 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Egg size is an important determinant of offspring weight and survival. However, despite extensive research on factors affecting egg dimensions, causes of egg variation are not fully understood. Between 2000 and 2016 we studied a population of little bitterns in western Poland, and examined a total of 529 eggs from 88 clutches. Furthermore, we conducted an extensive literature search for data on clutch and egg size from other populations of the species to test hypotheses explaining the relationships between these variables and latitude. Little bitterns in western Poland laid big eggs but also produced large clutches compared to most other studied populations of the species. The ratio of largest to smallest egg calculated for egg volume was 1.75 for all studied eggs, and up to 1.35 within clutches, suggesting a huge variation between and within clutches in the studied population. Egg dimensions were highly intercorrelated, but egg size was not related to clutch size in the study population. The comparison of data from different populations of the species revealed that egg volume increased significantly with latitude and was positively correlated with clutch size. These results support the embryonic temperature hypothesis and clutch cooling rates hypothesis, but contradict the optimal egg dimensions hypothesis.
Introduction
Egg size is an important determinant of nestling weight, and often correlates with future offspring survival (Williams Citation1994; Silva et al. Citation2007; Krist Citation2011). Although it is a frequently studied trait, the causes and consequences of its variation remain poorly understood (Christians Citation2002). Egg dimensions and egg weight can be affected by many environmental factors, such as weather conditions (Väisänen Citation1977; Ojanen Citation1983), calcium availability (Bańbura et al. Citation2010), laying date (Bancroft Citation1984), laying order (Ojanen Citation1983; Orłowski et al. Citation2016), and female body mass and age (Desrochers & Magrath Citation1993; Nager & Zandt Citation1994). Furthermore, egg dimensions may vary considerably both within and between clutches and within and between years (Flint et al. Citation1992; Arendt Citation2004; Riehl Citation2010).
Figure 1. Relationship between within-clutch mean egg length and egg width in the little bittern studied in western Poland
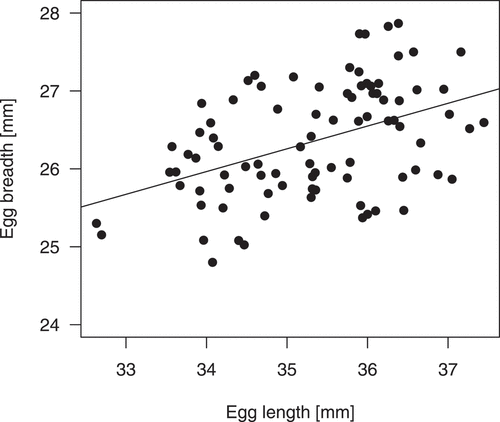
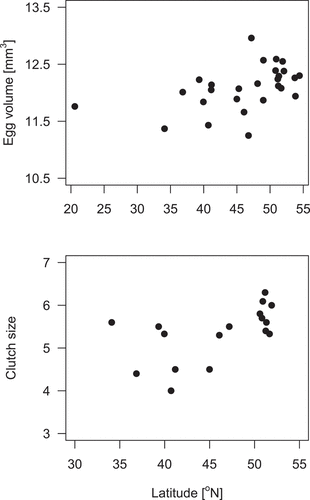
Figure 2. Relationships between latitude and mean egg volume (upper graph) and latitude and mean clutch size (lower graph) based on the data from different populations of little bitterns
Several hypotheses to explain the relationships between egg and clutch size have been proposed so far (). The optimal egg dimensions hypothesis predicts a trade-off between the number of eggs and their size (Lack Citation1967; Smith & Fretwell Citation1974; Blackburn Citation1991). It assumes that because of nutrient limitations for a breeding female, any increase in clutch size should be compensated for by a decrease in egg size. According to Smith and Fretwell (Citation1974), such a relationship should be observed especially in species laying large clutches and those without parental care. In contrast, the food availability hypothesis predicts a positive relationship between egg size and clutch size, assuming that populations occupying better habitats are able to lay larger clutches and at the same time produce larger eggs (Hõrak et al. Citation1995). Other hypotheses provide explanations for the relationship between egg size and temperature (). The embryonic temperature hypothesis suggests that the egg mass should increase in species with cooler egg temperatures to offset associated increases in the energetic requirements of embryos (Martin Citation2008). Finally, the clutch cooling rates hypothesis predicts that in lower ambient temperatures larger clutch sizes should be expected to compensate for higher egg cooling rates (Reid et al. Citation2000).
Table I. Hypotheses predicting relationships between egg and clutch size, and between egg/clutch size and ambient temperatures
The little bittern Ixobrychus minutus is a representative of the heron family, breeding in reedbeds of the Palaearctic, Africa and Madagascar. Birds nesting in temperate regions migrate to Africa or southern Asia. Due to living in a difficult-to-explore habitat and its secretive lifestyle, the species is regarded as the least studied among the Palaearctic herons (Pardo-Cervera et al. Citation2010). Nests of the species take the form of platforms built of reed stems, and contain 4–9 eggs (Cramp & Simmons Citation1977).
The aim of the current study was twofold: (1) to describe the variation in clutch size and egg dimensions in the population of little bitterns studied in western Poland, and to test for the factors that may affect egg size and clutch size; (2) to analyse eggs and clutches from different populations of the species, and to test the hypotheses explaining the relationships between latitude, clutch size and egg volume.
Methods
Study population
The study was carried out in May–August of 2002–2006 and 2008–2016 in western Poland on Lake Łoniewskie (51°54ʹN, 16°41ʹE) and four fish ponds located ca. 8 km away, near the village of Zgliniec (51°58ʹN, 16°43ʹE). Lake Łoniewskie is a shallow, post-glacial lake (145 ha), with the emergent vegetation covering ca. 30 ha, including chiefly common reed Phragmites australis, narrowleaf cattail Typha angustifolia, common cattail T. latifolia and sedges Carex sp. The fish ponds (total area of 46 ha, range 4.3–18.8 ha) are used for fish production, but not intensively managed: about 50% of their area is covered by emergent vegetation (mostly common reed and common and narrowleaf cattail).
Nest searches were conducted mostly from the banks of water bodies. In the first study period the main methods involved locating vocalising males, and observations of bird movements within the reedbed, which resulted in subsequent searches through vegetation in potential nesting places. In the latter period of the study (2009–2016) we additionally employed systematic searches through patches of vegetation (at least 4 times monthly), where little bitterns had bred in earlier study years. A total of 88 nests (on average 6.29 per year, range: 2–13) containing 529 eggs were found throughout the study period (75 on Lake Łoniewskie and 13 on nearby fish ponds), including 70 early (probably first) clutches and 18 late clutches (replacement clutches or second broods). They were visited every 3 days. About 80% of nests survived until hatching and 75% produced fledglings.
For each nest, clutch size and laying phenology were recorded, and the length (L) and width (W) of each egg were measured in mm to the nearest 0.1 mm. All measurements were taken by the same researcher (JS). If a nest with eggs was found, we backdated the first-egg laying date, assuming one egg was laid per day, and that incubation started on the day of laying the second egg and lasted for the following 19 days (Pardo-Cervera et al. Citation2010; Filipiuk & Kucharczyk Citation2016). If a nest with a full clutch was found and it did not survive until hatching we applied the procedure used by Mayfield (Citation1975) to estimate the date of nest-failure and the clutch initiation date.
Other populations
We performed an extensive literature survey to locate all published information on the recorded clutch sizes of little bitterns, as well as their egg dimensions, using Google Scholar. We searched for the terms “Ixobrychus minutus” and “little bittern”, but we restricted the search to the nominate subspecies I. m. minutus. Afterwards, we carefully browsed through published papers reporting the species egg dimensions and clutch sizes. We found 27 studies reporting egg dimensions, and 17 studies with information on clutch size, published between 1879 and 2018. For each study site we extracted information on its coordinates from the published source, if such data were provided. Otherwise, we assessed the centre of the study area based on its description in the publication (). Because 10 older data sources did not provide information about the years of data collection, we could not extract the temperatures corresponding with the study years. We used latitude instead, assuming a negative correlation between latitude and temperature (Feulner et al. Citation2013).
Table II. Descriptive statistics of the four basic egg measurements. The data in the 2nd and 3rd columns (n = 88) were calculated using average values per clutch (e.g. first the average egg length was calculated for all eggs from each clutch, and later mean length was calculated using average values from all clutches) while the data in the last column refer to egg variability (ranges) in the sample of all analysed eggs (n = 529)
Table III. Results of MMM and LMMs with factors predicted to influence little bittern egg size (n = 529 eggs, 88 nests). Egg length and egg width were analysed together in one MMM. Models for volume and shape index were univariate LMMs. For fixed variables we report estimate ± SE. Variance, SD and likelihood ratio test are given for random variables. All p > 0.05, unless otherwise specified
Table IV. Egg dimensions and clutch size in different populations of little bittern. Mean values of egg length and width (and their ranges in parentheses), number of measured eggs (n) and clutch size (with n values representing number of nests) in different study populations. Statistics were calculated for all eggs (not for means per nest). Egg volume was calculated based on mean population measurements, using the formula by Hoyt (Citation1979)
Statistical analysis
Egg volume (cm3) was estimated following the equation presented by Hoyt (Citation1979):
We calculated the shape index, or the sphericity index, following Hõrak et al. (Citation1995) as:
(W/L) * 100
We calculated the within-clutch coefficient of variation of egg measurements following the equation
where s is the standard deviation and Ῡ is the mean of egg measurements of the clutch. To avoid bias of the calculated coefficient of variation due to small sample sizes (Sokal & Rohlf Citation1995), we applied the following correction:
where CV* is the bias-adjusted coefficient of variation and n is the clutch size. We calculated the mean ratio of largest to smallest egg based on egg volume, both for eggs from single clutches and for all eggs in the population, following Christians (Citation2002).
The variation in egg characteristics was examined with multivariate mixed models (MMM) and linear mixed models (LMM) using R (version 3.4.1) software (R Development Core Team Citation2017). We built MMM with egg length and egg width as response variables. Clutch size and date of laying of the first egg (1 = 1 May) were introduced as fixed variables, while study year and nest identity were random factors. The model assumed unstructured covariance matrices, following the guidelines of Brommer et al. (Citation2019). This analysis was performed using the sommer R package (Covarrubias-Pazaran Citation2018). The other two egg size characteristics (i.e. egg volume and egg shape) were calculated from egg length and width, so we did not introduce them in the MMM. However, we analysed these variables with two univariate LMMs using the nlme R package (Pinheiro et al. Citation2017). In these models egg volume and egg shape were response variables and the study year and nest identity were introduced as random factors, while clutch size and date of laying of the first egg were fixed variables. The statistical significance of random variables was evaluated with a likelihood ratio test. Following the recommendations of Forstmeier and Schielzeth (Citation2011), we present full models.
Results
Intra-population analysis
The clutch size of little bittern (n = 88 nests) ranged from 4 to 8 eggs (laid between 9 May and 21 July) with an average of 6.01 (± 0.085) eggs. We did not find significant differences in clutch size between the study years (Kruskal–Wallis chi-squared = 11.42, df = 14, p = 0.653).
The mean values of four egg traits (egg length, width, volume and shape index) and their minima and maxima were calculated from the clutch means as well as from each individual egg (). The most variable trait was egg volume (mean CV* = 10.11 ± 5.03 SD (standard deviation), range: 2.15–28.1), followed by the egg shape index (CV* = 6.32 ± 2.48, range: 2.21–12.43), the egg length (CV* = 5.69 ± 2.62, range: 1.33–13.64) and finally the egg width (CV* = 3.74 ± 1.91 range: 0.58–9.84). No significant differences were found between within-clutch coefficients of variation for different clutch size classes (Spearman rank correlation, all p > 0.05).
Egg characteristics (length, width, volume and shape index) were not related to clutch size or laying date (). There was a small between-year variation relative to the within-year variation, while the between-nest variation was statistically significant (). Egg length and egg width were correlated (r = 0.44, p < 0.001, n = 88 nests; ). The ratio of largest to smallest egg (calculated based on egg volume) was 1.75 for all the analysed eggs (n = 529). The same ratio calculated for each clutch separately varied from 1.03 to 1.35 (mean = 1.12 ± 0.003 SE). The within-clutch ratio of largest to smallest egg did not correlate with clutch size (rs = −0.18, p = 0.865, n = 88).
Inter-population analysis
The analysis of the data from different populations () revealed that little bitterns produced significantly larger clutches (rs = 0.51, p = 0.036, n = 17) and laid larger eggs (rs = 0.57, p = 0.002, n = 27) () with increasing latitude. As a result, we found a significant correlation between egg volume and clutch size, despite a relatively small sample size (n = 17 populations, rs = 0.65, p = 0.007).
Discussion
Intra-population analysis
In comparison with other populations of little bittern studied so far (), birds from our study population laid one of the largest clutch sizes, but at the same time produced large eggs, exceeded only by the populations in Switzerland and Ukraine. The former result is not surprising as clutch size usually increases with latitude (Lack Citation1947; Ashmole Citation1963; Hõrak et al. Citation1995), and most study sites of little bittern were located farther south compared to our population (). We did not detect a significant difference in clutch sizes between study years. However, as annual sample sizes were low, this could have limited the power to detect between-year effects.
We did not find a significant relationship between egg size and clutch size in our study population of little bitterns (cf. Surmacki et al. Citation2003; Polak Citation2010; Golawski & Mitrus Citation2018). Therefore, the results of our study at the population level do not support the predictions of the optimal egg dimensions hypothesis (Lack Citation1967; Smith & Fretwell Citation1974).
Both egg dimensions and clutch size exhibited quite a large variability in our study population. The ratio of largest to smallest egg of 1.75 found in our study population indicates that the largest egg in the population was 75% bigger than the smallest. This value lies above the mean calculated for 39 species by Christians (Citation2002), showing that eggs of little bittern are relatively variable compared to those of other species. The same ratio calculated for each clutch separately produced much smaller values (mean of 1.116), which is consistent with observations from other species (Christians Citation2002). However, its maximum value of 1.35 (indicating that the largest egg in a clutch was 35% bigger than the smallest) is one of the highest reported so far (Christians Citation2002; Arendt Citation2004; Riehl Citation2010). The within-clutch ratio did not correlate with clutch size, suggesting that large egg-size variation was not an effect of having a greater number of eggs in the nest.
We found important differences in the variation of egg traits: the greatest variation was recorded for egg volume, and the lowest for egg width. Low variation in egg width seems to be a norm in birds (Ojanen Citation1983; Bańbura & Zieliński Citation1990; Surmacki et al. Citation2003) as this variable is constrained by the female oviduct diameter (Van Noordwijk et al. Citation1981). However, an increase in egg volume may be achieved by increasing egg length, which is less limited by physiological or mechanical conditions, and egg length was indeed much more variable in our study population.
Egg length and width were correlated in our study population, which is a common phenomenon in birds (e.g. Grant Citation1982; Hõrak et al. Citation1995; Polak Citation2010; but see Surmacki et al. Citation2003). At the same time, we did not find a significant effect of year on egg measurements and clutch size, in contrast to some other studies (Swennen & Van der Meer Citation1992; Surmacki et al. Citation2003; but see Nager & Zandt Citation1994; Polak Citation2010). This may have resulted from small annual sample sizes and/or relatively stable environmental conditions across years.
Inter-population analysis
We found a positive relationship between egg volume and clutch size among different populations of little bitterns in line with some earlier research conducted on other species (Coulson Citation1963; Ojanen et al. Citation1979; Hõrak et al. Citation1995). In contrast, many previous studies have provided evidence that egg size and clutch size are inversely correlated (Greig-Smith et al. Citation1988; Blackburn Citation1991; Song et al. Citation2016), which was explained by a trade-off between these traits (Smith & Fretwell Citation1974). Another hypothesis was proposed by Martin (Citation2008), who argued that laying larger eggs in cooler climates may reflect a compensation for the increased energetic requirements of embryos. The same may be true for larger clutches, which may help to maintain a higher temperature among eggs (Reid et al. Citation2000). As we found significant correlations between latitude and egg size (volume) and between latitude and clutch size, our results support the hypothesis of increased energetic requirements of embryos at higher latitudes (Martin Citation2008) as well as the clutch cooling rates hypothesis (Reid et al. Citation2000).
In conclusion, we found large differences in egg sizes within clutches in our study population of little bitterns, as well as important differences in the variation of egg traits. The greatest variation was recorded for egg volume, and the lowest for egg width (the latter probably reflects physical constraints related to oviduct diameter). The analysis of data from different populations supports the embryonic temperature hypothesis (Martin Citation2008) and clutch cooling rates hypothesis (Reid et al. Citation2000), but not the optimal egg dimensions hypothesis (Smith & Fretwell Citation1974).
Acknowledgements
We thank Norbert Dudziak for his help in the field. We are grateful to Józef Hordowski for the information about some data sources, Ihor Shydlovsky for translation of some texts, and Vital Sakhon for his help in accessing some literature published in Russian. We also thank Dr Boudjéma Samraoui for some additional data on his study population of little bittern.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Andronov VA, Ardamatskaya TB, Artyoohin YB. 2011. Ptitsi Rossii i sopryedyel’nih ryegionov: Pyelikanoobrazniye, Aistoobrazniye, Flamingoobrazniye. Moskva: Tovarishshyestvo naoochnih izdaniy KMK.
- Arendt WJ. 2004. A quarter century of variation in color and allometric characteristics of eggs from a rain forest population of the Pearly-eyed Thrasher (Margarops fuscatus). Caribbean Journal of Science 40:204–217.
- Ashmole NP. 1963. The regulation of numbers of tropical oceanic birds. Ibis 103:458–473.
- Baker ECS. 1935. The nidification of birds of the Indian empire. London: Taylor & Francis.
- Bańbura J, Zieliński P. 1990. Within-clutch repeatability of egg dimensions in the Black-headed Gull Larus ridibundus. Journal für Ornithologie 131(3):305–310. DOI: 10.1007/BF01641002.
- Bańbura M, Sulikowska-Drozd A, Kaliński A, Skwarska J, Wawrzyniak J, Kruk A, Zieliński P, Bańbura J. 2010. Egg size variation in Blue tits Cyanistes caeruleus and Great tits Parus major in relation to habitat differences in snail abundance. Acta Ornithologica 45(2):121–129. DOI: 10.3161/000164510X551264.
- Bancroft GT. 1984. Patterns of variation in size of Boat-tailed Grackle Quiscalus major eggs. Ibis 126(4):496–509. DOI: 10.1111/j.1474-919X.1984.tb02076.x.
- Blackburn TM. 1991. An interspecific relationship between egg size and clutch size in birds. Auk 108:973–977.
- Brommer J, Class B, Covarrubias-Pazaran G. 2019. Multivariate mixed models in ecology and evolutionary biology: Inferences and implementation in R. EcoEvoRxiv. 12 December. DOI: 10.32942/osf.io/hs38a.
- Christians JK. 2002. Avian egg size: Variation within species and inflexibility within individuals. Biology Reviews 77:1–26.
- Coulson JC. 1963. Egg size and shape in the Kittiwake (Rissa tridactyla) and their use in estimating age composition of populations. Journal of Zoology 140:211–226.
- Covarrubias-Pazaran G. 2018. Software update: Moving the R package sommer to multivariate mixed models for genome-assisted prediction. bioRxiv. DOI: 10.1101/354639.
- Cramp S, Simmons KEL. 1977. Handbook of the birds of Europe, the Middle East and North Africa: The birds of the Western Palearctic. Oxford: Oxford University Press.
- David A, Stermin AN, Sevianu E. 2018. Clutch size and egg repeatability in three elusive bird species: Little Bittern (Ixobrychus minutus), Little Crake (Zapornia parva) and Water Rail (Rallus aquaticus) from north-west Romanian populations. Studia Universitatis Babeş-Bolyai Biologia 63(1):81–88. DOI: 10.24193/subbbiol.2018.1.07.
- Desrochers A, Magrath RD. 1993. Age-specific fecundity in European Blackbirds (Turdus merula): Individual and population trends. Auk 110:255–263.
- Fazili MF, Shah GM, Jan U, Ahangar FA. 2010. On some breeding parameters of Little Bittern at Haigam wetland, Kashmir (India). Berkut 19:74–80.
- Feulner G, Rahmstorf S, Levermann A, Volkwardt S. 2013. On the origin of the surface air temperature difference between the hemispheres in Earth’s present-day climate. Journal of Climate 26(18):7136–7150. DOI: 10.1175/JCLI-D-12-00636.1.
- Filipiuk M. 2018. Breeding biology of the Little Bittern Ixobrychus minutus (L., 1766) on fish ponds in the Lublin region. Dissertation. UMCS.
- Filipiuk M, Kucharczyk M. 2016. A puzzling case of successful double-brooding in the Little Bittern Ixobrychus m. minutus. Journal Für Ornithologie 104(2):182–187. DOI: 10.5253/arde.v104i2.a3.
- Flint PL, Sedinger JS, Wells Jr JV. 1992. Reproductive implications of egg-size variation in the Black Brant. The Auk 109(4):896–903. DOI: 10.2307/4088164.
- Flis A. 2013. Population status and breeding biology of little bittern Ixobrychus minutus on the fishponds in Janowskie Forests Lanscape Park (SE Poland). Chronmy Przyrode Ojczysta 2:96–105.
- Forstmeier W, Schielzeth H. 2011. Cryptic multiple hypotheses testing in linear models: Overestimated effect sizes and the winner’s curse. Behavioral Ecology and Sociobiology 65(1):47–55. DOI: 10.1007/s00265-010-1038-5.
- Glutz von Blotzheim UN, Bauer KM. 1987. Handbuch der Vögel Mitteleuropas. Vol. 1. Wiesbaden: Aula.
- Goebel H. 1879. Vögel des Kreises Uman, Government Kiew, mit besonderer Rücksicht auf ihre Zugverhältniss. Buchdruckerbi der k. St. Perersburg: Akademie der Wissenschaften.
- Golawski A, Mitrus C. 2018. Weather conditions influence egg volume repeatability in clutches of the Red-backed Shrike Lanius collurio. Zoological Studies 57. DOI: 10.6620/ZS.2018.57-02.
- Grant PR. 1982. Variation in the size and shape of Darwin’s finch eggs. The Auk 99(1):15–23. DOI: 10.2307/4086017.
- Grattini N. 2011. Dati sulla biologia riproduttiva del Tarabusino Ixobrychus minutus nel Parco Sovra-comunale di. San Lorenzo (Pegognaga, Mantova). Alula 18:59–65.
- Greig-Smith PW, Feare CJ, Freeman EM, Spencer PL. 1988. Causes and consequences of egg-size variation in the European Starling Sturnus vulgaris. Ibis 130(1):1–10. DOI: 10.1111/j.1474-919X.1988.tb00950.x.
- Gudina A. 2007. Rare and insufficiently known birds of the Eastern Ukraine. Zaporozhye: Dneprovskiy metallurg.
- Hellebrekers WPJ. 1950. Measurements and weights of eggs of birds on the Dutch list. Leiden: E.J. Brill.
- Hõrak P, Mänd R, Ots I, Leivits A. 1995. Egg size in the Great tit Parus major: Individual, habitat and geographic differencess. Ornis Fennica 7:97–114.
- Hoyt DF. 1979. Practical methods of estimating volume and fresh weight of bird eggs. Auk 96:73–77.
- Klimov SM, Sarichev VS, Nedosekin VU, Abramov AV, Zemlyanuchin AI, Vengerov PD, Numerov AD, Melnikov MV, Sitnikov VV, Shubina JE. 1998. Layings and eggs’ sizes of the birds inhabiting the basin of the Verhny Don. Lipetsk: LGPI.
- Knysh NP. 2001. Notes about rare and insufficiently known birds of the forest-steppe part of Sumy region. Berkut 10:1–19.
- Krist M. 2011. Egg size and offspring quality: A meta-analysis in birds. Biology Reviews 86:692–716.
- Lack D. 1947. The significance of clutch-size. Part I – Intraspecific variations. Ibis 89(2):302–352. DOI: 10.1111/j.1474-919X.1947.tb04155.x.
- Lack D. 1967. The significance of clutch-size in waterfowl. Wildfowl 18:125–128.
- Lysenkov EV, Lapshin AS, Spiridonov SN. 2003. The birds of Mordovia: Oological and nest’s sizes of materials. Saransk: Morodovian State Teacher’s Training Institute named after M. E. Evseviev.
- Makatsch W. 1974. Die Eier der Vögel Europas. Vol. 1. Radebeul: Neumann Verlag.
- Martin TE. 2008. Egg size variation among tropical and temperate songbirds: An embryonic temperature hypothesis. Proceedings of the National Academy of Sciences 105(27):9268–9271. DOI: 10.1073/pnas.0709366105.
- Martínez-Abraín A. 1994. Notes on the biology of Little Bittern Ixobrychus m. minutus during the breeding season in eastern Spain. Ardeola 41:169–171.
- Mayfield HF. 1975. Suggestions for calculating nest success. Wilson Bulletin 87:456–466.
- Mlikovský J. 2006. Egg size in birds of southern Bohemia: An analysis of Rudolf Prázn’s collection. Sylvia 42:112–116.
- Nager RG, Zandt HS. 1994. Variation in egg size in great tits. Ardea 82:315–328.
- Nikiforov MY, Yaminskiy BV, Shklyarov LP. 1989. Ptitsi Byeloroossii. Spravochnik-opryedyelityel’ gnyezd i yaits. Minsk: Visheyshaya shkola.
- Ojanen M. 1983. Effects of laying sequence and ambient temperature on the composition of eggs of the great tit Parus major and the pied flycatcher Ficedula hypoleuca. Annales zoologici Fennici 20:65–71.
- Ojanen M, Orell M, Väisänen RA. 1979. Role of heredity in egg size variation in the Great tit Parus major and the Pied Flycatcher Ficedula hypoleuca. Ornis Scandinavica 10(1):22–28. DOI: 10.2307/3676340.
- Orłowski G, Hałupka L, Pokorny P, Klimczuk E, Sztwiertnia H, Dobicki W. 2016. Variation in egg size, shell thickness, and metal and calcium content in eggshells and egg contents in relation to laying order and embryonic development in a small passerine bird. The Auk 133(3):470–483. DOI: 10.1642/AUK-16-16.1.
- Pardo-Cervera F, Sørensen IH, Jensen C, Ruiz X, Sánchez-Alonso C. 2010. Breeding biology of the Little Bittern Ixobrychus minutus in the Ebro delta (NE Spain). Ardeola 57:407–416.
- Paulussen W, Bax J. 1957. Ixobrychus minutus (L.). Gerfaut 47:308.
- Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. 2017. nlme: Linear and nonlinear mixed effects models. R package version 3.1-131. Available: https://CRAN.R-project.org/package=nlme.
- Polak M. 2010. Clutch and egg size variation in the coot Fulica atra breeding on fishponds in eastern Poland – Test of the optimal egg dimensions hypothesis. Acta Zoologica Cracoviensia - Series A: Vertebrata 53A(1):35–40. DOI: 10.3409/azc.53a_1-2.35-40.
- Pyetrosyan SO, Pyetrosyan OS. 1997. Oologiya i nidologiya ptits Armyenii. Erevan: Arhityektoora.
- R Development Core Team. 2017. R: A language and environment for statistical computing. Available: http://www.R-project.org/.
- Reid JM, Monaghan P, Ruxton GD. 2000. Resource allocation between reproductive phases: The importance of thermal conditions in determining the cost of incubation. Proceedings of the Royal Society of London. Series B: Biological Sciences 267(1438):37–41. DOI: 10.1098/rspb.2000.0963.
- Riehl C. 2010. Egg ejection risk and hatching asynchrony predict egg mass in a communally breeding cuckoo, the Greater Ani (Crotophaga major). Behavioral Ecology 21(4):676–683. DOI: 10.1093/beheco/arq038.
- Samraoui F, Nedjah R, Boucheker A, Alfarhan AH, Samraoui B. 2012. Breeding ecology of the Little Bittern Ixobrychus minutus in northeast Algeria. Bird Study 59(4):496–503. DOI: 10.1080/00063657.2012.733335.
- Silva MC, Boersma PD, Mackay S, Strange I. 2007. Egg size and parental quality in thin-billed prions, Pachyptila belcheri: Effects on offspring fitness. Animal Behaviour 74(5):1403–1412. DOI: 10.1016/j.anbehav.2007.01.008.
- Smith CC, Fretwell SD. 1974. The optimal balance between size and number of offspring. The American Naturalist 108(962):499–506. DOI: 10.1086/282929.
- Sokal RR, Rohlf FJ. 1995. Biometry: The principles and practise of statistics in biological research. New York: WH Freeman and Co.
- Song S, Chen J, Jiang B, Liu N. 2016. Variation in egg and clutch size of the Black Redstart (Phoenicurus ochruros) at the northeastern edge of the Qinghai-Tibetan Plateau. Avian Research 7(1). DOI: 10.1186/s40657-016-0055-0.
- Surmacki A, Stępniewski J, Zduniak P. 2003. Repeatability of egg dimensions within the clutches of Bearded tit Panurus biarmicus. Acta Ornithologica 38(2):123–128. DOI: 10.3161/068.038.0209.
- Swennen C, Van der Meer J. 1992. Variation in egg size of common eiders. Ardea 80:363.
- Väisänen RA. 1977. Geographic variation in timing of breeding and egg size in eight European species of waders. Annales zoologici Fennici 14:1–25.
- Van Noordwijk AJ, Keizer LCP, Van Balen JH, Scharloo W. 1981. Genetic variation in egg dimensions in natural populations of the Great tit. Genetica 55(3):221–232. DOI: 10.1007/BF00127206.
- Williams TD. 1994. Intraspecific variation in egg size and egg composition in birds: Effects on offspring fitness. Biological Reviews 69(1):35–59. DOI: 10.1111/j.1469-185X.1994.tb01485.x.
