Abstract
In this paper, a live hectocotylus, found inside a stranded female Argonauta argo shell, allowed us to describe the behaviour of this copulatory arm and add new information on its morphology. The hectocotylus penile filament is contained in a special membranous pocket or sac, almost completely transparent, extending from the apex of the basal part to about one-third of the central section. The hectocotylus assumes a folded position to protect the penile filament in the membranous sac. This is probably extracted only when copulation occurs. Our observations seem to suggest that the shell may have the secondary function of storing and protecting the hectocotyli received by males until the copulation. The observations confirmed the high vitality and endurance of the hectocotylus after detachment from the A. argo male.
Introduction
Argonautidae are pelagic octopuses, commonly named argonauts or paper nautiluses, distributed in tropical and temperate oceans (Finn Citation2013) and characterised by a peculiar biology and ecology. This family includes only four species, belonging to a unique genus: Argonauta argo Linnaeus, 1758, Argonauta hians Lightfoot, 1786, Argonauta nodosus Lightfoot, 1786, Argonauta nouryi Lorois, 1852. They have sometimes been observed in swarms or in association with jellyfish (Heeger et al. Citation1992) or salps (Banas et al. Citation1982) and are an important food item for large pelagic fish (Romeo et al. Citation2012; Finn Citation2014).
Similar to other pelagic octopods, belonging to the Superfamily Argonautoidea (i.e., Alloposidae, Tremoctopodidae and Ocythoidae), argonauts display evident sexual dimorphism, with large females and dwarf males (Finn Citation2014). In some species of Argonauta, the size proportion between males and females is up to 1/8 in length and 1/600 in weight (Finn Citation2009).
Due to the presence of special integumental glands on the first dorsal pair of arms, Argonauta females secrete a calcified structure (improperly called a shell), which has the function of a brood case (Finn Citation2014). These dorsal arms are expanded as broad membranous webs and usually cover the outside faces of the shell (Finn Citation2014). Power (Citation1856) observed that Argonauta spp. are able to repair their shell when it is damaged, although females are not able to entirely reconstruct their shell and even then, never abandon this structure during their life (Power Citation1856). This is an important skill because this structure is used for the eggs’ deposition and care until hatching (Power Citation1856; Laptikhovsky & Salman Citation2003).
However, dwarf males lack a shell and have an extremely large copulatory arm (left arm of the third pair), named a hectocotylus, which is developed in a specialised sac under the male’s eye (Nesis Citation1977; Finn Citation2014). The hectocotylus is made up of three parts: a basal spermatophore reservoir, a central section equipped with suckers, and distally, a long penile filament (Beesley Citation1998; Sukhsangchan & Nabhitabhat Citation2007), which until now was considered not to be contained in a special sac (Mangold et al. Citation2016; Alejo-Plata & Martínez Santiago Citation2020). Males produce a unique spermatophore, hosted in a specific hectocotylus cavity (Naef Citation1923). During reproduction the hectocotylus detaches and becomes an active and autonomous spermatophore carrier (Naef Citation1923). According to the present knowledge, the detached hectocotylus reaches and remains within the female mantle cavity to achieve fecundation (Hanlon & Messenger Citation1996; Boyle & Rodhouse Citation2005; Bello Citation2009; Alejo-Plata & Martínez Santiago Citation2020). The hectocotylus can remain active for some time (Mangold et al. Citation2016).
As observed by some authors, the reproductive strategy of Argonauta is characterised by an extended spawning period and the continuous asynchronous production of eggs (Nishimura Citation1968; Nesis Citation1977, Citation1996; Rocha et al. Citation2001; Alejo-Plata & Martínez Santiago Citation2020).
Most data on Argonauta spp. regards female individuals, whereas finding males is very rare. Moreover, little information is available on the mating system and reproductive behaviour of Argonauta spp. (Finn Citation2014; Alejo-Plata & Martínez Santiago Citation2020), although in the past, this issue was strongly debated by several authors who had formulated different hypotheses (e.g., Costa Citation1841; Kölliker Citation1846; Müller Citation1851; Knight Citation1856; Power Citation1856). Indeed, originally, the male hectocotylus was mistaken for a parasitic worm affecting female individuals (Delle Chiaje Citation1825; Cuvier Citation1829), named Trichocephalus acetubularis by Delle Chiaje (Citation1825) and, later, Hectocotyle argonautae by Cuvier (Citation1829). Later, Costa (Citation1841) and Müller (Citation1851) understood that it was a reproductive arm of an argonaut male, while Kölliker (Citation1846) hypothesised that H. argonautae was instead the male individual of A. argo.
Study of the biological and ecological traits of argonauts has attracted scientists for the last two centuries. The difficulty in finding males in the wild is the main reason for poor knowledge of the mating and reproduction phases of these species. Therefore, the aim of the present paper was to provide new information on these aspects through the description of observations on a hectocotylus, found alive inside a shell of A. argo. New findings on its behaviour and morphology are reported here.
Materials and methods
On 30 December 2020 at about 6.40 a.m. an argonautid shell without the female individual was found on the beach in the Sicilian coast of the Strait of Messina (central Mediterranean Sea; ), during a routine survey aimed to monitor mesopelagic fauna stranding on the beach, a characteristic phenomenon of this area due to the particular hydrodynamic system (Battaglia et al. Citation2017). Despite a first check, the shell seemed apparently empty and, considering its fragility, was stored in a plastic box without water.
Figure 1. The hectocotylus found inside the shell of Argonauta argo on 30 December 2020 in the Strait of Messina (central Mediterranean Sea)
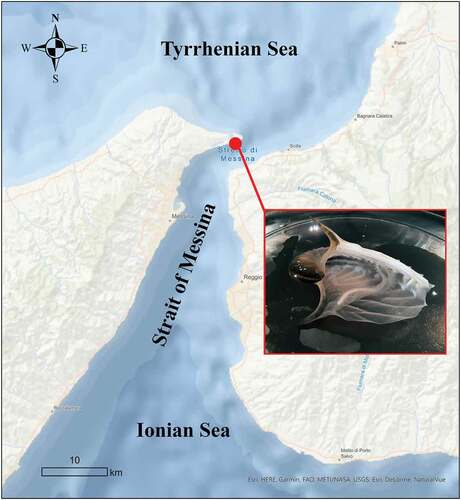
Although the shell remained without water until 2 p.m., surprisingly, when it was examined, a hectocotylus still alive and located inside the shell’s inner apex was discovered. For this reason, the sample was immersed in salt water for further analyses. The behaviour of the hectocotylus was carefully observed from 2 p.m. to 8 p.m. and a smartphone was used to take photos and videos. The shell length (ShL) was measured to the nearest 0.1 mm, according to Finn (Citation2013).
Results
The hectocotylus was found inside an argonaut shell of 34.0 mm ShL (). It persisted for about 7 hours without water and, when it was immersed (together with the shell) in salt water, it appeared very active. For the entire period of observation, the hectocotylus never abandoned the shell. Some attempts were made to extract it from the brood case, but it was strongly adhered to the shell wall by suckers, and it also showed a tendency to take refuge inside the inner apex of the brood case; this is the main reason it was not observed when the shell was first collected. The shell was also inspected to detect the presence of eggs, but no trace of them was found. The hectocotylus displayed an autonomous behaviour, and it seemed to react to dangerous situations (i.e., tweezer manipulation) managing to escape and often finding refuge in the innermost part of the shell.
Often, the hectocotylus was moving around inside the brood case. During this behaviour, it left the basal portion (containing the spermatophore) attached between the shell apex and lips (),(b)), while it checked the internal and external shell surface, and clinging to the shell ears (i.e. lateral extensions of shell axis beyond the shell surface) ()). The curved shape of the basal spermatophore reservoir seemed to fit the inner cavity of the shell apex.
Figure 2. Photo sequence of hectocotylus movements and behaviour. From (a)–(c): hectocotylus movements inside the shell; from (d)–(f): the hectocotylus was separated from the shell; from (g)–(i): the hectocotylus comes back to the brood case
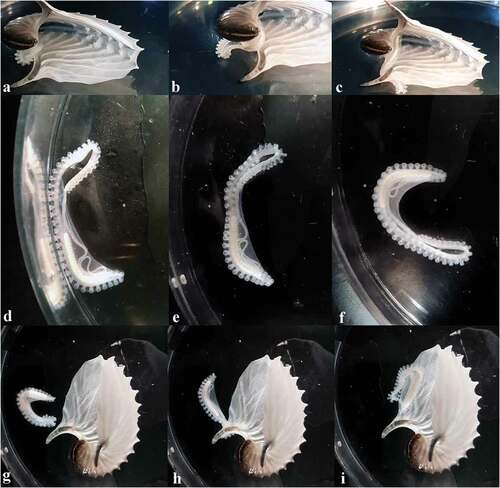
After several hours, it was possible to separate the hectocotylus from the shell, and it alone was placed on a plate containing sea water. Immediately, it started “walking” on its suckers on the plate margin ()) and then around the plate ()); at the end the hectocotylus stopped its movements ()). This allowed us to clearly observe its structure, confirming what was seen during the period of observation. Contrary to current knowledge, the hectocotylus never extracted the penile filament but assumed a folded position to protect the penile filament into a particular membranous pocket or sac, almost completely transparent, which extended from the apex of the basal part (e.g., near the spermatophore reservoir) to about the first portion of the central section (one-third of this section). The central section was then folded back on itself and the long penile filament was wrapped into the sac ().
After 10 minutes, the shell was again placed the plate ()), and the hectocotylus suddenly reacted, approaching the brood case ()) and going back inside it ()). Then, it again began to explore the shell.
At 19:30, during another attempt to separate the hectocotylus from the shell, the penile filament was accidentally extracted from its sac by tweezer. After this, the hectocotylus was unable to put the penile filament into the sac again, and it became less active. Finally, the hectocotylus was preserved in alcohol 80.
Discussion
Our observations allowed us to elucidate the morphology and behaviour of the A. argo hectocotylus. Mating in A. argo has been debated for the last two centuries but still needs further investigation. Several hypotheses were proposed on this aspect, dating back to 1825, when Delle Chiaje described the hectocotylus as a parasitic worm. Even when the studies of Costa (Citation1841), Kölliker (Citation1846) and Müller (Citation1851) allowed us to state the identity of the hectocotylus as a reproductive arm of a male Argonauta, scientists tried to understand the dynamics of the approach between males and females during mating. Since the detached arm was active, originally it was thought that it could freely swim to search for the female, but this hypothesis seems unlikely (Biagi Citation1978; Iliffe Citation1982; Orenstein & Wood Citation1996; Bello Citation2009). Indeed, our observations underline the importance of the hectocotylus suckers as tactile organs; when the male encounters the female, they establish contact. and this arm attaches, probably to the shell or female’s mantle, via suckers. The hectocotylus analysed in this study was always attached to the shell, moving around its walls via suckers without abandoning this structure.
Several authors reported that, after detachment, the hectocotylus autonomously moves towards the female mantle cavity and remains there until the female allows fertilisation (Iliffe Citation1982; Orenstein & Wood Citation1996; Norman Citation2003; Alejo-Plata & Martínez Santiago Citation2020). Based on our findings, we can hypothesise that the hectocotylus may also be stored within the shell until the female’s eggs complete maturation. This is supported by some points: i) if the hectocotylus had been stored inside the mantle cavity, it would have been lost along with the female specimen when it was stranded; ii) the hectocotylus shape seems to be adapted to the shell’s inner apex, where it found refuge each time we tried to separate it from the brood case; and iii) in the past, we also found other hectocotyli inside other shells (in some cases two on the same female), but they were already inactive. This implies that the shell has a protective function for eggs and embryos until hatching, but also a secondary function consisting of the storage and protection of hectocotyli received by males until the eggs reach maturity. The shell may also provide a substrate for the hectocotylus to release when males encounter females. Moreover, intact males have been also found within female shells (Naef Citation1923; Finn Citation2014). Unquestionably, this hypothesis needs the support of further observations on other specimens. However, hectocotyli have also been observed inside the mantle cavity of females (Finn Citation2014), but these individuals were likely ready for copulation.
Although some authors suggested that the male may be able to regenerate the reproductive arm (Steenstrup Citation1856; Imperadore & Fiorito Citation2018), another possibility is that, once the male detaches the hectocotylus, it dies (Nesis Citation1977; Iliffe Citation1982; Orenstein & Wood Citation1996; Norman Citation2003). It cannot be excluded that the male may also be eaten by the female, who would thus obtain additional energy to complete egg maturation, as occurs in other animals (Elgar & Schneider Citation2004; Hanlon & Forsythe Citation2008; Ibánez & Keyl Citation2010).
Our observations on hectocotylus morphology allowed us to revise the current knowledge, highlighting that the hectocotylus assumes a folded position to protect the penile filament in a membranous sac, until it reaches the mantle cavity to achieve copulation. This is the first time that such a feature has been observed; moreover, in the past, authors reported and illustrated that the hectocotylus penile filament was not contained in a special sac (Müller Citation1851; Brehm & Pechuel-Loesche Citation1893; Jatta Citation1896; Mangold Citation1989; Bello Citation2009; Finn Citation2013; Mangold et al. Citation2016; Alejo-Plata & Martínez Santiago Citation2020), probably because these authors observed hectocotyli already inside the mantle cavity, where the penile filament is usually everted to complete its function.
The hectocotylus motility and autonomous movements should be further investigated. It is still unclear how the hectocotylus is able to react to external mechanical and chemical stimuli, to find refuge in the inner apex of the shell when it is threatened, and to establish a relationship with the female.
Finally, we would underline the relevance of the Strait of Messina for the study of A. argo. Since the XIX century, studies on this species have been mainly based on individuals collected here (e.g., Delle Chiaje Citation1825; Owen Citation1839; Kölliker Citation1846; Power Citation1856), and now we had the opportunity to contribute to the knowledge of this species thanks to the finding of this important sample from the same location.
Conclusions
The scientific observations carried out on the hectocotylus of A. argo allowed us to add new information on the morphology and behaviour of this copulatory organ as well as in general on this species.
In summary, the following points were highlighted:
The hectocotylus penile filament is contained in a special membranous pocket or sac, almost completely transparent, extending from the apex of the basal part to about one-third of the central section. It is probably extracted only when copulation occurs.
The hectocotylus assumes a folded position to protect the penile filament in the membranous sac.
Although the primary shell’s function is to protect the eggs and embryos until hatching, a secondary function is to store and protect the hectocotyli received by males until copulation.
The observations confirmed the high vitality and endurance of the hectocotylus after detachment from the male A. argo, even in extreme conditions, which is an added value for the reproductive success of this species.
Supplemental Material
Download MP4 Video (112.6 MB)Supplemental Material
Download MP4 Video (51 MB)Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- Alejo-Plata MDC, Martínez Santiago N. 2020. The reproductive strategy of Argonauta nouryi (Cephalopoda: Argonautidae) in the Mexican South Pacific. Molluscan Research 40(3):205–213.
- Banas PT, Smith DE, Biggs DC. 1982. An association between a pelagic octopod, Argonauta sp. Linnaeus 1758, and aggregate salps. Fishery Bulletin U.S 80:648–650.
- Battaglia P, Ammendolia G, Cavallaro M, Consoli P, Esposito V, Malara D, Rao I, Romeo T, Andaloro F. 2017. Influence of lunar phases, winds and seasonality on the stranding of mesopelagic fish in the Strait of Messina (Central Mediterranean Sea). Marine Ecology 38(5):e12459. DOI: 10.1111/maec.12459.
- Beesley PL, Ross GJB, Wells A., CSIRO. 1998. Mollusca: The southern synthesis. Australian Government Publishing Service, CSIRO, Canberra, Australia.
- Bello G. 2009. I maschi dei polpi olopelagici. Naturalmente 22(1):46–48.
- Biagi V. 1978. Sul rinvenimento e la cattura di un esemplare vivente di Argonauta argo L. femmina nel golfo di Baratti (Piombino) e osservazioni sull’animale vivente in acquario. Conchiglie 14:119–134.
- Boyle P, Rodhouse P. 2005. Synopsis of living cephalopod families. Ecology and fisheries. Blackwell Science Ltd, Oxford, UK. pp. 464.
- Brehm AE, Pechuel-Loesche E. 1893. Brehms Tierleben. Allgemeine Kunde des Tierreichs. Niedere Tiere. pp 716
- Costa OG. 1841. Note sur le prétendu parasite de l’Argonauta argo. Annales des Sciences Naturelles partie zoologie 2s 16:184–187.
- Cuvier G. 1829. Mémoire sur un ver parasite d’un nouveau genre (Hectocotylus octopodis). Annales des Sciences Naturelles 16:147–156.
- Delle Chiaje S. 1825. Nota sul mollusco dell’Argonauta argo e su una nuova specie di epizoo che vi ospita. In: Memorie su la storia e notomia degli animali senza vertebre del Regno di Napoli. Battelli C. & Comp., Napoli, II. pp. 219–227.
- Elgar MA, Schneider JM. 2004. Evolutionary significance of sexual cannibalism. Advances in the Study of Behavior 34(4):135–163.
- Finn JK. 2009. Systematics and biology of the argonauts or ‘paper nautiluses’ (Cephalopoda: Argonautidae). PhD thesis. Department of Zoology, School of Life Sciences, Faculty of Science, Technology and Engineering, La Trobe University, Bundoora, Australia. pp. 548
- Finn JK. 2013. Taxonomy and biology of the argonauts (Cephalopoda: Argonautidae) with particular reference to Australian material. Molluscan Reserch 33(3):43–222.
- Finn JK. 2014. Family Argonautidae. In: Jereb P, Roper CFE, Norman MD, Finn JK, editors. Cephalopods of the world. An annotated and illustrated catalogue of cephalopod species known to date. Volume 3. Octopods and Vampire Squids, FAO Species Catalogue for Fishery Purposes. No. 4. Vol. 3. Rome: FAO. pp. 228–237.
- Hanlon RT, Forsythe JW. 2008. Sexual cannibalism by Octopus cyanea on a Pacific coral reef. Marine and Freshwater Behaviour and Physiology 41(1):19–28. DOI: 10.1080/10236240701661123.
- Hanlon RT, Messenger JB. 1996. Cephalopod Behaviour. Cambridge, UK: Cambridge University Press.
- Heeger T, Piatkowski U, Möller H. 1992. Predation on jellyfish by the cephalopod. Argonauta Argo. Marine Ecology Progress Series 88:293–296. DOI: 10.3354/meps088293.
- Ibánez CM, Keyl F. 2010. Cannibalism in cephalopods. Reviews in Fish Biology and Fisheries 20(1):123–136. DOI: 10.1007/s11160-009-9129-y.
- Iliffe TM. 1982. Argonaut: Octopus in a parchment shell. Sea Frontiers 28:224–228.
- Imperadore P, Fiorito G. 2018. Cephalopod tissue regeneration: Consolidating over a century of knowledge. Frontiers in Physiology 9:593. DOI: 10.3389/fphys.2018.00593.
- Jatta G. 1896. I Cefalopodi viventi nel golfo di Napoli. Fauna und Flora des Golfes von Neapel, Berlin: R. Friedländer & Sohn 23:1–264.
- Knight C. 1856. The English Cyclopedia. A new dictionary of universal knowledge. Natural history, vol. 4. London: Bradbury and Evans.
- Kölliker A. 1846. II. Some Observations upon the Structure of two new Species of Hectocotyle, parasitic upon Tremoctopus violaceus, D.Ch., and Argonauta Argo, Linn.; with an Exposition of the Hypothesis that these Hectocotylæ are the Males of the Cephalopoda upon which they are found. Transactions of the Linnean Society of London 20(1):9–21.
- Laptikhovsky V, Salman A. 2003. On reproductive strategies of the epipelagic octopods of the superfamily Argonautoidea (Cephalopoda: Octopoda). Marine Biology 142(2):321–326. DOI: 10.1007/s00227-002-0959-6.
- Mangold KM. 1989. Organes génitaux. In: Mangold KM, editor. Traité de Zologie, Anatomie, Systématique, Biologie. Tome V. Cephalopodes. Masson. Paris. pp. 459–552.
- Mangold KM (1922-2003), Vecchione M, Young RE. 2016. Argonautidae Tryon, 1879. Argonauta Linnaeus 1758. paper nautilus. Version 16 November 2016 (under construction) in The Tree of Life Web Project. Available: http://tolweb.org/. Accessed Jan 2021 18.
- Müller H. 1851. Note sur les argonautes male ei les hectocotyles. Annales des Sciences Naturelles Zoologie 3S 16(3):132–134.
- Naef A. 1923. Cephalopoda. Fauna und Flora des Golfes von Neapel. Monograph 35
- Nesis KN. 1977. The biology of paper nautiluses, Argonauta boettgeri and A. hians (cephalopods, octopoda) in the western Pacific Ocean and the seas of the East Indian archipelago. Zoologicheskiy Zhurnal (Russian Zoological Journal) 56:1004–1014.
- Nesis KN. 1996. Mating, spawning, and death in oceanic cephalopods: A review. Ruthenica 6(1):23–64.
- Nishimura S. 1968. Glimpse of the biology of Argonauta argo Linnaeus (Cephalopoda: Octopodida) in the Japanese waters. Publications of the Seto Marine Biological Laboratory 16(1):61–70. DOI: 10.5134/175488.
- Norman M. 2003. Cephalopods: A World Guide. Conch-Books, Hackenheim, Germany.
- Orenstein M, Wood B. 1996. Paper Nautilus (Argonauta argo) Marine Invertebrates of Bermuda. Available: http://www.thecephalopodpage.org/MarineInvertebrateZoology/Argonautaargo.html Accessed Jan 2021 18.
- Owen R. 1839. On the paper Nautilus Argonauta Argo. Proceedings of the Zoological Society of London 7:35–48.
- Power J. 1856. Observations physiques sur le Poulpe de l’Argonauta Argo. Imprimerie Charles de Morgues Freres. Paris. pp. 30.
- Rocha F, Guerra Á, González ÁF. 2001. A review of reproductive strategies in cephalopods. Biological Reviews of the Cambridge Philosophical Society 76(3):291–304. DOI: 10.1017/S1464793101005681.
- Romeo T, Battaglia P, Pedà C, Perzia P, Consoli P, Esposito V, Andaloro F. 2012. Pelagic cephalopods of the central Mediterranean Sea determined by the analysis of the stomach content of large fish predators. Helgoland Marine Research 66(3):295–306. DOI: 10.1007/s10152-011-0270-3.
- Steenstrup J. 1856. Die Hectocotylenbildung bei den Cephalopoden. Archival Naturegesch 22: 211–257.Translated in 1857. Hectocotylus-formation in Argonauta and Tremoctopus explained by observation on similar formations in the Cephalopoda in general. Annals and Magazine of Natural History 20:81–116. DOI: 10.1080/00222935709487882.
- Sukhsangchan C, Nabhitabhat J. 2007. Embryonic development of muddy paper nautilus, Argonauta hians Lightfoot, 1786, from Andaman Sea, Thailand. Agriculture and Natural Resources 41(3):531–538.
