Abstract
Twenty-four individuals of Albunea carabus (Linnaeus, 1758), a species rarely reported in the Mediterranean Sea, have recently been collected in the Tyrrhenian Sea (Italy) in hydraulic dredge surveys carried out to assess local razor clam stocks. They were found in sandy bottoms at depths of 1–6 m in association with the commercial bivalves Ensis minor and Chamelea gallina and the decapods Diogenes pugilator, Portumnus latipes and Liocarcinus vernalis. The distribution of the species in the Italian seas is described based on a careful search of the available records. Notes on its reproductive biology and autoecology are also included.
Introduction
Albunea carabus (Linnaeus, 1758) is the sole species of the family Albuneidae recorded in the Mediterranean Sea, even though fossil records of the genus Albunea in Italy date back to the Eocene (Beschin & de Angeli Citation1984). The species has albeit seldom been recorded in the Mediterranean Sea from the Alboran Sea (western Mediterranean) (Zariquiey Álvarez Citation1961) to the Levant Sea (eastern Mediterranean) (Aharoni Citation1937; Corsini-Foka & Kalogirou Citation2013) and in the eastern Atlantic from the Algarve (Portugal) (Pereira et al. Citation2008) to the Gulf of Guinea (Boyko Citation2002).
In August 2016, a specimen was collected in the Gulf of Salerno (Tyrrhenian Sea) during a hydraulic dredge survey, conducted by our research group to estimate the status of razor clam, Ensis minor (Chenu, 1843) stocks. Given the rarity of the find, photographs of the specimen were circulated to professional clam fishers to ask whether they had already encountered the species. We learned that A. carabus is locally called “fortunato” (lucky in Italian), because of the fishers’ belief that kissing and returning to the sea live specimens found in the catch will secure a good clam catch in the near future. Publication of the finding was postponed in hopes that scheduled surveys, soon to be conducted along the Tyrrhenian coast, would provide additional specimens. In the meantime, an accurate search was conducted for published records of the species in the Italian seas and for specimens held in the Decapod collections of some Italian museums.
We report all these new records of A. carabus, review the published records in the Italian seas, some of which had been overlooked in previous reviews, and provide some notes on its biology and ecology.
Materials and methods
Scientific surveys, conducted to assess razor clam stocks in the southern Tyrrhenian Sea, were carried out in 2016 and 2018 using professional vessels equipped with a hydraulic dredge (Lucchetti et al. Citation2016; Vasapollo et al. Citation2020). Hydraulic dredges like those used in our surveys consist of a metal cage, 3 m in width, whose walls are made of metal rods spaced 7 mm apart; the dredge is mounted on two lateral sledge runner skids to control digging into sediment (7–15 cm for razor clam). A hose connected to the vessel supplies pressurized water to a series of nozzles. The lower front of the cage is fitted with a scraper blade with nozzles oriented forward; water ejected through the nozzles fluidizes the sand and dislodges the marine organisms living in sediment, facilitating their collection. Another series of nozzles, placed under the roof of the dredge and oriented downward and backward, have the function of washing out sand and smaller organisms, facilitating towing of the gear. At the end of the tow, the dredge is hauled on board, its content is dropped into a collecting box and clams are hand-picked (Lucchetti & Sala Citation2012). In our surveys, a sampler consisting of a rectangular steel frame (40 cm long, 18 cm high) with an attached net bag (stretched mesh size, 14 mm), was fitted to the front of the dredge to collect undersize razor clams and the associated fauna, which are seldom retained by the dredge.
Forty-four stations were sampled in the Gulf of Salerno in 2016. The whole sampler catch was frozen and subsequently sorted in the laboratory, where the first specimen of A. carabus was picked and identified according to the key and descriptions in Boyko (Citation2002). The further dredge surveys, conducted in 2018 from the mouth of the Tiber River to the southern part of the Gulf of Salerno to investigate the density of razor clams, involved sampling of 57 stations at a depth of 1 to 5 m. The whole catch of the dredge was examined for A. carabus specimens while hand-picking the commercial size razor clams. There were 24 A. carabus specimens, which were refrigerated and taken to the laboratory. Here they were sex-sorted according to gonopore position and pleopod morphology and measured for carapace length (cl), i.e., the distance from the tip of the rostrum to the midpoint of the posterior concavity of the carapace. Unless otherwise stated, all specimens, stored in 80% ethanol, have been deposited in the collection of the Museo civico di Storia naturale in Verona under catalog numbers of the first author’s collection that includes another specimen collected in the past with the same fishing gear.
The search of the catalogs at the Museo regionale di Scienze naturali in Torino and the Museo civico di Storia naturale in Milano failed to locate any specimen, whereas two specimens are present in the collections of the Museo di Storia naturale dell’Università in Firenze, MZUF (G. Innocenti, pers. comm.), and another one is kept in the private exhibit of the Museo della Vita nelle Acque in Russi (RA) (R. Gattelli, pers. comm.).
Results
Systematics
Order DECAPODA Latreille, 1817Family ALBUNEIDAE Stimpson, 1858Genus Albunea Weber, 1795
Species Albunea carabus (Linnaeus, 1758)()
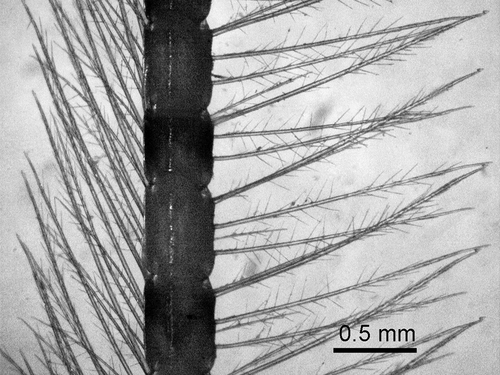
Figure 1. Albunea carabus. Specimens caught in a single night tow with hydraulic dredge in the Gulf of Salerno in 2018

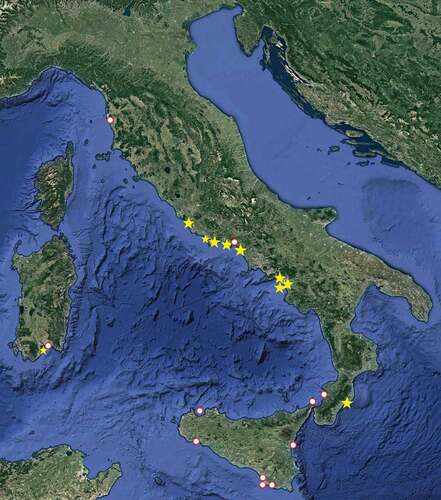
Figure 2. Records of Albunea carabus in the Italian seas: circles, literature records; stars, present material
Material examined
Tyrrhenian Sea: Gulf of Salerno (40°24.60ʹN – 14°51.22ʹE), 6 m, Aug. 4, 2016, 1 F cl. 19.0 mm (D2110); Gulf of Salerno (40°36.84ʹN – 14°50.90ʹE), 3 m, Aug. 30, 2018, night sampling, 5 M cl. 14.4–18.0 mm, 4 F cl. 20.5–24.5 mm (D2113); Gulf of Salerno (2 tows merged) (40°26ʹN – 14°58ʹE), 2–3 m, Aug. 30, 2018, 5 M cl. 14.0–17.5 mm, 5 F cl. 19.1–20.8 mm (D2114); off Baia Domizia (41°09.74ʹN – 13°49.93ʹE), 2 m, May 9, 2018, 1 F cl. 19.0 mm (D2111); Gulf of Gaeta (41°15.96ʹN – 13°09.22ʹE), 2 m, May 6, 2018, 1 F cl. 19.6 mm (D2115); Gulf of Gaeta (41°13.36ʹN – 13°31.24ʹE), 1 m, May 6, 2018, 1 F cl. 18.6 mm (D2116); off Torvaianica, 3 m, May 1993, coll. G. Iocca, razor clam hydraulic dredge, 1 F cl. 21.4 mm (D1624); off Torvaianica (41°33.77ʹN – 12°31.27ʹE), 3 m, Jun. 26, 2018, 1 F cl. 18.4 mm (CNR-IRBIM Ancona, reference collection).
Ionian Sea: Roccella Jonica, near the mouth of the Barruca River (38°18.4ʹN – 16°22.1ʹE), 5 m, Nov. 21, 2004, coll. A. Martino, in trammel net, 1 M cl. 19.0 mm, preserved dry in the exhibition of the “Museo della Vita nelle Acque” Russi; Gulf of Catania, Feb. 2017, coll. D. Scuderi, stranded ashore, 2 F cl. 17.9–19.6 mm, dried specimens subsequently stored in ethanol (D2112).
Since our specimens () match the detailed descriptions and illustrations provided by Zariquiey Álvarez (Citation1961) and Boyko (Citation2002), no further details are given here.
Albunea carabus was collected at depths of 1.5 to 3 m at 7 (12%) of the 57 stations sampled in 2018, which are characterized by medium-fine sands; coarser fraction was significant only in some station close to the shore. The benthic community associated with A. carabus included the commercial bivalves E. minor (Chenu, 1843), Chamelea gallina (Linnaeus, 1758) and Donax trunculus (Linnaeus, 1758), the gastropod Tritia mutabilis (Linnaeus, 1758), the echinoderm Echinocardium cordatum (Pennant, 1777), the decapods Diogenes pugilator (Roux, 1829), Liocarcinus vernalis (Risso, 1827) and Portumnus latipes (Pennant, 1777), and the sipunculid worm Sipunculus nudus (Linnaeus, 1766). It can be ascribed to the biocoenosis of infralittoral “well-sorted fine sands” as defined by Pérès and Picard (Citation1964).
The literature search found the first mention of A. carabus in the Italian seas as Hippa symnista (Petagna Citation1792, pp. 413–414). Petagna followed the systematic arrangement of taxa proposed in “Mantissa insectorum” (Fabricius, Citation1787). Petagna noted that all the species of the genus Hippa were exotic except H. symnista, which he described with the following diagnosis: “Thorax semicylindricus, lateribus inaequalibus, auctis spina antrorsum spectante. Margo anticus obtusus, serratus, ciliatus. Antennae, utrinque pilis ciliatae. Cauda articulis sex. Brachia inermia. Manus cordatae, pilose. Pollex subulatus, arcuatus. Pedes octo. Plantae bifidae, ciliatae”. The description allows Petagna’s H. symnista to be identified with the species currently known as A. carabus, not with Corystes cassivelaunus (Pennant, 1777, Pl. 7), which original figure is quoted in the synonymy of H. symnista in the “Mantissa insectorum”. Fabricius (Citation1793) subsequently realized to have mixed up two species under the name symnista and in the new “Entomologia Systematica emendata” he omitted the reference to Pennant in the description of Hippa symnista and named Pennant’s species Hyppa dentata.
The second mention of A. carabus was by Rafinesque-Schmaltz (Citation1814), who reported it as Albunea symnista from Sicily, without precise locality. Zava et al. (Citation2019) have suggested that it may have been found near Messina.
Only two specimens had been collected near Catania (Philippi Citation1857, p. 129; Magrì Citation1911, p. 40) until February 2017 when thousands of specimens were found stranded on the beach south of the town (Scuderi et al. Citation2017, Citation2019). Additional records from the seas around Sicily have been published by Giacobbe and Spanò (Citation1996), Spanò et al. (Citation1999), Pipitone and Arculeo (Citation2003) and Zava et al. (Citation2019). The species has also been collected in the Gulf of Cagliari (Sardinia) (Mura & Corda Citation2011). In the eastern Tyrrhenian Sea, where hydraulic dredgers harvest razor clams since the late 1960s, the capture off Formia of four specimens by a dredger was reported by Moncharmont (Citation1969); another specimen, reported in the present study, was caught with the same gear off Torvaianica in 1993.
All the records of A. carabus in the Italian seas, including those from our surveys and those in the literature, are summarized in and . Possible records based only on photographs, posted online, have not been considered.
Table I. Summary of previous publications, in chronological order, and present records of Albunea carabus in the Italian seas (estimated geographic coordinates in italics)
Discussion
The range of A. carabus includes the eastern Atlantic Ocean, from southern Portugal (Pereira et al. Citation2008) to the Gulf of Guinea and Macaronesia (Boyko Citation2002), and the Mediterranean Sea. Rubió and Holthuis (Citation1976), while reporting some new collections from the Catalan coast, provided a list of previous Mediterranean records known to them. The list was partially updated by Pereira et al. (Citation2008) and Zava et al. (Citation2019), who reported new captures from the Algarve (Portugal) and southern Sicily, respectively. Most records are based on one or a small number of specimens. Before our Tyrrhenian records, most A. carabus specimens in the western Italian seas had been collected at sites around Sicily. The two specimens caught with trammel net in the southern Ligurian Sea (Silvestri et al. Citation2001) represent the northernmost record in the Mediterranean, since Boyko (Citation2001) provided evidence that Hippa caerulea (Risso Citation1816), described in Nice (France), was based on a gnathid isopod and was not a synonym of A. carabus, as suggested by Holthuis (Citation1977). The specimen herein reported from Roccella Jonica (Ionian coast of Calabria) is, to the best of our knowledge, the easternmost record in Italy.
A. carabus does not seem to live in the Adriatic Sea, since no specimen has been collected in over 40 years of extensive sampling of its coastal sandy bottoms with hydraulic dredges targeting razor clam (Froglia Citation1975; Del Piero et al. Citation1984) and venus clam (Froglia Citation2000). One specimen has been reported, without comment, in a study of the stomach contents of poor cod, Trisopterus capelanus (Lacepède, 1800) sampled with a bottom trawl net in the eastern Adriatic Sea at a depth of 50–175 m (Dulčič & Dulčič Citation2004). The small size of the fish (total length <14 cm), the fishing depth, and the difficulty in identifying material at various stages of digestion in stomach contents let us suppose that a misidentification occurred. Indeed, all our samples and most of the literature records are from shallow sandy bottoms (depth <10 m). The deepest record in the literature (50 m) regards a specimen caught in a professional shrimp trap off Rhodes, Aegean Sea (Corsini-Foka & Kalogirou Citation2013), whereas the specimens on which Lucas (Citation1853) described Albunea guerinii, a junior synonym of A. carabus, were collected at 30–40 m in the Bay of Algiers (southern Mediterranean).
The long antennulae of A. carabus, which are almost three times the length of the carapace, bear two rows of plumose setae (); when paired, they act as a snorkel, facilitating the flow of water into the branchial chamber while the animal lies buried in sediment (Garstang Citation1897). This feature allows A. carabus to bury quite deep in the sand. This behaviour makes it less vulnerable to the sampling gears used in scientific studies, and probably led to its presumed rarity. The increased number of records in recent years are related to accidental captures by professional fishers who, as in past centuries, continue to add to our knowledge of marine biota. At least four of the Italian records are based on specimens caught by trammel nets and gill nets, which are commonly set in the evening and retrieved early in the morning. It has been suggested (Zava et al. Citation2019) that A. carabus may be active on the sediment surface during the night and get entangled in the nets. This habit would explain our catch of nine specimens (density 1/33 m2) in a single late-night tow, but the capture of the species in only 12% of the sampled stations may also point out a patchy distribution.
Two massive stranding episodes, recorded in March 1954 and February 2017, respectively, in the Bay of Algiers (Piguet Citation1955) and on a beach in Catania (Scuderi et al. Citation2017, Citation2019), indicate that A. carabus is actually far from rare in Mediterranean coastal sandy habitats. The two episodes followed heavy rain with flooding of local rivers; on both occasions, stormy seas washed ashore thousands of dead or moribund A. carabus specimens, which were probably forced out of the sediment by a local reduction in salinity due to the exceptional river runoff.
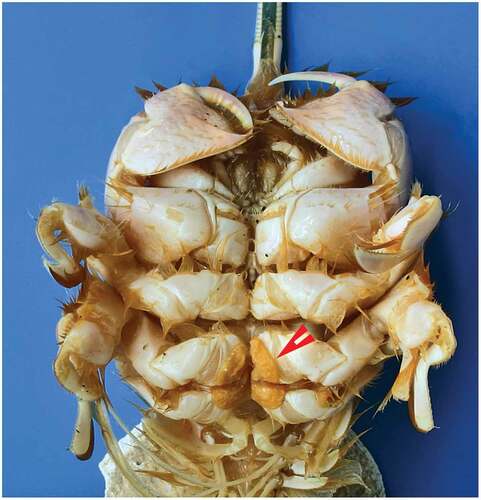
Almost no information is available on A. carabus reproduction. Ovigerous females seem to be highly elusive. In his monograph, Boyko (Citation2002) mentions a single ovigerous female among the 22 females of A. carabus examined in museum collections. The review of all Mediterranean records disclosed only two ovigerous females, collected, respectively, near the strait of Messina in October 1992 (Giacobbe & Spanò Citation1996) and off the Balearic Islands in June 1987 (Garcia Socias & Gracia Citation1988). The “ovigerous” female collected near Marina di Ragusa in May 2017, reported by Zava et al. (Citation2019, )), actually does not carry eggs on the pleopods, but an orange spermatophore mass attached to the sternal region.
In our samples collected in the Gulf of Salerno in August 2018, one female (cl. 20.6 mm) still had some egg envelopes attached to the setae of pleopods, whereas four other females (cl. 19.1–21.1 mm) had a yellowish striated sperm mass attached to the coxae of the 3rd and 4th pairs of pereopods, like the female collected in May 2018 off Baia Domizia (). A similar spermatophore-containing structure was described by Subramoniam (Citation1984) in A. symnista and has been observed in several other Albunea species (Boyko Citation2002). Albuneid larvae identified as A. carabus, the only member of the family found in the Mediterranean, have been collected off the Algerian coast mainly in summer (Seridji Citation1988). These scattered observations suggest that the reproductive season of A. carabus extends from late spring to early autumn.
At present, several other aspects of the life history and ecology of this beautiful and elusive species still remain unknown.
Acknowledgements
We are indebted to the crews of the commercial vessels for their support and to the students and personnel of CNR – IRBIM who kindly helped with laboratory activities. We thank G. Innocenti, Museo di Storia Naturale dell’Università di Firenze (MZUF) and R. Gattelli, Museo “La Vita nelle Acque” in Russi, for sharing information on the specimens of A. carabus in the collections under their care, We are grateful to Word Designs (www.silviamodena.com) for the language revision.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aharoni I. 1937. Données zoogéographiques sur la fauna de la Palestine et des contrées adjacentes par rapport à celles de la Péninsule Ibèrique. XII Congrés International de Zoologie, 15–21 Septembre 1935, Lisbonne. pp. 1132–1137.
- Beschin C, de Angeli A. 1984. Nuove forme fossili di anomura Hippidea: Albunea cuisiana sp.n. e Albunea lutetiana sp.n. Lavori Società Veneziana di Scienze Naturali 9(1):93–105.
- Boyko CB. 2001. The identity of Hippa caerulea Risso, 1816: An isopod in mole crab’s disguise. Crustaceana 74(1):115–122. DOI: 10.1163/156854001505299.
- Boyko CB. 2002. A worldwide revision of the recent and fossil sand crabs of the Albuneidae Stimpson and Blepharipodidae, new family (Crustacea: Decapoda: Anomura: Hippoidea). Bulletin of the American Museum of Natural History 272:1–396. DOI: 10.1206/0003-0090(2002)272.
- Corsini-Foka M, Kalogirou S. 2013. First record of Albunea carabus (Linnaeus, 1758) (Decapoda: Anomura: Hippoidea) in the Aegean Sea. Cahiers de Biologie Marine 54(2):297–299. DOI: 10.21411/CBM.A.AD3E1818.
- Del Piero D, Orel G, Valli G, Vio E, Froglia C. 1984. Aspetti e problemi della biologia e pesca di Ensis minor (Chenu) nel golfo di Trieste. Nova Thalassia 4:173–191. [ 1980].
- Dulčič J, Dulčič Z. 2004. Feeding habits of the Mediterranean poor cod Trisopterus minutus capelanus (Lacepède) (Pisces: Gadidae) from the Eastern Central Adriatic. Annales, Series Historia Naturalis 14(2):189–196.
- Fabricius JC. 1787. Mantissa Insectorum. Sistens eorum species nuper detectas adiectis characteribus genericis, differentiis specificis, emendationibus, observationibus. Tom.1. Hafniae: Impensis C.G. Proft. pp. xx+1–348.
- Fabricius JC. 1793. Entomologia Systematica Emendata et Aucta. Secundum Classes, Ordines, Genera, Species, Adjectis Synonimis, Locis, Observationibus, Descriptionibus. Tom.2. Hafniae: Impensis C.G. Proft. pp. viii+1–519.
- Froglia C. 1975. Osservazioni sull’accrescimento di Chamelea gallina (L.) ed Ensis minor (Chenu) nel medio Adriatico. Quaderni del Laboratorio di Tecnologia della Pesca 2(1):37–48.
- Froglia C. 2000. Il contributo della ricerca scientifica alla gestione della pesca dei molluschi bivalvi con draghe idrauliche. Biologia Marina Mediterranea 7(4):71–82.
- Garcia Socias L, Gracia F. 1988. Nuevas aportaciones a la fauna de Crustácea Decápoda de las Islas Baleares. Boletín de la Sociedad de Historia Natural de Baleares 32:47–56.
- Garstang W. 1897. On some modifications of structure subservient to respiration in Decapod Crustacea which burrow in sand with some remarks on the utility of specific characters in the genus Calappa, and the description of a new species of Albunea. The Quarterly Journal of the Microscopical Society, N.S. 40(2):211–232, pls 12–14.
- Giacobbe S, Spanò N. 1996. New records of Albunea carabus (L., 1758) (Decapoda, Anomura) in the Mediterranean Sea. Crustaceana 69(6):719–726. DOI: 10.1163/156854096X00736.
- Holthuis LB. 1977. The Mediterranean decapod and stomatopod Crustacea in A. Risso’s published works and manuscripts. Annales du Muséum d’Histoire Naturelle de Nice 5:37–88.
- Lucas H. 1853. Mélanges et nouvelles, Albunea. Revue et Magasin de Zoologie pure et appliquée Ser.2, 5:45–47, pl. 1.
- Lucchetti A, Sala A. 2012. Impact and performance of Mediterranean fishing gear by side-scan sonar technology. Canadian Journal of Fisheries and Aquatic Sciences 69(11):1806–1816. DOI: 10.1139/f2012-107.
- Lucchetti A, Vasapollo C, Virgili M. 2016. Indagine sull’abbondanza della risorsa cannolicchio nel mar Tirreno. Rapporto finale Convenzione tra MIPAF e CNR-ISMAR di Ancona per la “Valutazione della pesca dei bivalvi nella fascia costiera compresa nelle 0,3 miglia nautiche”. CUP J52I15003990001: pp. 73.
- Magrì F. 1911. I Crostacei Decapodi del Compartimento marittimo di Catania. Atti Accademia Gioenia di Scienze naturali, Catania Ser.5, 4(14):1–46.
- Moncharmont U. 1969. Sulla presenza di Albunea carabus (L., 1758) nel Mediterraneo (Crustacea, Decapoda, Anomura). Pubblicazioni della Stazione Zoologica di Napoli 37:434–440.
- Mura M, Corda S. 2011. Crustacea Decapoda in the Sardinian channel: A checklist. Crustaceana 84(5–6):667–687. DOI: 10.1163/001121611X572823.
- Pereira AM, Rufino MM, Gaspar MB. 2008. First record of Albunea carabus (Decapoda, Anomura, Hippidea) from the Algarve coast, South Portugal. Marine Biology Research 4(3):236–238. DOI: 10.1080/17451000801907955.
- Pérès JM, Picard J. 1964. Nouveau manuel de bionomie benthique. Recueil des Travaux de la Station marine d’Endoume 31(47):5–137.
- Petagna V. 1792. Institutiones Entomologicae. Tomus 1. Neapoli: Typis Cajetani Raymundi. pp. xii+439.
- Philippi RA. 1857. Abrote, ein neues Geschlecht der Crustaceen, aus der Familie der Hippaceen. Archiv für Naturgeschichte 23(1):124–129, pl. 8.
- Piguet P. 1955. Présentation des echantillons d’un petit Crustacé décapode anomoure Albunea carabus L. (= Albunea Guerini Lucas). Bulletin de la Société d’Histoire naturelle de l’Afrique du Nord 46:14–15.
- Pipitone C, Arculeo M. 2003. The marine Crustacea Decapoda of Sicily (central Mediterranean Sea): A checklist with remarks on their distribution. The Italian Journal of Zoology 70:69–78. DOI: 10.1080/11250000309356498.
- Rafinesque-Schmaltz CS. 1814. Précis des découvertes et travaux somiologiques de Mr. C.S. Rafinesque-Schmaltz entre 1800 et 1814. Ou choix raisonné de ses principales découvertes en Zoologie et en Botanique, pour servir d’introduction à ses ouvrages futurs. Palerme: Royale Tipographie Militaire. pp. 55.
- Risso A. 1816. Histoire naturelle des Crustacés des environs de Nice. Paris: Libraire Grecque-Latine-Allemande. pp. 175, 3 pls.
- Rubió M, Holthuis L. 1976. New records of Albunea carabus (L.) from Spanish waters (Decapoda, Anomura, Hippidea). Thalassia Jugoslavica 8(1):41–47. [ 1972].
- Scuderi D, Villari A, Angelico M. 2017. A massive record of the rare ‘mole crab’ Albunea carabus (Decapoda: Anomura: Hippoidea) along the sandy coasts of Catania. Bulletin of the Entomological Society of Malta 9:43.
- Scuderi D, Villari A, Angelico M. 2019. Massive beaching record along the sandy coast of Catania (E Sicily) of the rare “mole crab” Albunea carabus (Linnaeus, 1758) (Decapoda Anomura Hippoidea). Biodiversity Journal 10(4):405–408.DOI:10.31396/Biodiv.Jour.2019.10.4.405.408.
- Seridji R. 1988. Some planktonic larval stages of Albunea carabus (L., 1758) (Crustacea, Decapoda, Anomura). Journal of Natural History 22(5):1293–1300. DOI: 10.1080/00222938800770791.
- Silvestri R, Righini P, Violani A. 2001. Incidental capture of Albunea carabus (L., 1758) (Decapoda, Anomura) in the northern Tyrrhenian Sea. Biologia Marina Mediterranea 8(2):630–632.
- Spanò N, Rinelli P, Ragonese S. 1999. The first find of Albunea carabus (Decapoda, Anomura) on the Southern Sicilian coasts (Strait of Sicily, Mediterranean Sea). In: Schram FR, von Vaupel Klein JC, editors. Crustaceans and the biodiversity crisis. Leiden: Brill. pp. 617–621.
- Subramoniam T. 1984. Spermatophore formation in two intertidal crabs Albunea symnista and Emerita asiatica (Decapoda: Anomura). The Biological Bulletin 166:78–95. DOI: 10.2307/1541432.
- Vasapollo C, Virgili M, Bargione G, Petetta A, De Marco R, Punzo E, Lucchetti A. 2020. Impact on macro-benthic communities of hydraulic dredging for razor clam Ensis minor in the Tyrrhenian Sea. Frontiers in Marine Science 7:14. DOI: 10.3389/fmars.2020.00014.
- Zariquiey Álvarez R. 1961. Decápodos españoles XV. Sobre un raro Crustáceo Decápodo: Albunea carabus (Linneo 1758). Investigacion Pesquera 19:103–110.
- Zava B, Insacco G, Corsini-Foka M. 2019. Additional findings of Albunea carabus (Linnaeus, 1758) (Decapoda, Anomura, Hippoidea) in Italian waters. Acta Adriatica 60(1):91–98. DOI: 10.32582/aa.60.1.10.