Abstract
Noah’s ark shellfish is a commercially important species in the Adriatic Sea, but its populations are overexploited. The objective of this study was to complete the information on reproduction for potential commercial breeding. Previous attempts at ark cultivation resulted in high post-transfer mortality due to mechanical stress from individuals’ detachment from the natural environment. A different approach involving the culture of broodstock for aquaculture operations should be considered. Accurate prediction of spawning events and comparison of the main spawning season and peak in several populations could help to identify a favorable location and period for the collection of hatched gametes and transfer to growing sites. Thus, females of two distinct populations from the Middle Adriatic Sea were investigated. The range of oocyte size at each gonadal developmental stage, the monthly distribution of oocyte frequency and the variation in the monthly mean value of oocyte diameter during 2013 and 2014 were determined. Gametogenesis was not fully synchronous between localities. The period of spawning in Pasman Channel lasts longer and new gametes develop faster. Based on the results we could estimate the periods of gamete release in the studied sites, which may indicate a favorable location and period for the collection of hatched gametes for aquaculture purposes.
Introduction
Histological techniques provide general information on gonad development. According to Lango-Reynoso et al. (Citation2000), there are several means of assessing gamete development in bivalves: (a) visual observation in relation to the relative size, shape and color of the gonads; (b) gonad index, i.e. the weight of the gonad relative to body weight (wet or dry); (c) mean oocyte diameter; or (d) developmental stages based on certain cytological characteristics. The present work aims to establish quantitative and qualitative parameters of oocyte sizes investigated in two Arca noae Linnaeus, 1758 (Bivalvia: Arcidae) populations along the Adriatic Sea of Croatia based on oocyte diameter analysis to study reproductive activity in females. According to defined developmental stages of the female gonads determined by Erk et al. (Citation2018) on the same samples, measurements of the oocyte diameters in one microscopic field were conducted. The results of oocyte diameter analysis and histological gonad examinations led us to propose a qualitative reproductive scale for help in accurate prediction of the spawning events important for potential commercial aquaculture operations.
The Noah’s ark shell is a representative of the family Arcidae, which includes over 200 species of marine bivalves. Many of these bivalves constitute an important component of the human diet. Distribution of A. noae covers the eastern Atlantic Ocean, the Mediterranean and Black seas, and the West Indies (Nordsieck Citation1969). This species lives attached by a solid byssus on rocks on all types of bottoms that contain hard substrate, at a depth ranging from a few meters to more than 100 m (Poppe & Goto Citation2000). Previous studies investigated Mediterranean and Adriatic Sea populations of this species. These studies include research on ecology (Marin & López Belluga Citation2005; Peharda & Morton Citation2006), functional morphology (Morton & Peharda Citation2008), population structure (Hrs-Brenko Citation1980; Bello & Paparella Citation2001; Peharda et al. Citation2002a, Citation2003, Citation2009), reproduction (Peharda et al. Citation2003, Citation2006; Bello et al. Citation2013; Ghribi et al. Citation2017), aquaculture potential (Župan et al. Citation2012, Citation2013, Citation2014; Peharda et al. Citation2013), nutritional composition (Ezgeta-Balić et al. Citation2012; Dupčić Radić et al. Citation2014), growth and longevity of the species (Peharda et al. Citation2002b, Citation2003; Puljas et al. Citation2015), changes in heavy metal concentrations in the bivalve tissues during the reproductive cycle (Erk et al. Citation2018) and the study of trace elements in shells (Kobelja et al. Citation2016).
Most of these authors agree that the observed Adriatic populations are overfished. Due to regulations and market demands, divers are targeting larger specimens, which may lead to a population being dominated by smaller individuals (Župan et al. Citation2013). As described by Peharda et al. (Citation2009) and confirmed by Bello et al. (Citation2013) and Ghribi et al. (Citation2017), A. noae is an obligate protandric hermaphrodite. Males dominate small shell length categories while females become predominant as size increases. All of the studies mentioned above indicate that females are harvested more than males, which can cause an imbalance in the species’ reproductive strategy and may serve as a warning that the present level of exploitation is unsustainable.
The commercial size of A. noae is 50 mm (Official Gazette 63/10). This size can be reached in 3 to 7 years of age, meaning that this species is of interest for commercial production (Župan et al. Citation2012). The basic criterion for broodstock aquaculture activity is a continuous and sufficient supply of hatched gametes. Experiments with induced spawning of A. noae have not yet been made, so one option in terms of aquaculture is to collect hatched gametes from natural populations and transfer them to growing sites (Župan et al. Citation2012). This option requires serious research with the aim of determining the most suitable locations, time and depths for collection. Successful gamete collection from the wild requires knowledge of the spawning patterns of the local population (Gosling Citation2003). Thus, the optimal period for natural gamete collection could be tracked with oocyte size analyses provided by histological research. Assuming that different spawning timings can be observed in the same bivalve species from two geographically separate sites and that the spawning period can vary between seasons (Peharda et al. Citation2003, Citation2006; Bello et al. Citation2013; Ghribi et al. Citation2017), detailed tracking of the gonad development in different populations is necessary when planning this activity.
The aim of this study is to complete the information about the reproductive cycle of A. noae from the Telascica Bay and the Pasman Channel based on the morphometric analysis of oocytes. The purpose of the research is to describe in detail the annual period of oocyte maturation, the dynamics and the speed of oocyte development and to accurately circumscribe the spawning period, to determine the most favorable period and location for collecting hatched gametes from nature to introduce A. noae into broodstock aquaculture.
Materials and methods
Specimens of Arca noae were collected randomly once per month during 11 months (from March 2013 to February 2014) by scuba divers from the subtidal zone of the Telascica Nature Park (43.915552N, 15.154746E) and Pasman Channel (43.979086N, 15.347368E). Individuals from the Pasman Channel were collected at a depth of 5–7 m, while individuals in the Telascica Bay were collected at a depth of 10–14 m. This bay is situated on the south-eastern part of the island Dugi otok, Croatia, and was proclaimed a nature park in 1988. The Croatian Ministry of Environmental and Nature Protection (UP/I-612-07/13-68/07; 517–07-2-1-1-13-2) approved the collection from this protected site. A total of 217 females collected from April 2013 to February 2014 at the two sampling sites were processed following routine histological procedures. Of the 217 females, 110 individuals were from the Telascica Bay and 107 from the Pasman Channel. The shell length of each individual was recorded (mean ± standard deviation [SD] at the Telascica Bay site: 8.23 ± 0.78 cm, n = 110; and at the Pasman Channel site: 7.76 ± 0.71 cm, n = 107). In the laboratory, each individual was opened and a piece of gonad tissue was dissected and fixed in 4% formaldehyde. Each sample was dehydrated in ethanol, embedded in paraffin (Histowax, Leica), sectioned at 5 µm, stained using hematoxylin and counterstained with eosin. Histological sections were examined at 100× and 400× magnification under an Axio Zeiss Imager M1 microscope and photographed with an AxioCamera (MRc5).
The analysis of gonadal developmental stages was performed according to the criteria of Peharda et al. (Citation2006). Measurement of oocyte diameter was performed using the computer program Axio Vision Release 482 SP3 (08–2013). Oocyte diameters of 10 individuals were measured for each month. Exceptions were made for the Pasman Channel in January 2014 and October 2013, where nine and eight individuals were measured, respectively. In the visual field, for each individual, the diameter of 20 oocytes with a clearly visible nucleus and nucleolus, confirming that the sections intersect the oocyte through the middle, was measured. Based on the obtained data, the range of oocyte size at each developmental stages of the gonads, the variation in the mean value of oocyte diameter and the monthly distribution of oocyte frequency during 2013 and 2014 were determined.
Analyses of the data were performed using STATISTICA, v. 8 (www.statsoft.com). A t-test was used to determine differences in monthly oocyte diameters between sites. Differences were considered statistically significant at p < 0.05.
Results
This study reports the measurements of oocyte diameters of the Noah’s Ark shellfish from two populations living along the Croatian Adriatic shoreline. A total of 4340 oocytes were measured, of which 2200 were from individuals sampled in the Telascica Bay, and 2140 from individuals sampled in the Pasman Channel. The measured oocyte diameters were distributed by sampling sites and months from April 2013 to February 2014 ().
Table I. Mean values ± standard deviations (x– (µm) ± SD), minima and maxima of oocyte diameters of Arca noae females from Telascica Bay and Pasman Channel, collected from April 2013 to February 2014
The total range of oocyte diameters was 5.85 to 87.22 μm. Qualitative and quantitative characteristics of oocytes allowed us to propose a reproductive scale based on oocyte diameter, consisting of four stages: (1) “early gametogenesis” with an oocyte diameter <40 µm; (2) “growing stage” with a diameter range of 40–70 µm; (3) “mature stage” with a diameter range of 70–90 µm or more; and (4) “degenerating stage” referring to unreleased mature oocytes that will be reabsorbed within the gonad by a lysis process, i.e. oocyte atresia (; ). The lowest mean oocyte diameter in the Telascica Bay was recorded in September, while in the Pasman Channel, the lowest mean value was recorded in October. The highest mean oocyte diameters for both sites were measured in June and July. In the Telascica Bay, a decrease in oocyte diameter from August to September was recorded, showing a spawning event during the two months of the reproductive cycle (). In the Pasman Channel, a decrease in oocyte diameter was recorded from July to October, indicating a prolonged spawning period.
Table II. Reproductive scale based on the oocyte diameter
Figure 1. Light photomicrographs of the different gonad development stages of Arca noae females. (A) Early stage, (B) growing stage and (C) mature stage. Scale bar: 100 µm
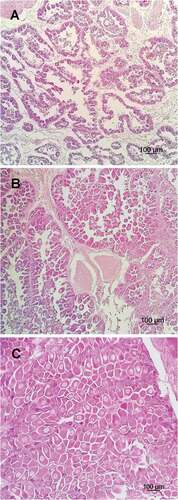
Figure 2. Mean diameter values of Arca noae oocytes collected from April 2013 to February 2014 in Telascica Bay and Pasman Channel
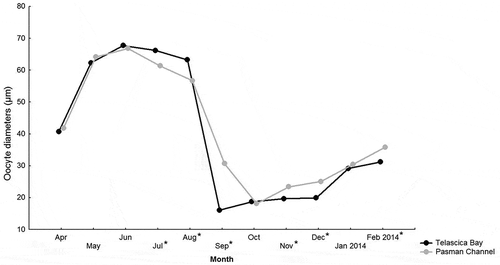
Frequency histograms of oocyte size () also showed a clearly defined pattern of seasonal gametogenic development in both sites, while comparisons between sites showed differences in reproductive strategies. Comparisons between oocyte diameters and sites reveal significant differences in measurements obtained for crucial month of spawning (p< 0.05) (). Intensive gametogenesis was found in the females from both sites from April until June. In Pasman Channel (the second site) spawning began earlier in July, since histology showed empty tubules or tubules filled with mature gametes in higher proportions. A delayed spawning stage, during August, was noticed in Telascica Bay (the first site). In the second site spawning lasted longer (from July until October), while in the first site the period of gamete release lasted only for two months (from August through September) (; ). The statistical analysis confirmed the noted differences in duration of spawning, since the t-test indicated statistically significant differences in oocyte diameter between the sites from July to October.
Figure 3. Size-frequency histograms of Arca noae oocyte diameters measured for females collected from April 2013 to February 2014 in (A) Telascica Bay and (B) Pasman Channel
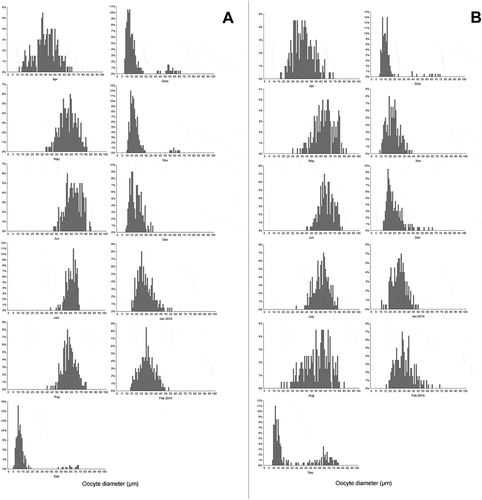
In Pasman Channel small oogonia cells and primordial oocytes were noticed in individuals collected in August, indicating the beginning of a new gametogenic cycle, while the spawning was still going on. A similar situation was noticed in individuals from Telascica Bay collected in September. During October specimens were at the post-spawning stage in both sites, with gonad tubules containing resorbed oocytes. The period of reabsorption lasted longer in Telascica Bay, and gametogenesis was slower there than in Pasman Channel (; ). The observed differences were also confirmed with statistical analysis, since the t-test indicated statistically significant differences in oocyte diameter between the sites from November through December, and during February when females from Pasman Channel had already reached an oocyte diameter of 40 µm.
Discussion
In the Adriatic Sea, unsustainable harvesting of Arca noae as edible species suggests aquaculture operations for human consumption, since it is noticed that the absence of large females as the basis of reproductive stocks will have a negative long-term effect on native ark populations (Peharda et al. Citation2002a, Citation2009, Citation2013; Župan et al. Citation2012, Citation2013). In addition, cultivation technology that includes detaching adult arks from the natural environment and cultivating them in nylon mesh nets is not optimal for this species, since Župan et al. (Citation2013) and Peharda et al. (Citation2013) recorded pronounced post-transfer mortality of individuals following the use of these techniques. This indicates that arks are extremely sensitive to manipulation and stress associated with removal from the natural substrate, suggesting that other methods for cultivation of arks should be considered. One of those methods is culture of broodstock for aquaculture operations. A necessary criterion for this method is obtaining commercial quantities of hatched gametes and/or larvae of the existing broodstock taken from well-known populations, while preconditions include detailed knowledge of the species’ reproductive strategy, including periods and pattern of reproduction.
With accurate prediction of the spawning events, gamete collection could be improved. The monthly distribution of oocyte frequency could show the period of gamete release, which in different populations may vary. Different reproductive patterns, as single- or two-peaked spawning, were described for the populations of the Adriatic Sea. Peharda et al. (Citation2006) showed that in the mid-eastern Adriatic Sea (Mali Ston Bay) this species reproduces via a single annual gametogenic cycle extending from October–November to April–May with one spawning peak in the summer (July and August). Bello and Paparella (Citation2001) noticed a similar pattern along the south-western Adriatic Sea (the coast of Bari), in contrast to investigations conducted in the northern Adriatic Sea (the Gulf of Trieste) where Valli and Parovel (Citation1981) found an extended spawning season from the spring to the autumn, with two spawning peaks (in March and September). Two spawning peaks per year were also reported for south-western Adriatic Sea populations (in the Gulf of Manfredonia; Bello & Paparella Citation2001).
The histological examination of the gonads and analysis of oocyte diameters conducted in this study indicate that A. noae individuals collected in 2013 and 2014 in Pasman Channel and Telascica Bay (the Middle Adriatic Sea) show a clearly defined pattern of seasonal gametogenic development but differences in seasonal spawning events important for aquaculture of broodstock. In general, the reproductive cycles of A. noae from Pasman Channel and Telascica Bay were characterized by an intensive gametogenesis during spring. In Pasman Channel, the ripe gonads were mostly recorded from late spring until late autumn, with a main spawning season from July until October. In Telascica Bay, ripe gonads were noticed in a shorter period from late spring until late summer, with a main spawning season in July and August.
Overall, A. noae in the Middle Adriatic Sea displayed inter-annual variation in their reproductive dynamics, namely in terms of spawning duration and intensity. The prolonged period of gamete release in Pasman Channel probably denotes multiple partial spawning events during a 4-month period, while the pattern observed in Telascica Bay indicates a single spawning peak.
Additionally, as confirmed in the present study, different sites can be characterized by distinct patterns of spawning, and this inter-site difference might be variable. For instance, depending on the population location, the timing, extent and frequency of spawning may vary. The comprehensive comparison of the main spawning season and peak in several populations highlights a general geographical gradient and latitudinal cline in the reproductive pattern and spawning features of the same species along their distributional ranges. Based on our results, we could estimate the periods of gamete release in the studied sites since gametogenesis was not fully synchronous between the localities. Analyses like this may indicate favorable periods for the collection of hatched gametes at different locations for aquaculture purposes. Currently, establishing a good knowledge base for aquaculture of broodstock is also important if we take into consideration Peterson’s (Citation2002) conclusion, that reducing adult bivalve spawning stock biomass by harvest, which causes lower recruitment, could eventually lead to effective elimination of commercial aquaculture in the area. Since it has already been noticed that the observed Adriatic populations are overfished and dominated by smaller individuals (Župan et al. Citation2013), the present study delivers valuable baseline information for the scientifically supported proposal of additional management measures for the sustainable harvesting of A. noae and future aquaculture development of this species.
Acknowledgements
This study is part of the second author’s master’s thesis through the Graduate Program in Biology and Chemistry of the Faculty of Science, University of Split. The authors thank the Laboratory for Histology and the Laboratory for Zoology (Department of Biology, Faculty of Science), which provided facilities for the development of this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Bello G, Paparella P. 2001. Struttura di popolazioni di Arca noae (Bivalvia, Arcidae) insediate su sustrati diversi nell’Adriatico meridionale. Atti della Società italiana di scienze naturali e del museo civico di storia naturale di Milano 141(2):175–185.
- Bello G, Paparello P, Corriero A, Santamaria N. 2013. Protandric hermaphroditism in the bivalve Arca noae (Mollusca: Arcidae). Mediterranean Marine Science 14(1):86–91. DOI:10.12681/mms.326.
- Dupčić Radić I, Carić M, Najdek M, Jasprica N, Bolotin J, Peharda M, Bratoš Cetinić A. 2014. Biochemical and fatty acid composition of Arca noae (Bivalvia: Arcidae) from the Mali Ston Bay, Adriatic Sea. Mediterranean Marine Science 15(3):520–531. DOI:10.12681/mms.436.
- Erk M, Ivanković D, Župan I, Čulin J, Dragun Z, Puljas S, Peharda Uljević M. 2018. Changes in the tissue concentrations of trace elements during the reproductive cycle of Noah’s Ark shells (Arca noae Linnaeus, 1758). Marine Pollution Bulletin 133:357–366. DOI:10.1016/j.marpolbul.2018.05.054.
- Ezgeta-Balić D, Najdek M, Peharda M, Blaûina M. 2012. Seasonal fatty acid profile analysis to 878 trace origin of food sources of four commercially important bivalves. Aquaculture 334–337(879):89–100. DOI:10.1016/j.aquaculture.2011.12.041.
- Ghribi F, Bello G, Zupa R, Passantino L, Santamaria N, El Cafsi M, Corriero A. 2017. Reproductive and tissue plasticity in Arca noae (Bivalvia: Arcidae). The European Zoological Journal 84(1):473–487. DOI:10.1080/24750263.2017.1368725.
- Gosling E. 2003. Bivalve Molluscs: biology, ecology and culture. Oxford, UK: Fishing New Books, Blackwell Publishing. pp 443.
- Hrs-Brenko M. 1980. Preliminary survey of populations of the bivalve Noah’s ark (Arca noae, Linné) in the northern Adriatic Sea. Aquaculture 21(4):357–363. DOI:10.1016/0044-8486(80)90071-X.
- Kobelja K, Nemet I, Župan I, Ŀulin J, Ronŀeviŀ S. 2016. Elemental profiling of Noah⿿s Ark shell (Arca noae , Linnaeus, 1758) by plasma optical spectrometry and chemometric tools. The Journal of Trace Elements in Experimental Medicine 38:157–164. DOI:10.1016/j.jtemb.2016.04.011.
- Lango-Reynoso F, Chávez-Villalba J, Cochard JC, Pennec ML. 2000. Oocyte size, a means to evaluate the gametogenic development of the Pacific oyster, Crassostrea gigas (Thunberg). Aquaculture 190(1–2):183–199. DOI:10.1016/S0044-8486(00)00392-6.
- Marin A, López Belluga MD. 2005. Sponge coating decreases predation on the bivalve Arca noae. Journal of Molluscan Studies 71(1):1–6. DOI:10.1093/mollus/eyh045.
- Morton B, Peharda M. 2008. The biology and functional morphology of Arca noae (Bivalvia: Arcidae) from the Adriatic Sea, Croatia, with a discussion on the evolution of the bivalve mantle margin. Acta Zoologica 89(1):19–28. DOI:10.1111/j.1463-6395.2007.00288.x.
- Nordsieck F. 1969. Die europäischen Meeresmuscheln (Bivalvia). Stuttgart: Fischer Verlag. pp 256.
- Peharda M, Bolotin J, Vrgoć N, Jasprica N, Bratoš A, Skaramuca B. 2003. A study of the Noah’s ark shell (Arca noae Linnaeus 1758) in Mali Ston bay, Adriatic Sea. Journal of Shellfish Research 22:705–709.
- Peharda M, Ezgeta-Balić D, Davenport J, Vrgoč N. 2013. The potential for aquaculture of the bearded horse mussel (Modiolus barbatus) and Noah’s Ark shell (Arca noae) in southern Croatia. Aquaculture International 21(3):639–653. DOI:10.1007/s10499-012-9598-1.
- Peharda M, Hrs–Brenko M, Onofri V, Lucić D, Benović A. 2002b. A visual census of bivalve distribution in the saltwater lake Malo jezero (Mljet National Park, South Adriatic Sea). Acta Adriatica 43:65–75.
- Peharda M, Mladineo I, Bolotin J, Kekez L, Skaramuca B. 2006. The reproductive cycle and potential protandric development of the Noah’s Ark shell, Arca noae L.: Implications for aquaculture. Aquaculture 252(2–4):317–327. DOI:10.1016/j.aquaculture.2005.07.007.
- Peharda M, Morton B. 2006. Experimental prey species preferences of Hexaplex trunculus (Gastropoda: Muricidae) and predator–prey interactions with the Black mussel Mytilus galloprovincialis (Bivalvia: Mytilidae). Marine Biology 148(5):1011–1019. DOI:10.1007/s00227-005-0148-5.
- Peharda M, Richardson CA, Onofri V, Bratoš A, Crnčević M. 2002a. Age and growth of the bivalve arca noae l. in the croatian adriatic sea. Journal of Molluscan Studies 68(4):307–310. DOI:10.1093/mollus/68.4.307.
- Peharda M, Stagličić N, Ezgeta D, Vrgoč N, Isajlović I, Krstulović-Šifner S. 2009. Distribution and population structure of Arca noae in Pašman channel. Ribarstvo 67:3–10.
- Peterson CH. 2002. Recruitment overfishing in a bivalve mollusc fishery: Hard clams (Mercenaria mercenaria) in North Carolina. Canadian Journal of Fisheries and Aquatic Sciences 59(1):96–104. DOI:10.1139/f01-196.
- Poppe GT, Goto Y. 2000. European seashells Voll. II Scaphopoda, Bivalvia, Cephalopoda. Hackenheim: Conch Books. pp 221.
- Puljas S, Peharda M, Župan I, Bukša F. 2015. Maximum recorded life span of Arca noae Linnaeus, 1758 in the marine protected area Telaščica, Adriatic Sea. Cahiers de biologie marine 56(2):163–168.
- Valli G, Parovel C. 1981. Aspects de la reproduction et de la biométrie chez Arca noae L. (Mollusca, Bivalvia). – Rapport et procès-verbaux des réunions. Commission International Pour l’Exploration Scientifique De La Mer Méditerraneé 27:135–136.
- Župan I, Peharda M, Dolenec T, Dolenec M, Žvab Rožić P, Lojen S, Ezgeta-Balić D, Arapov J. 2014. Aquaculture Assessment of Noah’s Ark (Arca noae Linnaeus, 1758) in The Central Adriatic Sea (Croatia). Journal of Shellfish Research 33(2):433–441. DOI:10.2983/035.033.0212.
- Župan I, Peharda M, Ezgeta-Balić D, Šarić T. 2012. Noah s Ark shell (Arca naoe Linnaeus, 1758) – What do we need to know for starting up its aquaculture. Croatian Journal of Fisheries 70:71–81.
- Župan I, Rogošić J, Šarić T, Kanski D. 2013. Transfer of Arca noae Linnaeus, 1758 from natural to different experimental farming conditions. Croatian Journal of Fisheries 71(4):187–191. DOI:10.14798/71.4.698.
