 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Temperature, an important factor in the ecological niche, determines the individual fitness, distribution and abundance of many spider species. Typically, spiders from different habitats have different thermal preferences, which vary depending on the season, time of day, and reproductive status. Selected temperatures may also depend on thermal acclimation of animals. The study aimed at determining preferred temperatures of females of two spider species, synanthropic Eratigena atrica and garden Araneus diadematus, exposed to low (14°C) and high (23°C) acclimation temperatures. The mean preferred temperature observed in a thermal gradient varied between the species: E. atrica selected 13.7 ± 1.0°C, while A. diadematus selected 22.5 ± 1.5°C, irrespective of the acclimation temperature. Accordingly, thermal niche overlaps between the species were significantly lower than predicted by the random distribution. E. atrica changed their thermal environment on average 12.8 ± 1.9 times during the test, which made them more mobile than A. diadematus, changing their thermal environment 2.7 ± 0.5 times. On the other hand, the thermal niche breadth of A. diadematus acclimated to warm temperature was wider than that of E. atrica. The female behaviour may be related to the selection of optimal conditions with regard to food availability (location for web construction), shelter and/or, in the case of E. atrica, embryonic development.
Introduction
Most animals are ectothermic, with body temperature reflecting their environmental conditions. Thus, environmental temperature determines the rate of their biochemical and physiological processes, affecting such functions as metabolism, development, growth, movement and reproduction, and having pronounced consequences at the organismal, community and ecosystem level (Sinclair et al. Citation2016). Nevertheless, ectothermic animals can control their temperature to some extent by using behavioural thermoregulation. Appropriate behaviour can help them avoid extremely high or low, potentially lethal temperatures and extends the period spent by an organism in physiologically optimal conditions (Dillon et al. Citation2009).
Thermoregulatory abilities of ectotherms are particularly important in the current world, undergoing global climate change: they may determine the potential of animal survival in the changing environment, spread to novel habitats and the outcome of interspecific interactions (Bozinovic et al. Citation2016). Many species suffer from current anthropogenic changes, including changes in the thermal environment. However, a number of organisms have adapted to live under human impact through the process of synathropication, and can benefit from artificially increased temperatures due to global warming, as well as in buildings and indoor facilities. These species should be able to select temperatures available in the anthropogenic environment to optimize their thermal conditions.
Many aspects of the thermal biology of ectotherms have been resolved using insect models (e.g. Drosophila sp.) that are easy to breed in captivity, have short life cycles and well known genetic structure. They also dominate in terrestrial ecosystems in terms of biomass and species richness (e.g. Dillon et al. Citation2009). Spiders are another group of arthropods constituting a good model for studying ectotherm biology. Spiders are the most ubiquitous insectivores in terrestrial ecosystems, reaching densities of more than 1000 ind. m−2 and strongly affecting invertebrate communities (Greenstone Citation1999; Nyffeler Citation2000). They are small (several mm to several cm, several mg to several g), which results in high surface to volume ratio, increasing sensitivity to temperature and susceptibility to water loss (Figueroa et al. Citation2010; Canals et al. Citation2015). Therefore, spiders are particularly suitable to study hypotheses on thermal adaptations of species living in various habitats and under variable environmental conditions (Alfaro et al. Citation2013a).
Thermal biology of spiders has been poorly studied; thermoregulatory behaviour, thermal tolerances and preferences have been determined for a small fraction of all spider species, despite the ongoing research and accumulation of new data on more and more spider taxa (e.g. Anthony et al. Citation2019; Barnes et al. Citation2019; Montes de Oca et al. Citation2020; Taucare-Rios et al. Citation2020; Schwerdt et al. Citation2020a). Such data are necessary to assess the suitability of habitats and understand the ecology of species (Hertz et al. Citation1993; Canals Citation1998; Schmalhofer Citation1999; Frick et al. Citation2007; Hanna & Cobb Citation2007; Veloso et al. Citation2012; Alfaro et al. Citation2013a).
As for all ectothermic organisms, spatial and temporal temperature fluctuations can negatively affect spider functioning and survival. Therefore, spiders exhibit a number of mechanisms helping them avoid thermal stress and maintain their optimum body temperature. They regulate their body temperature behaviourally, for example by seeking sunlight or shade, retreating to burrows, taking a certain position on the web, and/or building webs with a certain orientation (to receive maximum sunlight). Body temperature can also be regulated morphologically, through body colouration, size, shape and integument structure. All these mechanisms modulate physical factors and allow spiders to adjust their body temperature relative to the environmental conditions (Humphreys Citation1987, Citation1991; Pulz Citation1987; Barghusen et al. Citation1997).
Spider metabolic rate varies with temperature (e.g. Schmalhofer Citation2011 and references therein). Consequently, all processes depending on metabolic rate are related to temperature, including embryogenesis, moulting, sexual maturation, life expectancy, and reproductive success (Pulz Citation1987). Temperature also affects female fertility and oviposition (Downes Citation1988; Li & Jackson Citation1996; Kiss & Samu Citation2002). Finally, a number of behaviours depend on environmental temperature, including courtship (Davis Citation1989), copulation (Costa & Sotelo Citation1984), microhabitat selection (Taucare-Rios et al. Citation2018), escape speed (Cobb Citation1994), location and structure of the web (Riechert & Tracy Citation1975; Barghusen et al. Citation1997; Hanna & Cobb Citation2006), latency to attack (Henschel et al. Citation1992), duration of prey capture sequences (Henschel et al. Citation1992) time allocation to foraging (Riechert & Tracy Citation1975; Turner et al. Citation1993), reaction and orientation time on the web (Hesselberg & Vollrath Citation2006), nest location (Hanna & Cobb Citation2006), and dispersal via ballooning (long distance) and rappelling (short distance) (Bonte et al. Citation2008). Nevertheless, the exact impact of temperature on organisms is difficult to estimate, as thermal conditions in the wild may interfere with other environmental factors, e.g. humidity, lighting, habitat structure, winds, and food availability (Pulz Citation1987).
Spiders from different environments may have very different thermal preferences (Pulz Citation1987; Schmalhofer Citation1999; Schwerdt et al., Citation2020b), and these preferences can change seasonally or circadianly (Canals et al. Citation1997; Schmalhofer Citation1999; Hanna & Cobb Citation2007; Veloso et al. Citation2012; Alfaro et al. Citation2013a; Taucare-Rios et al. Citation2020). Moreover, spider thermal preferences may vary depending on reproductive status, sex, developmental stage, nutrition or hydration (Sevacherian & Lowrie Citation1972; Humphreys Citation1987).
Based on the above observations, we investigated thermal preferences of two European spider species, Eratigena atrica (C. L. Koch 1843) (Agelenidae) and Araneus diadematus Clerck 1758 (Araneidae), which differ in behaviour and occupied habitats. E. atrica, a synanthropic spider found near residential and farm buildings from spring to autumn, weaves funnel webs in shady places. Apart from waiting on the web, many individuals leave the web in search of prey (personal observations). At the end of summer (August/September), they reach sexual maturity, mate and then relocate into sheds, garages and basements, where females lay eggs. The oviposition period starts in November and lasts until the end of winter (March). Females lay several egg sacs, from which the next generation emerges. In the next spring, young spiders migrate to outdoor habitats where they continue their development (Jacuński et al. Citation1999). In contrast, A. diadematus is a typical orb-web weaver inhabiting forests, meadows and gardens. They mature between July and August, mate throughout August and at the beginning of September (Johannesen & Toft Citation2002). Females lay a single egg sac 2–6 weeks after the copulation, usually in October. Females die soon after and embryos overwinter in the cocoon. Juveniles appear in spring and develop throughout the summer (Smith Citation1984; Vollrath Citation1987).
Based on the differences between the two species, we hypothesised that pre-ovipositional E. atrica females would select a significantly lower temperature than A. diadematus. In autumn, immediately before egg laying, E. atrica move to buildings, where they should select places providing them with safety (they are relatively easy to spot due to their large size), food sources (insects) and good conditions for embryo development. Such microhabitats, located in utility rooms, farm buildings, sheds, etc., are usually cooler in winter than those in apartments and houses inhabited by humans, but still warmer than outdoor habitats (personal observations). On the other hand, we hypothesized that A. diadematus females would select a higher temperature, which under field conditions would represent the sunny locations most frequently visited by insects, i.e. food sources. Such places would allow them to obtain sufficient energetic resources to maximize their reproductive success. Moreover, on sun-exposed webs, they experience relatively high circadian temperature fluctuations, which suggests their adaptation to such variable conditions. Therefore, we also expected that A. diadematus would have a wider thermal niche (range of occupied temperatures) than E. atrica. We also assumed that an acclimation temperature would affect thermal preferences of females, which would select temperatures close to their acclimation conditions. Comparison between these two spider species would significantly contribute to understanding thermal preferences of ectothermic organisms during their pre-ovipositional period (after copulation, but before oviposition), specifically in the context of synanthropic species.
Materials and methods
We investigated thermal preferences of two spider species collected in the vicinity of Toruń (18°37ʹE; 53°02ʹN) and Chełmża (18°36ʹE; 53°12ʹN) (Poland). Female Eratigena atrica were collected near residential and farm buildings in the second half of September 2017, while female Araneus diadematus were sampled at the end of August 2018 on meadows, directly from their webs. Females of both species were collected at the time when they reach sexual maturity and can mate with males. This was important for unifying the conditions of the experiment. In total, 28 females of E. atrica and 36 females of A. diadematus were collected. The collected spiders were immediately transported to a dark laboratory (to standardize conditions for all experimental animals) with the temperature of 20–22°C and placed in 250 cm3 glass containers. The vessels contained water-soaked cotton balls to ensure proper humidity. Several holes in the lids allowed ventilation. In the containers, spiders constructed webs (though A. diadematus were unable to build a fully shaped orb web) from which they readily collected food (a Tenebrio molitor larva provided twice a week).
Females of E. atrica began to lay egg sacs 6–10 weeks after collection. Tests on their thermal preferences began in mid-December 2017, approximately in the middle of their oviposition period. Thus, during the tests, they were still pre-ovipositional and laid egg sacs in 10–14 day intervals. Tests on A. diadematus started in early October 2018, 5–6 weeks after their collection. Females of this species did not lay egg sacs before testing, but did it 4–7 days after testing (and then died within 1–2 weeks), thus they were also pre-ovipositional during the tests. To confirm that the eggs of both species were fertilized (thus the females had been inseminated in the wild before their collection), the cocoons were dissected and randomly selected eggs were immersed in paraffin oil making the chorion transparent. This made it possible to confirm the presence of a developing embryo in the examined egg sacs.
Before the tests, spider size was estimated on the basis of a random subsample of 10 individuals of each species, using the width and length of their prosoma. The prosoma in E. atrica was 6.2 ± 0.8 mm (mean ±SD) long and 8.2 ± 0.8 mm wide, while in A. diadematus these dimensions were 5.6 ± 0.7 mm and 6.9 ± 1.1 mm, respectively. Thus, the within species size variability in our study was very low and interspecific differences reflected natural variability between the studied species (Roberts Citation1995).
Our experiments were conducted in a thermal gradient. This allowed us to standardize all other factors potentially affecting spider choice (e.g. humidity, photoperiod, food availability, structural constraints of web building, etc.) at a constant level, so that temperature was the only factor driving their selection.
Before the experiment in the thermal gradient, spiders of both species were randomly divided into two groups. One group of E. atrica (16 females) and one group of A. diadematus (20 females) was kept in an air-conditioned room at 23°C for seven days (warm acclimation), while the other group (12 females of E. atrica and 16 females of A. diadematus) was exposed to 14°C (cold acclimation). The acclimation temperatures were selected as we intended to (1) establish the lower acclimation temperature contrasting with the higher one, but at the same time avoiding lethal values (12°C was found lethal to E. atrica embryos; Napiórkowska et al. Citation2018), (2) use conditions similar to those naturally experienced by spiders during their reproductive period. In Toruń, Poland, temperatures (daily min.-max.) in August, September and October are: 12.7–24.1°C, 8. 9–18.6°C and 4.9–13.0°C, respectively (World Meterological Organization (WMO): https://worldweather.wmo.int/en/pilot.html; retrieved on 21 September 2020). Among these values, maximum daily temperatures, experienced by spiders during their foraging activity, are the most important, as they are responsible for peaks of insect (i.e. the most common spider prey) activity. The selected acclimation temperatures also fitted the range found in winter in synanthropic locations, where the temperature may vary from values just a few °C higher than outdoors to more than 20°C in rooms occupied by humans (personal observations). The day before being placed in the gradient, the spiders were fed with one T. molitor larva to standardize their hunger level. Spiders were introduced individually into the gradient at 11 a.m. and deposited in the centre of the chamber (18–19°C). Each spider was used only once. After each trial the gradient was cleaned with ethanol to ensure that no pheromones would influence the behaviour of the next individual.
The test equipment was kept in an air-conditioned room (with the temperature of 16°C), illuminated with 6.0 lx infra-red LED lamps. No light of the visible spectrum was used to exclude the potential effect of unequal illumination of the arena and standardize conditions for both species. The system recording selected ambient temperatures (producer: Andrzej Zienkiewicz Zakład Remontowo-Montażowy Aparatury Laboratoryjnej, Toruń, Poland) consisted of four 1 × 0.07 × 0.035 m (L x W x H) computer-controlled aluminium chambers, each covered by a transparent plexiglass sheet. One end of each chamber was cooled by a cryostat K21 E20 (GK Sondermaschinenbau GmbH – Labortechnik Medingen), whereas the opposite end was simultaneously heated by an electric thermostat, which generated a linear temperature gradient, ranging from 3 to 42°C. The temperature distribution in each chamber was constantly monitored by 16 thermocouples disposed at equal distances along its floor and connected to a computer recording the data by means of Grad-K software (designed by Andrzej Zienkiewicz Zakład Remontowo-Montażowy Aparatury Laboratoryjnej, Toruń, Poland) ().
Figure 1. Experimental set-up for recording of thermoregulatory behaviour. A – thermal gradient chamber; B – fluid chambers; C – cryostat; D – thermostat; E – recording keeper; F – computer, G – thermocouples, H – position of spider introduction at the beginning of the test; Lred – red light and Cam – video camera (redrawn and modified after Grodzicki & Caputa Citation2012)
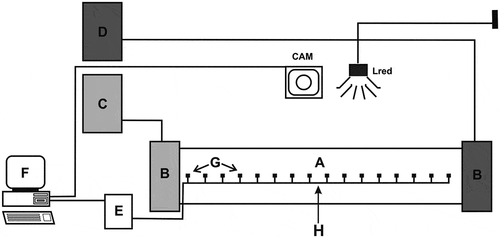
The animals were subjected to 24-h recording of their thermal behaviour. The chambers were photographed every 30 min. using a Sony Handycam HDR-SR5E video camera located above the experimental arena. Thus, for each individual, a series of 48 frames was obtained. The spider’s positions along the gradient in consecutive frames were measured using ImageJ 1.52i software (Wayne Rasband, National Institutes of Health, USA, http://imagej.nih.gov/ij). Then, each spider’s position in the gradient was associated with a current temperature using a linear intrapolation when the animal was located between two thermocouples. Finally, the following response variables were determined for each replicate (i.e. a single individual’s record): (1) the mean temperature (Tmean) selected by a spider (an average of all temperatures at which a spider was observed in consecutive frames); (2) the most common temperature (Tmode) selected by a spider (the middle value of a 2°C-wide temperature interval in which a spider spent the longest time during the test); (3) amplitude of temperatures (Tmax-min) selected by a spider (a difference between the highest and lowest temperature in which a spider occurred during the test); (4) the number of position changes (translocations of an animal between consecutive frames with a temperature change of at least 1°C). Moreover, we pooled all the replicates (separately for each species and acclimation temperature) to calculate frequencies of temperatures occupied by animals (1°C intervals). We calculated thermal niche breadths using a standardized Levin index:
where: n is the number of temperature intervals selected by animals, pi is the frequency of animal occurrences at temperature i
The index ranges from 0 to 1, with values below and above 0.6 considered as indicating stenothermic and eurythermic organisms, respectively (Alfaro et al. Citation2013b).
Moreover, we calculated thermal niche overlaps between species and acclimation temperatures using a Pianka index:
where: n is the number of temperature intervals selected by animals, p1i and p2i are the frequencies of occurrences of animals from two compared groups at temperature i.
The index ranges from 0 (no overlap) to 1 (full overlap) (Alfaro et al. Citation2013b).
Tmean was analysed using a General Linear Mixed Model including (1) species and (2) acclimation temperature as fixed factors, (3) their interaction, as well as (4) experimental series (groups of four spiders tested simultaneously in the four gradient chambers, nested in species and acclimation temperature) and (5) individual (for which 48 consecutive measurements were taken, nested in experimental series, species and acclimation temperature) as random factors. The response variable in this analysis consisted of 48 consecutive temperatures measured for each spider. Tmode and Tmax-min were analysed using General Linear Mixed Models including (1) species and (2) acclimation temperature as fixed factors, (3) their interaction and (4) experimental series (nested in species and acclimation temperature) as a random factor. Normality and homoscedasticity assumptions of the models were confirmed using a Shapiro-Wilk and Levene test, respectively. The number of position changes by spiders was analysed using a Generalized Linear Mixed Model with a negative binomial distribution suitable for overdispersed count data (variance > mean) and log link function, using the same design as that described above for Tmode and Tmax-min.
Pairwise differences in thermal niche overlap and niche breadth between species (at a given acclimation temperature) and between acclimation temperatures (within species) were tested using randomization tests with random assignments of particular individuals to experimental groups. The actual index values were compared with distributions obtained on the basis of 1000 random replicates to determine the probability of obtaining the measured values by chance (using Microsoft Excel 2013 spreadsheets). The results were considered as significant at p < 0.05.
Results
Tmean selected by spiders () varied between the species, whereas neither acclimation temperature nor an interaction of both factors affected spiders’ selection (). E. atrica spiders selected Tmean (±SE) = 13.7 ± 1.0°C (range: 2.2–24.6°C). A. diadematus individuals selected Tmean = 22.5 ± 1.5°C (range: 2.4–38.1°C).
Table 1. Analyses of the impact of spider species and acclimation temperature (Tacclim) on (a) the selected temperature (Tmean), (b) the most common selected temperature (Tmode), (c) the amplitude of selected temperatures (Tmax-min) and (d) the number of relocations between different temperatures. All models included a random effect Experimental series (nested in Species and Tacclim), the Tmean model also included a random effect Individual (nested in Experimental series, Species and Tacclim). The results for the random effects are not shown. Asterisks indicate statistically significant results (P < 0.05)
Figure 2. Frequencies of temperatures occupied by the two spider species acclimated to low (14°C) or high (23°C) temperature conditions (vertical bars), as well as their mean and most commonly selected temperatures (horizontal bars, ±SE). Niche breadths and overlaps are calculated as standardized Levin and Pianka indices, respectively. Different letters indicate significant differences in selected temperatures (GLM) and niche breadth (randomization tests). Asterisks indicate non-random niche overlaps (randomization tests)
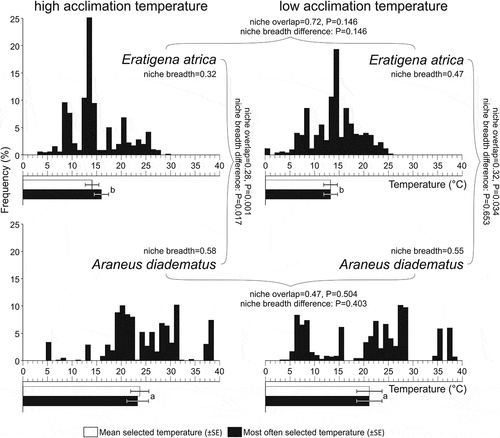
Tmode selected by spiders () was very similar to Tmean. It varied between the species, whereas the effects of acclimation temperature and the interaction of both factors were non-significant (). E. atrica and A. diadematus spiders spent most of their exposure time at Tmode = 13.7 ± 1.0°C (range: 3–25°C) and 22.2 ± 1.7°C (range: 3–39°C), respectively.
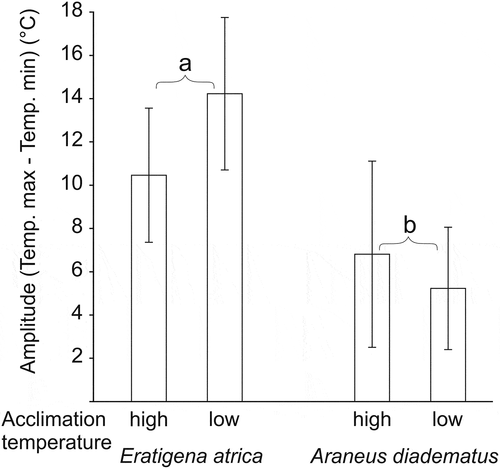
Tmax-min selected by spiders () varied between the species, but was unaffected by acclimation temperature (). During the test, E. atrica spiders visited a wider range of temperatures (mean ±SD: 12.1 ± 6.7°C) than A. diadematus (6.1 ± 7.4°C).
Figure 3. Mean amplitude of temperatures (maximum vs. minimum value noted during 24 h of exposure) selected by the two spider species acclimated to low (14°C) or high (23°C) temperature conditions. Different letters indicate significant differences in temperature amplitude (GLM). Error bars indicate standard deviations
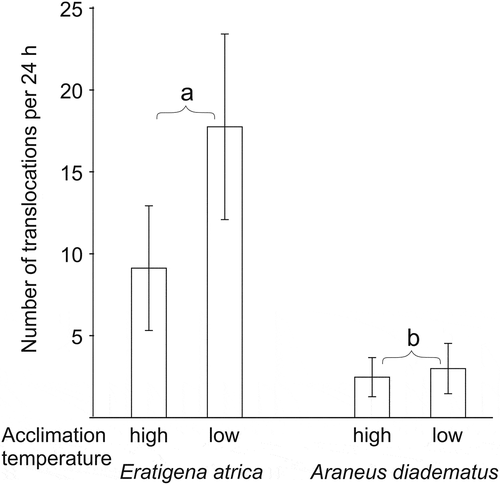
E. atrica individuals changed their thermal environment on average 12.8 ± 10.2 times during the test (), being more mobile than A. diadematus, which moved 2.7 ± 2.7 times during the test. Again, neither acclimation temperature nor an interaction of both factors significantly affected spider movements (). Both species moved freely on the chamber floor, but did not climb the walls or ceiling. They produced threads in the chambers, although they did not construct webs typical for the species.
Figure 4. Mean number of translocations between different temperature zones exhibited during 24 h of exposure by the two spider species acclimated to low (14°C) or high (23°C) temperature conditions. Different letters indicate significant differences in number of translocations (GLM). Error bars indicate standard deviations
Both species had relatively narrow thermal niches, with Levin indices ranging from 0.32 (warm-acclimated E. atrica) to 0.58 (warm-acclimated A. diadematus). The species differed significantly from each other in their niche breadth after warm acclimation, but not after the cold acclimation (). Niche overlaps between the species were significantly lower than expected on the basis of the random distribution (0.28 and 0.32 for warm- and cold-acclimated animals) ().
Discussion
In our study, females of the two different spider species had different thermal preferences, as shown by the difference in their selected temperatures and low level of thermal niche overlap. The result was expected, given that the investigated spiders have different ecology and habitats. In accordance with our hypothesis, the average temperature selected by females of E. atrica was lower than that selected by females of A. diadematus.
The thermal preference of 14°C displayed by E. atrica fits the normal range of temperatures occurring in their winter synanthropic environment, which can vary from values just a few °C higher than outdoors to those typical for heated rooms occupied by humans, depending on the distance to heating appliances (personal observations). Perhaps, females of E. atrica have an intrinsic inclination for moving to places with their preferred temperature, such as basements, attics and farm buildings, where the temperature in winter is cooler than in apartments and houses directly inhabited by humans, yet higher than outdoors. Such places are suitable for female survival until laying all the eggs produced during the reproductive season. Moreover, their preferred temperature is probably still sufficiently high for embryo development, which is particularly important as, unlike other developmental stages, spider embryos are incapable of behavioural thermoregulation and are thus susceptible to changes in environmental temperature (Li & Jackson Citation1996; Hanna & Cobb Citation2006). Moreover, spiders may migrate to buildings in search for food, which in winter becomes less available in outdoor habitats. Places uninhabited directly by humans are less frequently visited and cleaned, thus safer for relatively large E. atrica spiders and their offspring, as well as for synanthropic insects, which constitute their natural food. Moreover, such places are more humid, which also facilitates their synanthropic inhabitants. Therefore, selecting cooler microhabitats inside buildings seems to be the optimal strategy for E. atrica.
Thus, the temperature selection by E. atrica seems to be a compromise between suitable thermal conditions for embryo and juvenile development on the one hand, and safety and food availability during egg laying on the other hand. This is important for E. atrica, as it commonly lays eggs in the direct vicinity of its web and hunting area (personal observations), thus, its microhabitat selection must be based on its suitability for both of these activities. Napiórkowska et al. (Citation2018) found that E. atrica embryos do not develop at 12°C, whereas their development rate increases above 22°C, accompanied by very low mortality. Unfortunately, 14°C, found here as preferred by the species, is outside of the range which was covered by the cited study. Perhaps, it may be a threshold at which the embryo development starts, which would confirm the above-mentioned compromise hypothesis. Nevertheless, this gap in knowledge should be filled in future research.
Other spider species are also known to prefer low temperatures. Sepủlveda et al. (Citation2014) observed that the synanthropic spider Dysdera crocata in Chile, whose habitat is similar to that of E. atrica, selected the temperature of 9°C in a thermal gradient. This temperature is adjusted to the preferences of the main prey species of Dysdera crocata, the woodlouse (Porcellio sp.), rather than to the optimal conditions for embryonal development of the spider (Pollard Citation1986; Canals et al. Citation2015). Other temperate zone spiders preferring low temperatures (14.4°C), similar to that selected by E. atrica, are Stemonyphantes lineatus (Linyphiidae) and Misumenops asperatus (Thomisidae) (Schmalhofer Citation1999; Table 2). However, they are not synanthropic: the former is a nocturnal sheet weaver, whereas the latter is a diurnal active hunter (flower crab spider). This suggests that thermal preferences of spiders may be similar across different habitats and circadian activities. In contrast, our results show that species originating from the same geographical area, but different habitats and having different activity modes, can considerably differ in their thermal niches.
Females of A. diadematus selected a much higher temperature in the gradient. Moreover, each individual had rather narrow thermal preferences, as shown by the low number of relocations within the gradient and low amplitude Tmax-min, though there was a considerable variability among individual females (resulting in a flat distribution in and relatively wide thermal niche). This species is a sit-and-wait orb spider. When these animals are removed from their webs, their most important activity is to find an optimum location to build a new one. The selection of an optimum site is based on a number of factors, including availability of food, exposure to sunlight (affecting activity of an ectothermic animal), structures facilitating web building, and/or presence of a suitable shelter (Rypstra Citation1983; Lubin et al. Citation1993; McReynolds Citation2000; Herberstein & Fleisch. Citation2003). The two former factors are directly associated with temperature, whereas the two latter features were kept constant in our setup, i.e. did not change along the entire gradient. Therefore, spiders in our experiment selected their microhabitat exclusively on the basis of temperature optimal for web building. In this context, studies on thermal preferences of A. diadematus females in the gradient are justified, although this apparatus is more frequently used in studies on thermal preferences of non-web building spiders (Schmalhofer Citation1999; Frick et al. Citation2007; Veloso et al. Citation2012; Sepủlveda et al. Citation2014). Our results show that such a gradient can also be used to study the behaviour of orb web spiders. Although A. diadematus females were unable to construct fully functional webs, they were active and translocated in the gradient, confirming that they could select a preferred temperature under experimental conditions.
Selecting an appropriate foraging site (Chmiel et al. Citation2000; Jakob et al. Citation2001) is crucial for spiders, as well-fed females can reproduce earlier (Pekár Citation2000) and lay more and/or larger eggs (Buddle Citation2000; Mayntz et al. Citation2003; Skow & Jakob Citation2003; Wherry & Elwood Citation2009). The temperature selected by A. diadematus in our experiment is optimum for their main prey: most insect species (Smith Citation1984; Eggs & Sanders Citation2013 and references therein), whose flight activity, mainly associated with foraging, depends on environmental temperature and light intensity (Pivnick & McNeil Citation1987; Herrera Citation1990; Totland Citation1994; Vicens & Bosch Citation2000). Most insects are less active or even unable to fly at a low temperature (Schmidt-Nielsen Citation1983), whereas with rising temperature, the activity of insects, particularly “sun lovers”, increases (Kevan & Baker Citation1983). Therefore, insect encounters with a spider’s web become more likely at higher temperatures. Orb spiders often move and rebuild their webs in another location (Uetz Citation1992; Nakata & Ushimaru Citation1999; Venner et al. Citation2000; Wherry & Elwood Citation2009) due to seasonal changes in weather (Schneider & Vollrath Citation1998) and/or availability of prey (McReynolds Citation2000) driven by temperature cues (Herberstein & Fleisch. Citation2003). Thus, A. diadematus is likely to face situations in which it is forced to use various environmental cues, including temperature, to locate a new appropriate site in the wild. As A. diadematus lays a single egg sac in crevices, under the bark or in low vegetation, rather than directly in its web (Burch Citation1979), it is unlikely that conditions for embryonic development were important for the temperature selection in our experiment, unless the females were about to lay their cocoon in a very short time. However, this did not happen before 4–7 days after the tests were finished, which makes searching for optimum conditions for offspring a less likely driver of female behaviour in the gradient.
Preferences similar to those shown by A. diadematus were exhibited by Agelena labyrinthica, a diurnally-active funnel web spider from the family Agelenidae (Schmalhofer Citation1999; Table 2). Moreover, similarly high temperatures (23.1°C) were selected by some non-web building spiders: Zelotes longipes (Gnaphosidae) and Philodromus aureolus (Philodromidae). Nevertheless, the comparative literature on thermal ecology of spiders, especially orb weavers and synanthropes from the temperate zone, is still limited.
To summarise, E. atrica probably used temperature cues to find suitable conditions for egg development and more sheltered locations with higher food availability. Araneus diadematus selected an optimum location for web building, thus a place with the optimum abundance of prey. Conditions for egg development seem particularly important for Eratigena atrica, whose feeding grounds are spatially associated with egg laying locations (personal observations). On the other hand, A. diadematus lay eggs outside their hunting web. Moreover, during our experiments, the females of this species were just forming their eggs before they were ready for oviposition, thus they needed optimum food conditions in the first place. Thus, the differences between these species could depend on different needs resulting from their life styles.
The range of temperatures selected by A. diadematus and its inter-individual variability was higher than in E. atrica, resulting in the wider thermal niche of the former. This supports our hypothesis and is associated with the fact that A. diadematus in outdoor conditions experiences much higher temperature fluctuations, to which is better adapted compared to the synanthropic species living in a more stable environment. The thermal niche overlap between the studied species was significantly lower than random. This can result from different habitats occupied by both species, suggesting that microhabitat selection during the reproductive period differs between the species and the probability of their occurrence in similar thermal conditions is very low. In contrast, Alfaro et al. (Citation2013b) found that the thermal niche overlap of the recluse spider Loxosceles laeta (Sicariidae) and the spitting spider Scytodes globula (Scytodidae) (both synanthropic species in Chile) was considerable, indicating similar thermal microhabitat choices and making the predation of the former species by the latter likely.
In the thermal gradient, females of E. atrica were more active than A. diadematus. These differences may result from their different hunting strategies. During the 24-hour observation, E. atrica changed their thermal environment 13 times on average. This is consistent with the behavioural pattern of E. atrica, which leave webs in search of prey. Therefore, their increased activity in the gradient could be associated with searching for prey and finally with providing the best conditions for offspring development. In contrast, females of A. diadematus changed thermal environment in the gradient only 3 times on average. This result is not surprising, given that A. diadematus is a typical “sit-and-wait” web-spinning species, moving on the web only when necessary. Similar observations were made by Frick et al. (Citation2007), who examined thermal preferences of Pardosa riparia (males and females with different reproductive status). They distinguished three types of behaviour: some individuals remained in one particular section, moving only a few centimetres, others moved between sections, while the majority spent some time in one section, left it and either re-entered or moved to another section with a similar temperature. The behaviour of E. atrica and A. diadematus was comparable to that.
Acclimation temperatures had no influence on Tmean, Tmode and activity of both species. In contrast to these results, other studies have demonstrated that an acclimation temperature may affect thermal preferences to some degree. Alfaro et al. (Citation2013a), studying two mygalomorph spiders, found that the larger species, Grammostola rosea, did not change its thermal preferences between different acclimation conditions (15 vs. 25°C), whereas the smaller species, Paraphysa parvula, responded to an increase in the acclimation temperature with an increase of its selected temperature by 2–3°C. Alfaro et al. (Citation2013a) used a longer acclimation period (3 weeks vs. 1 week in our study). Nevertheless, similar to our study, Sevacherian and Lowrie (Citation1972) did not observe the impact of a 3-week acclimation on thermal preferenda of spiders Pardosa sierra and P. ramulosa.
Since in our study acclimation temperatures had no significant effect on spider behaviour in the gradient, a question arises whether the acclimation period in our experiment was sufficient. It is known that spiders transferred from lower to higher acclimation temperatures and vice versa increase or decrease their metabolic rate, which affects their behaviour (Pulz Citation1987). Such reactions are triggered when the individuals are exposed to changing thermal conditions long enough, i.e. several days or weeks, rather than hours (Rohr et al. Citation2018 and references therein). In our study, females were acclimated for a week (following Jumbam et al. Citation2008), before a test in the thermal gradient, which is within the accepted standard of 5–8 days of acclimation used in thermal testing (Klok & Chown Citation2003; Deere & Chown Citation2006; Terblanche et al. Citation2006). Therefore, it can be concluded that E. atrica and A. diadematus are not sensitive to acclimation and their selection is independent of short-term experiences. Moreover, this demonstrates that different dates of collecting of both species are unlikely to affect their preferences in our experiment, confirming that we observed real interspecific differences.
Our results contribute to an increased understanding of the thermal behaviour of ectothermic animals, specifically during the reproductive phase of the lifecycle. Additionally, our study adds to the growing body of knowledge of synanthropic species, a group that it is still relatively poorly understood with respect to their thermal ecology. Synanthropes are often believed to benefit from the global changes induced by humans, but they may also suffer from excessive heating and abrupt temperature fluctuations. Thermal behaviour is a useful tool to study potential impact of global warming on the body temperature of ectotherms, shaping their metabolic rate and thus affecting all life processes and changing sensitivity to other environmental stressors. We show that ectothermic spiders are capable of fine tuning to the local conditions and selecting a proper thermal microhabitat across a range of available temperatures, which may give them advantage in the changing environment.
Our results show that species co-occurring in the same geographical area can differ considerably in their thermal niches and preferences. Such complexity should be taken into account during creation of species distribution models (SDMs). These tools are useful in forecasting changes in species distribution in the light of global climate changes, including on the one hand organisms threatened by extinction and, on the other hand, species that are invasive and/or medically important. Such models have been created for some spider species (e.g. Dias et al. Citation2011; Krehenwinkel & Tautz Citation2013; Canals et al. Citation2016; Mammola Citation2017; Wang et al. Citation2018; Hazzi & Hormiga Citation2021). Nevertheless, our results emphasise that the models may perform poorly when only wide scale geographical temperature data are considered. Instead, the presence of local microclimates, including those created by human impact, must be taken into account, especially in the case of synanthropic species, capable of finding suitable microhabitats in a generally unsuitable environment.
Supplemental Material
Download MS Excel (100.3 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
Additional information
Funding
References
- Alfaro C, Figueroa DP, Torres H, Veloso C, Venegas F, Canals L, Canals M. 2013a. Effect of thermal acclimation on preferred temperatures in two mygalomorph spiders inhabiting contrasting habitats. Physiological Entomology 38:20–25. DOI: 10.1111/j.1365-3032.2012.00853.x.
- Alfaro C, Veloso C, Torres-Contreras H, Solis R, Canals M. 2013b. Thermal niche overlap of the corner recluse spider Loxosceles laeta (Araneae; Sicariidae) and its possible predator, the spitting spider Scytodes globula (Scytodidae). Journal of Thermal Biology 38:502–507. DOI: 10.1016/j.jtherbio.2013.08.003.
- Anthony S, Buddle CHM, Høye TT, Sinclair BJ. 2019. Thermal limits of summer-collected Pardosa wolf spiders (Araneae: Lycosidae) from the Yukon Territory (Canada) and Greenland. Polar Biology 42:2055–2064. DOI: 10.1007/s00300-019-02580-7.
- Barghusen LE, Claussen DL, Anderson MS, Bailer AJ. 1997. The effects of temperature in the web-building behaviour of the common house spider, Achaearanea tepidariorum. Functional Ecology 11:4–10. DOI: 10.1046/j.1365-2435.1997.00040.x.
- Barnes CL, Blay NW, Wilder SM. 2019. Upper thermal tolerances of different life stages, sexes, and species of widow spiders (Araneae, Theridiidae). Journal of Insect Physiology 114:10–14. DOI: 10.1016/j.jinsphys.2019.02.004.
- Bonte D, Travis JMJ, De Clercq N, Zwertvaegher I, Lens L. 2008. Thermal conditions during juvenile development affect adult dispersal in a spider. Proceedings of the National Academy of Sciences of the USA 105(44):17000–17005. DOI: 10.1073/pnas.0806830105.
- Bozinovic F, Sabat P, Rezende E, Canals M. 2016. Temperature variability and thermal performance in ectotherms: Acclimation, behaviour, and experimental considerations. Evolutionary Ecology Research 17:111–124.
- Buddle CM. 2000. Life history of Pardosa moesta and Pardosa mackenziana (Araneae, Lycosidae) in central Alberta, Canada. Journal of Arachnology 28:319–328. DOI: 10.1636/0161-8202(2000)028[0319:LHOPMA]2.0.CO;2.
- Burch TL. 1979. The importance of communal experience to survival for spiderlings of Araneus diadematus (Araneae: Araneidae). Journal of Arachnology 7:1–18.
- Canals M. 1998. Thermal ecology of small animals. Biological Research 31:367–374.
- Canals M, Solis R, Valderas J, Ehrenfeld M, Cattan PE. 1997. Preliminary studies on temperature selection and activity cycle of Chilean vectors of the Chagas disease. Journal of Medical Entomology 34:11–17. DOI: 10.1093/jmedent/34.1.11.
- Canals M, Taucare- Rios A, Brescovit AD, Peńa-Gomez F, Bizama G, Canals A, Moreno L, Bustamante R. 2016. Niche modelling of the Chilean recluse spider Loxosceles laeta and araneophagic spitting spider Scytodes globula and risk for loxoscelism in Chile. Medical and Veterinary Entomology 30(4):383–391. DOI: 10.1111/mve.12184.
- Canals M, Veloso C, Solis R. 2015. Adaptation of the spiders to the environment: The case of some Chilean species. Frontiers in Physiology 6:1–9. DOI: 10.3389/fphys.2015.00220.
- Chmiel K, Herberstein ME, Elgar MA. 2000. Web damage and feeding experience influence web site tenacity in the orb-web spider Argiope keyserlingi Karsch. Animal Behaviour 60:821–826. DOI: 10.1006/anbe.2000.1541.
- Cobb VA. 1994. Effects of temperature on escape behavior in the cribellate spider, Oecobius annulipes (Araneae, Oecobiidae). The Southwestern Naturalist 39:391–394.
- Costa FG, Sotelo Jr. JR. 1984. Influence of temperature on the copulation duration of Lycosa malitiosa Tullgren (Araneae, Lycosidae). Journal of Arachnology 12:273–277.
- Davis D. 1989. The effect of temperature on the courtship behavior of the wolf spider Schizocosa rovneri (Araneae: Lycosidae). The American Midland Naturalist 122:281–287. DOI: 10.2307/2425914.
- Deere JA, Chown SL. 2006. Testing the beneficial acclimation hypothesis and its alternatives for locomotor performance. The American Naturalist 168(5):630–644. DOI: 10.1086/508026.
- Dias MA, Simó M, Castellano I, Brescovit AD. 2011. Modeling distribution of Phoneutria bahiensis (Araneae: Ctenidae): An endemic and threatened spider from Brazil. Zoologia 28(4):432–439. DOI: 10.1590/S1984-46702011000400004.
- Dillon ME, Wang G, Garrity PA, Huey RB. 2009. Review: Thermal preference in Drosophila. Journal of Thermal Biology 34(3):109–119. DOI: 10.1016/j.jtherbio.2008.11.007.
- Downes MF. 1988. The effect of temperature on oviposition interval and early development in Theridion rufipes Lucas (Araneae, Theridiidae). Journal of Arachnology 16:41–45.
- Eggs B, Sanders D. 2013. Herbivory in spiders: The importance of pollen for orb- weavers. PLoS ONE 8(11):e82637. DOI: 10.1371/journal.pone.0082637.
- Figueroa DP, Sabat P, Torres-Contreras H, Veloso C, Canals M. 2010. Participation of book lungs in evaporative water loss in Paraphysa parvula, a migalomorph spider from Chilean Andes. Journal of Insect Physiology 56(7):731–735. DOI: 10.1016/j.jinsphys.2010.01.001.
- Frick H, Kropf C, Nentwig W. 2007. Laboratory temperature preferences of the wolf spider Pardosa riparia (Araneae: Lycosidae). Bulletin of the British Arachnological Society 14(1):45–48.
- Greenstone MH. 1999. Spider predation: How and why we study it. Journal of Arachnology 27:333–342.
- Grodzicki P, Caputa M. 2012. Photoperiod influences endogenous rhythm of ambient temperature selection by the honeybee Apis melifera. Journal of Thermal Biology 37:587–594. DOI: 10.1016/j.jtherbio.2012.07.005.
- Hanna CJ, Cobb VA. 2006. Effect of temperature on hatching and nest site selection in the Green lynx spider, Peucetia viridans (Araneae: Oxyopidae). Journal of Thermal Biology 31:262–267. DOI: 10.1016/j.jtherbio.2005.10.004.
- Hanna CJ, Cobb VA. 2007. Critical thermal maximum of the green lynx spider, Peucetia viridans (Araneae, Oxyopidae). Journal of Arachnology 35:193–196. DOI: 10.1636/SH06-01.1.
- Hazzi NA, Hormiga G. 2021. Morphological and molecular evidence support the taxonomic separation of the medically important Neotropical spiders Phoneutria depilata (Strand, 1909) and P. boliviensis (F.O. Pickard-Cambridge, 1897) (Araneae, Ctenidae). ZooKeys 1022:13–50. DOI: 10.3897/zookeys.1022.60571.
- Henschel JR, Ward D, Lubin YD. 1992. The importance of thermal factors for nest-site selection, web construction and behaviour of Stegodyphus lineatus (Araneae: Eresidae) in the Negev Desert. Journal of Thermal Biology 17:97–106. DOI: 10.1016/0306-4565(92)90005-Z.
- Herberstein ME, Fleisch. AF. 2003. Effect of abiotic factors on the foraging strategy of the orb-web spider Argiope keyserlingi (Araneae: Araneidae). Austral Ecology 28:622–628. DOI: 10.1046/j.1442-9993.2003.t01-1-01319.x.
- Herrera CM. 1990. Daily patterns of pollinator activity, differential pollinating effectiveness, and floral resource availability, in a summer-flowering Mediterranean shrub. OIKOS 58:277–288. DOI: 10.2307/3545218.
- Hertz P, Huey R, Stevenson R. 1993. Evaluating temperature regulation by field-active ectotherms: The fallacy of the inappropriate question. The American Naturalist 142:796–818. DOI: 10.1086/285573.
- Hesselberg T, Vollrath F. 2006. Temperature affects both web spider response time and prey escape speed. Bulletin of the British Arachnological Society 13(7):275–280.
- Humphreys WF. 1987. Behavioral temperature regulation. In: Nentwig W, editor. Ecophysiology of Spiders. Germany: Springer-Verlag. pp. 56–65.
- Humphreys WF. 1991. Thermal behaviour of the small spider (Araneae: Araneidae: Araneinae) on horizontal webs in semi-arid Western Australia. Behavioral Ecology and Sociobiology 28:47–54. DOI: 10.1007/BF00172138.
- Jacuński L, Napiórkowska T, Tesznar L. 1999. Breeding of the spider Tegenaria atrica C.L. Koch. Przegląd Zoologiczny XLIII(1–2):111–116.
- Jakob EM, Poter AH, Uetz GW. 2001. Site fidelity and the costs of movement among territories: An example from colonial web-building spiders. Canadian Journal of Zoology 79:2094–2100. DOI: 10.1139/z01-179.
- Johannesen J, Toft S. 2002. A test for reproductive separation of alternate generations in a biennial spider, Araneus diadematus (Araneae, Araneidae). Journal of Arachnology 30:65–69. DOI: 10.1636/0161-8202(2002)030[0065:ATFRSO]2.0.CO;2.
- Jumbam KR, Terblanche JS, Deere JA, Somers MJ, Chown SL. 2008. Critical thermal limits and their responses to acclimation in two sub-Antarctic spiders: Myro kerguelenensis and Prinerigone vagans. Polar Biology 31:215–220. DOI: 10.1007/s00300-007-0349-0.
- Kevan PG, Baker HG. 1983. Insects as flower visitors and pollinators. Annual Review of Entomology 28:407–453. DOI: 10.1146/annurev.en.28.010183.002203.
- Kiss B, Samu F. 2002. Comparison of autumn and winter development of two wolf spider species (Pardosa, Lycosidae, Araneae) having different life history patterns. Journal of Arachnology 30:409–415. DOI: 10.1636/0161-8202(2002)030[0409:COAAWD]2.0.CO;2.
- Klok CJ, Chown SL. 2003. Resistance to temperature extremes in sub-Antarctic weevils: Interspecific variation, population differentiation and acclimation. Biological Journal of the Linnean Society 78:401–414. DOI: 10.1046/j.1095-8312.2003.00154.x.
- Krehenwinkel H, Tautz D. 2013. Northern range expansion of European populations of the wasp spider Argiope bruennichi is associated with global warming–correlated genetic admixture and population-specific temperature adaptations. Molecular Ecology 22:2232–2248. DOI: 10.1111/mec.12223.
- Li D, Jackson RR. 1996. How temperature affects development and reproduction in spiders: A review. Journal of Thermal Biology 21(4):245–274. DOI: 10.1016/0306-4565(96)00009-5.
- Lubin Y, Ellner S, Kotzman M. 1993. Web relocation and habitat selection in a desert widow spider. Ecology 74(7):195–1928.
- Mammola S. 2017. Modelling the future spread of native and alien congeneric species in subterranean habitats — The case of Meta cave-dwelling spiders in Great Britain. International Journal of Speleology 46(3):427–437. DOI: 10.5038/1827-806X.46.3.2134.
- Mayntz D, Toft S, Vollrath F. 2003. Effects of prey quality and availability on the life history of a trap-building predator. Oikos 101:631–638.
- McReynolds CN. 2000. The impact of habitat features on web features and prey capture of Argiope aurantia (Araneae, Araneidae). Journal of Arachnology 28:169–179. DOI: 10.1636/0161-8202(2000)028[0169:TIOHFO]2.0.CO;2.
- Montes de Oca L, Pérez-Miles F, Clavijo-Baquet S. 2020. The reproductive period of tarantulas is constrained by their thermal preferences (Araneae, Theraphosidae). Journal of Thermal Biology 92:102665. DOI: 10.1016/j.jtherbio.2020.102665.
- Nakata K, Ushimaru A. 1999. Feeding experience affects web relocation and investment in web threads in an orb-web spider, Cyclosa argenteoalba. Animal Behaviour 57:1251–1255. DOI: 10.1006/anbe.1999.1105.
- Napiórkowska T, Kobak J, Napiórkowski P, Templin J. 2018. The effect of temperature and light on embryogenesis and post-embryogenesis of the spider Eratigena atrica (Araneae, Agelenidae). Journal of Thermal Biology 72:26–32. DOI: 10.1016/j.jtherbio.2017.12.003.
- Nyffeler M. 2000. Ecological impact of spider predation: A critical assessment of Bristowe’s and Turnbull’s estimates. Bulletin of the British Arachnological Society 11(9):367–373.
- Pekár S. 2000. Webs, diet, and fecundity of Theridion impressum (Araneae: Theridiidae). European Journal of Entomology 97:47–50. DOI: 10.14411/eje.2000.012.
- Pivnick KA, McNeil JN. 1987. Diel patterns of activity of Thymelicus lineola adults (Lepidoptera: Hesperiidae) in relation to weather. Ecological Entomology 12(2):197–207. DOI: 10.1111/j.1365-2311.1987.tb00998.x.
- Pollard SD. 1986. Prey capture in Dysdera crocata (Araneae: Dysderidae), a long fanged spider. New Zealand Journal of Zoology 13:149–150. DOI: 10.1080/03014223.1986.10422656.
- Pulz R. 1987. Thermal and water relations. In: Nentwig W, editor. Ecophysiology of Spiders. Germany: Springer-Verlag. pp. 26–55.
- Riechert SE, Tracy CR. 1975. Thermal balance and prey availability: Basis for a model relating web site characteristics to spider reproductive success. Ecology 56:265–284. DOI: 10.2307/1934960.
- Roberts MJ. 1995. Spiders of Britain & Northern Europe. London: HarperCollinsPublishers.
- Rohr JR, Civitello DJ, Cohen JM, Roznik EA, Sinervo B, Dell AI. 2018. The complex drivers of thermal acclimation and breadth in ectotherms. Ecology Letters 21:1425–1439.
- Rypstra AL. 1983. The importance of food and space in limiting web-spider densities; a test using field enclosures. Oecologia (Berlin) 59:312–316. DOI: 10.1007/BF00378855.
- Schmalhofer VR. 1999. Thermal tolerances and preferences of the crab spiders Misumenops asperatus and Misumenoides formosipes (Araneae, Thomisidae). Journal of Arachnology 27:470–480.
- Schmalhofer VR. 2011. Impacts of temperature, hunger and reproductive condition on metabolic rates of flower-dwelling crab spiders (Araneae: Thomisidae). Journal of Arachnology 39:41–52. DOI: 10.1636/Hi09-103.1.
- Schmidt-Nielsen K. 1983. Animal physiology: Adaptation and environment. England: Cambridge University Press.
- Schneider JM, Vollrath F. 1998. The effect of prey type on the geometry of the capture web of Araneus diadematus. Naturwissenschaften 85:391–394. DOI: 10.1007/s001140050521.
- Schwerdt L, De Villalobos AE, Ferretti N. 2020a. Warming is here: Using locomotor performance to infer thermal parameters and vulnerability for an endemic Argentinean tarantula Grammostola vachoni. Ecological Entomology. DOI: 10.1111/een.12997.
- Schwerdt L, De Villalobos AE, Pérez-Miles F, Ferretti N. 2020b. Thermal preferences and effects of temperature on fitness parameters of an endemic Argentinean tarantula (Grammostola vachoni). Canadian Journal of Zoology 98(2):134–141. DOI: 10.1139/cjz-2019-0180.
- Sepủlveda R, Taucare-Rios A, Veloso C, Canals M. 2014. Thermal preference of Dysdera crocata C. L. Koch 1838 (Araneae: Dysderidae). Journal of Arachnology 42:299–302. DOI: 10.1636/B13-87.1.
- Sevacherian V, Lowrie D. 1972. Preferred temperature of two species of lycosid spiders, Pardosa sierra and P. ramulosa. Annals of the Entomological Society of America 65:111–114. DOI: 10.1093/aesa/65.1.111.
- Sinclair BJ, Marshall KE, Sewell MA, Levesque DL, Willett CS, Slotsbo S, Dong Y, Harley CDG, Marshall DJ, Helmuth BS, Huey RB. 2016. Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures? Ecology Letters 19:1372–1385. DOI: 10.1111/ele.12686.
- Skow CD, Jakob EM. 2003. Effects of maternal body size on clutch size and egg weight in a pholcid spider (Holocnemus pluchei). Journal of Arachnology 31:305–308. DOI: 10.1636/01-85.
- Smith RB. 1984. Aspects of the feeding ecology of the garden cross-spider, Araneus diadematus Clerck. MSc thesis. The University of British Columbia.
- Taucare-Rios A, Veloso C, Bustamante RO. 2018. Thermal niche conservatism in an environmental gradient in the spider Sicarius thomisoides (Araneae: Sicariidae): Implications for microhabitat selection. Journal of Thermal Biology 78:298–303. DOI: 10.1016/j.jtherbio.2018.10.018.
- Taucare-Rios A, Veloso C, Canals M, Bustamante RO. 2020. Daily thermal preference variation of the sand recluse spider Sicarius thomisoides (Araneae: Siciariidae). Journal of Thermal Biology 87:102465. DOI: 10.1016/j.jtherbio.2019.102465.
- Terblanche JS, Klok CJ, Krafsur ES, Chown SL. 2006. Phenotypic plasticity and geographic variation in thermal tolerance and water loss of the tsetse Glossina pallidipes (Diptera: Glossinidae): Implications for distribution modeling. American Society of Tropical Medicine and Hygiene 74:786–794. DOI: 10.4269/ajtmh.2006.74.786.
- Totland Ø. 1994. Influence of climate, time of day and season, and flower density on insect flower visitation in Alpine Norway. Arctic, Antarctic, and Alpine Research 26(1):66–71. DOI: 10.2307/1551879.
- Turner JS, Henschel JR, Lubin YD. 1993. Thermal constraints on prey-capture behaviour of a burrowing spider in a hot environment. Behavioral Ecology and Sociobiology 33:35–43. DOI: 10.1007/BF00164344.
- Uetz GW. 1992. Foraging strategies of spiders. Trends in Ecology & Evolution 7:155–158. DOI: 10.1016/0169-5347(92)90209-T.
- Veloso C, Luhr D, Marfull R, Torres-Contreras H, Figueroa Pérez D, Sabat P, Canals M. 2012. Characterization of the thermal micro-environment of Paraphysa parvula Pocock 1903 (Araneae: Theraphosidae), a spider from the Chilean Andes. Journal of Arachnology 40:34–38. DOI: 10.1636/B10-46.1.
- Venner S, Pasquet A, Leborgne R. 2000. Web-building behaviour in the orb-weaving spider Zygiella x-notata: Influence of experience. Animal Behaviour 59:603–611. DOI: 10.1006/anbe.1999.1327.
- Vicens N, Bosch J. 2000. Weather-dependent pollinator activity in an Apple Orchard, with special reference to Osmia cornuta and Apis mellifera (Hymenoptera: Megachilidae and Apidae). Environmental Entomology 29(3):413–420. DOI: 10.1603/0046-225X-29.3.413.
- Vollrath F. 1987. Foraging, growth and reproductive success. In: Nentwig W, editor. Ecophysiology of spiders. Germany: Springer-Verlag. pp. 357–370.
- Wang Y, Casajus N, Buddle C, Berteaux D, LarriveÂe M. 2018. Predicting the distribution of poorly-documented species, Northern black widow (Latrodectus variolus) and Black purse-web spider (Sphodros niger), using museum specimens and citizen science data. PLoS ONE 13(8):e0201094. DOI: 10.1371/journal.pone.0201094.
- Wherry T, Elwood RW. 2009. Relocation, reproduction and remaining alive in an orb-web spider. Journal of Zoology 279:57–63. DOI: 10.1111/j.1469-7998.2009.00590.x.
