Abstract
The Afrotropical genus Zonitodema is recorded for the first time from Madagascar, and the species Zonitodema madagascarensis sp. nov. is described. Some taxonomic remarks are presented, including the new synonymy Zonitis nigricollis Beauregard, 1890 = Zonitodema collaris (Laporte de Castelnau, 1840), and the possible new synonymy Zonitis abyssinica Fairmaire, 1882 = Zonitodema collaris (Laporte de Castelnau, 1840). Moreover, new faunistic records are published and a new key to the species is given. Finally, biogeographic remarks on the blister beetles of Madagascar are included.
1. Introduction
The genus Zonitodema was described by Péringuey (Citation1909) to gather some species from southern and eastern Africa previously referred to the genera Zonitis Fabricius, 1775 and Nemognatha Illiger, 1807. Since then, additional species have been described and Kaszab (Citation1954b) published a key with the description of other new species and some synonymies. Bologna and Pinto (Citation2002) and Bologna et al. (Citation2018) included Z. posoka Wellman, 1908 in the genus, previously confused with Z. parentalis Péringuey, Citation1909, and François (Citation2018) summarised the taxonomic history of the genus, in which he included 15 species, proposing a new synonymy and describing a new species from Tanzania.
This genus is distributed in Sub-Saharan Africa, and only two species [Z. erythraea (Pic, 1909) and Z. francoisi (Pic, 1909)] are marginally distributed in the Gulf of Guinea, while most of the remaining species are spread from Ethiopia to South Africa. In the framework of a wide study on the Afrotropical Nemognathinae, we considered additional material of this genus and surprisingly found a new species, described here, from Madagascar. The blister beetle fauna of Madagascar is mostly unknown. The only two works devoted to this island were published by Paulian (Citation1956) and Kaszab (Citation1965), who recorded 12 species in seven genera of Meloidae.
As pointed out by Bologna and Pinto (Citation2002), it is difficult to morphologically separate Zonitodema and Nemognatha, but most species of the first genus have metallic green or blue elytra, a triangular elongate head and relatively elongate maxillary galeae. However, we have molecular evidence that Zonitodema represents an independent lineage within Nemognathinae (Riccieri et al., in preparation).
The aim of this paper is to (i) report the presence of the genus Zonitodema in Madagascar, describing a new species; (ii) point out a few taxonomic aspects of other species, including new synonymies; (iii) publish some faunistic records and a new key to the species; and (iv) discuss the biogeographic origin of Meloidae from this island.
2. Material and methods
The following abbreviations used in the text represent the studied collections (presented in alphabetical order): HNHM = Hungarian Natural History Museum, Budapest, Hungary; MABC = M. A. Bologna, Università Roma Tre, Rome, Italy; MCZR = Museo civico di Zoologia, Rome, Italy; MNHN = Muséum National d’Histoire Naturelle, Paris, France; RMCA = Royal Museum of Central Africa, Tervuren, Belgium; SKC = Stanislav Krejcik, Unicov, Czech Republic; ZMHB = Museum für Naturkunde der Humboldt-Universität, Berlin, Germany.
For this study we examined 42 adult specimens: 3 exx. Zonitodema bimaculatithorax (MABC, RMCA); 18 exx. Z. collaris (MABC, RMCA, SKC); 3 exx. Z. erythraea (MABC, MCZR, HNHM); 4 exx. Z. madagascarensis (holotype and 2 paratypes RMCA, 1 paratype MABC); 2 exx. Z. nigrithorax (MABC); 1 ex. Z. notatithorax (HNHM); 1 ex. Z. parentalis (MABC); 2 exx. Z. ruficeps (MABC, SKC); 8 exx. Z. viridipennis (MABC, SKC, ZMHB).
The morphological terminology of male genitalia agrees with Bologna et al. (Citation2013). Morphological study was carried out using a Visionary Digital LK Lab System (Visionary Digital, Palmyra, VA) equipped with a Canon EOS 6D mark II dSLR camera and an MP-E 65 mm f/2.8 1–5× lens (Canon, Tokyo, Japan); this device allowed us to automatically capture stacks of images on different focal planes, which were modified in Adobe Photoshop CS6 and combined with the Helicon Focus 7 software.
3. Taxonomic accounts
3.1. Description of the new species
Zonitodema madagascarensis sp. nov.
LSID: urn:lsid:zoobank.org:act:
Type material
Holotype ♂, and 1 ♂ 1 ♀ paratypes (RMCA), 1 ♂ paratype (MABC), all labelled “coll. Mus. Tervuren, Madagascar: Ranomandry, XII.52/I.53 ex coll. Breuning”.
Diagnosis
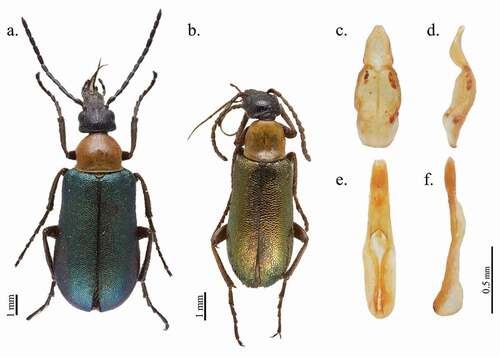
The new species is characterised by metallic green elytra, orange unicolor pronotum subcampaniform, dark head, antennae and legs, elongated maxillary galeae with the apical setated portion about twice as long as the maxillary palpi, temples scarcely rounded externally. This is the only Zonitodema known from Madagascar, which differs from its congeners by the following combination of characters: (a) from Z. hayekae Kaszab, 1954 and Z. rufipennis Pic, 1939 by their non-metallic, yellow-orange elytra; (b) from Z. friedmani François, Citation2018 by its dark brown, almost black elytra; (c) among the remaining species with metallic green-blue elytra, from Z. ruficeps (Péringuey, 1888) by its head (except uncommon variation), and by antennomere I and legs orange; from Z. erytraea (Pic, 1909) by their legs and scutellum orange; from Z. nigrithorax Pic, 1929, Z. francoisi (Pic, 1909), and uncommon individuals of Z. collaris (Laporte de Castelnau, 1840) (nigricollis form; see below), by their black pronotum; from Z. notatithorax Pic, 1912 and Z. bimaculatithorax Kaszab, 1954 by their pronotum with two black spots at base; from Z. proxima (Péringuey, 1892) by its ventral integuments totally black or dark brown; from Z. posoka Wellman, 1908, Z. parentalis Péringuey, Citation1909 and Z. viridipennis (Fabricius, 1794) by their maxillary lobes about 1/3 longer than maxillary palpi; finally, from Z. collaris, which has similar colour pattern and elongate maxillary lobes, by the pronotum which in the new species is distinctly narrower at the middle and especially in front on the apical third, temples more parallel and less rounded externally, antennae distinctly shorter, reaching the first third of elytra rather than almost the middle ((a–b)).
Description
Length: 5.4–7.1 mm. Body ()) dark brown with metallic green elytra, head black, antennae and tarsi lighter, pronotum light orange and end of abdomen orange, scutellum dark. Whole body completely covered with light hairs, longer and denser ventrally.
Head sub-rectangular, slightly longer than wide, posteriorly slightly convex, medially and anteriorly flat, frons almost depressed with two small oblique depressions near the base of antennae; surface with punctures distanced, relatively deep, median longitudinal portion smooth, extended until the middle of eye. Temples sub-parallel, vaguely widened posteriorly, slightly shorter than eye. Eyes slightly bulging. Clypeus sub-rectangular, transverse, with punctures as on frons, anterior third smooth and less deep. Labrum narrower than clypeus, sub-oval, slightly longer than wide, anterior margin slightly transverse, smooth and lightened. Mandibles quite short, slightly extended beyond the labrum. Maxillary palpi quite short, palpomere I short, slightly widened at apex, II–IV elongate, II ca. 1.3× as long as III, about as long as IV. Maxillary galeae very long and slender, apical setated portion ca. twice as long as maxillary palpi. Antennae quite short, reaching the first third of elytra, with antennomeres rather flattened and broadened, especially last five, I–VI slender, antennomeres I–II and IV–VI similar in size and shape, and slightly widened at apex, III longer than others; antennomeres VII–X progressively shorter, broader and shorter than others, XI similar in shape to but longer than X.
Pronotum as wide as head, about as long as wide at base, slightly campaniform, broader at base, depressed along base, especially on lateral basal angles. Punctation dispersed and fine (especially on centre where a well visible longitudinal median line is present), sparser than on head and on rest of body, surface shining. Scutellum small and rounded at apex with same punctures as elytra. Elytra longer than their combined breadth, 1.5× as wide as pronotum, parallel-sided from their base to posterior third, then narrowing; punctures distinctly denser than on head, almost coalescent; humeri distinct but not prominent. Ventrally with punctation sparser and smaller than dorsally; mesosternum quite wide with pointed apex; metasternum with median longitudinal smooth line without hairs and punctation, with slightly acute apex. Legs quite robust, femora thick, tibiae as long as femora of corresponding legs, tarsi longer than tibiae; penultimate tarsomere of all legs distinctly shorter than others; metatibial spurs sub-spatulate, inner one smaller; pro- and mesotibial spurs thin and pointed.
Male genitalia with phallobase not distinctly widened in middle in ventral view ()), slightly wider than gonoforceps in its mid portion; gonoforceps sub-conically narrowed in the basal 2/3 and narrowed apically in ventral view ()), forming two short lobes; in lateral view ()) slender, barely recurved dorsally; aedeagus () narrow and slender, almost straight, not recurved in lateral view, without hooks.
Sexual dimorphism
Last two male visible ventrites orange, the remaining ones black; in female last three ventrites orange. Last male ventrite divided, that of female slightly emarginate.
3.2. Taxonomic notes
Zonitodema abyssinica (Fairmaire, Citation1882).
Described as Zonitis twice by Fairmaire (Citation1882, Citation1883) and included in this genus also by Kaszab (Citation1954a), who subsequently (Kaszab Citation1963) referred it to Zonitodema without any comment.
The description of this species overlaps completely that of Z. collaris and of its synonyms Nemognatha caerulans Fairmaire, 1887 from Tanzania, and Zonitodema fahrei Péringuey, Citation1909 from Botswana and Zimbabwe. Temples in some specimens from Ethiopia are slightly narrower than in others from Zambia and Zimbabwe. Pending an examination of the types of all taxa, we consider Z. abyssinica a putative synonym of Z. collaris.
2. Zonitodema bimaculatithorax Kaszab, 1954.
This species is scarcely known, but we examined the male monotypus, labelled “Van Semeren, Makueni, Ukamna, 11.47” (HNHM), which corresponds to the specimens from southern Ethiopia recorded by Bologna (Citation1978). Moreover, we examined another specimen from the type locality, recorded by Bologna (Citation1980) and now at RMCA.
3. Zonitodema collaris (Laporte de Castelnau, 1840).
We examined one specimen (RMCA) identified by Kaszab in 1966 as Z. collaris ab. nigricollis. Moreover, we studied one specimen of Z. collaris from southern Ethiopia (see below) (MABC), mixed with four belonging to the typical form, which has the pronotum deeply dark, almost black, except along the margins, which are dark orange. The character used by Beauregard (Citation1890) to describe Zonitis nigricollis simply represents a colour variation of the pronotum. Since we could not find other relevant morphological differences, we propose the following new synonymy: Zonitis nigricollis Beauregard, 1890 = Zonitodema collaris (Laporte de Castelnau, 1840) syn. nov.
4. Zonitodema ruficeps (Péringuey, 1888).
The colouration of the head is typically red, but we examined one specimen (SKC; see below) with the head subopaque dark, although not distinctly black.
3.3. New faunistic records
After the revision published by Kaszab (Citation1954b), scarce additional faunistic records have been published by Kaszab (Citation1955a, Citation1956, Citation1960, Citation1961), Ferreira (Citation1965), Bologna (Citation1978, Citation1980, Citation1990, Citation2000), De Moor (Citation1978), Bologna and Pinto (Citation2002), Bologna et al. (Citation2018). As the distribution of the species of Zonitodema is scarcely known, the addition of the following new data obtained from the examination of some collections will improve the faunistic knowledge of this genus, even though these new records do not represent a significant range extension (localities are indicated according to labels):
“Zonitodema bimaculatithorax. Ethiopia. 1 ex., Gemu Gofa, nr. Konso, iv. 1994, Werner (MABC). Tanzania. 1 ex. Korogwe, 4.ix.1985 A. Zocchi (MABC)”.
“Zonitodema collaris. Ethiopia. 5 exx., 11 km See Goro, 1.v. 2013 S. Prespl (MABC); 1 ex., Gibbie Gorge, 5000 ft, viii. 1971, R.O.S. Clarke (MABC). Zambia. 1 ex., Mogoye, II.1990, Minetti leg. (MABC); 2 exx., Mkushi env., 16–18.12.2004, M. Snizek (SKC). Zimbabwe. 2 exx., 26 km SW Harare, 22–23.12.1998 A. Kudrna (MABC)”.
“Zonitodema erythraea. Cameroon. 2 exx., Yomandé, viii.1953 (MABC; MCZR). 1 ex., N’Kongsamba, 1.xi.56, J. Cantalombe (HNHM)”.
“Zonitodema nigrithorax. Zimbabwe. 2 exx., 26 km SW Harare, 22–23.12.1998 A. Kudrna (MABC)”.
“Zonitodema notatithorax. Tanzania. 1 ex., D.O. Afrika, Mehemer leg. (HNHM)”.
“Zonitodema parentalis. South Africa. 1 ex., Eastern Cape, Addo, 60 m, 33°32ʹ24” S 25°40ʹ81” E, 17.xi.2006, E. Colonnelli (MABC)”.
“Zonitodema ruficeps. South Africa. 1 ex., Northern cape, SW Springbook, 4.xi.1999 M. Halada (MABC); 1 ex., Northern Cape, Springbook env., 15.10.1999 M. Snizek (SKC)”.
“Zonitodema viridipennis. South Africa. 3 exx., Free State, 4 km S Zastron, m 1700, 30°19’S 27°06ʹE, 3.xii.1995 A. De Biase (MABC); 1 ex., Free State, Villens env., 15.1.2001 M. Snizek (SKC); 1 ex., Free State, N Fikcsburg R.70, 17.1.2001 M. Snizek (SKC); Natal/Free State, Botha’s pass, 1500–1750 m, 27°38’S 29°42ʹE, 13.xii.1995 A, De Biase (MABC); 2 exx., Transvaal, Bonnefoi (ZMHB)”.
3.4. Key to the species
Resulting from the new descriptions and the taxonomic changes proposed here and by François (Citation2018), 15 species of Zonitodema are now recognised. The key to the species published by Kaszab (Citation1954b) must be upgraded with the new taxa and synonymies; we propose the following new key to identify all species.
1 Elytra and pronotum yellow, head black, without metallic reflections2
1’ Elytra metallic, black, dark brown, blue or green, pronotum orange3
2 Legs yellow but trochanters, coxae, tibio-femoral junction, apex of tibiae slightly dark, tarsomeres apically darker. Maxillary galeae with apical setated portion distinctly shorter than maxillary palpi. Pronotum parallel on sides, finely and densely punctured, surface shagreened and shining (South Africa: Kwa-Zulu/Natal)hayekae
2’ Legs black, antennae, palpi and ventral side dark brown; last two urites yellow, like the previous ones in male. Maxillary galeae with the apical setated portion longer than maxillary palpi. Pronotum rounded, shining, with punctures fine and sparse (South Africa: Western Cape; Tanzania) rufipennis
3 Head red (dark red in some individuals), mouthparts yellow-red (but apex of mandibles, last two maxillary palpomeres, and last labial palpomere dark), pronotum, scutellum, ventral side and legs yellow-red; antennomeres II–XI dark; tarsi and procoxae dorsally brown; fore portion of metasternite slightly dark. Head wide, temples distinctly widened posteriorly to eyes, frons flat and roughly and densely punctuated; maxillary galeae with the apical setated portion only slightly longer than maxillary palpi. Pronotum transverse, with wide punctures; elytra green, with punctures dense and wide, transversely confluent (South Africa: Northern and Western Cape)ruficeps
3’ Head black, uncommonly dark brownish but with ventral side of temples almost black4
4 Legs, ventral side, pronotum, and scutellum yellow-red; antennomeres brown-red but last ones darker or brown-black. Head black or rarely brown-red dorsally and darker ventrally, distinctly narrower than pronotum, temples elongate and posteriorly quite gibbose, punctures sparse and wide, denser anteriorly. Pronotum scarcely wider than long, with punctures sparse and very thin. Elytra blue-green, with punctures wide and dense, scarcely confluent (Nigeria; Cameroon; D.R. Congo; Ethiopia; Eritrea) erythraea
4’ Antennae and legs black, thoracic sternites black, urites totally or partially red 5
5 Pronotum unicolourous orange 6
5’ Pronotum orange with black or black-brown spots 12
6 Elytra metallic dark brown 7
6’ Elytra metallic green or blue 8
7 Ventral side totally dark brown. Head with punctures very dense and deep, almost rugose; maxillary galeae with apical setated portion only slightly longer than maxillary palpi. Pronotal punctures fine and sparse (South Africa: Kwa-Zulu/Natal, Mpumalanga) proxima
7’ Ventral side with last two (male) or three (female) urites orange. Head with punctures sparse and deep, not rugose; maxillary galeae with apical setated portion very long, as long as antennae, distinctly longer than maxillary palpi. Pronotum punctures coarse and deep, sparse (Tanzania) friedmani
8 Maxillary galeae with apical setated portion only slightly or at maximum about 1/3 longer than maxillary palpi, pointed apical portion not longer than the basal shining portion 9
8’ Maxillary galeae very elongate and slender, apical setated portion ca. twice as long as maxillary palpi 11
9 Maxillary galeae with apical setated portion about 1/3 longer than maxillary palpi, the pointed apical portion distinctly longer than last palpomere; head with punctures dense and coarse, in the middle longitudinally impunctate. Pronotum punctures sparse and fine; elytra blue-green with wide and dense punctures, confluent (Tanzania; South Africa: Kwa-Zulu Natal, Mpumalanga, North West province, Free State, Western Cape) viridipennis
9’ Maxillary galeae with apical setated portion only slightly longer than maxillary palpi, the pointed apical portion shorter than last palpomere; head with punctures coarse and deep, but sparse. Pronotum punctures very sparse and fine; elytra green or bluish, with coarse and dense punctures, confluent 10
10 Pronotum as wide as long, sub-oval, distinctly narrower on fore sides, punctures coarse and sparse (Angola, Namibia) posoka
10’ Pronotum wider than long, more transverse, progressively curved on fore sides, punctures very sparse and fine (Tanzania; South Africa: Kwa-Zulu Natal, Mpumalanga, North West province, Free State, Eastern Cape, Western Cape).....parentalis
11 Temples parallel and scarcely rounded externally, antennae short, reaching first third of elytra. Pronotum not distinctly widened in the middle or on the fore third (Madagascar) madagascarensis
11’ Temples rounded externally, antennae elongate, reaching almost the middle of elytra. Pronotum distinctly rounded and transverse in the middle and anteriorly (for synonyms see Taxonomic notes). (Eritrea, Ethiopia, Somalia, Uganda, Rwanda, Kenya, Tanzania, Zambia, Zimbabwe, Botswana)collaris
12 Pronotum orange with two black rounded spots at base 13
12’ Pronotum black or dark-brown, with sides sometimes lighter 14
13 Pronotum transverse, distinctly convex, sides distinctly rounded, punctures coarse, deep and irregularly distributed, surface among punctures minutely shagreened and consequently almost shining; middle longitudinal line deep. Head coarsely and densely punctured, without impunctate surface; maxillary galeae very elongate and slender, apical setated portion ca. twice as long as maxillary palpi (Uganda, Tanzania, D.R. Congo) notatithorax
13’ Pronotum ca. as long as wide, sides less rounded, punctures fine and sparse, surface among punctures smooth and shining; middle longitudinal line shallow. Head coarsely but sparsely punctured, with a small impunctate surface. Maxillary galeae very elongate and slender, apical setated portion more than twice as long as maxillary palpi and fine (Ethiopia, Kenya, Tanzania)bimaculatithorax
14 Head with punctures coarse and dense, sparser in middle; maxillary galeae elongate and narrow, less than twice as long as the maxillary palpi. Pronotum rounded on sides with punctures sparse and fine, middle longitudinal line deep (Uganda, Kenya, Tanzania, D.R. Congo, Zimbabwe)..nigrithorax
14’ Head with punctures coarse but sparse, largely impunctate medially; maxillary galeae very long and narrow, distinctly more than twice as long as maxillary palpi. Pronotum narrower, even if with distinctly rounded sides, with punctures extremely sparse and fine, middle longitudinal line indistinct (syn. brittoni Kaszab) (Nigeria, D.R. Congo, Zambia)francoisi
4. Conclusive remarks
4.1. Biogeography of Malagasy Meloidae
The genus Zonitodema is a strictly Afrotropical element, mainly distributed in eastern and southern Africa. Its presence in Madagascar supports the great biogeographic affinities of this island with the African continent, as discussed below, whereas no relationships with the Indian subcontinent can be evidenced. No information on larval biology and morphology are available, but we suppose that in this genus larvae are phoretic as all other genera of the tribe Nemognathini (Bologna & Pinto Citation2001).
The blister beetle fauna of Madagascar was monographically studied by Kaszab (Citation1965), who listed 12 species belonging to the genera Cyaneolytta Péringuey, Citation1909; Lydomorphus Fairmaire, Citation1882 (as Cylindrothorax Escherich, 1896); Meloe Linnaeus, 1758; Zonitis Fabricius, 1775; Zonitoschema Péringuey, Citation1909; Nemognatha Illiger, 1807; and Synhoria Kolbe, 1897. No additional taxa have been described or recorded from this island. All these genera are widely distributed in Africa and marginally also in India, but the Malagasy species, all endemic and mostly belonging to distinct lineages, show affinities with Afrotropical rather than with Indian groups of species.
Cyaneolytta caeruleata (Fairmaire, 1895) represents an isolated lineage (Kaszab Citation1953); the three species of Lydomorphus belong to the endemic group of rufopleuralis as defined by Kaszab (Citation1955b, Citation1965 as Cylindrothorax); Meloe chevrolati (Coquerel, 1851) belongs to the Afrotropical subgenus Afromeloe, revised by Bologna and Pinto (Citation1998), and shows relationships with species from eastern African mountains (Tanzania and Kenya) (Salvi et al., in preparation); as for the four species until now referred to Zonitis, the taxonomy is not resolved (Bologna & Molfini, in preparation), because all Afrotropical Zonitis actually belong to Palaestra or to possible new genera; Zonitoschema squalida (Fairmaire, 1899) seems to be related to an Afrotropical group of species (Bologna et al., in preparation); and Nemognatha proboscidea (Fairmaire, 1902) is virtually unknown, while Synhoria betsimisaraka Paulian, Citation1956 appears related to the southern African S. testacea (Fabricius, 1781) (Bologna, unpublished). Finally, Zonitodema madagascarensis seems related to Z. collaris from eastern and southern Africa.
Considering that the larvae of almost all Malagasy genera are adapted to phoresy (Bologna & Pinto Citation2001), their distribution in Madagascar can be explained by long-jump dispersal events by phoresy on bees from continental Africa. The isolation after dispersal of the ancestors and subsequent speciation explains the endemicity of all species and can support the hypothesis of relatively recent events of dispersion. The only exception could be that of those species currently ascribed to “Zonitis”, which have unclarified taxonomic definition and relationships, and could represent a new endemic genus originated by an older colonisation and isolation.
The only two problematic cases are those of Cyaneolytta and Lydomorphus. Larvae of several Afrotropical and Indian Cyaneolytta are phoretic on Coleoptera Carabidae (Bologna et al. Citation1990; Di Giulio et al. Citation2003), but this biological specialisation seems derived from a basal condition of phoresy on Apoidea, repeatedly evolved in Meloidae (Bologna & Pinto Citation2001). Actually, Selander (Citation1987) considered the triungulin of C. fryi (Wollaston, 1861) from West Africa to be phoretic (and parasitoid) on bees, and this supports the possibility that a similar larval biology could be typical also of the Malagasy Cyaneolytta. The larval biology and hosts of the genus Lydomorphus are unknown; larvae of only seven species are available to us (only one described by Bologna & Aloisi Citation1992) and all have a “lyttine” morphology, without specialisation to phoresy. Selander (Citation1991) placed this genus in the Meloini, presumably because he assumed larval phoresy due to the presence of the genus in Madagascar. This hypothesis is not demonstrated, but the possibility that derived biological conditions can be present in this genus is plausible. On the other hand, we could suppose a dispersion by flying in the adult stage, step by step, through the Mozambique Channel to Madagascar.
Our biogeographic hypothesis of a colonisation of Madagascar by dispersal for blister beetles (possibly through phoresy, as already proposed for other islands; Bologna & Marangoni Citation1990) fits with those already proposed to explain the biogeographic history of some Malagasy vertebrates (Samonds et al. Citation2013), which colonised this macro-island by dispersal through the Mozambique Channel partially during the late Miocene and mostly during the Plio–Pleistocene (e.g. Crocodylus; Hyppopotamus; bird species of Dicruridae, Motacillidae, Nectarinidae, Pycnonotidae etc.; some bats). In our opinion, the Meloidae dispersal could have occurred also by stepping stones through the Comoro archipelago or the Davie Ridge.
Acknowledgements
We thank the curator of Coleoptera of the Royal Museum of Central Africa (Tervuren, Belgium), who supported our study of the Meloidae collection of this institution during a Synthesis project.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Beauregard H. 1890. Les Insectes Vésicants. Paris: Alcan.
- Bologna MA. 1978. Alcuni Meloidi dell’Africa orientale e meridionale e descrizione di una specie nuova (Coleoptera, Meloidae). Quaderni dell’Accademia nazionale dei Lincei, Roma 243(1):137–189.
- Bologna MA. 1980. Recherches en Afrique de l’Institut de Zoologie de l’Université de L’Aquila (Italie). V. Coléoptères Meloidae du Kenya et de Tanzanie. Revue de Zoologie africaine 94:921–935.
- Bologna MA. 1990. Faunistica e zoogeografia dei Meloidae (Coleoptera) della Somalia. Biogeographia, (n.s.) 13:375–457.
- Bologna MA. 2000. Meloidae (Coleoptera: Tenebrionoidea). In: Kirk-Spriggs AH, Marais E, editors. Daures -biodiversity of the Brandberg Massif, Namibia. Vol. 9. Cimbebasia: Memoir. pp. 201–208.
- Bologna MA, Aloisi G. 1992. Systematics of Lydomorphus Fairmaire 1882, with a description of the first instar larva of L. dusaulti (Dufour 1821) (Coleoptera Meloidae). Tropical Zoology 5:55–71. DOI: 10.1080/03946975.1992.10539182.
- Bologna MA, Aloisi G, Vigna Taglianti A. 1990. Phoretic association of some African Cyaneolytta with Carabids, and morphology of first instar larvae in Meloini (Coleoptera, Meloidae). Tropical Zoology 3:159–180. DOI: 10.1080/03946975.1990.10539459.
- Bologna MA, Amore V, Pitzalis M. 2018. Meloidae of Namibia (Coleoptera): Taxonomy and faunistics with biogeographic and ecological notes. Zootaxa 4373(1):1–141. DOI: 10.11646/zootaxa.4373.1.1.
- Bologna MA, Marangoni C. 1990. Dispersal, dispersion and phoresy in blister beetles fauna (Coleoptera, Meloidae) of Eastern Mediterranean and other islands. Accademia nazionale dei Lincei, Atti Convegno dei Lincei, 85, “Biogeographical Aspects of Insularity”. pp. 345–366.
- Bologna MA, Pinto JD. 1998. A review of the Afrotropical species of Meloe Linnaeus, 1758 (Coleoptera Meloidae) with descriptions of first instar larvae, a key to species and an annotated catalogue. Tropical Zoology 11:19–59. DOI: 10.1080/03946975.1998.10539352.
- Bologna MA, Pinto JD. 2001. Phylogenetic studies of the Meloidae (Coleoptera), with emphasis on the evolution of phoresy. Systematic Entomology 26:33–72. DOI: 10.1046/j.1365-3113.2001.00132.x.
- Bologna MA, Pinto JD. 2002. The old World genera of Meloidae (Coleoptera): A key and synopsis. Journal of Natural History 36:2013–2102.
- Bologna MA, Turco F, Pinto JD. 2013. The Meloidae (Coleoptera) of Australasia: A generic review, descriptions of new taxa, and a challenge to the current definition of subfamilies posed by exceptional variation in male genitalia. Invertebrate Systematics 27:391–427. DOI: 10.1071/IS12054.
- De Moor FC. 1978. Botswana Meloidae (Coleoptera). Occasional papers of the national museums and monuments of Rhodesia. (B) Natural Sciences 6:80–127.
- Di Giulio A, Aberlenc HP, Vigna Taglianti A, Bologna MA. 2003. Definition and description of larval types of Cyaneolytta (Coleoptera Meloidae) and new records on their phoretic association with Carabidae (Coleoptera). Tropical Zoology 16:165–187. DOI: 10.1080/03946975.2003.10531193.
- Fairmaire L. 1882. Diagnoses de Coléoptères Abyssins. Le Naturaliste 4(9):68.
- Fairmaire L. 1883. Descriptions de Coléoptères nouveaux ou peu connus récoltés par M. Raffray en Abyssinie. Annales de la Société entomologique de France 3(6):89–112.
- Ferreira MC. 1965. Familia Meloidae; in Catalogo dos Coleopteros de Angola. Revista de Entomologia de Mocambique 8:788–817.
- François M. 2018. Description of a new species of Zonitodema Péringuey (Coleoptera: Meloidae), with synonymic notes on the genus. Israel Journal of Entomology 48:99–104. DOI: 10.5281/zenodo.2388809.
- Kaszab Z. 1953. Revision der Meloiden-Gattung Cyaneolytta Pér. (Col.). Annales Historico-Naturales Musei Nationalis Hungarici, (s.n.) 4:81–93.
- Kaszab Z. 1954a. Die aethiopischen Arten der Gattung Zonitis Fabr. (Coleoptera Meloidae). Revue de Zoologie et Botanique africaine 50:17–28.
- Kaszab Z. 1954b. Über die Arten der Meloiden-Gattung Zonitodema Péinguey (Coleoptera). Proceedings of the Royal Entomological Society of London, (B) 1954:191–196.
- Kaszab Z. 1955a. Contribution à l’étude de la faune entomologique du Ruanda-Urundi (Mission P. Basilewsky 1953). XIX. Coleoptera Meloidae. Annales du Musée Royale du Congo, Tervuren, 8° (Zoologie) 56:189–198.
- Kaszab Z. 1955b. Die Arten der Meloiden-Gattung Cylindrothorax Escher. (Coleoptera). Annales Historico-Naturales Musei Nationalis Hungarici, (s.n.) 6:225–258.
- Kaszab Z. 1956. Coleoptera Meloidae. In: Hanstrom B, Brinck P, Rudebeck G, editors. South African animal life. Results of the Lund University expedition in 1950–1951, Vol. 3. Stockholm: Almquist and Wiksell. pp. 273–294.
- Kaszab Z. 1960. Mission zoologique de l’I.R.S.A.C en Afrique orientale (P. Basilewsky et N. Leleup, 1957). Annales du Musée Royale du Congo, Tervuren, 8° 88:269–286.
- Kaszab Z. 1961. Über die von Dr. E. Haaf in Ost-Afrika gesammelten Meloiden, nebst Beschreibung einern neuen Coryna-Art aus Somaliland (Coeleoptera). Entomologische Arbeiten der Museum G. Frey 12:365–372.
- Kaszab Z. 1963. Studien über Meloiden (Coleoptera). Annales Historio-naturales Musei nationalis Hungarici, Pars Zoologica 55:335–346.
- Kaszab Z. 1965. Die Meloiden Madagaskars (Coleoptera). Acta Entomologica Musei nationalis Pragae 36:393–402.
- Paulian R. 1956. Les Meloidae malgaches (Coleoptera). Le Naturaliste Malgache 8:203–207.
- Péringuey L. 1909. Descriptive catalogue of the Coleoptera of South Africa. Family Meloidae. Transactions of the Royal Society of South Africa 1:165–297, 4 pls. DOI: 10.1080/00359190909520035.
- Samonds KE, Godfrey LR, Ali JR, Goodman SM, Vences M, Sutherland MR, Irwin MT, Krause DW. 2013. Imperfect isolation: Factors and filters shaping Madagascar’s extant Vertebrate Fauna. Plos One 8(4):e62086. DOI: 10.1371/journal.pone.0062086.
- Selander RB. 1987. Behavioral observations in Cyaneolytta and description of the triungulin larva of C. fryi (Coleoptera: Meloidae). Journal of the Kansas Entomological Society 60:288–304.
- Selander RB. 1991. On the nomenclature and classification of the Meloidae (Coleoptera). Insecta Mundi 5:65–94.