Abstract
Anal papillae of caddisflies are peripheral organs responsible for osmoregulation and detoxification. Investigation of morphological abnormalities in the anal papillae of Hydropsyche angustipennis enriched with using SEM-EDX analysis (scanning electron microscopy-energy dispersive X-ray analysis), was used to assess heavy metal pollution levels in urban streams receiving surface runoff. Heavy metal ions not previously detected in water and tissue samples by commonly used methods (e.g., AAS) were detected using SEM-EDX method. Analysis of heavy metal in the anal papillae revealed the presence of 11 elements (Fe>Mo>Mn≥Al>Cu>Pb≥Ni>Co≥As≥Ti≥V). Morphological irregularities were most frequently observed in larvae from the most contaminated streams. Most of the individuals collected from streams flowing through the city center and included in the sewage system had morphological abnormalities (~70%), contrary to the reference site (~10%). In pale, normal-shaped papillae the quantity of heavy metals was almost 10 times lower compared to darkened papillae. The present study confirms that SEM-EDX microscopy is an effective method as a support of standard heavy metal bioassays, especially if there is a necessity to detect trace elements in very small amounts of the tested material.
1. Introduction
The assessment of pollution impact on aquatic ecosystems is usually based on both physical and chemical analysis of water quality and biomonitoring of living organisms. However, the emphasis in the evaluation of the environment pollution of surface water has been recently shifted to the monitoring of bioindicator species (Rochfort et al. Citation2000; Wright Citation2000; Siddig et al. Citation2016). Among the traditionally used bioindices, the majority are founded on taxonomic differentiation of aquatic invertebrates or multispecies assemblages’ sensitivity in response to human-induced stressors (Kefford et al. Citation2011). Also morphological (Olaifa et al. Citation2004) or behavioural (Johnson et al. Citation1993; Marqués et al. Citation2003) changes in comprehensively studied species, that are caused by modifications of environment quality, may turn out to be as exploitable as commonly used biotic indices (Tszydel et al. Citation2015). The use of individual taxons as one-species universal bioindicators is increasing considering the assessment of aquatic pollution.
One of the main purposes of the current policy, e.g. Water Framework Directive (Directive 2000/60/EC in EU) is the protection and improvement of aquatic environment in all types of surface waters. The protection programme also applies to highly modified, polluted urban streams, usually inhabited only by eurytopic species (Bonada et al. Citation2005). The wide tolerance range of these organisms limits their usefulness in pollution assessment (Hilty & Merenlender Citation2000). On the other hand, such less sensitive species, resistant to substantial changes of water quality, may be used for detection of dangerous pollutants, e.g. heavy metals, as non-destructive environmental bioindicators (Macedo-Sousa et al. Citation2008).
Evaluation of heavy metal pollution is often based on the assumption that metals can accumulate in organism tissues, which reflect the environment contamination (Cain et al. Citation2004; Gharbi et al. Citation2012). Such bioaccumulation-based methods may also effectively track elements, which are often in very low concentrations in the environment (Tszydel et al. Citation2015). However, the obtained results may not reflect the water quality conditions correctly, because organisms show diverse levels of assimilation, detoxification, metal tolerance, and active absorption/removal of particular elements (Cain et al. Citation2004). Discrepancies may appear when heavy metals are being excessively assimilated from sediment (Dukowska et al. Citation2012; Grzybkowska et al. Citation2015), adsorbed on the body surface (Vuori & Kukkonen Citation1996) or accumulated in subsequent trophic levels (Rainbow Citation2002; Watanabe et al. Citation2008). Higher levels of heavy metal concentration are observed in early larval stages, in which the detoxification system is not fully developed. On the other hand, several larval stages in the life cycle result in lower levels of contamination (Buchwalter & Luoma Citation2005).
Morphological abnormalities of bioindicator species among aquatic macroinvertebrates offer another useful tool for pollution assessment, reflecting metal exposure conditions via element concentrations in organism tissues (Vuori & Kukkonen Citation1996). Body deformations should be clearly visible, easy to compare with not contaminated individuals and irreversible even after ceasing the pollution, but simultaneously not lethal (therefore, they are often referred to as “sublethal effects”) (Vuori Citation1994; Carls et al. Citation1999; Baby et al. Citation2010). Depending on the species, such changes may consist of irregularities, e.g. in the head capsule structure, mouthparts, tracheal gills or anal papillae (Kefford et al. Citation2011). In case of caddisflies, morphological abnormalities are usually connected with changes in water chemistry and based on the analysis of tracheal gills and anal papillae structure (Vuori & Kukkonen Citation1996). Larvae of Hydropsychidae seem to be a promising tool in biomonitoring of surface waters, especially for streams flowing through urbanized areas (Bonada et al. Citation2005; Tszydel et al. Citation2015). They are widely distributed and resistant to chemical and physical modifications of stream environments. The presence of heavy metals in water manifests itself in their accumulation in larvae tissues as well as in anal papillae abnormalities such as darkening and constricting (Vuori & Kukkonen Citation1996; Tszydel et al. Citation2015). The degree of such morphological irregularities in response to water pollution changes gradually, which can be particularly informative (Vuori & Kukkonen Citation1996).
In our previous studies, we have also observed suggested correlation between heavy metal contamination in water and tissue samples, and sublethal effects leading to morphological changes in the anal papillae (Tszydel et al. Citation2015, Citation2016). However, due to the small size of the anal papillae (2–3 mm), it has not been tested for heavy metal accumulation so far. In the present study, our aim was to directly confirm that this particular organ (anal papillae) has the ability to deposit heavy metals depending on the level of contamination. The method most often used for tissue analysis, such as AAS (Atomic Absorption Spectrometry) was not possible to apply in this research due to the small size of the tested organ. Thus, we used SEM-EDX analysis technique enabling qualitative identification of all trace elements in the tested sample, even such small sizes. By SEM-EDX microscopy analysis, we were also able to compare particular parts of the anal papillae e.g. pale with darkened ones. We supplemented the analysis of particular elements presence (Fe, Mo, Mn, Al, Cu, Pb, Ni, Co, As, Ti, V) with visual observations of anal papillae. We aimed to prove that this organ can serve as an effective tool in water quality assessment, especially at the screening stage, as its state can be verified intravitally and indicates the effect through accumulation of contaminants, which could form the basis for further research.
2. Materials and methods
2.1. Study area
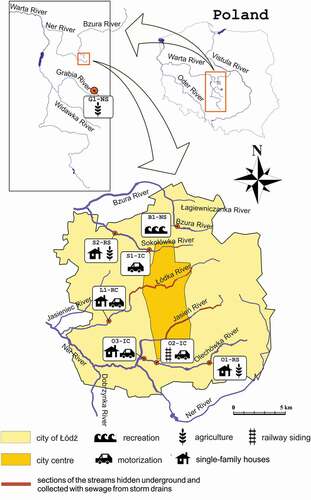
The study was conducted in streams located in the city of Łódź (central Poland). In contrast to most other large cities, Łódź is not situated on a single large river, but has a channel network consisting of almost 30 small streams. Sampling sites were established so that they do not differ in terms of basic environmental parameters, but only in the degree of human-mediated stream bed modification and the level of water pollution (). Study has been done on eight sampling sites, two control and six polluted (under impact). Detailed characteristics of the sampling sites were included in Tszydel et al. (Citation2015). Seven out of the eight sampling sites were located within the city (on the rivers Bzura, Sokołówka, Łódka and Olechówka). Two reference sites (as a control) were assigned for the river network of the Łódź agglomeration, one located on the outskirts of the Łódź city, in the Bzura River (B1-NS), and the other one in the Grabia River running outside the urban area (G1-NS) (). Anthropogenic alterations of streambeds as well as potential sources of pollution are shown in .
Figure 1. Study area and location of the sampling sites. Graphic symbols show the type of pollution being the main source of heavy metals in the studied rivers. Sampling sties were encoded as follows: the first letter of the code is the name of a stream (B – Bzura, S – Sokołówka, L – Łódka, O – Olechówka and G – Grabia), the next is a number of the station, the third mark means the degree of riverbed naturalness (N – natural, R – regulated, I–isolation by covering the bottom with concrete and/or bricks). The last letter indicated the relation of the river to the municipal sewage system (C- stream included in the sewage system, S – stream in areas of separate sewage system)
2.2. Heavy metal analysis in anal papillae
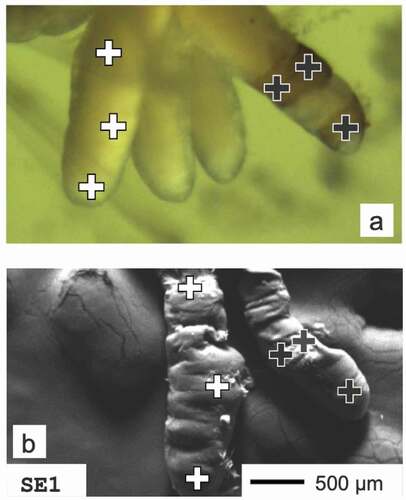
Samples were taken once a month from March till June 2011, just after winter thaws and spring heavy rains. At each sampling site 30 larvae individuals of H. angustipennis in the full-grown, fifth instar were randomly taken (collected from stones and available objects immersed in water; hand sorted). Specimens were rinsed twice with deionized water in order to remove contamination from the body surface and stored in 70% ethanol. In the laboratory, before heavy metal evaluation, larvae were photographed (Nikon SMZ 1000 and OptaTech HD digital camera) for anal papillae morphological comparisons (). In H. angustipennis there are always four anal papillae. In unpolluted streams, this organ is invisible, hidden in the last (9th) abdomen segment (Edington & Hildrew Citation1995). In response to increasing contamination, papillae start to grow and begin to protrude outside as white or pale finger-shaped structures (marked as PALE; ) to function more efficiently (Wichard Citation1976). Larvae with partially darkened anal papillae were marked as COLRAP ± () and those completely darkened as COLRAP (). Presence of pale papillae, as well as its darkening and structural changes, were assigned as morphological abnormalities of the anal papillae. For each category, mean values of the percentage of larvae with irregularities compared to all collected specimens were indicated for a particular sampling term and site. Only the most advanced/last larvae stage (5th instar) was taken into the analysis. Larvae stages were identified based on our experience, however, in case of any doubts, we used the keys: Edington and Hildrew (Citation1995) as well as Hildrew and Morgan (Citation1974), where particular species ranges of the head capsule and other diagnostic features for each larval stadium are provided. In the temperate climate zone, the 5th instar stage is present in the water the longest and the larvae collected for the study were the last molting at least 3 months earlier (the species has an annual life cycle). Thus, the process of larval molting did not influence the assessment of darkening. After visual observation and taking a photograph of the larvae, the last abdominal body segments were dissected for SEM-EDX analysis. From each sampling site we have randomly selected 10 individuals with visible anal papillae (at least one papillae). Samples were then placed in 2% glutaraldehyde and dehydrated in graded ethanol concentrations (30–96%) and acetone. In order to remove water from the samples and avoid deformation, critical point dryer (POLARON CPD), was used. For microscope examination, the samples were coated with carbon and gold in a vacuum sprayer (JEOL JEE 4 C vacuum evaporator). Papillae were then mounted to the pins with double-sided adhesive carbon tape, keeping their position parallel to the pin surface. The approximate thickness of papillae at the analysis points is only 1 mm in diameter and thus it is not limiting the SEM-EDX analysis or causing analysis errors. Elemental analyses were carried out with FEI QUANTA 250 scanning electron microscope (FEI Company) equipped with energy-dispersive X-ray spectroscopy module (EDX) (Grabka et al. Citation2015). Measurements were carried out at three different measuring points (), then the results were averaged for individual categories of sublethal effects. In case of papillae classified as COLRAP and COLRAP ±, only darkened places were analyzed. The samples were analysed using always the same tilt angle – 0° (approx. 90° take-off angle) and with the accelerating voltage set to 30kV. We used 0.1% as the detection limit for the EDX analysis (Goldstein et al. Citation2003), where Wt% is concentration of the element in terms of the mass fraction of that element in the sample. EDX detectors collected a spectrum for every pixel of the frame. The spectra were then processed into a set of element intensity maps and analysed using MultiSpec© (Biehl & Landgrebe Citation2002), a multispectral image analysis software, in which a clustering algorithm is used to identify and quantify groups of mutually exclusive chemical compositions. EDX analysis is generally used for qualitative assessment of particular elements, however, the method can have wider application (Goldstein et al. Citation2003).
Figure 2. Location of anal papillae and their abnormalities. Sublethal effects were analyzed in larvae of H. angustipennis, where (a) PALE - anal papillae visible, (b) COLRAP± one to four anal papillae partially darkened, (c) COLRAP - anal papillae completely darkened
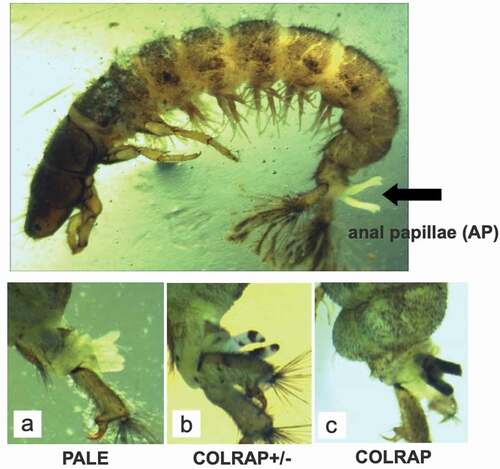
Figure 3. Measuring points of H. angustipennis anal papillae. (a) Upper photo was taken with the digital camera, (b) lower photo comes from SEM microscopy
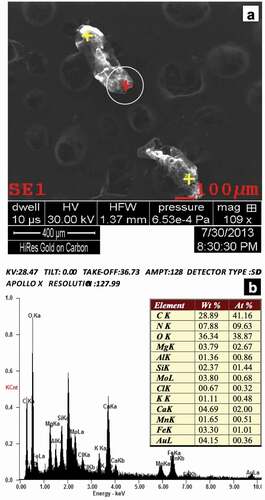
In our case, the system provided information on the qualitative chemical composition of a sample, showing the weight percentage and atomic percentage for each element of interest in a given point on the SEM image (). For each point, three measurements were made and those from which a repetitive spectrum was obtained were implemented. Before the EDX analysis of the biological material tested, the microscope operating conditions and microanalytical parameters were set. These included specifying microscopic parameters related to detector geometry and system calibration. Calibration was performed whenever the curve in the HPD (Halographic Peak Deconvolution) profile did not coincide with the spectrum profile for the main peaks. SEM-EDX was calibrated using a copper/aluminium reference pin. The calibration method uses spectral lines for AlK and CuK. To identify the spectrum, the HPD function was used to collect the spectra and generate a theoretical spectrum for comparison with the resulting set of spectra. Only the peaks in which the collected peaks were superimposed on the theoretical peaks were accepted ().
2.3. Data analysis
All analyses were performed with the use of STATISTICA software system version 10 (StatSoft Inc. Citation2011). Kolmogorov–Smirnov test with Lilliefors correction was used to test for normality. To standardize and stabilize the distribution of variance, the logarithmic transformation of variables (log10 (x + 1)) was made (Elliott Citation1977; Sokal & Rohlf Citation2005). One-way ANOVA test (preceded by Levene’s test) was performed in comparisons: between concentration of heavy metals in the anal papillae as well as deformities of anal papillae. In all statistical analyses, the level of significance was equal to p < 0.05 (Zar Citation1999).
3. Results
3.1. Sublethal effects of anal papillae
Analysed individuals had various stages of irregularities. Subsequent darkening of the anal papillae was observed as well as larvae with two out of four papillae darkened. In some specimens all papillae were partially darkened – only at the top of the papillae or black rings (circuited in black) around the papillae (, ). In larvae with all papillae darkened completely, also structural deformation (rugosity/shrinking) of the papillae was observed, which is the final mark of degradation (). Such changes were recorded in larvae from the L1-RC and O3-IC sampling sites. At the reference sites (B1-NS and G1-NS), in almost half of the individuals the anal papillae were not even protruded outside the last abdominal segment (). Only a small percentage of larvae had partially (COLRAP ±) or completely darkened (COLRAP) papillae (8–11% and less than 10%, respectively). An opposite situation was observed in samples from streams flowing through the city center and included in the sewage system, where most individuals had morphological abnormalities. In larvae collected from the Rivers Sokołówka and Olechówka (S1-IC and S2-NS, O1-RS and O2-IC, respectively), less than 30% of specimens had no changes in the anal papillae, while this value for larvae at the L1-RC sampling site amounted to only 2%. Mean values of the observed abnormalities differed among sampling sites within each category: PALE (FANOVA(7,24) = 3.7705 and pANOVA<0.0068), COLRAP ± (FANOVA(7,24) = 9.1353 and pANOVA<0.0000) and COLRAP (FANOVA(7,24) = 2.2101 and pANOVA<0.0498) (). The values for individual categories in did not sum up to 100%, as some of the larvae did not have any visible/exposed anal papillae.
3.2. Heavy metals in anal papillae
Analysis of heavy metal in the anal papillae revealed the presence of 11 elements. Summarised mean values of the percentage of heavy metals in the anal papillae cuticula did not exceed 10% at any of the sampled stations (). Heavy metal domination in anal papillae was presented in increasing order Fe>Mo>Mn≥Al>Cu>Pb≥Ni>Co≥As≥Ti≥V (). The lowest values for most elements were recorded for reference sites flowing beyond the city center (G1-NS and B1-NS). Iron reached maximum values (13%) in papillae from larvae collected at the S1-IC sampling site and dominated in the papillae of larvae from O2-IC and L1-RC (). Aluminium was detected in larvae papillae at all sampling sites (highest values L1-RC), and it was the only element recorded at the reference site G1-NS, although sometimes below the accepted detection limit. Most of the investigated elements, even nickel, were observed in the anal papillae collected at the sampling site localized near the railway siding O2-IC. Considering the mean values of heavy metal concentrations in the anal papillae, sampling sites differed significantly (FANOVA(7,51) = 5.7109 and pANOVA<0.0001), which results from discrepancy among values of Fe, Mo, Mn and Al and the total surface load of heavy metals (). In both pale and darkened papillae, similar elements were recorded, however, in different concentrations and dominance (FANOVA(2,56) = 20.0931 and pANOVA<0.0000) (). For protruded, but not darkened papillae (PALE) mean values were ten times lower compared to completely darkened ones (COLRAP). In the case of partially and totally darkened papillae, the order of leading heavy metals was similar and as follows: Fe>Mn>Mo>Al and Fe>Mo>Mn>Al, respectively. For PALE, the order was different: Al>Cu>Ni>Fe>Pb ().
Table I. Mean values of heavy metals accumulated in anal papillae. Mean values (min-max) of the percentage of heavy metals (Wt – weight ratio) in H. angustipennis anal papillae at the sampling sites. F values represent the results of one-way ANOVA test. Significant differences (p) among sampling sites were marked in bold
4. Discussion
It was commonly recorded that heavy metal concentration in the tissues of hydropsychid larvae corresponds to the contamination of water and sediment at sites where the source of pollution was urbanization, industry (Vuori & Kukkonen Citation1996; Cain et al. Citation2006; Poteat & Garland Citation2013) or agriculture (Girgin et al. Citation2010). H. angustipennis, like other representatives of this family (Moore et al. Citation1991; Chiba et al. Citation2011), are metallotolerant organisms and capable of accumulating heavy metals in the body tissues even if the elements in the environment are in very low concentrations (Tszydel et al. Citation2015, Citation2016). Although there is usually a positive relationship between the presence of heavy metals in the water and the same elements accumulated in the body tissues, it may not always be true for all elements detected. The presence of a particular heavy metal in the larval bodies may result from the rate of assimilation of the element and the removal efficiency when its concentration becomes toxic (Buchwalter & Luoma Citation2005; Cain et al. Citation2006), the duration of exposure to pollution (Tszydel et al. Citation2015, Citation2016) and the feature of bottom sediments (Stephansen et al. Citation2016). Exposed to pollution, organisms respond in various ways. For aquatic insect larvae, which possess anal papillae, the organ serves as a regulatory and supportive in detoxification. Their epithelium is covered with a structure of highly concentrated, specialized cells for osmoregulation via active and passive transport of ions (Kefford et al. Citation2011). In these cells, heavy metals can be bonded and precipitated by metallothioneins (Darlington & Gower Citation1990; Mason & Jenkins Citation1995). The observed darkening could also be the result of increasing concentration of melanophores (Radhakrishnan et al. Citation2000). Darkening and deformation of anal papillae due to water contamination appear when heavy metal and metaloid concentrations in the environment exceed the physiological ion exchange ability (Vuori & Kukkonen Citation1996). Morphological abnormalities in the anal papillae of Hydropsyche spp. in response to water pollution, also in contact with heavy metals, were observed under laboratory conditions (Vuori & Kukkonen Citation1996; Cain et al. Citation2000) as well as in the field (Tszydel et al. Citation2015). Analysis of such deformities in natural conditions can be applied especially when contamination persists for several days, regardless of its concentration (Rainbow Citation2007), although even short-time high concentrations of heavy metals can cause morphological abnormalities (Vuori Citation1994). The observed deformities of the anal papillae progress gradually, dependent on the intensity of contamination, and are irreversible (Vuori Citation1994). Aquatic invertebrates have other structures with a ‘long memory‘ of water pollution as the epithelium of the middle intestine, the Malpighian tubules or adipose tissue (Johnson et al. Citation1993) but only anal papillae, as an external organ, are possible to investigate intravitally. The appearance of visible changes in the anal papillae of H. angustipennis larvae may be the first sign for further environmental investigation.
Physicochemical research conducted on streams within the Łódź city revealed the presence of various heavy metals in the water samples, Zn>Cu>Pb>Cd (Trawczyńska et al. Citation2009; Tszydel et al. Citation2015, Citation2016; Sakson et al. Citation2018), in the bottom sediments, Zn>Cu>Pb>Mn>Ni>Cr>Cd (Wagner et al. Citation2007; Urbaniak et al. Citation2008; Dukowska et al. Citation2012; Grzybkowska et al. Citation2015), and in the body tissues of aquatic insects, Fe>Zn>Pb>Cu>Mn>Ni>Cd>Cr (Grzybkowska et al. Citation2015; Tszydel et al. Citation2015, Citation2016). In the present study, in anal papillae Al, Mo, Co, As, Ti and V were also recorded. None of these elements were detected in the previously mentioned studies, thus it is difficult to assess if they were available in the water, might contribute by food uptake, or are the result of omitting these elements at the stage of setting up the analysis device or of trace amounts not exceeding the detection threshold. However, in the mentioned studies different methodology was applied (AAS with flame atomization or non-flame atomization in a graphite tube atomizer). Taking into account that SEM-EDX is a qualitative method it is not possible to directly compare the results with quantitative methods such as AAS. Nevertheless, based on the obtained results, element dominance can be ordered, and this dominance trend can be compared. The results are usually presented as a percentage of a given element in the examined tissue, which in the case of anal papillae should be understood as the dominance of the occurrence in the environment (Krantzberg & Stokes Citation1990), which the larva was trying to neutralize in the body (Darlington & Gower Citation1990). Some elements, even in very low concentrations, may be toxic to aquatic organisms, and therefore their detection is important from the monitoring point of view. This may be the case of aluminum, arsenic and vanadium, which even in a low concentration in water, can be toxic and may contribute to various types of abnormalities in aquatic organisms (Rainbow Citation2007).
SEM-EDX microscopy confirmed that the darkened and deformed papillae had higher concentrations of heavy metals than the pale one. This explains why the sublethal effects observed in these organs are more common in the contaminated waters (Tszydel et al. Citation2015). SEM-EDX method proved to be a valuable support in environmental studies, especially the one in which caddisfly larvae H. angustipennis meet most of the expectations and can serve as a heavy metal bioindicator in urban streams. Using the representative of the same species, at the same stage of development, which has been exposed for a long time to contaminated water, the possible discrepancies of bioaccumulation (Buchwalter et al. Citation2007; Ratia et al. Citation2012; Tszydel et al. Citation2015) and underestimation related to increased temporary metallotolerance (Brix et al. Citation2005, Citation2011) will not have such a significant impact on the objectivity of water quality assessment. Although SEM-EDX does not give quantitative results, it allows to indicate to which heavy metals and metalloids the body has been exposed. In anal papillae, heavy metals are accumulated in an inactive form, while analyzing these elements in the tissues is burdened by their active management in the body. The SEM-EDX method was confirmed to be valuable also for various freshwater (Lavilla et al. Citation2010; Kheirallah Citation2015), marine (Chan et al. Citation2012) and terrestrial insects (Borowska & Pyza Citation2011). It seems that this method could be particularly useful in the screening study, in which it enables to identify all trace elements present in the sample and indicate the direction for further detailed studies. The SEM-EDX analysis could also indicate a point short-term discharge of sewage, which cannot always be captured by taking a water sample for analysis, as after ceasing the pollution accumulated elements will be possible to trace in detoxification organs.
Acknowledgements
We are grateful to Marcin Markowski for providing help in the laboratory analysis. We thank Łukasz Głowacki for the language edition of our manuscript. The study was financed by the Mayor of Łódź from the budget of The City of Lodz (research project No. ED.VII.4346 G–17/2009 and 2010). The study was supported also by the internal Grant from the University of Lodz. The authors declare no conflicts of interest. All data needed to evaluate the conclusions in the paper are present in the paper. Additional data related to this paper may be requested from the authors.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Baby J, Raj JS, Biby ET, Sankarganesh P, Jeevitha MV, Ajisha SU, Sheeja S, Rajan SS. 2010. Toxic effect of heavy metals on aquatic environment. International Journal of Biological and Chemical Sciences 4:939–952.
- Biehl L, Landgrebe D. 2002. MultiSpec a tool for multispectral–hyperspectral image data analysis. Computers & Geosciences 28(10):1153–1159. DOI: 10.1016/S0098-3004(02)00033-X.
- Bonada N, Vives S, Rieradevall M, Prat N. 2005. Relationship between pollution and fluctuating asymmetry in the pollution-tolerant caddisfly Hydropsyche exocellata (Trichoptera, Insecta). Archiv für Hydrobiologie 162(2):167–185. DOI: 10.1127/0003-9136/2005/0162-0167.
- Borowska J, Pyza E. 2011. Effects of heavy metals on insect immunocompetent cells. Journal of Insect Physiology 57(6):760–770. DOI: 10.1016/j.jinsphys.2011.02.012.
- Brix KV, DeForest DK, Adams WJ. 2011. The sensitivity of aquatic insects to divalent metals: A comparative analysis of laboratory and field data. Science of the Total Environment 409(20):4187–4197. DOI: 10.1016/j.scitotenv.2011.06.061.
- Brix KV, DeForest DK, Burger M, Adams WJ. 2005. Assessing the relative sensitivity of aquatic organisms to divalent metals and their representation in toxicity datasets compared to natural aquatic communities. Human and Ecological Risk Assessment: An International Journal 11(6):1139–1156. DOI: 10.1080/10807030500278628.
- Buchwalter DB, Cain DJ, Clements WH, Luoma SN. 2007. Using biodynamic models to reconcile differences between laboratory toxicity tests and field biomonitoring with aquatic insects. Environmental Science & Technology 41(13):4821–4828. DOI: 10.1021/es070464y.
- Buchwalter DB, Luoma SN. 2005. Differences in dissolved cadmium and zinc uptake among stream insects: mechanistic explanations. Environmental Science & Technology 39(2):498–504. DOI: 10.1021/es0404421.
- Cain DJ, Buchwalter DB, Luoma SN. 2006. Influence of metal exposure history on the bioaccumulation and subcellular distribution of aqueous cadmium in the insect Hydropsyche californica. Environmental Toxicology and Chemistry 25(4):1042–1049. DOI: 10.1897/05-255R.1.
- Cain DJ, Carter JL, Fend SV, Luoma SN, Alpers CN, Taylor HE. 2000. Metal exposure in a benthic macroinvertebrate, Hydropsyche californica, related to mine drainage in the Sacramento River. Canadian Journal of Fisheries and Aquatic Sciences 57(2):380–390. DOI: 10.1139/f99-260.
- Cain DJ, Luoma SN, Wallace WG. 2004. Linking metal bioaccumulation of aquatic insects to their distribution patterns in a mining-impacted river. Environmental Toxicology and Chemistry 23(6):1463. DOI: 10.1897/03-291.
- Carls MG, Rice SD, Hose JE. 1999. Sensitivity of fish embryos to weathered crude oil: Part I. Low-level exposure during incubation causes malformations, genetic damage, and mortality in larval pacific herring (Clupea pallasi). Environmental Toxicology and Chemistry 18(3):481–493. DOI: 10.1002/etc.5620180317.
- Chan I, Tseng L-C, Kâ S, Chang C-F, Hwang J-S. 2012. An experimental study of the response of the Gorgonian Coral Subergorgia suberosa to polluted seawater from a former coastal mining site in Taiwan. Zoological Studies 51:27–37.
- Chiba WAC, Passerini MD, Tundisi JG. 2011. Metal contamination in benthic macroinvertebrates in a sub-basin in the southeast of Brazil. Brazilian Journal of Biology 71:391–399.
- Darlington ST, Gower M. 1990. Location of copper in larvae of Plectrocnemia conspersa (Curtis) (Trichoptera) exposed to elevated metal concentrations in a mine drainage stream. Hydrobiologia 196(1):91–100. DOI: 10.1007/BF00008896.
- Dukowska M, Michałowicz J, Grzybkowska M. 2012. Influence of natural organic matter and metal accumulation in sediment on riverine macrobenthic assemblages. Polish Journal of Ecology 60:351–362.
- Edington JM, Hildrew AG, editors. 1995. A key to the caseless caddis larvae of the British Isles with notes on their ecology. Cumbria: Freshwater Biological Association No53, The Ferry House.
- Elliott JM, editors. 1977. Some methods for the statistical analysis of samples of benthic invertebrates. 2 ed. Westmorland, UK, Ambleside: Freshwater Biological Association.
- Gharbi H, Sowlat MH, Mahvi AH, Mahmoudzadeh H, Arabalibeik H, Kershavarz M, Kerimzadeh N, Hassani G. 2012. Development of a dairy cattle drinking water quality index (DCWQI) based on fuzzy inference systems. Ecological Indicators 20:228–237.
- Girgin S, Kazanci N, Dügel M. 2010. Relationship between aquatic insects and heavy metals in an urban stream using multivariate techniques. International Journal of Environmental Science & Technology 7(4):653–664. DOI: 10.1007/BF03326175.
- Goldstein J, Newbury DE, Joy DC, Lyman CE, Echlin P, Lifshin E, Sawyer L, Michael JR, editors. 2003. Scanning electron microscopy and X-ray microanalysis. New York: Kluwer Academic/Plenum Publishhers.
- Grabka D, Raczyńska-Żak M, Czech K, Słomkiewicz PM, Jóźwiak MA. 2015. Modified halloysite as an adsorbent for prometryn from aqueous solutions. Applied Clay Science 114(321):321–329. DOI: 10.1016/j.clay.2015.06.012.
- Grzybkowska M, Dukowska M, Michałowicz J, Leszczyńska J. 2015. Trace metal concentrations in free-ranger, tube-dweller chironomid larvae and a weakly polluted fluvial sediment. Oceanological and Hydrobiological Studies 44(4):445–455. DOI: 10.1515/ohs-2015-0042.
- Hildrew AG, Morgan JC. 1974. The taxonomy of the British Hydropsychidae (Trichoptera). Journal of Entomology Science 43:217–229.
- Hilty J, Merenlender A. 2000. Faunal indicator taxa selection for monitoring ecosystem health. Biological Conservation 92(2):185–197. DOI: 10.1016/S0006-3207(99)00052-X.
- Johnson RK, Wiederholm T, Rosenberg DM. 1993. Freshwater biomonitoring using individual organisms, populations and species assemblages of benthic macroinvertebrates. In: Rosenberg DM, Resh VH, editors. Freshwater biomonitoring and benthic macroinvertebrates. New York: Chapman and Hall. pp. 40–125.
- Kefford BJ, Reddy-Lopata K, Clay K, Hagen T, Parkanyi O, Nugegoda D. 2011. Size of anal papillae in chironomids: Does it indicate their salinity stress? Limnologica 41(2):96–106. DOI: 10.1016/j.limno.2010.09.004.
- Kheirallah DA. 2015. Ultrastructure biomarker in Anisops sardeus (Heteroptera Notonectidae) for the assessment and monitoring of water quality of Al-Mahmoudia Canal,Western Part of Nile Delta, Egypt. Journal of Bioscience and Applied Research 1(6):326–334. DOI: 10.21608/jbaar.2015.106043.
- Krantzberg G, Stokes PM. 1990. Metal concentrations and tissues distribution in larvae ofChironomus with reference to x-ray microprobe analysis. Archives of Environmental Contamination and Toxicology 19(1):84–93. DOI: 10.1007/BF01059816.
- Lavilla I, Rodriguez-Liñares G, Garrido J, Bendicho C. 2010. A biogeochemical approach to understanding the accumulation patterns oftrace elements in three species of dragonfly larvae: Evaluation as biomonitors. Journal of Environmental Monitoring 12(3):724. DOI: 10.1039/b920379f.
- Macedo-Sousa JA, Gerhardt A, Brett CMA, Nogueira AJA, Soares AMVM. 2008. Behavioural responses of indigenous benthic invertebrates (Echinogammarus meridionalis, Hydropsyche pellucidula and Choroterpes picteti) to a pulse of Acid Mine Drainage: A laboratorial study. Environmental Pollution 156(3):966–973. DOI: 10.1016/j.envpol.2008.05.009.
- Marqués MJ, Martínez-Conde E, Rovira JV. 2003. Effects of zinc and lead mining on the benthic macroinvertebrates of a fluvial ecosystem. Water, Air, and Soil Pollution 148(1/4):363–388. DOI: 10.1023/A:1025411932330.
- Mason AZ, Jenkins KD. 1995. Metal detoxification in aquatic organisms. In: Tessier A, Turner DR, editors. Metal speciation and bioavailability in aquatic systems. New York, NY, USA: John Wiley. pp. 479–606.
- Moore JN, Luoma SN, Peters D. 1991. Downstream effects of mine effluent on an intermontane riparian system. Canadian Journal of Fisheries and Aquatic Sciences 48(2):222–232. DOI: 10.1139/f91-030.
- Olaifa FG, Olaifa AK, Onwude TE. 2004. Lethal and sublethal effects of copper to the African Cat fish (Clarias gariepnus). African Journal of Biomedical Research 7:65–70.
- Poteat MD, Garland T, Fisher NS, Wang WX, Buchwalter DB. 2013. Evolutionary patterns in trace metal (Cd and Zn) efflux capacity in aquatic organisms. Environmental Science & Technology 47(14):7989–7995. DOI: 10.1021/es401368u.
- Radhakrishnan MV, Hemalatha S, Paul VI. 2000. Effect of cadmium chloride on the melanophores of Channa striatus (Bloch). Indian Journal of Fisheries 47:135–141.
- Rainbow PS. 2002. Trace metal concentrations in aquatic invertebrates: Why and so what? Environmental Pollution 120(3):497–507. DOI: 10.1016/S0269-7491(02)00238-5.
- Rainbow PS. 2007. Trace metal bioaccumulation: Models, metabolic availability and toxicity. Environment International 33(4):576–582. DOI: 10.1016/j.envint.2006.05.007.
- Ratia H, Vuori K-M, Oikari A. 2012. Caddis larvae (Trichoptera, Hydropsychidae) indicate delaying recovery of a watercourse polluted by pulp and paper industry. Ecological Indicators 15:217–226.
- Rochfort Q, Grapentine L, Marsalek J, Brownlee B, Reynoldson T, Thompson S, Milani D, Logan C. 2000. Using benthic assessment techniques to determine combined sewer overflow and stormwater impacts in the aquatic ecosystem. Water Quality Research Journal 35(3):365–398. DOI: 10.2166/wqrj.2000.025.
- Sakson G, Brzezinska A, Zawilski A. 2018. Emission of heavy metals from urban catchment into receiving water and possibility of its limitation on the example of Lodz city. Environmental Monitoring and Assessment 190(5):1–15. DOI: 10.1007/s10661-018-6648-9.
- Siddig AAH, Ellison AM, Ochs A, Villar-Leeman C, Lau MK. 2016. How do ecologists select and use indicator species to monitor ecological change? Insights from 14 years of publication in ecological indicators. Ecological Indicators 60(223):223–230. DOI: 10.1016/j.ecolind.2015.06.036.
- Sokal RR, Rohlf F, editors. 2005. Biometry: The principles and practice of statistics in biological research. 4 ed. San Francisco: WH Freeman & Co.
- StatSoft Inc. 2011. STATISTICA (data analysis software system) version 10. Available: www.statsoft.com
- Stephansen DA, Nielsen AH, Hvitved-Jacobsen T, Pedersen ML, Vollertsen J. 2016. Invertebrates in stormwater wet detention ponds - Sediment accumulation and bioaccumulation of heavy metals have no effect on biodiversity and community structure. The Science of the Total Environment 566–567:1579–1587.
- Trawczyńska A, Tołoczko W, Niewiadomski A. 2009. The content of trace elements in the water of the upper course of the Bzura River. [abstract in English]. Environmental Protection and Nature Research 40:491–496.
- Tszydel M, Markowski M, Majecki J. 2016. Larvae of Hydropsyche angustipennis (Trichoptera, Hydropsychidae) as indicators of stream contamination by heavy metals in Łódź agglomeration. Zootaxa 4138(1):127–138. DOI: 10.11646/zootaxa.4138.1.5.
- Tszydel M, Markowski M, Majecki J, Błońska D, Zieliński M. 2015. Assessment of water quality in urban streams based on larvae of Hydropsyche angustipennis (Insecta, Trichoptera). Environmental Science and Pollution Research 22(19):14687–14701. DOI: 10.1007/s11356-015-4638-9.
- Urbaniak M, Zieliński M, Wesołowski W, Zalewski M. 2008. PCBs and heavy metals contamination in bottom sediments from three reservoirs of different catchment characteristics. Polish Journal of Environmental Studies 17:941–949.
- Vuori K-M. 1994. Rapid behavioural and morphological responses of hydropsychid larvae (Trichoptera, Hydropsychidae) to sublethal cadmium exposure. Environmental Pollution 84(3):291–299. DOI: 10.1016/0269-7491(94)90141-4.
- Vuori K-M, Kukkonen J. 1996. Metal concentrations in Hydropsyche pellucidula larvae (Trichoptera, Hydropsychidae) in relation to the anal papillae abnormalities and age of exocuticle. Water Research 30(10):2265–2272. DOI: 10.1016/0043-1354(96)00109-1.
- Wagner I, Izydorczyk K, Drobniewska A, Frątczak W, Zalewski M. 2007. Inclusion of ecohydrology concept as integral component of systemic in urban water sources management. Proceedings of the International Symposium of New Directions in Urban Water Management, Paris, UNESCO Headquarter. pp. 1–9.
- Watanabe K, Monaghan MT, Takemon Y, Omura T. 2008. Biodilution of heavy metals in a stream macroinvertebrate food web: Evidence from stable isotope analysis. Science of the Total Environment 394(1):57–67. DOI: 10.1016/j.scitotenv.2008.01.006.
- Wichard W. 1976. Morphologische Komponenten bei der Osmoregulation von Trichopteren larven. Proceedings of the 1st International Symposium on Trichoptera, Malicky H, editors. The Hague: Dr Junk. pp. 171–177.
- Wright JF. 2000. An introduction to RIVPACS. In: Wright JF, Sutcliffe DW, Furse MT, editors. Assessing the biological quality of fresh waters: RIVPACS and other techniques. Cumbria: Freshwater Biological Association. pp. 1–24.
- Zar JH, editor. 1999. Biostatistical analysis. 4 ed. Upper Saddle River: Prentice Hall.

![Figure 5. Mean values of the percentage [%] of larvae with abnormalities of H. angustipennis anal papillae at the sampling sites](/cms/asset/612d5d58-47bc-4fc4-a3a0-83c03c4c6c15/tizo_a_1931490_f0005_oc.jpg)
![Figure 6. Mean values of the percentage [%] domination of heavy metals in anal papillae, depending on the degree of their deformations](/cms/asset/2912aea1-7b67-493e-ba62-cb5ab1e41aa7/tizo_a_1931490_f0006_oc.jpg)