Abstract
Lithistid demosponges are well known from limestone caves of karstic origin in the Mediterranean Sea. However, they have never been reported from submarine caves of volcanic origin in the South Pacific. Here, we describe and provide DNA barcodes for four new lithistid demosponges including one new genus. All species grew on basaltic rocks inside lava tubes on Nuku Hiva Island (Marquesas Islands) and Tahiti Iti peninsula on Tahiti Island (Society Islands) in French Polynesia. Three of the species have rhizoclone desmas as choanosomal skeletons and belong to the family Scleritodermidae (Microscleroderma miritatarata sp. nov. and Microscleroderma lava sp. nov.) and Siphonidiidae (Gastrophanella basaltica sp. nov.). The new genus Levispongia gen. nov. belongs to the family Corallistidae. The new species Levispongia meyeri gen. nov. sp. nov. has dicranoclone desmas, complex dichotrianes with strongly spinose upper surfaces of the cladome and microstyles as the only microscleres. Phylogenetic relationships of these new species are discussed and compared with other material from the Caribbean and Central to the West Pacific Ocean.
urn:lsid:zoobank.org:pub:844E385C-9A92-4F1B-B85C-9C1E319CD13F
Introduction
Submarine caves share several ecological features which often resemble that of deep-sea habitats including darkness, hydrodynamic, geochemical and faunal compositions (Harmelin & Vacelet Citation1997). Although differences in pressure, temperature and habitat sizes exist (Moldovan et al. Citation2019), these submarine caves are often colonized by deep-sea invertebrates like gastropods, annelids, crustaceans and sponges (Bussotti et al. Citation2006; Gerovasileiou et al. Citation2015; Culver & Pipan Citation2019). In particular, the abundance of demosponges represents an important element of submarine cave communities (Bibiloni et al. Citation1989; Gerovasileiou & Voultsiadou Citation2012). Since most of these caves are accessible by SCUBA diving, they provide a unique opportunity to study various aspects of deep-sea sponge biology and systematics (Vacelet Citation1996). However, we still know little about the diversity and connectivity of sponges from different cave systems around the world, especially how many of these species are cryptic or relict, present as a living fossil. Sponge cave biodiversity has been well studied in the Mediterranean Sea (e.g. Gerovasileiou et al. Citation2016). The Mediterranean submarine caves are all of karstic origin (limestones) and are a large biodiversity reservoir of sponge species that are otherwise only recorded from the deep-sea (Gerovasileiou & Voultsiadou Citation2012). In particular, the occurrence of a polyphyletic group of demosponges historically called lithistids (Pisera & Lévi Citation2002a; Schuster et al. Citation2015) seems to be highly abundant and thrive in these cave systems (Pérez et al. Citation2004; Manconi et al. Citation2006; Manconi & Serusi Citation2008; Pisera & Vacelet Citation2011; Pisera & Gerovasileiou Citation2018). Lithistids are characterized by an articulated choanosomal skeleton composed of siliceous desmas. Molecular systematics placed the majority of these desma-bearing sponges into the order Tetractinellida Marshall Citation1876 (Schuster et al. Citation2015). In oceans today, lithistids are normally found in deep-waters of the tropics (Carvalho et al. Citation2015; Schuster et al. Citation2021), however, some species are also found in shallow waters (Pomponi et al. Citation2001). It is postulated that the majority of these sponges were originally shallow water sponges that moved into deeper waters as silica concentrations decreased in shallow water (Pisera Citation2004). Few records exist of lithistid cave fauna occurring outside the Mediterranean Sea (Gerovasileiou et al. Citation2016; Gómez et al. Citation2021). Some lithistids were described from shallow crevices in coral reefs of Madagascar (Vacelet & Vasseur Citation1965), from the central western Atlantic in Belize and Fernando de Noronha (Muricy & Minervino Citation2000), and a few more were observed in a large submarine cave on Palawan in the Philippines, which represents the only known record of cave lithistids so far in the Pacific Ocean (Pisera & Vacelet Citation2006). In all investigated caves, the dominant lithistid species seem to belong to rhizomorine families like Scleritodermidae Sollas, Citation1888, Azorizidae Sollas, Citation1888 Siphonidiidae Von Lendenfeld, Citation1903 and dicranoclone family Corallistidae Sollas, Citation1888. All of which are usually recorded from deep-waters. The observed morphological features from all these various cave lithistids show high affinities to those of the Mesozoic of Europe (Łukowiak et al. Citation2014; Frisone et al. Citation2016; Świerczewska-Gładysz Citation2016), which would suggest that most of these species represent a relict fauna with probable Tethys origin as already predicted for other non-cave lithistids (Reid Citation1967; Manconi Citation2011) and recently tested with molecular clocks for the lithistid genus Vetulina Schmidt, Citation1879 (Sphaerocladina Schrammen Citation1924) (Schuster et al. Citation2018).
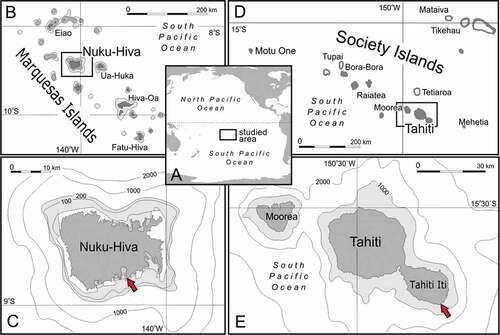
In this integrative taxonomy study, we describe for the first time four new lithistid demosponges and one new genus from two shallow submarine caves in French Polynesia: Ekamako Cave at the southern coast of Nuku Hiva (Marquesas Islands) and Te Pari Cave on Tahiti Iti peninsula (Tahiti island, Windward Islands, Society Islands) (). In contrast to the above-mentioned limestone caves, the Polynesian cave systems are formed by large lava flows and are of volcanic origin. This is an important finding not only because they grow on these basaltic volcanic rocks inside lava tubes, but also due to the fact that the new genus and species described herein represent the first records of lithistids from this extreme isolated archipelago in French Polynesia. Thus, our results will be of profound interest for further biogeographical and systematic studies on this evolutionary important demosponge group.
Material and methods
Specimen collection and morphological investigations
Lithistid sponges from Marquesas were collected by SCUBA diving in the 80 m long lava tube called Ekamako, on Nuku Hiva southern shore (Marquesas Islands) (): Research Vessel (R/V) called Alis, cruise BSMPF-1 (Debitus Citation2009), site MNH03, 8°56.173’S, 140°05.593ʹW, 11 m, 29/08/2009 (). They grew from 10 meters (m) distance from the entrance of the cave and continued to appear deeper into the cave (Pérez et al. Citation2012). Other sponges in this cave were the non-lithistid demosponges Xestospongia sp. (6–10 m), Dactylospongia elegans (6–10 m distance from the entrance), Suberea sp. (6–10 m distance from the entrance), Epipolasis sp. (6–10 m distance from the entrance) and Cinachyrella sp. (8 m distance from the entrance), some of them probably represent new species too, but await formal description.
The sponges from Tahiti Iti peninsula were also collected by SCUBA diving from a lava tube inside the Te Pari Cave (Tahiti Island, Windward Islands, Society Islands) (). For more details on the cruise see Debitus (Citation2013). The entrance of this cave is about 10 m wide and its sandy bottom lies at 12 m depth. It forms a right angle 25 m after the cave mouth and is another 180 m long (measured with a hand depth sounder). It is a regular 6–8 m wide cylinder, that narrows and rises towards the end. The sides and bottom (after the turn) are formed of irregular basaltic rocks. The cave ends in a narrow, rocky, chaotic pipe, inaccessible for divers. The roof at the end of the cave is characterized by an air pocket as it is above sea level. The presence of a few dead leaves indicated freshwater inflows, probably during heavy rains. The top water column temperature in the cave was around 26°C, while the lower bottom was warmer around 28°C, and the open sea temperature was 30°C. This cooler top water temperature would confirm the inflow of freshwater, thus keeping the upper layer of the cave water cooler. Further indication for freshwater inflow is given by the observation of some cave openings that are close to trails where locals observed salty water coming out during rough seas and killing the surrounding plants, however, no light could be observed inside the cave. Moreover, the water at the surface in the back (end) of the cave tasted less saline than throughout the rest of the cave, which again indicates the inflow of freshwater from the surrounding environments. This cave represents a hot spot of diversity of sponges in Tahiti and concentrates almost half of the total observed species in our surveys (26 species including lithistids, most of them new to science) (personal observation by the author C. Debitus).
Worth mentioning is that the first 25 m into the cave (before the turn) concentrate most of the non-lithistid sponge species of which some are already described and published such as the calcareous Leucetta chagosensis Dendy, Citation1913 and Ascandra cf. crewsi (Klautau et al. Citation2020) and many more demosponge species that await formal descriptions (Chondrosia corticata, Psammocinia sp., Suberea ianthelliformis, Spirastrella sp., Chondrosia sp., Tedania sp., Hyatella sp., Dysidea sp., Psammoclema sp., Haliclona spp., Halichondria sp., Chondrilla sp., Cinachyrella sp., Luffariella sp., Rhabdastrella sp.). The lithistids were found further inside the cave on the walls and roof of the cave, with Microscleroderma lava sp. nov. first, after which Gastrophanella basaltica sp. nov. and Levispongia meyeri gen. nov. sp. nov. followed further inside the cave with a few other demosponges on the rocky blocks on the cave floor (Suberea sp., Halichondria sp., Dysidea sp., Haliclona sp., Tedania sp., Plakinastrella sp.).
All lithistid specimens were preserved in 70–80% ethanol and are deposited within the “Zoothèque” of the Natural History Museum in Paris (MNHN). SEM stubs and small pieces of the holotypes and paratypes are stored at the Polish Academy of Sciences, Institute of Paleobiology in Warsaw, Poland (ZPAL). In addition, DNA samples are available at the Bavarian State Collection for Palaeontology and Geology (BSPG) in Munich, Germany. DNA barcodes are registered at the Sponge Barcoding Database (SBD) https://www.spongebarcoding.org/ and new sequences (28S, C1-D2 region) from this study are deposited at the National Center for Biotechnology Information (NCBI). New species are registered in ZooBank (http://zoobank.org/urn:lsid:zoobank.org:pub:844E385C-9A92-4F1B-B85C-9C1E319CD13F.)
Morphological features of the specimens were analyzed through Scanning Electron Microscopy (SEM). In detail, pieces of the ectosome and choanosome of the sponges were cut and either directly mounted on stubs or digested in nitric acid (HNO3) first, in order to remove any organic material, followed by several washing steps with distilled water and twice 70–80% ethanol before a final fixation in 99% ethanol (Pisera & Pomponi Citation2015). Dried spicules, zygosed skeletons and chemically untreated surfaces were mounted on stubs using Carbon-Leit-C (PLANO, Germany) and covered with platinum for examination on a Philips L-20 SEM at the Institute of Paleobiology in Warsaw, Poland.
Molecular Systematics
For the assessment of the molecular relationships of the new species and the new genus Levispongia gen. nov. among other lithistids, we performed two separate phylogenetic analyses. One for the family Corallistidae where the new genus belongs to, and another for the suborder Spirophorina Bergquist & Hogg, Citation1969 that includes all new rhizomorine species from this study. In detail, we used the C1-D2 region of the 28S rDNA gene (~800 bp) that has been shown in previous studies to resolve relationships on species level (see Schuster et al. Citation2015, Citation2018). For Microscleroderma lava sp. nov. (LR656090) and the two Microscleroderma miritatarata sp. nov. (LR656088, LR656089) 28S sequences already exist and were extracted from Schuster et al. (Citation2021). For Gastrophanella basaltica sp. nov. and Levispongia meyeri gen. nov. sp. nov., new sequences of the same gene were obtained following the molecular barcoding protocol in Schuster et al. (Citation2015, Citation2021).
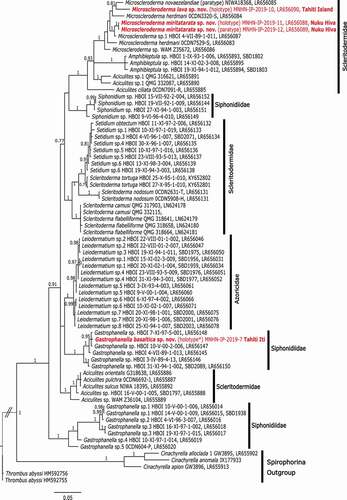
For a better overview, we reduced the sequence alignment of Schuster et al. (Citation2021) to the family Corallistidae which includes representative species from all six valid genera (Awhiowhio Kelly, Citation2007, Corallistes Schmidt, Citation1870, Herengeria Lévi & Lévi, Citation1988, Isabella Schlacher-Hoenlinger, Pisera & Hooper, Citation2005, Neophrissospongia Pisera & Lévi, Citation2002b and Neoschrammeniella Pisera & Lévi, Citation2002b) and aligned to this the new sequence Levispongia meyeri gen. nov. sp. nov. using AliView v.1.26 (Larsson Citation2014). A reduced alignment from Schuster et al. (Citation2021) including Scleritodermidae, Siphonidiidae and Azoricidae was used to calculate the phylogenetic tree. Since in this study the new Microscleroderma species were already included, only Gastrophanella basaltica sp. nov. was added using the same program as above.
Bayesian inference (BI) of phylogenies was executed separately for each dataset using the MrBayes 3.2.2 plugin on the CIPRES Science gateway (Miller et al. Citation2010). The best fit evolutionary model (GTR+G + I) was selected according to the results of JModelTest2 (Darriba et al. Citation2012) plugin using the CIPRES Science Gateway v.3.3 (Miller et al. Citation2010). Two concurrent runs of four Metropolis-coupled Markov-chains Monte Carlo (MCMC) for 100,000,000 generations were run and stopped when the average standard deviation of split frequencies reached below 0.01. The first 25% of the sampled trees were discarded as burn-in for further analyses. FigTree v1.4.2 (Rambau Citation2009) and Inkscape 1.0 was used to visualize the trees.
Unfortunately, we could not sequence the independent mitochondrial barcoding marker COI for Gastrophanella basaltica sp. nov. and Microscleroderma miritatarata sp. nov., most likely because of the presence of intronic regions within COI. These intronic regions seem to be widespread among rhizomorine taxa (see Schuster et al. Citation2017) and it was detected in the new species Microscleroderma lava sp. nov. (COI = LT628364, synonym Microscleroderma sp.3, in Schuster et al. Citation2017). In fact, this species harbors two intron insertions (ORF 723 and ORF 870) within its most conserved COI barcoding region. Therefore, we suspect that also Microscleroderma miritatarata sp. nov., contain such an intron, which awaits to be sequenced in a follow-up study.
Results
Species descriptions
Porifera Grant, Citation1836
Class Demospongiae Sollas, Citation1885
Subclass Heteroscleromorpha Cárdenas et al., Citation2012
Order Tetractinellida Marshall, Citation1876
Suborder Astrophorina Sollas, Citation1887
Family Corallistidae Sollas, Citation1888
Genus Levispongia gen. nov.
Etymology
This genus name is given to honor Prof. Claude Lévi for his achievements in sponge science.
Type species: Levispongia meyeri gen. nov. sp. nov.
Diagnosis
Massive to fan-shaped corallistid with complex dichotrianes that show a strongly spinose upper surface of their cladome and microscleres that are microstyles and microrhabds; no streptaster of any kind.
Remarks
The new genus is close to Neophrissospongia as it shares very similar desmas with a central core, complex dichotriaenes with spinose or tuberculated upper surface of the cladome and microstyles among microscleres. However, it lacks any kind of streptasters, but instead has two types of spinose microrhabds. Also, the habitus of this new species is different from most, but not all Neophrissospongia species, which are vase or foliose in shape. Such differences usually are treated to characterize different genera (see for example Racodiscula; Zittel, Citation1878 and Theonella; Gray, Citation1868; (Pisera & Lévi Citation2002c)). The only other close corallistid species, attributed earlier to Neophrissospongia, is N. microstylifera Lévi & Lévi, Citation1983 from New Caledonia, as a result we propose to transfer this species from the genus to the new genus Levispongia gen. nov.
Levispongia meyeri sp. nov.()
Diagnosis
Massive to fan-shaped sponge composed of cylindrical units that are more or less fused; dichotriaenes are complex and have strongly spinose upper surface of cladome; desmas are dicranoclones with central core and are strongly tuberculated; larger tubercules are ornated by small tubercules; microscleres as spinose microstyles and two types of spinose microrhabds; one is thin, long and curved, the second is short, massive and often irregular.
Type material
Holotype: MNHN-IP-2019-2 (field number P515/1), Tahiti Island, Society Islands, French Polynesia, basaltic lava tube on island fall, Station 30, 17°52.657’S, 149°09.330ʹW, 6 m, SCUBA, Coll. Cécile Debitus, Merrick Ekins 14/04/2013. Paratypes: MNHN-IP-2019-3, MNHN-IP-2019-4, MNHN-IP-2019-5, MNHN-IP-2019-6 (field numbers P515/2, P515/3, P515/4 and P516A respectively), same collection details as the holotype.
Type locality
Tahiti Iti peninsula, Tahiti Island, Society Islands, French Polynesia ().
Distribution
Only known from type locality.
Habitat
Lava tube cave, attached to basaltic rocks.
Description
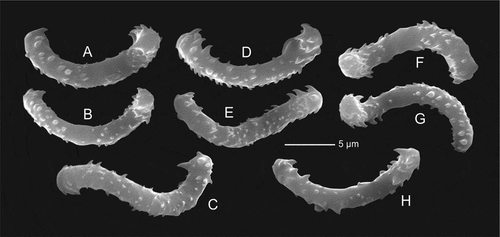
Morphology, the examined sponges have the shape of cylindrical almost erected subunits that are fused and evenly anastomosing along most of their length, each with its own osculum (). The body plus side growths of the largest sponge (holotype, MNHN-IP-2019-2) is 13 cm wide x 10 cm high and about 2 cm thick, )). The individual cylinders are up to 10 cm long and about 0.5–1 cm in diameter. The top of cylinders is rounded with a small 1–3 mm centrally located osculum. There are about seven of such cylindrical subunits present in the holotype (–)) with numerous outgrowths on sides at the base. At the base of the fan, those cylindrical subunits have an irregular orientation, are shorter and only partly fused (,)). The two smaller paratypes ()) are of the same growth form but smaller in size (6 cm wide x 3.5 cm height and are 1–1.5 cm thick, and 7 × 5 cm and 1 cm thick). The smallest specimen is just 2 × 1.5 in size and formed by the partial fusion of only three cylinders. Texture, stony and hard. Surface, even, but not entirely smooth due to the rugged surface of the choanosomal skeleton protruding in many places from below the ectosome. The external surface of the paratype (MNHN-IP-2019-6) is covered very sparsely by inhalant rounded openings that are about 200 µm in diameter; the other internal side is covered with very densely distributed slit to rounded openings that are evenly distributed over the whole surface. These openings are separated only by dichotriaene branches. Colour, is cream white in life, beige in ethanol preservative. Ectosomal skeleton, contains rhabd microscleres and the cladome part of the dichotriaene megascleres. The cladomes are strongly spinose on the upper surface with 184–218 μm in diameter. The rhabdomes of the dicotriaene are 218–358 μm long and 33.8–48.1 μm in diameter and penetrate into the choanosome (–)). Choanosomal skeleton composed of zygosed dicranoclone desmas () and microstyles. Megascleres, desmas that are massive arched shaped with a central core from which several arms (four to six) branch from the underside of the arch and both the core and the arms are strongly covered with irregular tubercles (). Young dicranoclone desmas are less tuberculated than mature ones ()). Microscleres, in the holotype are spined microstyles that occur in the choanosome and that are 77.6–93.6 μm long and 3.09–4.56 μm in diameter at the top (–)); short and thick microrhabds that are concentrated in the ectosome and that are 24.4–32.7 μm long and 11.3–17.3 μm in diameter (–); ()), as well as longer slightly curved rhabds that are 47.1–53.6 μm long and 6.73–9.83 μm in diameter (–)).
Etymology
Named after Jean-Yves Meyer, research delegate of French Polynesia and an active conservation biologist in French Polynesia who helped in various logistical and administrative ways during our sampling field trips.
DNA barcodes
We sequenced partial 28S (C1-D2 region ~800 bp) of the holotype. GeneBank accession number: MZ313226, Sponge Barcoding Project (SBP) number: 2323.
Remarks
Habitus of this new species is unique within the family Corallistidae, in addition to the set of microscleres that contains no streptasters. The only other described corallistid species that lacks streptasters is Neophrissospongia microstylifera from New Caledonia. The species N. microstylifera differs by having only microstyles and microstrongyles. It differs also in growth form by being massive conical. On the other hand, we are aware of an undescribed lithistid sponge from Western Australia (WAM Z35946, LR656117, ) that has a similar set of microsclere characters (pers. observation A. Pisera) and is molecularly sister (3 bp difference) to the new Levispongia gen. nov. species (). We therefore suggest to synonymize the species WAM Z35946 (LR656117) with the new genus Levispongia gen. nov. sp. (). Due to major differences in the composition of microscleres and the species specific habitus, we assign the new described species L. meyery sp. nov. and L. gen. nov. sp. (WAM Z35946) together with N. microstylifera to the new genus Levispongia that is sister to the genus Neophrissospongia (). The species N. microstylifera (0CDN7083-J, LR656096) will be N. cf. microstylifera until further investigations and comparison.
Figure 2. Levispongia meyeri gen. nov. sp. nov. (a,b): Paratype specimen MNHN-IP-2019-3; (a): lateral view; (b): top view. (c–e): Holotype specimen MNHN-IP-2019-2; (c): top view, (d,e): lateral views
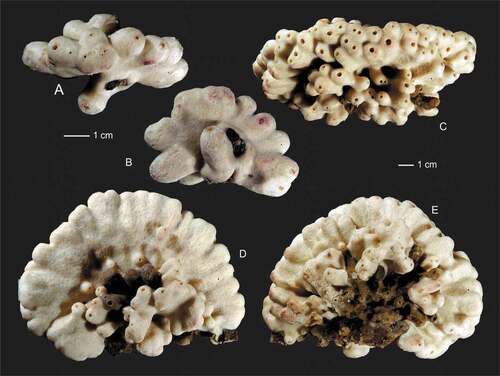
Figure 3. Choanosomal desma skeleton of the holotype Levispongia meyeri gen. nov. sp. nov. (MNHN-IP-2019-2). (a–d): Details of choanosmal desmas skeleton formed by tuberculated dicranoclones. (e–f): details of the desma skeleton with young and less tuberculated spicules inserted
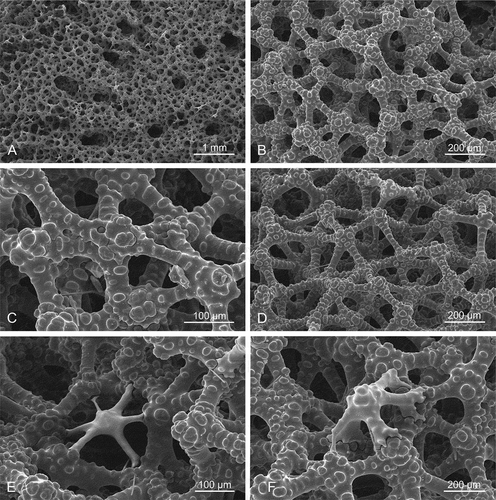
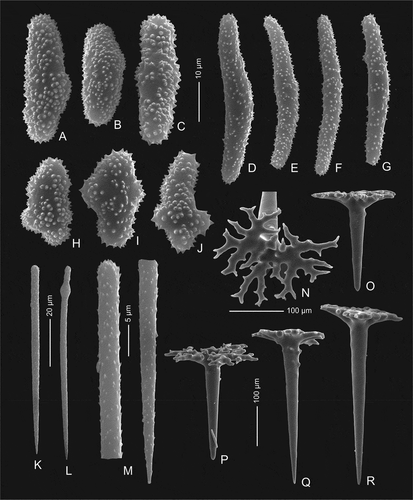
Figure 4. Mega- and microscleres of holotype Levispongia meyeri gen. nov. sp. nov. (MNHN-IP-2019-2). (a–c) & (h–j): short thick spined microrhabds. (d–g): long slightly curved and spined microrhabds. (k–m): microstyles. (n–r): dichotriaenes in various views
Suborder Spirophorina Bergquist & Hogg, Citation1969
(Note: status change and disused)
Family Siphonidiidae Von Lendenfeld, Citation1903
Genus Gastrophanella Schmidt, Citation1879
Type species
Gastrophanella implexa Schmidt, Citation1879
Diagnosis
Following Pisera & Lévi, Citation2002d, clavate to cylindrical to globular to massive lobate to vase- to ear-shaped sponge. No special ectosomal spicules; desmas are rhizoclones. Choanosomal spicules are smooth staples and/or exotylostyles with ornamented to smooth heads.
Gastrophanella basaltica sp. nov.(, ,)
Diagnosis
Irregular mass of cylindrical subunits with additional lateral tubes or curved shallow ear-shaped fan with two clearly different surfaces. Microscleres are exotylostyles in the ectosomal part of the sponge and densely distributed and oriented perpendicularly to the surface.
Type material
Holotype specimen MNHN-IP-2019-7 (field number P516/2), Tahiti Iti peninsula, Tahiti Island, Society Islands, French Polynesia, Station 30, 17°52.657’S, 149°09.330ʹW, 6 m, basaltic Te Pari lava tube on Island fall, SCUBA, Coll. Cécile Debitus, Merrick Ekins 14/04/2013. Paratypes: specimen MNHN-IP-2019-8 (field number P533) same collection details as the holotype; paratype specimen MNHN-IP-2019-9 (field number P554), basaltic Te Pari lava tube on Tahiti Iti peninsula, Tahiti Island, Society Islands, French Polynesia, Station 30, Coll. Cécile Debitus, 15/12/2015.
Type locality
Te Pari lava tube cave, Tahiti Iti Peninsula, Tahiti island, Society Islands.
Distribution
Only known from type locality Tahiti Iti Island.
Habitat
On basaltic rocks in lava tube caves.
Description
Morphology of the holotype (specimen MNHN-IP-2019-7) is an irregular mass of cylindrical subunits (or fan-like with additional lateral tubes). Each tube is fused at the base or along most of its length and has an apical osculum of 1 to 3 mm in diameter (,)). The sponge is 6 cm wide and 4.5 cm high and has about 20 tubes ranging from 3 mm to 10 mm in diameter ()). Texture, stony and hard. Color in life is cream white and beige to light brownish in ethanol. Surface, mostly inhalant; these areas are separated by narrow, slightly elevated flat ridges that are smooth. Short sinuous subdermal canals are visible on the surface that run from the top of tubes downward. Inhalant areas are rough on the surface of the narrow, short skeletal ridges that run in all directions; between them are sparsely distributed depressions with ostia. This entire surface is hispid as it is covered with very short protruding microsclere spicules. One of the paratype specimens (MNHN-IP-2019-9, –)) differs considerably in morphology by being a curved shallow ear-shaped fan with two clearly different surfaces, one is concave, has a lot of irregularities and is rough with subdermal short skeletal ridges visible on the surface (the same as inhalant areas in the holotype MNHN-IP-2019-7) between each ridge membranes ostias are visible that measure about 0.05–0.1 m in diameter. This concave side has exotylostyles that are densely distributed and oriented perpendicularly to the surface. The other side that is convex bears numerous (about 30) raised oscula that are 1–2 mm in diameter. The oscula are located on low (up to 5 mm high) conical outgrowths. Oscula approaches the upper margin of the sponge from the convex side. The oscula membrane is armed with tangentially embedded exotylostyles. Ectosomal skeleton, with exotylostyles that are densely distributed and oriented perpendicularly to the surface ()). On the concave side, the margin bears numerous sinuous subdermal furrows that are visible through the dermal membrane. Choanosomal skeleton is composed of a very dense network of rhizoclone desmas with blunt outgrowths bearing tiny spines (). Megascleres are rhizoclone desmas only. Microscleres are exotylostyles that are 207–249 μm long with spinose heads ()). The head of the exotylostyles are 10.9–14.2 μm long and 12.3–14.3 μm in diameter, whilst the rhabd is 9.41–11.1 μm in diameter at the top ()).
Etymology. named after the basaltic rocks on which it grows inside lava tube cave.
DNA barcodes. We sequenced partial 28S (C1-D2 region ~800 bp) of the holotype. GeneBank accession number: MZ313225, Sponge Barcoding Project (SBP) number: 2324.
Remarks. Gross morphology of the sponge resembles that of Levispongia meyeri gen. nov. sp. nov., for which it could be mistaken for in the field, however, it has different spicules: rhizoclones instead of dicranoclones, and microscleres as spiny exotylostyles. To date, three Gastrophanella species are known from the Atlantic namely G. implexa Schmidt, Citation1879, G. cavernicola Muricy & Minervino, Citation2000 and G. stylifera; Mothes & Silva, Citation1999, however, the latter one might be likely a junior synonym of G. implexa (see Pisera & Lévi Citation2002d). One species is known from a cave in the Mediterranean Sea G. phoeniciensis Perez, Vacelet, Bitar & Zibrowius, Citation2004 and another one G. mammilliformis Burton, Citation1929 was recorded from South African waters, with no precise location given (Burton Citation1929). Until now, the only known Gastrophanella from the central Eastern Pacific coast of Mexico was G. primore Gómez, Citation1998. Our new species Gastrophanella basaltica sp. nov. will add the second species of this genus in the Pacific. Its habitus is similar to G. cavernicola from Fernando de Noronha, Brazil because both species form a mass of cylindrical tubes, but G. cavernicola is rather massive while our species is rather fan shaped. Another similar species from the Atlantic (Brazil) is G. stylifera Mothes and Silva, Citation1999 which is tube shaped thus also similar. Molecularly (28S C1-D2, ) G. basaltica sp. nov. is closely related (2 bp difference) to species from Honduras and Curacao () that were first considered to belong to the genus Siphonidium in Schuster et al. (Citation2021). Based on our current morphological and molecular barcodes of the G. basaltica sp. nov. in this study we propose the reallocation of all species (LR656148, LR656147, LR656145, LR656146, LR656150) to the genus Gastrophanella ().
Figure 5. Outer growth form of new rhizomorine lithistids. (a,b): Paratype (MNHN-IP-2019-12) of Microscleroderma miritatarata sp. nov. (c): Holotype (MNHN-IP-2019-11) of Microscleroderma miritatarata sp. nov. (d–f): Paratype (MNHN-IP-2019-9) of Gastrophanella basaltica sp. nov. (g,h): Holotype (MNHN-IP-2019-7) of Gastrophanella basaltica sp. nov. (i–k): Holotype (MNHN-IP-2019-10) of Microscleroderma lava sp. nov
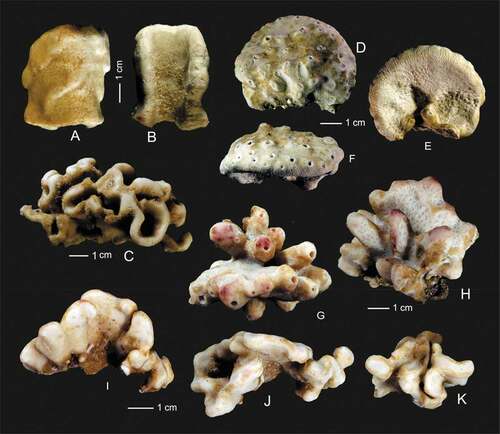
Figure 6. Ectosomal surface and choanosomal skeleton of Gastrophanella basaltica sp. nov., (holotype MNHN-IP-2019-7). (a): Ectosomal natural surface with protruding exotylostyles. (b–d): details of choanosomal rhizomorine desmas
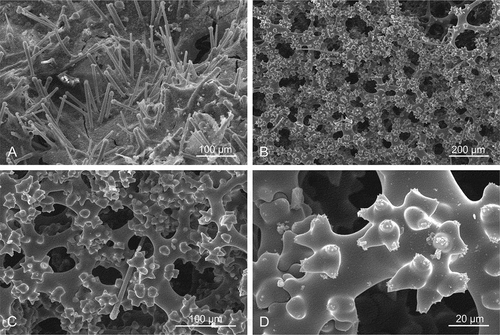
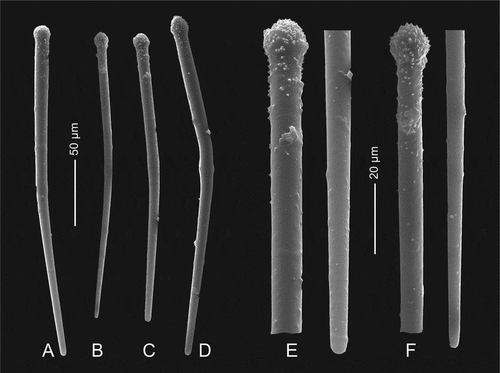
Figure 7. Microscleres of Gastrophanella basaltica sp. nov. (holotype MNHN-IP-2019-7). (a–d): exotylostyle microscleres. (e,f): details of spinose heads of exotylostyles
Family Scleritodermidae Sollas, Citation1888
Genus Microscleroderma Kirkpatrick, Citation1903
Diagnosis
Adopted from Pisera and Lévi 2002. This genus can be vase or foliate in shape with hairy oxeas protruding from the ectosome; Microscleres are sigmaspires.
Synonymy
Microscleroderma sp. 3 (Schuster et al. Citation2017, 2019).
Diagnosis
Microscleroderma with laminar folded slightly compressed growth morphology. Surface covered with long hair-like oxeas; microscleres as thick spinose sigmaspires.
Type material
Holotype MNHN-IP-2019-10 (field number P498 composed of several fragments of various size), Tahiti Iti peninsula, Tahiti Island, Society Islands, French Polynesia, 17°52.657’S, 149°09.330ʹW, 6 m, Station 30, basaltic lava tube Te Pari on Island fall, SCUBA, Coll. Cécile Debitus, Merrick Ekins, 14/04/2013.
Comparative material
Microscleroderma miritatarata sp. nov. MNHN-IP-2019-10 and MNHN-IP-2019-11 (see below).
Type locality
Tahiti-Iti peninsula, Tahiti Island
Distribution
French Polynesia
Habitat
On basaltic rock in lava tube cave Te Pari
Description
Morphology, three larger and three very small fragments of the same laminar sponge were examined. The larger fragments show several folds that are slightly compressed ()). The size of the largest fragment is about 4 cm long, 2.5 cm in wide and 3 cm high. The thickness of the folds is 3 to 5 mm. Ectosomal surface is even and strongly hispid on both sides; there are evenly and densely occurring depressions with ostia openings that are about 0.4–0.5 mm in diameter. Choanosomal skeleton, is evenly and densely covered by canal openings that are 200–300 μm in diameter and contains rhizoclone desmas. Megascleres are dense heavily spined rhizoclones ()), where the spines are divided many times. On the surface, long hair-like oxeas are visible ()). Microscleres as relatively thick spinose sigmaspires that are 13.0–16.0 μm long and 2.02–2.76 μm in diameter ()).
Etymology
Named after its occurrence in caves formed by lava flows.
DNA barcode
28S (C1-D2 region): LR656090 (Schuster et al. Citation2021); CO1: LT628364, intron ORF 723 + 870 (Schuster et al. Citation2017).
Remarks
This species is most similar to Microscleroderma lamina Perez, Vacelet, Bitar & Zibrowius, Citation2004 from the submarine cave in Lebanon (Eastern Mediterranean Sea), however, its desmas are rather tuberculated than spinose, sigmaspires are more S-shaped and spiral, as well as much smaller and less thick than in M. lava sp. nov.
In habitus M. lava sp. nov. resembles also M. novaezelandiae Kelly, Citation2007 from New Zealand, but its outer wall is considerably thicker and rhizoclone desmas are different. The sizes of the sigmaspires are similar, albeit being slightly smaller and less thick (stout). The differences mentioned here between our Tahiti material and the one from New Zealand, as well as their geographical distance between them, support the morphological difference that they are different species. Notable that features of M. novaezelandiae are not well illustrated in detail, making any closer comparison difficult. Molecularly these two species differ by 28 bp difference in the 28S C1-D2 region ().
Figure 8. Microscleroderma lava sp. nov. (holotype MNHN-IP-2019-10). (a,b): natural surface with ectosomal hair-like oxeas randomly distributed. (c–f): Details of the choanosomal skeleton composed of rhizoclone desmas forming a dense network
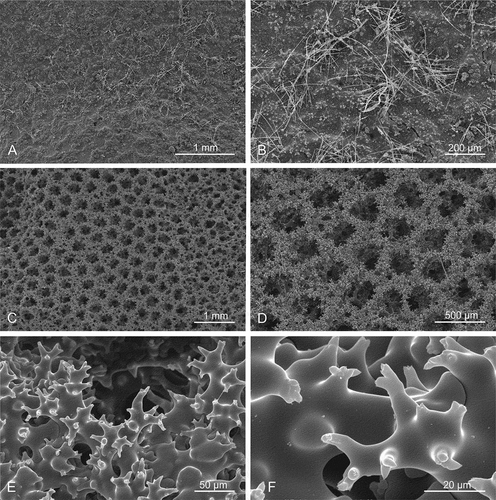
Microscleroderma miritatarata sp. nov.(–), )
Synonymy
Microscleroderma sp. 1 LR656088, LR656089 (Schuster et al. Citation2021)
Diagnosis
Microscleroderma with a complex mass of folded laminae, microoxeas in the choanosomal part, long hair-like oxeas partly bundled on top of the surface.
Type material
Holotype specimen MNHN-IP-2019-11 (field number P112), Nuku Hiva Island, Marquesas Islands, French Polynesia, 8°46.173’S, 140°05.593ʹW, 6 m, Station MNH03, Expedition BSMPF-1, long Ekamako cave, sandy bottom, SCUBA, Coll. Eric Folcher, 29/08/2009; Paratype specimen MNHN-IP-2019-12 (field number P109) same collection details as the holotype.
Comparative material
Microscleroderma lava sp. nov. MNHN-IP-2019-10 (see above).
Distribution
Nuku Hiva Island, Marquesas Islands, French Polynesia.
Habitat
Deep inside caves, sandy bottom.
Description
Morphology, the holotype (MNHN-IP-2019-11) is a complex mass of folded laminae that is 7.5 cm large and about 4 cm wide and 3 cm high. The laminae are 5–6 mm wide and have rounded margins (
Etymology
Named after Miri Tatarata, the head of the environment department in French Polynesia.
DNA barcodes
28S (C1-D2 region): LR656088, LR656089 (Schuster et al. Citation2021).
Remarks
This species is most similar to the new species Microscleroderma lava sp. nov. from Tahiti Iti, however, it differs in having longer, thinner and more spined sigmaspires. It also differs in the gross morphology. Another morphologically close species is M. lamina from the Lebanon coast of the Mediterranean Sea (Pérez et al. Citation2004), but their geographic distance makes their con-specificity very unlikely. Molecularly our new species M. miritatarata sp. nov. is sister to Microscleroderma sp. 1 (LR656087) from Martinique (
Figure 10. Surface of Microscleroderma miritatarata sp. nov. (a,b): Holotype specimen MNHN-IP-2019-11. (c,d): Paratype specimen MNHN-IP-2019-12 with D showing the membrane around ostium with several sigmaspire microscleres embedded
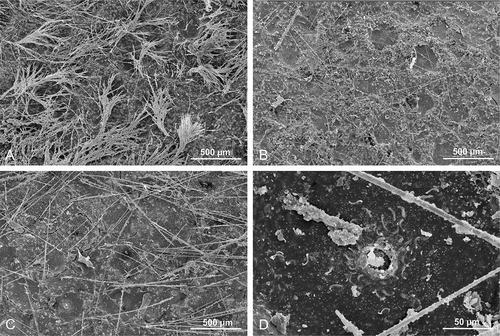
Figure 11. Choanosomal skeleton of Microscleroderma miritatarata sp. nov. Details of rhizoclone desmas of the holotype specimen MNHN-IP-2019-11. (a–c): represent one side of the surface. (d–f): represents the opposite side of the surface
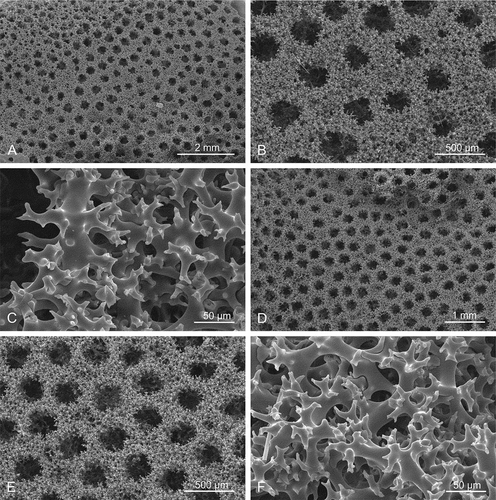
Figure 12. Choanosomal skeleton of Microscleroderma miritatarata sp. nov. Details of rhizoclone desmas of the paratype specimen MNHN-IP-2019-12. (a–c): represent one side of the surface. (d–f): represents the opposite side of the surface
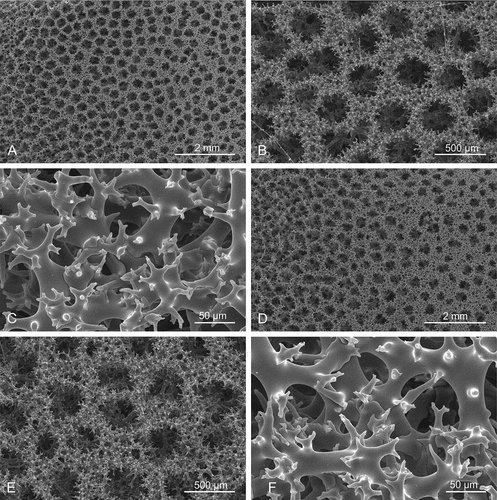
Figure 13. Sigmaspires of Microscleroderma miritatarata sp. nov. (a–e): Sigmaspires of holotype specimen MHNH-IP-2019-11. (f–j): Sigmaspires of paratype specimen MHNH-IP-2019-12
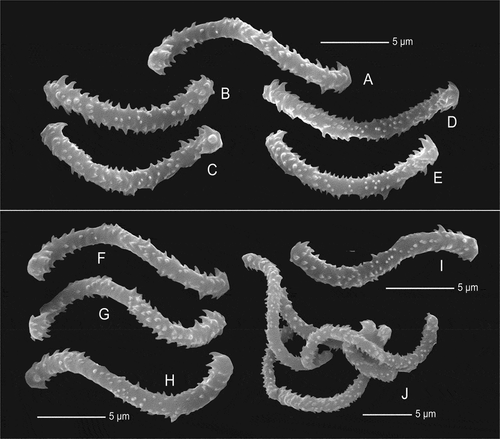
Figure 14. Bayesian inference (BI) reconstruction of the 28S rDNA gene (C1-D2 region) showing the relationship of Levispongia meyeri sp. nov. (in red) to other genera within the family Corallistidae. Bayesian posterior probability values are indicated for clades >0.75 otherwise not given. Numbers following the taxon names are collection numbers or NCBI Genbank accession numbers
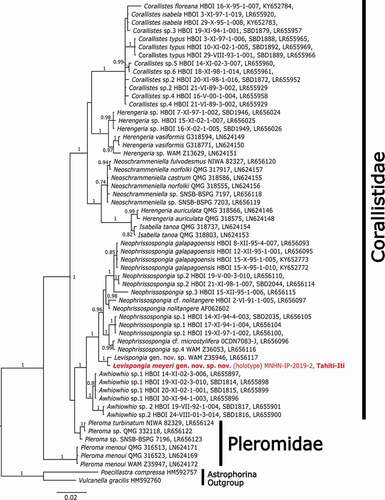
Figure 15. Bayesian inference (BI) reconstruction of the 28S rDNA gene (C1-D2 region) showing the relationship of the new species (in red) to other spirophorin species. Bayesian posterior probability values are indicated for clades >0.75 otherwise not given. Numbers following the taxon names are collection numbers or NCBI Genbank accession numbers
Discussion and summary
In this study, we report for the first time the occurrence of lithistids in French Polynesia and subsequently increase the current knowledge on Pacific lithistids. Three new species of rhizomorines were described i.e. Microscleroderma lava sp. nov., Microscleroderma miritatarata sp. nov. and Gastrophanella basaltica sp. nov. One new corallistid species Levispongia meyeri gen. nov. sp. nov., which is also a new genus based on the morphological and the phylogenetic species concept, was also described.
Microscleroderma miritatarata sp. nov. was found in the cave Ekamako on Nuku Hiva Island (Marquesas Islands) (
Overall, the Tahiti lithistid fauna with three new species belonging to three different families seems to be more diverse than the Marquesas with only one species. However, compared to the observed diversity in the Western Pacific (unpublished data A. Pisera and M. Ekins) and Eastern Pacific e.g. the Gálapagos Archipelago (Schuster et al. Citation2018) and Cocos Island (unpublished data A. Schuster) both faunas show a much lower diversity. This finding would fit well with the general pattern of decreasing diversity from the West and East continental areas towards the Central Pacific (Zezina Citation1997, Citation2001; Bitner Citation2006). In addition, the Marquesas Islands and Tahiti are one of the most isolated archipelagos in our oceans with no additional islands in between that would facilitate dispersal of organisms. Also, water current patterns, which play an essential role in dispersal of marine organisms, are generally further away from this archipelago (Springer Citation1982; Richer De Forges et al. Citation1999). Interestingly, a very similar assemblage of lithistids (Microscleroderma, Gastrophanella and corallistids) was reported from a submarine cave in the Philippines (Pisera & Vacelet Citation2006). In conclusion, this study provides the first to investigate the cave lithistid fauna in one of the most remote marine areas, the French Polynesian Archipelago by providing descriptions and barcodes of this unique cave specimens. Our phylogenetic analyses (
Acknowledgements
We thank the Research and Environment offices of French Polynesia for their help and support, and IRD for funding the R/V Alis field trip. This research work was funded by the Netbiome project (ANR-11-EBIM-0006) “POMARE” and grants from French Polynesian Authorities and Délégation à la Recherche-France in Tahiti (projects Marquesas and Biopolyval). AS received funding from VILLUM FONDEN [Grant No. 16518]. AP was supported by statutory funds of the Polish Academy of Sciences and partly by NSC Poland, grant no. 2016/21/B/ST10/02332. The authors would like to warmly thank Lionel Hertrich who discovered the Te Pari cave and guided us for its exploration, and Peva Levi for his observations above this cave and help for the underwater cave exploration. Aleksandra-Hołda Michalska (Institute of Paleobiology, Warsaw) is thanked for help with preparation of figures. Prof. Dr Dirk Erpenbeck and Prof. Dr Gert Wörheide from the Department of Earth and Environmental Sciences, LMU München, Munich, Germany are thanked for providing the sequences of two of the new species for this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Bergquist PR, Hogg JJ. 1969. Free amino acid patterns in Demospongiae: A biochemical approach to sponge classification. Cahiers De Biologie Marine 10(2):205–220.
- Bewley CA, Debitus C, Faulkner DJ. 1994. Microsclerodermins A and B. antifungal cyclic peptides from the lithistid sponge Microscleroderma sp. Journal of the American Chemical Society 116(17):7631–7636. DOI: 10.1021/ja00096a020.
- Bibiloni MA, Uriz MJ, Gili JM. 1989. Sponge communities in three submarine caves of the Balearic Islands (Western Mediterranean): Adaptations and faunistic composition. Marine Ecology 10(4):317–334. DOI: 10.1111/j.1439-0485.1989.tb00076.x.
- Bitner MA. 2006. First record of brachiopods from the Marquesas Islands, French Polynesia, South Central Pacific. Pacific Science 60(3):417–424. DOI: 10.1353/psc.2006.0016.
- Burton M. 1929. Description of South African sponges collected in the South African marine survey. Part II. The ‘Lithistidae’, with critical survey of the desma-forming sponges. Fisheries and Marine Biological Survey Division, Union of the South Africa Report. Special Report 7:7–9.
- Bussotti S, Terlizzi A, Fraschetti S, Belmonte G, Boero F. 2006. Spatial and temporal variability of sessile benthos in shallow Mediterranean marine caves. Marine Ecology Progress Series 325:109–119. DOI: 10.3354/meps325109.
- Cárdenas P, Pérez T, Boury-Esnault N. 2012. Sponge systematics facing new challenges. In: Becerro MA, Uriz MJ, Maldonado M, Turon X, editors. Advances in sponge science: Phylogeny, systematics, ecology, Vol. 61. Advances in Marine Biology. Amsterdam, Boston, Heidelber, London, New York, Oxford, Paris, San Diego, San Francisco, Singapore, Sydney, Tokyo: Elsevier. pp. 79–209.
- Carvalho FC, Pomponi SA, Xavier JR. 2015. Lithistid sponges of the upper bathyal of Madeira, Selvagens and Canary Islands, with description of a new species of Isabella. Journal of the Marine Biological Association of the United Kingdom 95(7):1287–1296. DOI: 10.1017/S0025315414001179.
- Culver DC, Pipan T. 2019. Adaptations to subterranean life. In: The biology of caves and other subterranean habitats. Oxford University Press. DOI: 10.1093/oso/9780198820765.001.0001.
- Darriba D, Taboada GL, Doallo R, Posada D. 2012. jModelTest 2: More models, new heuristics and parallel computing. Nature Methods 9:772. DOI: 10.1038/nmeth.2109.
- Debitus C. 2009. BSMPF-1 cruise. RV Alis. DOI: 10.17600/9100030.
- Debitus C. 2013. Tahiti Iti cruise. RV Alis. DOI: 10.17600/13100040.
- Dendy A. 1905. Report on the sponges collected by Professor Herdman at Ceylon, in 1902. In: Herdman WA, editor. Report to the Government of Ceylon on the Pearl Oyster Fisheries of the Gulf of Manaar, Vol. 3 (Supplement 18). London: Royal Society. pp. 103–104 & pls I–XVI.
- Dendy A. 1913. The Percy Sladen trust expedition to the Indian Ocean in 1905 (V). I. Report on the calcareous sponges collected by HMS ‘Sealark’ in the Indian Ocean. Transactions of the Linnean Society of London 2(16):1–29.
- Frisone V, Pisera A, Preto N. 2016. A highly diverse siliceous sponge fauna (Porifera: Hexactinellida, Demospongiae) from the Eocene of north-eastern Italy: Systematics and palaeoecology. Journal of Systematic Palaeontology 14(11):949–1002. DOI: 10.1080/14772019.2015.1132015.
- Gerovasileiou V, Chintiroglou C, Vafidis D, Koutsoubas D, Sini M, Dailianis T, Issaris Y, Akritopoulou E, Dimarchopoulou D, Voultsiadou E. 2015. Census of biodiversity in marine caves of the eastern Mediterranean Sea. Mediterranean Marine Science 16(1):245–265. DOI: 10.12681/mms.1069.
- Gerovasileiou V, Chintiroglou CC, Konstantinou D, Voultsiadou E. 2016. Sponges as ‘living hotels’ in Mediterranean marine caves. Scientia Marina 80(3):279–289. DOI: 10.3989/scimar.04403.14B.
- Gerovasileiou V, Voultsiadou E. 2012. Marine caves of the Mediterranean Sea: A sponge biodiversity reservoir within a biodiversity hotspot. PLoS One 7(7):e39873. DOI: 10.1371/journal.pone.0039873.
- Gómez P. 1998. First record and new species of Gastrophanella (Porifera: Demospongiae: Lithistida) from the central East Pacific. Proceedings of the Biological Society of Washington 111(4):774–780.
- Gómez P, Calderón-Gutiérrez F, González-Gándara C, De Los Angeles Rojas-Terán M. 2021. New species of Microscleroderma and Amphibleptula (Demospongiae, Tetractinellida, Scleritodermidae) from two contrasting marine environments. Journal of the Marine Biological Association of the United Kingdom 1–11. DOI: 10.1017/S0025315421000114.
- Grant RE. 1836. Animal Kingdom. In: Todd RB, editor. The cyclopaedia of anatomy and physiology, Vol. 1. London: Sherwood,Gilbert, and Piper. pp. 107–118. 1–813.
- Gray JE. 1868. Note on Theonella, a new genus of coralloid sponges from Formosa. Proceedings of the Zoological Society of London 3:565–566.
- Harmelin JG, Vacelet J. 1997. Clues to deep-sea biodiversity in a nearshore cave. Vie et milieu 47(4):351–354.
- Kelly M. 2007. The Marine Fauna of New Zealand: Porifera: Lithistid Demospongiae (rock sponges). NIWA Biodiversity Memoir 121:1–100.
- Kirkpatrick R. 1903. Descriptions of South African Sponges. Part II. Marine Investigations in South Africa 2(14):171–180, pl. IV. p. 173.
- Klautau M, Lopes MV, Guarabyra B, Folcher E, Ekins M, Debitus C. 2020. Calcareous sponges from the French Polynesia (Porifera: Calcarea). Zootaxa 4748(2):261–295. DOI: 10.11646/zootaxa.4748.2.3.
- Larsson A. 2014. AliView: A fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 30(22):3276–3278. DOI: 10.1093/bioinformatics/btu531.
- Lévi C, Lévi P. 1983. Eponges Tétractinellides et Lithistides bathyales de Nouvelle-Calédonie. Bulletin du Muséum national d’Histoire naturelle (4) 5(1):114–116.
- Lévi C, Lévi P. 1988. Nouveaux Spongiaires Lithistides bathyaux à affinités crétacées de la Nouvelle-Calédonie. Bulletin du Muséum national d’Histoire naturelle 10(A, 2):241–263.
- Łukowiak M, Pisera A, Schlögl J. 2014. Bathyal sponges from the late early miocene of the Vienna Basin (central Paratethys, Slovakia). Paläontologische Zeitschrift 88:263–277. DOI: 10.1007/s12542-013-0197-x.
- Manconi R. 2011. Order lithistida. In: Pansini M, Manconi R, Pronzato R, editors. Porifera I. Calcarea, Demospongiae (partim), Hexactinellida, Homoscleromorpha. Fauna d’Italia, XLVI, Vol. 24. Bologna: Edizioni Calderini-II Sole. pp. 367–392.
- Manconi R, Serusi A. 2008. Rare sponges from marine caves: Discovery of Neophrissospongia nana nov. sp. (Demospongiae, Corallistidae) from Sardinia with an annotated checklist of Mediterranean lithistids. ZooKeys 4(4):71–87. DOI: 10.3897/zookeys.4.39.
- Manconi R, Serusi A, Pisera A. 2006. A new Mediterranean ‘lithistid’ sponge, Aciculites mediterranea sp. nov. (Porifera: Demospongiae) from a dark marine cave in Sardinia. Journal of the Marine Biological Association of the UK 86(4):691–698. DOI: 10.1017/S0025315406013580.
- Marshall W. 1876. Ideen über die Verwandtschaftsverhältnisse der Hexactinelliden. Zeitschrift für wissenschaftliche Zoologie 27(1):113–136.
- Miller MA, Pfeiffer W, Schwartz T 2010. Creating the CIPRES science gateway for inference of large phylogenetic trees. Gateway Computing Environments Workshop, New Orleans, USA: 1–8. DOI: 10.1109/GCE.2010.5676129.
- Moldovan OT, Kováč Ľ, Halse S. 2019. Cave ecology. Springer Nature Switzerland. DOI: 10.1007/978-3-319-98852-8.
- Mothes B, Silva CMM. 1999. Esponjas com desmas do Atlântico Sul-Brasileiro (Porifera, Demospongiae) com duas novas espécies. Iheringia (Zoologia) 86:125–136.
- Muricy G, Minervino JV. 2000. A new species of Gastrophanella from central western Atlantic, with a discussion of the family Siphonidiidae (Demospongiae: Lithistida). Journal of the Marine Biological Association of the United Kingdom 80(4):599–605. DOI: 10.1017/S0025315400002411.
- Pérez T, Chevaldonne P, Corbari L, Poupin J, Heros V, Starmer J, Buge B, Albenga L, Pachoud G, Morvan J, Bouchet P 2012. Endémisme et hotspots de biodiversité des milieux marins de l’archipel des Marquises - un enjeu de conservation pour la Polynésie dans le Pacifique. Leg 3, CNRS UMR 7263, rapport de campagne: 1–75.
- Pérez T, Vacelet J, Bitar G, Zibrowius H. 2004. Two new lithistids (Porifera: Demospongiae) from a shallow eastern Mediterranean cave (Lebanon). Journal of the Marine Biological Association of the UK 84(1):15–24. DOI: 10.1017/S0025315404008859h.
- Pisera A. 2002. Fossil lithistids: An overview. In: Hooper JNA, Van Soest RWM, editors. Systema Porifera. A guide to the classification of sponges. New York, Boston: Kluwer Academic/ Plenum Publishers. pp. 388–402.
- Pisera A. 2004. What can we learn about siliceous sponges from Palaeontology. Bollettino dei musei e degli istituti biologici dell’Universita di Genova 68:55–69.
- Pisera A, Gerovasileiou V 2018. Lithistid demosponges from submarine caves of Crete Island (Eastern Mediterranean Sea): Is their occurrence controlled by water silicate content? IV International Symposium for Anchialine Ecosystems Lanzarote.
- Pisera A, Lévi C. 2002a. ‘Lithistid’ Demospongiae. In: Hooper JNA, Van Soest RWM, editors. Systema Porifera. A guide to the classification of sponges. New York, Boston: Kluwer Academic/ Plenum Publishers. pp. 299–301.
- Pisera A, Lévi C. 2002b. Family Corallistidae Sollas, 1888. In: Hooper JNA, Van Soest RWM, editors. Systema Porifera. A guide to the classification of sponges. New York, Boston: Kluwer Academic/ Plenum Publishers. pp. 312–320.
- Pisera A, Lévi C. 2002c. Family Theonellidae Lendenfeld, 1903. In: Hooper JNA, Van Soest RWM, editors. Systema Porifera. A guide to the classification of sponges. New York, Boston: Kluwer Academic/ Plenum Publishers. pp. 327–337.
- Pisera A, Lévi C. 2002d. Family Siphonidiidae Lendenfeld, 1903. In: Hooper JNA, Van Soest RWM, editors. Systema Porifera. A guide to the classification of sponges. New York, Boston: Kluwer Academic/ Plenum Publishers. pp. 338–343.
- Pisera A, Pomponi SA. 2015. New data on lithistid sponges from the deep Florida shelf with description of a new species of Theonella. Journal of the Marine Biological Association of the UK 95(7):1297–1309. DOI: 10.1017/S0025315414001477.
- Pisera A, Vacelet J 2006. New lithistid sponges from a submarine cave in Philippines: Affinities and evolutionary significance. 7th International Sponge Symposium, Biodiversity, Innovation, Sustainability Rio de Janeiro, Brazil.
- Pisera A, Vacelet J. 2011. Lithistid sponges from submarine caves in the Mediterranean: Taxonomy and affinities. Scientia Marina 75(1):17–40. DOI: 10.3989/scsimar.2011.75n1017.
- Pomponi SA, Kelly M, Reed JK, Wright AE. 2001. Diversity and bathymetric distribution of lithistid sponges in the tropical western Atlantic region. Bulletin of the Biological Society of Washington 10:344–353.
- Qureshi A, Colin PL, Faulkner DJ. 2000. Microsclerodermins F-I, antitumor and antifungal cyclic peptides from the lithistid sponge Microscleroderma sp. Tetrahedron 56(23):3679–3685. DOI: 10.1016/S0040-4020(00)00286-6.
- Rambau A 2009. FigTree, a graphical viewer of phylogenetic trees. http://tree.bio.ed.ac.uk/software/figtree/.
- Reid REH. 1967. Tethys and the zoogeography of some modern and Mesozoic Perifera. In: Adams CG, Ager DV, editors. Aspects of tethyan biogeography. London: Systematics Association, Vol. 7. pp. 171–181.
- Richer De Forges B, Poupin J, Laboute P. 1999. La campagne Musorstom 9 dans l’archipel des îles Marquises (Polynésie française). Compte rendu et liste des stations. In: Crosnier A, editor. Résultats des campagnes Musorstom vol. 20, Vol. 180. Paris: Mémoires du Museum National d’Histoire Naturelle. pp. 9–29.
- Schlacher-Hoenlinger MA, Pisera A, Hooper JNA. 2005. Deep-sea “lithistid” assemblages from the Norfolk Ridge (New Caledonia), with description of seven new species and a new genus (Porifera, Demospongiae). Zoosystema 27(4):649–696.
- Schmidt O. 1870. Grundzüge einer Spongien-Fauna des atlantischen Gebietes. Leipzig: Verlag von Wilhelm Engelmann. pp. 1–88.
- Schmidt O. 1879. Die Spongien des Meerbusen von Mexico (Und des caraibischen Meeres). Abteilung I. Heft I. In: Reports on the dredging under the supervision of Alexander Agassiz, in the Gulf of Mexico, by the USCSS ‘Blake’. Gustav Fischer. Jena. pp.1–32, pls I–IV.
- Schrammen A. 1924. Die Kieselspongien der oberen Kreide von Nordwestdeutschland. III. 767 und letzter Teil. Monographien zur Geologie und Paläontologie 1:1–159.
- Schuster A, Erpenbeck D, Pisera A, Hooper JNA, Bryce M, Fromont J, Wörheide G. 2015. Deceptive desmas: Molecular phylogenetics suggests a new classification and uncovers convergent evolution of lithistid demosponges. PLoS One 10(1):e116038. DOI: 10.1371/journal.pone.0116038.
- Schuster A, Lopez JV, Becking LE, Kelly M, Pomponi SA, Wörheide G, Erpenbeck D, Cárdenas P. 2017. Evolution of group I introns in Porifera: New evidence for intron mobility and implications for DNA barcoding. BMC Evolutionary Biology 17:1–21. DOI: 10.1186/s12862-017-0928-9.
- Schuster A, Pisera A, Kelly M, Bell LJ, Pomponi SA, Wörheide G, Erpenbeck D. 2018. New species and a molecular dating analysis of Vetulina Schmidt, 1879 (Porifera: Demospongiae: Sphaerocladina) reveal an ancient relict fauna with Tethys origin. Zoological Journal of the Linnean Society 184(3):585–604. DOI: 10.1093/zoolinnean/zlx114.
- Schuster A, Pomponi SA, Pisera A, Cárdenas P, Kelly M, Wörheide G, Erpenbeck D. 2021. Systematics of ‘lithistid’ tetractinellid demosponges from the Tropical Western Atlantic – Implications for phylodiversity and bathymetric distribution. PeerJ 9:e10775. DOI: 10.7717/peerj.10775.
- Sollas WJ. 1885. A classification of the sponges. Annals and Magazine of Natural History 5 16(95):395. DOI: 10.1080/00222938509459901.
- Sollas WJ. 1887. Sponges. In: Black AC, editor. Encyclopaedia Britannica, 9th ed. Edinburgh. Encyclopaedia Britannica. pp. 412–429.
- Sollas WJ. 1888. Report on the Tetractinellida collected by H.M.S. Challenger, during the years 1873-1876. Zoology 25(part 63):1–458, pl. 1–44.
- Springer VG. 1982. Pacific plate biogeography, with special reference to shorefishes. Smithsonian Contributions to Zoology 367:1–182. DOI: 10.5479/si.00810282.367.
- Świerczewska-Gładysz E. 2016. Early Campanian (Late Cretaceous) Pleromidae and Isoraphiniidae (lithistid Demospongiae) from the Łódź-Miechów Synclinorium (central and southern Poland): New data and taxonomic revision. Cretaceous Research 71:40–62. DOI: 10.1016/j.cretres.2016.11.007.
- Vacelet J. 1996. Deep-sea sponges in a Mediterranean cave. In: Uiblein F, Ott J, Stachowitsch M, editors. Deep-sea and extreme shallow-water habitats: Affinities and adaptations. Biosystematics and Ecology Series 11:299–312.
- Vacelet J, Vasseur P. 1965. Spongiaires des grottes et surplombs des récifs de Tuléar (Madagascar). Recueil des Travaux de Station Marine d’Endoume-Marseille hors Serie 2–4:71–123.
- Van Soest RWM, Boury-Esnault N, Hooper JNA, Rützler K, de Voogd NJ, Alvarez B, Hajdu E, Pisera A, Manconi R, Schönberg C, Klautau M, Kelly M, Vacelet J, Dohrmann M, Díaz M-C, Cárdenas P, Carballo JL, Ríos P, Downey R, Morrow CC 2019. World Porifera Database. Gastrophanella Schmidt, 1879. Accessed at: http://www.marinespecies.org/porifera/porifera.php?p=taxdetails&id=171200 2020 November 16.
- Von Lendenfeld R. 1903. Porifera. Tetraxonia. In: Schulze FE, editor. Das Tierreich. Vol. 19. Berlin: Friedländer. pp. vi–xv, 1–168.
- Zezina ON. 1997. Biogeography of the bathyal zone. In: Gebruk AV, Southward EC, Tyler PA, editors. The biogeography of the oceans, Vol. 32. Advances in Marine Biology. London: Academic Press. pp. 389–426.
- Zezina ON. 2001. Global surface-water circulation and the main features of brachiopod biogeography. In: Brunton CHC, Cocks LRM, Long SL, editors. Brachiopods past and present, Vol. 63. Systematics Association Special Volume Book. London. pp. 102–107.
- Zittel KA. 1878. Studien über fossile Spongien. II. Lithistidae. Abhandlungen der Mathematisch-Physikalischen Classe der Königlich-Bayerischen Akademie der Wissenschaften 13(1):65–154.