a single protein spot while a heterozygous phenotype c’1c’2 is composed of a double protein spot. The histone H1.c’ isoforms have different in-gel locations in relation to the neighbouring subtype H1.b. The isoform H1.c’1 is placed closer to the subtype H1.b while the isoform H1.c’2 is located farther from it. Likewise, we also observed a shift of H1.c’ isoforms in relation to the faster migrating subtype H1.d, having a similar position in relation to subtype H1.c. Thus, the same rate of electrophoretic mobility and various in gel-placing of histone H1.c’ isoforms point to a difference in their net charges.
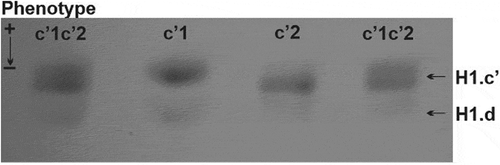
A confirmation of the disparity between histone H1.c’ isoforms uncovered in the second-dimension polyacrylamide gel was obtained after their electrophoresis in the acetic acid-urea polyacrylamide gel, effective in the separation of proteins based on a difference in their net charge. As shown in , migration of histone H1.c’ isoforms corresponds to lower (phenotype c’1), higher (phenotype c’2) and intermediate (phenotype c’1c’2) electrophoretic mobility. In general, both localisation and migration rate of histone H1.c’ in the polyacrylamide gels prove that this is a polymorphic protein composed of two isoforms (H1.c’1 and H1c’2) possessing different net charge.
Figure 2. One-dimensional electrophoretic pattern of Muscovy duck histone H1.c’ isoforms. Different rates of migration correspond to low (isoform H1.c’1 – phenotype c’1), high (isoform H1.c’2 – phenotype c’2) and intermediate (isoform H1.c’1 and H1.c’2 – phenotype c’1c’2) electrophoretic mobility
Among the avian H1 histones, bi-allelic H1 subtypes differing in molecular weight can often be found, such as grey partridge histone H1.a’ (Kowalski et al. Citation2008) and H1.b (Kowalski et al. Citation2011a), and rarely occurring ones that vary in abundance, such as quail histone H1.a (Pałyga Citation1998) and H1.d (Kowalski et al. Citation2015). Less frequent are histone H1 isoforms identified based on a difference in their net charges. Examples are chicken, duck (Górnicka-Michalska et al. Citation2014) and quail (Kowalski & Knaga Citation2017) isoforms of histone H1.a. Occasionally, tri-allelic H1 subtypes, such as duck histone H1.b (Pałyga et al. Citation2000) and H1.z (Kowalski et al. Citation2004), displaying a set of isoforms with different molecular weight and different net charge, are encountered.
In such a broad spectrum of avian histone H1 polymorphic variation, however, it is rare to find subtypes displaying a varied set of the isoforms in different species (Kowalski Citation2018). The type of histone H1.c’ polymorphism identified in this work based on an isoform-specific net charge is dissimilar to that detected due to an isoform-specific molecular weight in the guinea fowl and pheasant (Kowalski Citation2019). Therefore, histone H1.c’ is an example of an H1 subtype displaying a set of allelic variants characteristic for a species. In this respect, histone H1.c’ can be considered peculiar because the only similar histone in this context is histone H1.c. This H1 subtype was identified as composed of the isoforms that have different net charges in the pheasant (Kowalski et al. Citation2010) and various molecular weights in the guinea fowl (Kowalski et al. Citation2011b).
Although the exact importance of H1 polymorphic variants is not a resolved question, some data indicate their specificity may be related to an individual impact on chromatin organisation and/or activity (Tanaka et al. Citation2005; Flanagan et al. Citation2016). In agreement with the notion that avian H1 isoforms differ in their sequences, as an effect of amino acid deletions and substitutions (Kowalski Citation2018), it can be predicted that they may display a varied propensity of binding DNA sequence and favour unlike the type of binding to nucleosomes (Gorkovets et al. Citation2018).
Characteristics of the Muscovy duck population
An estimation of the Muscovy duck population structure was initiated with an evaluation of the sex proportion of the birds. The distribution of histone H1.c’ phenotypes between the sexes is comparable (p > 0.05); however, females comprised the majority of the population (). The other parameters characterising the Muscovy duck population are listed in .
Table II. The Muscovy duck population structure estimated with histone H1.c’ polymorphic locus
Participation of histone H1.c’ phenotypes in the population assessed based on their frequencies revealed that phenotypes c’1 (observed 0.38 and expected 0.27) and c’2 (observed 0.35 vs expected 0.22) are overrepresented, while the phenotype c’1c’2 (observed 0.27 vs expected 0.5) is underrepresented. This reflects a difference (p > 0.05) in the distribution of histone H1.c’ phenotypes in the population. An observed deficiency of heterozygotes is a probable cause of population departure from Hardy–Weinberg equilibrium (p < 0.05) and a tendency to inbreeding, measured by the inbreeding coefficient (F = 0.46) and the inbreeding rate (HI = 0.27). The total population differentiation (δT = 0.51) indicates that about half of the duck individuals are genetically different, whereas PIC (= 0.5) shows that the intermediate genetically diverse population was assessed based on the average polymorphic H1.c’ locus. Thus, histone H1.c’ can be considered effective in the evaluation of genetic diversity and suitable for exploring avian populations.
The structure of the Muscovy duck population identified through the histone H1.c’ polymorphic locus does not fully resemble other avian breeding populations recognised based on the various polymorphic H1 subtypes. These populations usually stay in conformity with the Hardy–Weinberg law and exhibit a low level of inbreeding resulting from slight deficiency of the heterozygotes. An example are quail populations analysed based on the polymorphic variants of histone H1.d and H1.z (Kowalski et al. Citation2015) and histone H1.a (Kowalski & Knaga Citation2017). They are found to agree with the Hardy–Weinberg principle (p > 0.05) and exhibit no appreciable inbreeding (F = 0.15−0.20). It seems that breeding practice and/or selection does not affect population status, maintaining the balanced parameters of its structure.
In contrast, wild populations of the birds are usually characterised by strong deviation from Hardy–Weinberg expectations and a loss of genetic diversity in response to inbreeding and drift. An example of such a case is a pheasant population, the structure of which was estimated based on the polymorphism of histone H5 (Kowalski Citation2016). Moreover, the structure of a guinea fowl population assessed through the polymorphisms of histone H1.c (Kowalski et al. Citation2011b) and H1.c’ (Kowalski Citation2019) was found to be similar. Both populations were devoid of heterozygous individuals, resulting in a departure from the Hardy–Weinberg law (p < 0.05) and the occurrence of inbreeding (F = 1). These cases are customarily observed in small closed populations exposed to environmental changes (Lens et al. Citation2000) and generally indicate a decline of genetic diversity (Neal Citation2002). As a consequence, decreasing resistance to disease (Spielamn et al. Citation2004) and accelerating reduction of fitness, tending towards extinction of the individuals (Neaves et al. Citation2015), might occur.
The reasons for the special status of the Muscovy duck population, manifested by an understated number of heterozygotes resulting in the occurrence of inbreeding, may be numerous. A probable cause of their appearance might be accidental events occurring uncontrollably at the time of selection for mating and the monitoring breeding programme in the parental generation, and the unbalanced acquisition of the samples. However, it cannot be excluded that disturbance of the Muscovy duck population structure is an effect of histone H1 subtypes’ ADP-ribosylation. As shown in previous work, histone H1 is a main target of ADP-ribosylation introduced in response to DNA damage (Kreimeyer et al. Citation1984; de Murcia et al. Citation1988). Generally, this is a beneficial event because histone H1 ADP-ribosylation relaxes chromatin architecture to permit the excision repair of damaged DNA (Aubin et al. Citation1983; de Murcia et al. Citation1986). But it is also known that intense modification affects many cellular functions and processes, e.g. transcription, replication, differentiation and proliferation, disruption of which can lead even to cell death (Hegedüs & Virág Citation2014). Moreover, a stress-activated ADP‐ribosylation (Grüning et al. Citation2010) can result in protein inhibition and/or inactivation in both physiological and pathological conditions (Corda & Di Girolamo Citation2003). When ADP-ribosylation occurs in the aftermath of DNA lesion, the DNA damage-induced effects can be reflected in the condition of the population. The genetic composition of the population might be modulated due to the alterations of DNA in both somatic and germ cells. A parental population can undergo changes in age structure and sex proportion, while a descendant population can be exposed to diminished viability and fertility, leading to a decrease in its average fitness (Mussali-Galante et al. Citation2013).
The appearance of modified H1 histones within a whole avian population is a very rare case, so far identified in the Muscovy duck only (Kowalski et al. Citation2009). Considering the abovementioned possible causes and effects of ADP-ribosylation, it can be assumed that it is not an accidental occurrence but rather provoked by specific factor(s) and/or circumstance(s) acting on the entire population. It is likely that the ADP-ribosylated H1 histones appear as an effect of DNA lesions caused by adverse environmental conditions. Because exposure to various pollutants can damage DNA (Mannes & Emslie Citation2002), and DNA-damaging effects have already been observed in bird populations residing in various terrestrial ecosystems (Bonisoli-Alquati Citation2014), it can be assumed that ADP-ribosylation of H1 histones appeared in the Muscovy duck individuals as a response to genotoxic stimuli occurring locally in the place of their raising.
Histone H1.c’ allelic variants and extent of histone H1 ADP-ribosylation
As shown in , the histone H1-ADP-ribose LHL linkage displays varying intensity in different histone H1 preparations. Based on quantification of LHL complex protein spots (), two ranges of their abundance were distinguished. The measurements of protein spot area and density revealed a statistically significant difference (p < 0.05) between low-intensity (mean area 1516.3 ± 227, mean density 316284.9 ± 47776.76) and high-intensity (mean area 5498.7 ± 466.14, mean density 1380419.6 ± 116471.34) LHL conjugate. The obtained values, showing a difference in LHL complex abundance of more than 4 times, reflect the disparate extent of histone H1 modification.
Figure 3. Quantification of LHL complex abundance assessed based on the area and density of their protein spots. The depicted values indicate a range of variation within low relative variability complexes (CV < 0.25) and the difference (p < 0.05) between high- and low-intensity complexes
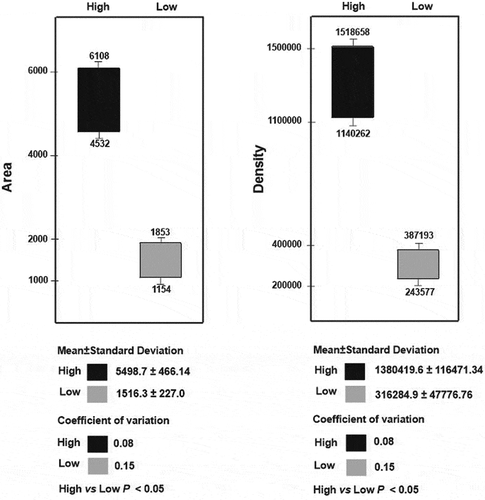
The juxtaposition of differently intense LHL conjugates with histone H1.c’ revealed a regular relationship between its phenotypes and the level of modification. Highly modified re H1 histones are coupled to phenotype c’1, while those connected with the phenotype c’1c’2 and c’2 have low modification. This distinction indicates a pronounced difference between the level of modification associated with phenotype c’1 and the extent of modification associated with the remaining H1.c’ phenotypes. This difference is also clear when a scatter of obtained values is considered. The CV values calculated for LHL conjugates of different intensities take a low dispersal around the mean, i.e. below the threshold value of 0.25, indicating their low degree of volatility. In the LHL conjugates appearing together with phenotype c’1, the values for area and density range from 4532 to 6108 and from 1,140,262 to 1,518,658, respectively. The corresponding values for the LHL complexes assigned to phenotypes c’2 and c’1c’2 (i.e. area, 1154–1853 and density, 243,577–387,193), are definitely lower ().
Based on this, it may be assumed there is a specific association between histone H1.c’ phenotypes and the extent of histone H1 subtypes’ ADP-ribosylation. To justify such a supposition, the relation between the intensity of LHL linkages and the phenotypes of another polymorphic H1 subtype, H1.z, was checked. The regular assignment of histone H1.c’ phenotypes to LHL complexes of different intensities was not confirmed for the polymorphic variants of histone H1.z. Almost the same number of individuals possessing the phenotype z1 display an association with both high and low intensity LHL complexes, similarly to the high and low extent assigned to the same number of individuals having phenotype z2. Furthermore, the individuals with phenotype z1z2 are correlated in almost equal numbers with high vs low extents of the LHL conjugate (). This shows that histone H1.z does not influence the extent of ADP-ribosylation in the allelic variant-specific mode. The observed state indicates, however, the opposite activity of histone H1.c’, the phenotypes of which might be individually engaged in establishing a modification status.
Table III. The phenotypes of histone H1.z assigned to the intensity of LHL complexes
The above supposition seems to be possible because histone proteins are not only the prime acceptors of ADP-ribose (Althaus et al. Citation1994; Hassa et al. Citation2006) but also effective regulators of the ADP-ribosylation reaction. It has been shown that histones H2A and H2B block poly(ADP-ribose) polymerase activity, the stimulation of which is triggered by histone H4 (Pinnola et al. Citation2007). Also, binding of histone poly(ADP-ribose) polymerase to histone H4 induces the prolonged activation of the enzyme (Thomas et al. Citation2019). Similar effects were observed in relation to histone H1. It was found to be the most effective activator of ADP-ribosylation reaction (Kun et al. Citation2004), but also an enhancer of both poly(ADP-ribose) polymerase (Rosidi et al. Citation2008) and poly(ADP-ribose) synthetase activity (Kawaichi et al. Citation1980).
The results confirming a stimulatory activity of histone H1 on the enzymes engaged in the ADP-ribosylation reaction come from studies on mammalian tissue, i.e. calf thymus (Huletsky et al. Citation1989) and rat pancreas (Huletsky et al. Citation1985); however, a similar occurrence is characteristic of bird erythrocytes. It has been shown that histone H1 from turkey erythrocytes may serve not only as an ADP-ribose acceptor but also as an activator of ADP-ribosyltransferase, which catalyses formation of ADP-ribose-protein adducts (Moss & Stanley Citation1981). Besides, histone H1 is able to stimulate an action of ADP-ribose synthetase that determines the initiation of ADP-ribosylation and subsequent elongation of a chain of ADP-ribose (Moss et al. Citation1982). It seems plausible that both reactions can take place in the Muscovy duck chromatin, assuming that histone H1 subtypes (H1.b and H5) and ADP-ribose create the LHL conjugate in the reaction catalysed by ADP-ribose polymerase, an activity the extent of which is individually designated by histone H1.c’ variant-dependent stimulation of ADP-ribose synthetase.
The data obtained in the current study clearly indicate that Muscovy duck histone H1 subtypes might be associated at all with regulation of ADP-ribosylation and, moreover, that participation in such a process can be attributed to some histone H1 polymorphic variants only. Regarding this, the level of ADP-ribosylation is not specified by histone H1.z allelic variants, in contrast to histone H1.c’1 and H1.c’2 which seem to be individually associated with high and low extent, respectively, of modification. This may mean that histone H1.c’1 is more potent than histone H1.c’2 in the activation of ADP-ribose transferase and/or ADP-ribose synthetase, responsible for the formation of ADP-ribose-histone linkages.
Conclusion
Muscovy duck histone H1.c’ is a heterogeneous protein consisting of variously charged isoforms (H1.c’1 and H1.c’2) existing as three phenotypes (c’1, c’2 and c’1c’2) in the population that remain under inbreeding (F = 0.46) and do not meet Hardy–Weinberg assumptions (p < 0.05). The observed breach of population equilibrium could have been evoked by a lesion of DNA, one consequence of which is an occurrence of ADP-ribosylated H1 histones. This may confirm an adverse effect of ADP-ribosylation affecting the whole duck population. Measurements of the area and intensity of LHL (histone H1-ADP ribose conjugate) protein spots indicate varying extent of modification, individually associated with polymorphic variants of histone H1 subtypes. While histone H1.z allelic variants were not assigned as a whole to concrete levels of modification, histone H1.c’ allelic variants were identified as individually associated with a particular extent of modification. A high level of modification is associated with histone H1.c’1, while low modification is characteristic of histone H1.c’2. The revealed relationships indicate that histone H1 subtypes are not functionally equivalent and may individually influence the chromatin processes, including a mechanism of the ADP-ribosylation reaction. Thus, the specificity of histone H1 allelic variants may concern differences in the propensity to regulate the extent of ADP-ribosylation, presumably due to varied stimulation of catalysing enzymes.
Disclosure statement
No potential conflict of interest was reported by the author.
Additional information
Funding
References
- Althaus FR, Hofferer L, Kleczkowska HL, Malanga M, Naegeli H, Panzeter PL, Realini CA. 1994. Histone shuttling by poly ADP ribosylation. Molecular and Cellular Biochemistry 138:53–59. DOI: 10.1007/BF00928443.
- Anderson JA, Churchill GA, Autrique JE, Tanksley SD, Sorrells ME. 1993. Optimizing parental selection for genetic linkage maps. Genome 1993:181–186. DOI: 10.1139/g93-024.
- Andrés M, Garcia-Gomis D, Ponte I, Suau P, Roque A. 2020. Histone H1 post-translational modifications: Update and future perspectives. International Journal of Molecular Science 21:5941. DOI: 10.3390/ijms21165941.
- Aredia F, Scovassi AI. 2014. Poly(ADP-ribose): A signaling molecule in different paradigms of cell death. Biochemical Pharmacology 92:157–163. DOI: 10.1016/j.bcp.2014.06.021.
- Aubin R, Fréchette A, de Murcia G, Mandel P, Lord A, Poirier GG. 1983. Correlation between endogenous nucleosomal hyper(ADPribosyl)ation of histone H1 and the induction of chromatin relaxation. EMBO Journal 2:1685–1693. DOI: 10.1002/j.1460-2075.1983.tb01643.x.
- Bonisoli-Alquati A. 2014. Avian genetic ecotoxicology: DNA of the canary in a coalmine. Current Zoology 60:285–298. DOI: 10.1093/czoolo/60.2.285.
- Corda D, Di Girolamo M. 2003. Functional aspects of protein mono-ADP-ribosylation. EMBO Journal 22:1953–1958. DOI: 10.1093/emboj/cdg209.
- de Murcia G, Huletsky A, Lamarre D, Gaudreau A, Pouyet J, Daune M, Poirier GG. 1986. Modulation of chromatin superstructure by poly(ADP)ribose synthesis and degradation. Journal of Biological Chemistry 261:7011–7017. DOI: 10.1016/S0021-9258(19)62715-8.
- de Murcia G, Huletsky A, Poirier GG. 1988. Modulation of chromatin structure by poly(ADP-ribosyl)ation. Biochemistry and Cell Biology 66:626–635. DOI: 10.1139/o88-072.
- Flanagan TW, Files JK, Casano KR, George EM, Brown DT. 2016. Photobleaching studies reveal that a single amino acid polymorphism is responsible for the differential binding affinities of linker histone subtypes H1.1 and H1.5. Biology Open 5:372–380. DOI: 10.1242/bio.016733.
- Fyodorov DV, Zhou B-R, Skoultchi AI, Bai Y. 2017. Emerging roles of linker histones in regulating chromatin structure and function. Nature Reviews Molecular Cell Biology 19:192–206. DOI: 10.1038/nrm.2017.94.
- Gorkovets TK, Armeev GA, Shaitan KV, Shaytan KH. 2018. Joint effect of histone H1 amino acid sequence and DNA nucleotide sequence on the structure of chromatosomes: Analysis by molecular modelling methods. Molecular Biology 73:82–87. DOI: 10.3103/S0096392518020025.
- Górnicka-Michalska E, Kowalski A, Pałyga J. 2014. Allelic isoforms of the chicken and duck histone H1.a. Cellular and Molecular Biology Letters 19:116–125. DOI: 10.2478/s11658-014-0182-8.
- Gregorius HR. 1988. The meaning of genetic variation within and between subpopulations. Theoretical and Applied Genetics 76:947–951. DOI: 10.1007/BF00273686.
- Grüning N-M, Lehrach H, Ralser M. 2010. Regulatory crosstalk of the metabolic network. Trends in Biochemical Sciences 35:220–227. DOI: 10.1016/j.tibs.2009.12.001.
- Hassa PO, Haenni SS, Elser M, Hottiger MO. 2006. Nuclear ADP-ribosylation reaction in mammalian cells: Where are we today and where are we going? Microbiology and Molecular Biology Reviews 70:789–829. DOI: 10.1128/MMBR.00040-05.
- Hegedüs C, Virág L. 2014. Inputs and outputs of poly(ADP)ribosylation: Relevance to oxidative stress. Redox Biology 2:978–982. DOI: 10.1016/j.redox.2014.08.003.
- Hergeth SP, Schneider R. 2015. The H1 linker histones: Multifunctional proteins beyond the nucleosomal core particle. EMBO Reports 16:1439–1453. DOI: 10.15252/embr.201540749.
- Hottiger MO. 2011. ADP-ribosylation of histones by ARTD1: An additional module of the histone code. FEBS Letters 585:1595–1599. DOI: 10.1016/j.febslet.2011.03.031.
- Hottiger MO, Hassa P, Lüscher B, Schüler H, Koch-Nolte F. 2010. Toward a unified nomenclature for mammalian ADP-ribosyltransferases. Trends in Biochemical Sciences 35:208–219. DOI: 10.1016/j.tibs.2009.12.003.
- Huletsky A, de Murcia G, Muller S, Hengartner M, Menard L, Lamarre D, Poirier G. 1989. The effect of poly(ADP-ribosyl)ation on native and H1-depleted chromatin. A role of poly(ADP-ribosyl)ation on core nucleosome structure. Journal of Biological Chemistry 264:8878–8886. DOI: 10.1016/S0021-9258(18)81875-0.
- Huletsky A, Niedergang C, Frechette A, Aubin R, Gaudreau A, Poirier GG. 1985. Sequential ADP-ribosylation pattern of nucleosomal histones. ADP-ribosylation of nucleosomal histones. European Journal of Biochemistry 146:277–285. DOI: 10.1111/j.1432-1033.1985.tb08650.x.
- Izzo A, Schneider R. 2016. The role of histone H1 modifications in the regulation of gene expression and chromatin dynamics. Biochimica at Biophysica Acta 1859:486–495. DOI: 10.1016/j.bbagrm.2015.09.003.
- Kalashnikova AA, Rogge RA, Hansen JC. 2016. Linker histone H1 and protein-protein interactions. Biochimica at Biophysica Acta 1859:455–461. DOI: 10.1016/j.bbagrm.2015.10.004.
- Kawaichi M, Ueda K, Hayaischi O. 1980. Initiation of poly(ADP-ribosyl) histone synthesis by poly(ADP-ribose) synthetase. Journal of Biological Chemistry 255:816–819. DOI: 10.1016/S0021-9258(19)86100-8.
- Kowalski A. 2016. A heterogeneity of the pheasant (Phasianus colchicus L.) erythrocyte histone H1 subtype H5. Comptes Rendus Biologies 339:357–363. DOI: 10.1016/j.crvi.2016.07.001.
- Kowalski A. 2018. Significance of avian linker histone (H1) polymorphic variation. Journal of Biosciences 43:751–761. DOI: 10.1007/s12038-018-9791-0.
- Kowalski A. 2019. A status of guinea fowl (Numida meleagris) and pheasant (Phasianus colchicus) population transferred from wildlife to the breeding assessed based on the histone H1.c’ polymorphic variants. Avian Biology Research 12:145–151. DOI: 10.1177/1758155919860351.
- Kowalski A, Knaga S. 2017. Evidence on the stability of histone H1.a polymorphic variants during selection in quail. Archives of Animal Breeding 60:145–151. DOI: 10.5194/aab-60-145-2017.
- Kowalski A, Markowski J. 2018. Brown hare’s (Lepus europaeus) histone H1 variant H1.2 as an indicator of anthropogenic stress. Archives of Environmental Contamination and Toxicology 75:576–584. DOI: 10.1007/s00244-018-0540-z.
- Kowalski A, Pałyga J. 2012a. Linker histone subtypes and their allelic variants. Cell Biology International 36:981–996. DOI: 10.1042/CBI20120133.
- Kowalski A, Pałyga J. 2012b. High-resolution two-dimensional polyacrylamide gel electrophoresis: A tool for identification of polymorphic and modified linker histone components. In: Magdeldin S, editor. Gel electrophoresis - principles and basics. Croatia: In Tech Open. pp. 117–136.
- Kowalski A, Pałyga J. 2016. Modulation of chromatin function through linker histone H1 variants. Biology of the Cell 108:339–356. DOI: 10.1111/boc.201600007.
- Kowalski A, Pałyga J, Górnicka-Michalska E. 2004. Identification of histone H1.z complements in a Muscovy duck (Cairina moschata L.) population. Comparative Biochemistry and Physiology Part B 137:151–157. DOI: 10.1016/j.cbpc.2003.10.016.
- Kowalski A, Pałyga J, Górnicka-Michalska E. 2008. Polymorphic isoforms of erythrocyte histone H1.a’ in a Grey partridge population. Journal of Agrobiology 25:125–127.
- Kowalski A, Pałyga J, Górnicka-Michalska E. 2009. Linker histone-like proteins in Muscovy duck (Cairina moschata L.) erythrocyte chromatin. Cell Biology International 33:344–351. DOI: 10.1016/j.cellbi.2009.01.002.
- Kowalski A, Pałyga J, Górnicka-Michalska E. 2010. Phenotypic variation of erythrocyte linker histone H1.c in a pheasant (Phasianus colchicus L.) population. Genetics and Molecular Biology 33:475–478. DOI: 10.1590/S1415-47572010000300016.
- Kowalski A, Pałyga J, Górnicka-Michalska E. 2011a. Linker histone H1.b is polymorphic in grey partridge (Perdix perdix). Zeitschrift fur Naturforschung C 66:296–304. DOI: 10.5560/ZNC.2011.66c0296.
- Kowalski A, Pałyga J, Górnicka-Michalska E. 2011b. Two polymorphic linker histone loci in Guinea fowl erythrocytes. Comptes Rendus Biologies 334:6–12. DOI: 10.1016/j.crvi.2010.10.006.
- Kowalski A, Pałyga J, Knaga S, Witkowski A. 2015. A shift in the erythrocyte histone H1 complement following selection in quail (Coturnix japonica). Czech Journal of Animal Science 60:105–115. DOI: 10.17221/8075-CJAS.
- Kreimeyer A, Wielckens K, Adamietz P, Hilz H. 1984. DNA repair associated ADP ribosylation in vivo. Modification of histone H1 differs from that of the principal acceptor proteins. Journal of Biological Chemistry 259:890–896. DOI: 10.1016/S0021-9258(17)43540-X.
- Kun E, Kirsten E, Mendeleyev J, Ordahl CP. 2004. Regulation of the enzymatic catalysis of poly(ADP)ribose polymerase by dsDNA, polyamines, Mg2+, Ca2+, histones H1 and H3 and ATP. Biochemistry 43:210–216. DOI: 10.1021/bi0301791.
- Lens L, Van Dongen S, Galbusera P, Schenck T, Matthysen E, Van De Castelle T. 2000. Developmental instability and inbreeding in natural bird populations exposed to different levels of habitat disturbance. Journal of Evolutionary Biology 13:889–896. DOI: 10.1046/j.1420-9101.2000.00232.x.
- Lowe A, Harris S, Ashton P. 2004. Ecological genetics: Design, analysis and application. Oxford: Blackwell Publishing.
- Mannes TJ, Emslie SD. 2002. An analysis of possible genotoxic exposure in adult and juvenile royal terns in North Carolina, USA. Waterbirds 24:352–360. DOI: 10.2307/1522065.
- Messner S, Hottiger MO. 2011. Histone ADP-ribosylation in DNA repair, replication and transcription. Trends in Cell Biology 21:534–542. DOI: 10.1016/j.tcb.2011.06.001.
- Millãn-Ariño L, Izquierdo-Bouldstridge A, Jordan A. 2016. Specificities and genomic distribution of somatic mammalian histone H1 subtypes. Biochimica at Biophysica Acta 1859:510–519. DOI: 10.1016/j.bbagrm.2015.10.013.
- Moss J, Stanley SJ. 1981. Histone-dependent and histone-independent forms of an ADP-ribosyltransferase from human and turkey erythrocytes. Proceedings of the National Academy of Sciences USA 78:4809–4812. DOI: 10.1073/pnas.78.8.4809.
- Moss J, Stanley SJ, Osborne JC Jr.. 1982. Activation of an NAD:Arginine ADP-ribosyltransferase by histone. Journal of Biological Chemistry 257:1660–1663. DOI: 10.1016/S0021-9258(19)68087-7.
- Mussali-Galante P, Tovar-Sánchez E, Valverde M, Rojas E. 2013. Genetic structure and diversity of animal populations exposed to metal pollution. Reviews of Environmental Contamination and Toxicology 227:79–106. DOI: 10.1007/978-3-319-01327-5_3.
- Neal D. 2002. Small population, genetic drift and inbreeding. In: Frankham R, Ballou JD, editors. Introduction to conservation genetics. New York: Cambridge University Press. pp. 116–134.
- Neaves LE, Eales J, Whitlock R, Hollingsworth PM, Burke T, Pullin AS. 2015. The fitness consequences of inbreeding in natural populations and their implications for species conservation – A systematic map. Environmental Evidence 4:5. DOI: 10.1186/s13750-015-0031-x.
- Neelin JM, Neelin EM, Lindsay DW, Pałyga J, Nichols CR, Cheng KM. 1995. The occurrence of a mutant dimerizable histone H5 in Japanese quail erythrocytes. Genome 38:982–990. DOI: 10.1139/g95-129.
- Niedergang CP, de Murcia G, Ittel ME, Pouyet J, Mandel P. 1985. Time course of polynucleosome relaxation and ADP-ribosylation. Correlation between relaxation and histone H1 hyper-ADP-ribosylation. European Journal of Biochemistry 146:185–191. DOI: 10.1111/j.1432-1033.1985.tb08637.x.
- Pałyga J. 1990. Variability of histone H1 in rabbit populations. International Journal of Biochemistry 22:1351–1361. DOI: 10.1016/0020-711X(90)90319-X.
- Pałyga J. 1998. Genes for polymorphic H1 histones are linked in the Japanese quail genome. Biochemical Genetics 36:93–103. DOI: 10.1023/A:1018756320718.
- Pałyga J, Górnicka-Michalska E, Kowalski A, Książkiewicz J. 2000. Natural allelic variation of duck erythrocyte histone H1.b. International Journal of Biochemistry and Cell Biology 32:665–675. DOI: 10.1016/S1357-2725(99)00153-3.
- Parseghian M. 2015. What is the role of histone H1 heterogeneity? AIMS Biophysics 2:724–772. DOI: 10.3934/biophy.2015.4.724.
- Pinnola A, Naumova N, Shah M, Tulin AV. 2007. Nucleosomal core histones mediate dynamic regulation of poly(ADP-ribose) polymerase 1 protein binding to chromatin and induction of its enzymatic activity. Journal of Biological Chemistry 282:32511–32519. DOI: 10.1074/jbc.M705989200.
- Poirier GG, Niedergang C, Champagne M, Mazen A, Mandel P. 1982. Adenosine diphosphate ribosylation of chicken-erythrocyte histone H1, H5 and high-mobility-group proteins by purified calf thymus poly(adenosine-diphosphate-ribose) polymerase. European Journal of Biochemistry 127:437–442. DOI: 10.1111/j.1432-1033.1982.tb06891.x.
- Roque A, Ponte I, Suau P. 2016a. Interplay between histone H1 structure and function. Biochimica at Biophysica Acta 1859:444–454. DOI: 10.1016/j.bbagrm.2015.09.009.
- Roque A, Ponte I, Suau P. 2016b. Post-translational modifications of the intrinsically disordered terminal domains of histone H1: Effects on secondary structure and chromatin dynamics. Chromosoma 126:83–91. DOI: 10.1007/s00412-016-0591-8.
- Rosidi B, Wang M, Wu W, Sharma A, Wang H, Iliakis G. 2008. Histone H1 functions as a stimulatory factor in backup pathways of NHEJ. Nucleic Acid Research 36:1610–1623. DOI: 10.1093/nar/gkn013.
- Spielamn D, Brook DW, Briscoe DA, Frankham R. 2004. Does inbreeding and loss of genetic diversity decrease disease resistance? Conservation Genetics 5:439–448. DOI: 10.1023/B:COGE.0000041030.76598.cd.
- Szerlong HJ, Herman JA, Krause CM, DeLuca JG, Skoultchi A, Winger QA, Prenni JE, Hansen JC. 2015. Proteomic characterization of the nucleolar linker histone H1 interaction network. Journal of Molecular Biology 427:2056–2071. DOI: 10.1016/j.jmb.2015.01.001.
- Tanaka M, Kihara M, Hennebold JD, Eppig JJ, Viveiros MM, Emerey BR, Carrell DY, Kirkman NJ, Meczekalski B, Zhou J, Bondy CA, Becker M, Schultz RM, Misteli T, De La Fuente R, King GJ, Adashi EY. 2005. H1FOO is coupled to the initiation of oocytic growth. Biology of Reproduction 72:135–142. DOI: 10.1095/biolreprod.104.032474.
- Thomas C, Ji Y, Wu C, Datz H, Boyle C, McLeod B, Patel S, Ampofo M, Currie M, Harbin J, Pechenkina K, Lodhi N, Johnson SJ, Tulin AV. 2019. Hit and run versus long-term activation of PARP-1 by its different domains fine-tunes nuclear processes. Proceedings of the National Academy of Sciences USA 116:9941–9946. DOI: 10.1073/pnas.1901183116.
- Wood C, Snijders A, Williamson J, Reynolds C, Baldwin J, Dickman M. 2009. Post-translational modifications of The linker histone variants and their association with cell mechanisms. FEBS Journal 276:3685–3697. DOI: 10.1111/j.1742-4658.2009.07079.x.
